Abstract
Purpose
The purpose of the study was to evaluate the feasibility of integrating Community Health Workers (CHWs) as part of the team leading diabetes group visits.
Methods
This was a randomized controlled study that integrated CHWs as part of the team leading diabetes group visits for low-income Hispanic adults (n = 50). Group visits met for 3 hours each month for a 6-month duration. Main measures included baseline and 6-month clinical outcomes (ie, A1C, lipids), concordance with 8 standard of care guidelines (ie, screens for cervical, breast, and colon cancer) from the US Preventive Task Force and American Diabetes Association, and participant acceptability.
Results
Compared to control participants, the intervention group resulted in significantly better clinical outcomes or guideline concordance for the following areas: target A1C levels, retinal eye exams, diabetes foot exams, mammograms, and urine microalbumin. Significantly more individuals in the control group gained weight, whereas a greater number of participants in the intervention group lost weight. Intervention participants found the group visits highly acceptable.
Conclusions
Integrating CHWs as part a comprehensive diabetes group visit program is a feasible and effective system-level intervention to improve glycemic control and achieve guideline concordance.
The prevalence of diabetes is escalating, estimated to increase from 8 to 15 per 1000 persons by 2030.1 To account for such increases, more than 52 000 additional primary care providers will be needed by 2025.2 These trends largely impact the annual US expenditure for diabetes, which surged from $171 billion to $245 billion (41%) during the years 2007 to 2012.3 As rates of diabetes continue to climb, it will become more important to achieve optimal disease-related outcomes including glycemic control and guideline concordance.
Compelling evidence suggests that glycemic control and adhering to preventive care reduces the risk of sequelae in type 2 diabetes.1,4–7 The American Diabetes Association (ADA) and the United States Preventive Task Force (USPTF) have compiled a set of standards of diabetes and preventive care.5,6 In spite of the evidence behind these measures, multiple investigators have reported that clinical outcomes and guideline concordance is suboptimal in patients with type 2 diabetes.5,8,9
There are increasing pressures of health care value, efficiency, and performance that parallel the shortage of care primary care providers.2,4 These have resulted in some health care systems utilizing disease-specific programs such as diabetes group visits.9 The general medical community agrees that group visits consist of education, goal development, an integrated primary care visit (eg, referrals, physical examination, medication reconciliation), signed confidentiality agreements, and reimbursement for services for 3 to 30 patients.10 Though diabetes self-management education (DSME) classes are similar to group visits, they do not include an integrated primary care visit and do not necessarily contain signed confidentiality agreements.10
There is strong evidence for group visit efficacy including for glycemic control and guideline concordance in a range of ethnicities and settings.9–16 In a recent systematic review of 17 diabetes group visits, participants resulted in improved A1C levels (−0.55%) and systolic blood pressure (−5.2 mmHg) when compared to usual care.17 Similarly, another systematic review with a meta-analysis found similar A1C outcomes (−0.46%) for group visit participants.18 Further, several randomized controlled trials have found a positive impact on guideline concordance as a result of group visits.9,19
The promising outcomes of group visits in the Hispanic community may provide answers to a seemingly bleak picture. There are 1.7 million Hispanics with undiagnosed diabetes and 16.1 million with prediabetes, contributing to an estimated two-fold increase in Hispanics with diabetes by 2030.20 Hispanics have a 66% greater risk of obtaining diabetes, experience worse morbidity, and have a 50% increased mortality rate when compared to non-Hispanics whites.4 Upon diagnosis, many have already suffered from disease sequelae including nephropathy, retinopathy, and myocardial infarctions.20
Complicating health care for many Hispanics are socioeconomic issues. Research suggests that social and economic variables are key determinants of health.21 Hispanics living in the United States have less education, higher poverty rates, and less access to care than non-Hispanic whites.21 Gaps in health care remain evident from the health systems to the community and for low-income minorities, cultural barriers, and limited resources often broaden these gaps.22,23 These data suggest that current modalities are insufficient to manage diabetes for low-income Hispanics and that there is urgency for new, creative methodologies.21,24
A substantial body of literature has shown that Community Health Workers (CHWs) bridge gaps in health care by serving as liaisons from the patient to the system.22,25–31 There is increasing evidence of the importance of CHWs for underserved populations with diabetes including Hispanic communities.22,26,28,32–34 Investigators from a study for 3 large Hispanic communities at risk for diabetes found that individuals reduced body mass index (BMI) (−0.91 kg/m2) and waist circumference (−1.56 in) after a 6-month CHW Diabetes Prevention Program lifestyle intervention.35 A large randomized controlled trial (n = 164) with African American and Latino adults that evaluated a CHW-involved community based participatory research intervention resulted in a 0.8% decrease in A1C.36 Further, CHW interventions have been found cost-effective, revealed by a study with Hispanic adults with diabetes in Laredo, Texas, that showed $33 319/QALY gained as a result of their involvement.37
Investigators have delineated the importance of CHWs as part of the health care leadership team, suggesting that this is an essential foundation to achieve the role as a health promoter, obtain sustainable change, and equip those served to improve their health.38 Though there are suggestions of integrating CHWs as part of leadership teams,32 there are no reported studies of this intervention in diabetes group visits.
