Abstract
Studying regeneration in animals where and when it occurs is inherently interesting and a challenging research topic within developmental biology. Historically, vertebrate regeneration has been investigated in animals that display enhanced regenerative abilities and we have learned much from studying organ regeneration in amphibians and fish. From an applied perspective, while regeneration biologists will undoubtedly continue to study poikilothermic animals (i.e., amphibians and fish), studies focused on homeotherms (i.e., mammals and birds) are also necessary to advance regeneration biology. Emerging mammalian models of epimorphic regeneration are poised to help link regenerative biology and regenerative medicine. The regenerating rodent digit tip, which parallels human fingertip regeneration, and the regeneration of large circular defects through the ear pinna in spiny mice and rabbits, provide tractable, experimental systems where complex tissue structures are regrown through blastema formation and morphogenesis. Using these models as examples, we detail similarities and differences between the mammalian blastema and its classical counterpart to arrive at a broad working definition of a vertebrate regeneration blastema. This comparison leads us to conclude that regenerative failure is not related to the availability of regeneration-competent progenitor cells, but is most likely a function of the cellular response to the microenvironment that forms following traumatic injury. Recent studies demonstrating that targeted modification of this microenvironment can restrict or enhance regenerative capabilities in mammals helps provide a roadmap for eventually pushing the limits of human regeneration.
Introduction
While regeneration refers to the replacement of a lost body part, the term also describes the repeated replacement of skin cells or shark’s teeth, the generation of a whole organism from part of an embryo, or in the broadest sense, the process of embryogenesis from a fertilized egg (the regeneration of an organism from a genome). These and other examples are classically typed as either physiological (homeostatic) or reparative regeneration, where physiological regeneration describes the regular replacement of cells and tissues during homeostasis and aging (e.g., epidermis, blood, shark’s teeth, etc.), and reparative regeneration occurs in response to injury (Morgan, 1901). Homeostatic regeneration is a ubiquitous property of vertebrates until that time when cells can no longer replace themselves and tissues and organs begin to fail. When considered in this context, all vertebrates exhibit a capacity for tissue regeneration. The enigma for regeneration biologists is that vertebrate species appear to be distributed along a continuum of reparative regenerative ability. As a biological problem, we still do not understand why some adult vertebrates can regenerate organs in response to damage while others heal similar injuries with scar tissue. Viewed as a clinical problem, although all tissues of the human body display some level of homeostatic regenerative ability, complex tissues and organs do not generally regenerate in response to injury. Can complex tissue regeneration be stimulated in non-regenerating species? These questions are the nexus of regenerative biology and medicine.
The cell is the basic unit of tissue regeneration. Accordingly, regeneration can occur at multiple levels of biological organization. In one dimension the smallest injury requires repair of a single cell (e.g., a severed axon). In another, severe trauma requires replacement of an entire organ de novo through the coordinated morphogenesis of millions of cells into distinct tissue types (e.g., a limb following amputation). Somewhere in between is the regeneration of variably complex structures with multi-cellular architecture and multiple functional units (e.g., ear punch, spinal cord resection, skeletal fracture, etc.). Regeneration in all of these examples is intimately tied to the regulated activation, coordinated growth, and differentiation of local cells, be they adult stem cells, or differentiated cells that undergo de-differentiation or reprogramming. How are resident cells activated and coordinated to regenerate organs?
Generally speaking, after blood loss is stemmed and an organism mounts defensive action from infection, local cells accumulate and produce new tissue. Among vertebrates, the process of regenerating a replacement organ in situ requires cell proliferation and is referred to as epimorphic regeneration (Morgan, 1901). This distinguishes it from regeneration that involves the reorganization of existing cells at the wound site to restore normal patterning prior to growth (i.e., morphallaxis). Appendage regeneration in salamanders and newts is the classic example of a complete and complex epimorphic response (Goss, 1969; Wallace, 1981). While amputating one lobe of the liver will also stimulate cell (hepatocyte) proliferation to restore organ function, the lobe itself does not regenerate. What distinguishes epimorphic regeneration from disorganized tissue regeneration is that a regenerating limb or tail forms from a transient proliferative mass called a blastema. While a similar response is not usually observed following appendage amputation in mammals, there do exist a few mammalian examples of epimorphic regeneration in which a blastema forms, and these can instruct the likelihood of enhancing regenerative capabilities in humans.