Purpose of the Study
To better understand the feasibility of integrating CHWs as part of the team leading a comprehensive diabetes group visit program, the authors conducted a randomized controlled feasibility study at a community clinic serving low-income Hispanics. Specifically, the study objectives included (1) evaluating the feasibility of integrating CHWs as part of the leadership team and (2) examining preliminary evidence of the efficacy to improve clinical outcomes and adhere to 8 ADA and USPTF guidelines. It was hypothesized that participants in the group visits (intervention) would find the program acceptable and have better clinical outcomes and guideline concordance when compared to those who received treatment as usual in the clinic (control).
Methods
Design and Intervention
A randomized controlled feasibility study with delayed control design among adult Hispanics receiving care through community clinics was utilized. For 6 months, the intervention group received 3-hour (Saturdays, 9 AM to 12 PM), monthly comprehensive diabetes group visits with CHWs integrated as part of the leadership team while the control group received treatment as usual in the clinic. After month 6, the control group received the intervention. Investigators randomized participants by block randomization to ensure equal sample sizes assigned to study arms.39
Integrating Community Health Workers as Leaders
A recent review demonstrated the importance of identifying potential CHW candidates with appropriate personality traits to decrease turnover.40 Taking this into account, the authors interviewed potential local Hispanic candidates with a commitment to working with underserved individuals and who had personality characteristics of flexibility, compassion, and determination. CHWs were recruited from the host site’s bilingual volunteers who live or work in the vicinity of the clinic. In the current study, CHWs were integrated as part of the team leading group visits in several areas including teaching large/small groups, meal planning, and scheduling.
CHW training
Published literature has consistently revealed the lack of ongoing CHW training.25,41,42 To ensure adequate support, the authors assisted CHWs in obtaining their Texas state certifications and with an academic institution affiliation (University of Texas, School of Public Health).43 During the study, CHWs received an orientation and ongoing training (n = 14 hours). The Texas Department of State Health Services approved trainings and materials, and CHWs sent post-training evaluations to them after each session.
The Principal Investigator of the study taught all CHW trainings using the text Living a Healthy Life With Chronic Conditions,44 an internationally recognized source targeting non–health care professionals (ie, CHWs) with strong evidence for efficacy.45 Its 19 chapters review topics including diabetes and behavioral strategies.45 This training was in addition to ad hoc access to the study physician.
CHW-participant contact in-between classes
Published studies have shown value of in-between visit monitoring for patients with diabetes.46,47 CHWs were assigned participants to contact each week in-between group visits. They called or sent text messages to patients regarding (1) weight loss (if indicated), (2) diet and medication adherence, and reminders (eg, appointments, assisting with transportation). Participants had the opportunity to ask questions or raise concerns, such as medication refills, so they would not have to wait until the next class to address them. This information was relayed to the study physician. The study physician contacted participants directly if clarifications or any urgent issue arose.
Participants
Investigators recruited participants from a growing, free 501(c) community clinic in southwest Houston with 98% Hispanic patients. The mean patient age at the clinic is 42.5 years, and one-third of patients have less than a high school diploma. To qualify for clinic services, an individual must be ≥150% of the federal poverty level and uninsured. Recruitment methods included provider and CHW referral, chart review, and word of mouth. Inclusion criteria included (1) Hispanic adults (≥18 years) and (2) a documented diagnosis of type 2 diabetes (ie, A1C ≥6.5%; 48 mmol/mol) or prediabetes (ie, A1C 5.7%-6.4%; 39-46 mmol/mol).5 Exclusion criteria included being a high-risk patient (eg, pregnancy, comorbidities requiring one-on-one provider attention).
Group Visit Structure
Table 1 illustrates the group visit structure and the educational topics. Labs and vitals were obtained (if applicable) on patient arrival. After, CHWs taught a 30-minute large group education based on Living a Healthy Life With Chronic Conditions.44 Then, the class divided into 3 for small groups and rotated every 30 minutes. At the end, the entire group gathered for a healthy meal. Small groups targeted: (1) medical management (ie, individual appointment with the physician), (2) social support (CHW-led to address physical barriers to health; ie, transportation, self-management skills), and mental health (CHW-led to address psychological barriers to diabetes; ie, anxiety or depression).
Table 1.
Agenda and Curriculum for the Study
| Time | Activity |
|---|---|
| Hour 1a | Obtain vitals, glucose check/labs (if indicated) |
| Hour 1b | CHW-led large group educational session |
| Hour 2a | Small group 1: Medical management |
| Hour 2b | Small group 2: Overcoming social barriers to care |
| Hour 3a | Small group 3: Overcoming psychological barriers to care |
| Hour 3b | Healthy meal and conclusions |
| Total | 3 hours per group visit, met monthly for 6 months |
| Monthly curriculum: Large group44 | |
| Month 1: Diabetes overview (chapters 1, 2, 18) | |
| Month 2: Healthy eating, preventive care (chapter 11) | |
| Month 3: Weight management, glucose control (chapters 12, 18) | |
| Month 4: Physical activity (chapters 6–8) | |
| Month 5: Improving communication with others about your disease (chapter 9) | |
| Month 6: Prevention of diabetes sequelae (chapters 13, 19) | |
Abbreviation: CHW, Community Health Worker.
Class size
Sample size recommendations for feasibility studies vary.48 The average size for studies involving continuous outcomes is 30 per group,49 though some suggest as few as 12.50 However, 30 per group has been proposed as a general guideline.51 In addition, published literature has shown high attrition rates in low-income settings.52,53 Accordingly, the authors aimed to recruit 60 participants (30 per group) for this feasibility study. With the exception of the large group education, participants would spend all of their didactic time in small groups, which would have a maximum of 10 participants if 100% recruitment and no attrition occurred. This allowed appropriate numbers for the study but individual attention for participants.
Other supplies
Intervention participants received a bathroom scale, glucometer, and a log to record daily weight, glucose, and medication adherence. The study physician determined the frequency/necessity of home glucose monitoring on an individual basis.5 Control subjects received bathroom scales and glucometers on request or if ordered by their health care provider in the clinic.
Patient consent
An informational meeting was held prior to the study to ensure that participants understood that they would receive health care in a group setting and obtain written consent. The need for individualized care is a recognized concern, and therefore the authors incorporated study designs from prior investigations.11,12,15 For example, the study physician met individually with patients at each visit and if subjects required more time or a private exam (eg, abdominal exam), they were scheduled for a one-on-one appointment. Medical concerns were directed to the study physician and not addressed by CHWs. The study received approval from Baylor College of Medicine Institutional Review Board.
Main Measures
Clinical outcomes
The authors obtained blood pressure and weight measurements at each visit. To collect weights, participants stood on a digital scale wearing no footwear and lightweight clothing. To obtain blood pressure values, the authors used an automatic digital monitor while participants were seated with their arm at a 90° angle. Investigators gathered baseline, 3-month, and 6-month A1C and lipid levels during the group visits. The clinic used this listed methodology for usual care. The authors obtained values from the control group by chart review. However, if control participants were not scheduled for a minimum of baseline and 6-month values, the authors made these appointments. Target A1C levels were not included for patients with prediabetes, but A1C data were measured to determine if any of these participants converted to diabetes during the study.
Concordance with guidelines
At month 6, the authors collected data on 8 standards of care per ADA and USPTF: (1) weight loss, (2) retinal screening, (3) comprehensive foot exam (ie, assessment of foot pulses, sensation, skin exam), (4) blood pressure, (5) urine microalbumin, and (6-8) cancer screening (breast, cervical, colorectal) by reviewing each individual’s medical record. Frequency and necessity of screening were determined per ADA and USPTF guidelines.5,6 Concordance was coded as a dichotomous yes or no.
Acceptability
At month 6, intervention participants completed a 10-point (1 = not, 5 = somewhat, 10 = extremely helpful) Likert scale survey created for the study to evaluate program acceptability. The survey evaluated participant impressions of CHWs as part of the group visit team, home measures (ie, bathroom scales), and receiving group health care. The authors defined acceptability as high (8-10), moderate (4-7), and low (1-3) based on published literature54 and totaled items to create an overall acceptability score (total range, 4-40).
Data Analyses
Analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina). Continuous variables were assessed for normality. For variables found to be non-normally distributed, appropriate nonparametric tests were used. Differences in baseline characteristics were conducted using t tests for continuous variables (ie, concordance) and chi-square tests for categorical variables (ie, weight loss). Mann-Whitney U and Wilcoxon two-sample tests were used for variables without normal distributions. Intention-to-treat analysis was used for missing clinical data using the most recent past value.
Linear mixed models (Proc Mixed) were used to evaluate treatment effects on change in A1C, BMI, blood pressure, and lipid levels. Treatment effects were examined by comparing each study arm at baseline and 6 months. Proc GLIMMIX was used for the non-normally distributed variables. A mean guideline score was computed using the number applicable for each participant as the divisor and adjusting for guidelines not applicable to all (ie, mammograms). Treatment acceptability data were analyzed by computing a total and item mean score and comparing to a priori established definitions of acceptability levels. Alpha was set at 0.05.