In this review we focus our attention on digit tip and ear hole regeneration as two examples of blastema-based epimorphic regeneration in mammals (Gawriluk et al., 2016; Joseph and Dyson, 1966; Muneoka et al., 2008). As described below, these two mammalian models present unique opportunities to discover the mechanisms that stimulate a regenerative response to injury in lieu of fibrotic healing. In focusing on the digit and ear pinna we do not discuss the broader regenerative abilities of the spiny mice primarily because full-thickness skin regeneration is not mediated by a blastema (Seifert et al., 2012; Seifert and Maden, 2014). Similarly, we have chosen to exclude the annual shedding and regrowth of deer antlers which represents an extreme example of physiological regeneration (Kierdorf et al., 2009). While some authors have suggested deer antler regeneration is blastema-mediated (Li, 2012) studies have shown that antler regeneration is nerve-independent and the antler bud displays characteristics that are distinct from an epimorphic blastema (Kierdorf et al., 2007; Kierdorf et al., 2009). Thus, while fascinating, in light of these differences and the fact that antler regeneration does not occur in response to injury, it is difficult to find parallels with other blastema-mediated regenerative responses in mammals. Ultimately, studying different types of regeneration reveals the diversity of regenerative processes. In our view, however, understanding the mechanisms that stimulate and regulate blastema formation is the key to moving from a deconstructive to constructive study of regeneration.
The vertebrate regeneration blastema
Several factors have emerged from the vertebrate regeneration literature as key components in all known examples of blastema-based epimorphic regeneration. These include: (1) formation of a specialized wound epidermis that functions to attract blastemal cells and maintain cell proliferation (Globus et al., 1980b; Thornton, 1957a; Thornton and Steen, 1962; Thornton and Thronton, 1965), (2) dependence on innervation and exposure to nerve or Schwann cell secreted factors (Farkas et al., 2016; Kumar et al., 2007; Mescher et al., 1997; Mullen et al., 1996; Singer, 1952), (3) formation of a pro-regenerative extracellular matrix (Calve et al., 2010; Gawriluk et al., 2016; Mailman and Dresden, 1979; Marrero et al., 2017; Onda et al., 1991; Satoh et al., 2012; Seifert et al., 2012; Tassava et al., 1996; Vinarsky et al., 2005), (4) deployment of major developmental signaling pathways (Stoick-Cooper et al., 2007), (5) physical interaction of cells from antonymic positions in three-dimensional space (Carlson, 1974; Cook and Seifert, 2016; Lheureux, 1975a, b), (6) recognition of uninjured versus new tissue and thus level-specific replacement of appropriate tissue to generate a complete organ and (7) a dependence on macrophages to initiate regeneration (Godwin et al., 2013; Petrie et al., 2014; Simkin et al., 2017). Together, these features contribute to, and support, blastema formation, without which regeneration will not occur. Against the backdrop of these features epimorphic regeneration involves two major transformations in response to injury: 1) mature tissue into a blastema and 2) a blastema into a regenerated organ. Viewed in this light, the blastema is the link between healing and morphogenesis.
How to broadly define a blastema?
Blastema: From Greek blastein – to sprout; ma – result of action
A blastema is a heterogeneous cell mass that through migration and proliferation transiently forms at the injury site and undergoes morphogenesis to form the missing organ. We use morphogenesis as it refers to organogenesis (i.e., organ development during embryonic development), but occurring in the adult form. The heterogeneous mass of cells is necessarily covered by epidermis (termed the wound epidermis) and thus the definition of a blastema includes this ectoderm-derived component. Historically, a vertebrate blastema was often defined as a mass of pluripotent cells where blastemal cells would contribute to all the of the regenerated structures. In reality, classic and modern lineage tracing techniques have shown that blastemal cells tend to respect developmental lineages during normal regeneration (Monaghan and Maden, 2013), although some evidence suggests that connective tissue fibroblasts over-contribute progeny to the blastema (Dunis and Namenwirth, 1977; Kragl et al., 2009; Muneoka et al., 1986). The blastema has also been classically defined by its developmental potential, i.e., what it transforms into distinguishes the blastema from any other mass of undifferentiated cells (e.g., tumor, granulation tissue, etc.). While this may be appropriate during development (nephrogenic blastema) or during salamander limb regeneration (limb blastema) where regeneration routinely occurs, this definition is problematic in approaching translational issues associated with regenerative failure. In this regard, it is important to modify this classic definition and try to define a blastema by functional attributes that are essential for establishing its’ developmental potential, rather than applying a descriptor of its ultimate developmental fate. A broad definition of this type allows us to identify key properties of a blastema that promote the transition from healing to morphogenesis and thus provides a basis for comparison across species or across distinct injury responses within a species. This conceptual framework is particularly useful when examining epimorphic regeneration in mammals where variation in regenerative ability exists between closely related species (ear pinna) and within an individual organ (digit). In these instances, the presence or absence of a blastema can help provide an explanation for regenerative success or failure.