Results
A total of 132 individuals were approached to enter the study, of which 62 (46.9%) agreed to participate. Participants were randomized to the intervention (n = 31) or wait list control (n = 31) groups. A total of 11 participants (6 intervention, 5 control) did not show (ie, attended orientation only), and 1 became pregnant (control), leaving 25 participants in the intervention group (n = 21 female) and 25 participants in the control group (n = 19 female) (Figure 1). Retention rates were similar between study arms (intervention: 76%, control: 68%) (P = .75).
Figure 1.
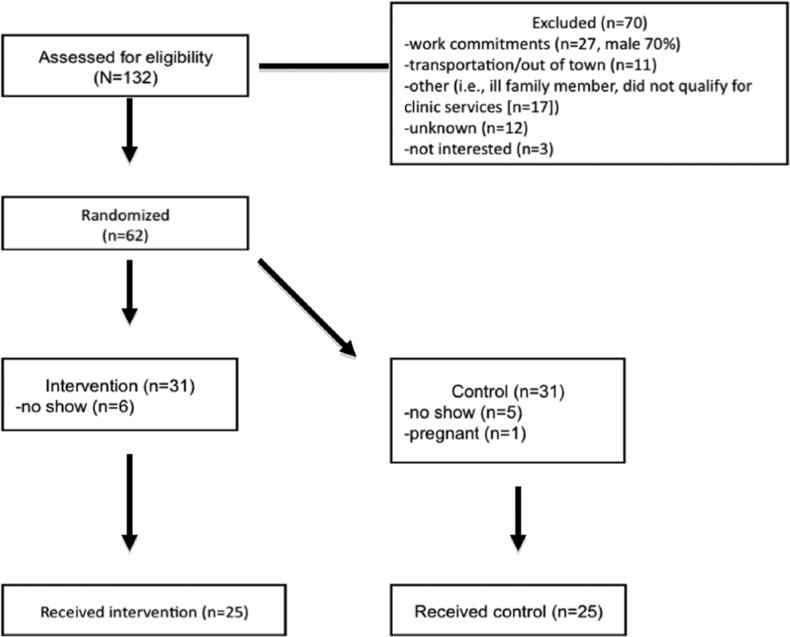
CONSORT protocol from a randomized controlled trial that accessed the feasibility of integrating Community Health Workers as part of the team leading a comprehensive diabetes group visit program (intervention) versus usual care (clinic visits-control) for low-income Hispanics.
There were no significant baseline differences between groups. This included age (intervention = 51.3 years, control = 48.0 years, P = .11), sex (intervention female: n = 21, control female: n = 19, P = .48), individuals with prediabetes (intervention: n = 4, control n = 9, P = .11), and treatment regimen: lifestyle modifications (intervention: 24.0%, control: 38.5%, P = .42), oral agents (intervention: 72.0%, control: 61.5%, P = .62), and insulin ± oral agents (intervention: 4%, control: 0.0%, P = .98). Similarly, there were no significant baseline clinical differences between groups including A1C (P = .57), lipids (total cholesterol: P = .56, HDL: P = .40, LDL: P = .13, triglycerides: P = .32), blood pressure (systolic: P = .42, diastolic: P = .57), and BMI (P = .47).
Clinical Outcomes
At 6 months, 57.1% (intervention) compared to 25.0% (control) achieved target A1C levels (P < .05). There were no other significant differences in clinical outcomes at 6 months. However, the intervention participants had more positive trends of clinical improvement than the control group. For example, the average BMI decreased for intervention participants, whereas it increased for the control group.
There were no individuals with prediabetes diagnosed with diabetes during the study in either group. Since 8% (2/25) of the control group compared to 80% (20/25) of the intervention group had data at 3 months, the authors did not compare groups at this interval. Table 2 illustrates the clinical outcomes by study arm.
Table 2.
Clinical Outcomes by Study Arm
| Variable | Intervention | Control | P |
|---|---|---|---|
| Achieved target A1C levelsa,b | 57.1% | 25.0% | <0.05 |
| A1C (%)a | |||
| Month 0 | 8.7 (±2.5) (72 mmol/mol) | 8.3 (±2.0) (67 mmol/mol) | .57 |
| Range | 5.4–14.2 | 6.1–13.0 | |
| Month 6 | 8.0 (±2.3) (64 mmol/mol) | 8.2 (±1.9) (62 mmol/mol) | .77 |
| Range | 5.8–12.5 | 5.8–12.4 | |
| Total cholesterol (mg/dL) | |||
| Month 0 | 192.2 (±34.1) | 185.6 (±39.1) | .56 |
| Month 6 | 187.2 (±41.1) | 180.6 (±31.2) | .55 |
| HDL cholesterol (mg/dL) | |||
| Month 0 | 47.0 (±10.8) | 44.6 (±6.0) | .40 |
| Month 6 | 48.2 (±12.3) | 43.7 (±7.8) | .14 |
| LDL cholesterol (mg/dL) | |||
| Month 0 | 111.7 (±28.7) | 99.0 (±26.4) | .13 |
| Month 6 | 108.7 (±35.5) | 97.8 (±24.0) | .22 |
| Triglycerides (mg/dL) | |||
| Month 0 | 171.0 (±100.6) | 186.3 (±76.9) | .32 |
| Month 6 | 166.9 (±81.9) | 195.6 (±95.0) | .30 |
| Systolic blood pressure (mmHg) | |||
| Month 0 | 134.8 (±17.0) | 132.2 (±21.0) | .42 |
| Month 6 | 131.9 (±25.1) | 128.8 (±15.8) | .89 |
| Diastolic blood pressure (mmHg) | |||
| Month 0 | 81.0 (±7.1) | 82.3 (±8.8) | .57 |
| Month 6 | 78.4 (±8.7) | 81.8 (±9.1) | .39 |
| Body mass index (kg/m2) | |||
| Month 0 | 33.0 (±5.8) | 34.3 (±5.9) | .47 |
| Month 6 | 32.2 (±5.2) | 34.6 (±6.7) | .40 |
Excludes patients with prediabetes.
A1C < 7% (53 mmol/mol); ≥65 years, A1C < 7.5% (58 mmol/mol).
Guideline Concordance
Guideline concordance by study arm is shown in Table 3. The intervention group resulted in better guideline concordance for: any weight loss (P < .01), retinal eye exam screening (P < .001), comprehensive foot exam (P < .001), urine microalbumin (P < .01), and mammogram screening (P < .01). The control group had a significant number of individuals who gained weight (P < .05). Groups were comparable in ≥5% weight loss (P = .68), target blood pressure (systolic: P > .999, diastolic: P = .56), and cervical (P = .08) and colorectal (P = .05) cancer screening.
Table 3.
Guideline Concordance by Study Arm
| Variable | Intervention (%) | Control (%) | P |
|---|---|---|---|
| Weight | |||
| ≥5% weight loss | 12.5 | 8.3 | .68 |
| Any weight loss | 88.0 | 48.0 | <.01 |
| Any weight gain | 8.0 | 36.0 | <.05 |
| Retinal eye exam | 90.5 | 13.3 | <.001 |
| Papanicolaou testing | 70.0 | 41.2 | .08 |
| Comprehensive foot exam | 57.1 | 0.0 | <.001 |
| Met target blood pressure | |||
| Systolic | 88.0 | 88.0 | >.999 |
| Diastolic | 96.0 | 92.0 | .56 |
| Urine microalbumin | 81.0 | 28.6 | <.01 |
| Mammography | 55.0 | 8.3 | <.01 |
| Colon cancer screening | 69.2 | 25.0 | .05 |
Acceptability
Intervention participants found the group visits highly acceptable with an average total score of 35.4 (SD = ±4.1) and an average item score of 8.8 (SD = ±1.0). The survey revealed high acceptability of CHWs as part of the group visit team (ie, calls in between the group visits) (mean = 9.7) and receiving health care in a group setting (mean = 10.0). The assistance of bathroom scales for weight loss and glucometers for glycemic control were moderately acceptable (mean = 8.0, 7.7, respectively).
Discussion
Type 2 diabetes is a progressive disease where system-level interventions are needed to improve clinical outcomes, minimize complications, and avoid preventable diseases.5,6,8,11,24 Findings support the feasibility of integrating CHWs into the diabetes group visit leadership team, an intervention that has not been reported to date. Though feasibility studies are not powered to assess efficacy,48 the authors found that a significant number of individuals in the intervention achieved target A1C levels, lost weight, and obtained screenings (retinal eye exam, foot exam, urine microalbumin, mammogram). The only significant finding for the control group was weight gain.
It is clear that glycemic control is critical to long-term outcomes in diabetes1,4,5 and that achieving target A1C levels is a pivotal marker, signifying disease sequelae risk reduction.5 Findings were similar to a 6-month feasibility study of rural African Americans where more group visit participants achieved target A1C levels (P < .05).55 Similarly, an urban African American study resulted in better target A1C levels in group visit individuals (P < .05).56 Several systematic reviews have also found that diabetes group visits have a positive impact on glycemic control.10,17,18
Findings of guideline concordance are also consistent with the literature. Two randomized controlled trials that evaluated group visits versus usual care found that the intervention group resulted in greater ADA and USPTF guideline concordance.9,19 A large body of evidence suggests the importance of preventive care for patients with diabetes.6,7 However, preventive care needs are difficult to address in traditional models due to the complicated nature of diabetes. The longitudinality of group visits and the involvement of CHWs in the current study allowed time to evaluate preventive care needs, follow up on orders, and provide structured didactics that include preventive care.
Furthermore, group visits are not only important for individuals already diagnosed with diabetes but also for those at risk. A growing body of literature, including the 2017 ADA guidelines, has revealed the importance of education for diabetes prevention.5,57,58 However, group visits have typically excluded individuals with prediabetes.16 By including those with prediabetes in the current study, susceptible individuals who have not succumbed to long-term sequelae received diabetes education and preventive care that may have been otherwise omitted.
Though multiple investigators have reported separately on efficacy of group visits and CHW involvement for diabetes self-management,9,14–16,28 the authors of the current study did not identify reported research that integrated CHWs as part of the diabetes group visit leadership team. In the current study, authors established several methods to integrate CHWs as part of group visit leadership that allowed participants to view them as well-respected, knowledgeable individuals. First, intense CHW job screening and obtaining state certifications resulted in improved retention. Also, ongoing trainings (n = 14 hours) were critical to establish a solid foundation in diabetes management, enabling CHWs to confidently teach participants. Examples of CHW integration included leading small groups, designing and preparing meals, and study recruitment.
Though other studies have utilized CHWs to follow-up with participants (ie, house calls),36 no reported studies detail a contact system for the duration of the study. However, investigators have showed improved outcomes from health care professionals, such as nurses, calling in between scheduled visits.59 Contacting patients weekly allowed CHWs to address participant concerns (eg, medication refills, scheduling reminders) immediately instead of waiting until subsequent group visits. This may explain why the intervention group resulted in superior guideline concordance.
Future Studies
There are several considerations that will be important for future studies. Due to the size of this feasibility study, there likely was not enough power to detect a difference in most clinical outcomes between groups. Also, 6 months is a short timeframe to assess clinical outcomes for a lifelong disease. To account for these issues, fully powered randomized controlled trials with longer longitudinal follow-up are needed.
Implications and Conclusions
With 415 million individuals suffering from diabetes worldwide,60 innovative changes in the current care models are imperative to avoid preventable disease sequelae and worsened stress on health care systems. This comprehensive group visit program that integrated CHWs as part the team leading group visits resulted in improved glycemic control and guideline concordance findings when compared to usual care. These data reveal that traditional models for diabetes are insufficient in addressing the complex needs of underserved minorities and highlight the potential of a system-level change with CHW group visit involvement.
Acknowledgments
National Institutes of Health. National Institute of Diabetes, Digestive, and Kidney Disorders. Federal Award Identification Number (FAIN) K23DK110341. Elizabeth Vaughan, DO, MPH (PI). The authors are grateful to the host clinic, the Community Health Workers, and the study participants. Presented at the North American Primary Care Research Group (NAPCRG) International Conference, Mexico, October 2015.
Footnotes
Conflicts of interest: None.
Contributor Information
Elizabeth M. Vaughan, Department of Medicine, Baylor College of Medicine, Houston, Texas.
Craig A. Johnston, Department of Health & Human Performance and the Texas Obesity Research Center, University of Houston, Houston, Texas.
Victor J. Cardenas, Department of Medicine, University of Texas Medical Branch, Galveston, Texas.
Jennette P. Moreno, Department of Pediatrics-Nutrition SDA/ARS Children’s Nutrition Research Center, Baylor College of Medicine, Houston, Texas.
John P. Foreyt, Department of Medicine, Baylor College of Medicine, Houston, Texas.
References
- 1.Centers for Disease Control and Prevention. Number of Americans with Diabetes Projected to Double or Triple by 2050. Atlanta, GA: Center for Disease Control; 2010. [Google Scholar]
- 2.Petterson SM, Liaw WR, Phillips RL, Rabin DL, Meyers DS, Bazemore AW. Projecting US primary care physician workforce needs: 2010-2025. Ann Fam Med. 2012;10(6):503–509. doi: 10.1370/afm.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care. 2012;36(4):1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. Atlanta: CDC; 2014. [Google Scholar]
- 5.American Diabetes Association. Standards of medical care in diabetes 2017. Journal of Clinical and Applied Research and Education. 2017;40(S1):S1–S134. [Google Scholar]
- 6.US Preventive Services Task Force. Final recommendations (screening) https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed October 6, 2017.
- 7.Stone NJ, Robinson J, Lichtenstein AH, et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2013 2013. http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437738.63853.7a. Accessed October 6, 2017.
- 8.Sriwijitkamol A, Moungngern Y, Vannaseang S. Attainment of American Diabetes Association clinical practice recommendations in 722 Thai type 2 diabetes patients. J Med Assoc Thai. 2011;94(1):S159–S167. [PubMed] [Google Scholar]
- 9.Clancy DE, Huang P, Okonofua E, Yeager D, Magruder KM. Group visits: promoting adherence to diabetes guidelines. J Gen Intern Med. 2007;22(5):620–624. doi: 10.1007/s11606-007-0150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke RE, OGrady ET. Group visits hold great potential for improving diabetes care and outcomes, but best practices must be developed. Health Affairs. 2012;31(1):103–109. doi: 10.1377/hlthaff.2011.0913. [DOI] [PubMed] [Google Scholar]
- 11.Clancy DE, Huang P, Okonofua E, Yeager D, Magruder MM. Group visits: Promoting adherence to diabetes guidelines. J Gen Intern Med. 2007;22(5):620–624. doi: 10.1007/s11606-007-0150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnett KG. Group medical visits: the future of healthcare? Glob Adv Health Med. 2015;4(6):6–7. doi: 10.7453/gahmj.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vachon GC, Ezike N, Brown-Walker M, Chhay V, Pikelny I, Pendergraft TB. Improving access to diabetes care in an inner-city, community-based outpatient health center with a monthly open-access, multistation group visit program. J Natl Med Assoc. 2007;99(12):1327–1336. [PMC free article] [PubMed] [Google Scholar]
- 14.Davis A, Sawyer DR, Vinci LM. The potential of group visits in diabetes care. Clin Diabetes. 2008;26(2):58–62. [Google Scholar]
- 15.Jones KR, Kaewluang N, Lekhak N. Group visits for chronic illness management. Nursing Economics. 2014;32(3):118–134. [PubMed] [Google Scholar]
- 16.Riley SB, Marshall ES. Group visits in diabetes care: a systematic review. Diabetes Educ. 2010;36(6):936–944. doi: 10.1177/0145721710385013. [DOI] [PubMed] [Google Scholar]
- 17.Edelman D, Gierisch JM, McDuffie JR, Oddone E, Williams JW. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30(1):99–106. doi: 10.1007/s11606-014-2978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185(13):E635–E644. doi: 10.1503/cmaj.130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clancy DE, Cope DW, Magruder KM, Huang P, Salter KH, Fields AW. Evaluating group visits in an uninsured or inadequately insured patient population with uncontrolled type 2 diabetes. Diabetes Educ. 2003;29(2):292–302. doi: 10.1177/014572170302900220. [DOI] [PubMed] [Google Scholar]
- 20.Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(1):6–12. doi: 10.1089/pop.2015.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morales LS, Lara M, Kington RS, Valdez RO, Escarce JJ. Socioeconomic, cultural, and behavioral factors affecting Hispanic health outcomes. J Health Care Poor Underserved. 2007;13(4):477–503. doi: 10.1177/104920802237532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia ME, Grant RW. Community Health Workers: a missing piece of the puzzle for complex patients with diabetes? J Gen Intern Med. 2015;30(7):878–879. doi: 10.1007/s11606-015-3320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peek ME, Cargill A, Huang ES. Diabetes health disparities. Med Care Res Rev. 2007;64(5):101S–156S. doi: 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Prato S, Giuseppe P, Miccoli R. Changing the treatment paradigm for type 2 diabetes. Diabetes Care. 2009;32(2):S217–S222. doi: 10.2337/dc09-S314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furino A. Community Health Worker National Workforce Study. Washington, DC: US Department of Health and Human Services; 2007. [Google Scholar]
- 26.Centers for Disease Control and Prevention. CDC’s Division of Diabetes Translation Community Health Workers/Promotores de Salud: Critical Connections in Communities. Atlanta, GA: Center for Disease Control; 2002. [Google Scholar]
- 27.Norris SL, Chowdhury FM, Van Le K, et al. Effectiveness of community health workers in the care of persons with diabetes. Diabet Med. 2006;23(5):544–556. doi: 10.1111/j.1464-5491.2006.01845.x. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Addressing Chronic Disease Through Community Health Workers: A Policy and Systems-Level Approach. 2nd. Atlanta, GA: Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 29.Colleran K, Harding E, Kipp BJ, et al. Building capacity to reduce disparities in diabetes: training community health workers using an integrated distance learning model. Diabetes Educ. 2012;38(3):386–396. doi: 10.1177/0145721712441523. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. Community Health Workers: What Do We Know About Them? Geneva: World Health Organization; 2007. [Google Scholar]
- 31.Perry H, Zulliger R. How Effective Are Community Health Workers? Baltimore, MD: Johns Hopkins University; 2012. [Google Scholar]
- 32.Shah M, Kaselitz E, Heisler M. The role of Community Health Workers in diabetes: update on current literature. Curr Diab Rep. 2014;13(2):163–171. doi: 10.1007/s11892-012-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmann U, Sanders D. Community Health Workers: What Do We Know About Them? The State of the Evidence on Programmes, Activities, Costs and Impact on Health Outcomes of Using Community Health Workers. Geneva: World Health Organization; 2007. [Google Scholar]
- 34.Collinsworth A, Vulimiri M, Snead C, Walton J. Community health workers in primary care practice: redesigning health care delivery systems to extend and improve diabetes care in underserved populations. Health Promot Pract. 2014;15(suppl 2):51S–61S. doi: 10.1177/1524839914539961. [DOI] [PubMed] [Google Scholar]
- 35.Ruggiero L, Oros S, Choi YK. Community-based translation of the diabetes prevention program’s lifestyle intervention in an underserved Latino population. Diabetes Educ. 2011;37(4):564–572. doi: 10.1177/0145721711411107. [DOI] [PubMed] [Google Scholar]
- 36.Spencer MS, Rosland AM, Kieffer EC, et al. Effectiveness of a Community Health Worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health. 2011;101(12):2253–2260. doi: 10.2105/AJPH.2010.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown HS, 3rd, Wilson KJ, Pagan JA, et al. Cost-effectiveness analysis of a community health worker intervention for low-income Hispanic adults with diabetes. Prev Chronic Dis. 2012;9:E140. doi: 10.5888/pcd9.120074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kane S, Kok M, Ormel H, et al. Limits and opportunities to community health worker empowerment: a multi-country comparative study. Soc Sci Med. 2016;164:27–34. doi: 10.1016/j.socscimed.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Suresh KP. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8–11. doi: 10.4103/0974-1208.82352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Kangovi S, Grande D, Trinh-Shevrin C. From rhetoric to reality—Community Health Workers in post-reform U.S. health care. N Engl J Med. 2015;372:2277–2279. doi: 10.1056/NEJMp1502569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spencer MS, Gunter KE, Palmisano G. Community Health Workers and their value to social work. Soc Work. 2010;55(2):169–180. doi: 10.1093/sw/55.2.169. [DOI] [PubMed] [Google Scholar]
- 42.Koskan AM, Friedman DB, Brandt HM, Walsemann KM, Messias DKH. Preparing promotoras to deliver health programs for Hispanic communities: training processes and curricula. Health Promot Pract. 2013;14(3) doi: 10.1177/1524839912457176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.University of Texas School of Public Health. Community Health Worker Certification/Continuing Education Program. 2017 https://sph.uth.edu/research/centers/tphtc/. Accessed September 15, 2017.
- 44.Lorig K, Holman H, Sobel D, Laurent D, Gonzalez V, Minor M. Living a Healthy Life With Chronic Conditions. 4th. Boulder, CO: Bull Publishing Company; 2012. [Google Scholar]
- 45.Stanford University. Chronic disease self-management program. 2017 http://patienteducation.stanford.edu/programs/cdsmp.html. Accessed September 15, 2017.
- 46.Hussein WI, Hasan K, Jaradat AA. Effectiveness of mobile phone short message service on diabetes mellitus management; the SMS-DM study. Diabetes Res Clin Pract. 2011;94(1):e24–e26. doi: 10.1016/j.diabres.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 47.Piette JD, Aikens J, Rosland AM, Sussman JB. Re-thinking the frequency of between-visit monitoring for patients with diabetes. Med Care. 2014;52(6):511–518. doi: 10.1097/MLR.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thabane L, Ma J, Cheng Ji, et al. A tutorial on pilot studies: the what, why and how. BMC Medical Research Methodology. 2010;10(1) doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Billingham SA, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med Res Methodol. 2013;13:104. doi: 10.1186/1471-2288-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Julious SA. Sample size of 12 per group rule of thumb for a pilt study. Pharmaceutica Stat. 2005;4:287–291. [Google Scholar]
- 51.Browne RH. On the use of a pilot sample for sample size determination. Stat Med. 1995;14(17):1933–1940. doi: 10.1002/sim.4780141709. [DOI] [PubMed] [Google Scholar]
- 52.Billingham SA, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med Res Methodol. 2013;13(104):1471–2288. doi: 10.1186/1471-2288-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuo CR, Quan J, Kim S, Tang AH, Heuerman DP, Murphy EJ. Group visits to encourage insulin initiation: targeting patient barriers. J Clin Nurs. 2017;26(11-12):1705–1713. doi: 10.1111/jocn.13577. [DOI] [PubMed] [Google Scholar]
- 54.Tariman JD, Berry DL, Halpenny B, Wolpin S, Schepp K. Validation and testing of the Acceptability E-scale for Web-based patient-reported outcomes in cancer care. Appl Nurs Res. 2012;24(1):53–58. doi: 10.1016/j.apnr.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bray P, Roupe M, Young S, Harrell J, Cummings DM, Whetstone LM. Feasibility and effectiveness of system redesign for diabetes care management in rural areas: the eastern North Carolina experience. Diabetes Educ. 2005;31(5):712–718. doi: 10.1177/0145721705280830. [DOI] [PubMed] [Google Scholar]
- 56.Reitz JA, Sarfaty M, Diamond JJ, Salzman B. The effects of a group visit program on outcomes of diabetes care in an urban family practice. J Urban Health. 2012;89(4):709–716. doi: 10.1007/s11524-012-9675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma J, Yank V, Xiao L, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173(2):113–121. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schillinger D, Wang F, Handley M, Hammer H. Effects of self-management support on structure, process, and outcomes among vulnerable patients with diabetes. Diabetes Care. 2009;32:559–566. doi: 10.2337/dc08-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.International Expert Committee. International Diabetes Federation Atlas. 2017 http://www.diabetesatlas.org. Accessed September 15, 2017.