In animals that possess regenerative capabilities, local amputation initiates the first transformation from mature tissue into a transient undifferentiated proliferative phase (blastema) that is followed by the second transformation where morphogenesis and re-differentiation replace the missing structures. The first transformation is a specialized wound healing response that ends with the formation of the blastema (Figure 1). This can be distinguished from a non-regenerative repair response where re-epithelialization is followed by reconstitution of a mature basement membrane, wound contraction, and the deposition of a densely layered fibrous scar tissue that defines regeneration-incompetence. During a regenerative response where the first transformation ends with the formation of a small mass of undifferentiated proliferating cells, the second transformation involves proliferative expansion this cell population, patterning, and ultimately the orderly differentiation of these cells into the multitude of cell types that make up the tissues of the replacement structure. In the context of regenerative failure, these two transforming events are generally viewed as distinct and independent biological problems. In other words, the study of mammalian regeneration involves two distinct and historically separate fields of inquiry: wound healing, which is largely focused on tissue healing that is non-regenerative and resolves in the formation of scar tissue, and tissue development, which is largely studied during embryogenesis. In regeneration competent models, the blastema sits at this interface making the study of its formation critical for understanding how regeneration is controlled.
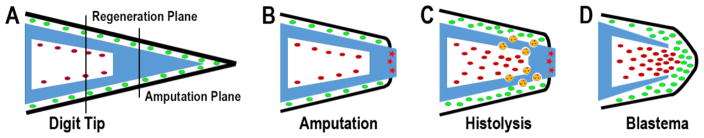
Figure 1. Schematic depicting formation of the regenerating mouse digit tip blastema.
(A) Cartoon of the mouse digit tip. The P3 bone (blue) is shown as triangular with a proximal base that contains a bone marrow region (white) and tapers to the distal tip. The bone marrow highly is vascularized (not shown) and hypocellular (red). Epidermis (black) and connective tissue cells (green) surround the P3 bone. Amputation of the digit tip does not damage the bone marrow. (B) Following amputation of the distal bone the epidermis heals onto the periosteum of the stump. The distal bone stump contains dead bone (red stars). (C) Histolysis of the stump is mediated by multinucleated osteoclasts (yellow) that erode the bone and creates a secondary amputation plane. This phase is associated with increased cell numbers in the connective tissue and bone marrow. (D) Once the secondary amputation is complete, the wound epidermis closes through the region of degraded bone and a blastema forms. Cells from the bone marrow and surrounding connective tissue participate in blastema formation. The P3 level that regenerates is proximal to the original level of digit amputation.
Whether or not morphogenesis is an emergent property of the blastema or something that must be stimulated is unknown. If the former, then discovering the ability to create a regeneration blastema may be the key to stimulating successful regeneration where it does not naturally occur (Goss, 1980). Focusing on the first transformation, it remains unresolved if pro-regenerative signals distinct from generalized injury signals exist to stimulate blastema formation (Sugiura et al., 2016; Tassava and Mescher, 1975). One way or another, the early response to injury during epimorphic regeneration involves acute inflammation that is concurrent with re-epithelialization and formation of the wound epidermis (Gauron et al., 2013; Godwin et al., 2013; Love et al., 2013; Mescher and Neff, 2005; Simkin et al., 2017). As the inflammatory response resolves, local histolytic activity, re-innervation, and extracellular matrix production facilitates the migration and accumulation of cells beneath the wound epidermis to form the nascent blastema (Calve et al., 2010; Singer, 1952; Vinarsky et al., 2005). In amphibians and fish, cells of the regenerating limb/fin are derived from multiple tissue types and lineage restricted progenitor cells have been clearly identified (Knopf et al., 2011; Kragl et al., 2009; Singh et al., 2012; Tu and Johnson, 2011). In addition to the variety of lineage restricted progenitor cells that make up the blastema, there is evidence that fibroblastic cells of the interstitial connective tissue are an important cell source for the blastema (Muneoka et al., 1986; Tank and Holder, 1979). The connective tissue of the dermis has been studied most extensively using cell markers coupled with skin transplantation. Such tissue grafting studies show that cells of the dermis over-contribute to the blastema, and that these cells participate in regenerating skeletal limb tissues (e.g., bone, cartilage, tendons) as well as re-forming the dermis (Dunis and Namenwirth, 1977; Kragl et al., 2009; Muneoka et al., 1986). Since there are a number of distinct cell types in dermal connective tissue, e.g., vascular, and perivascular cells, and heterogeneity among fibroblasts (Driskell et al., 2013; Rinkevich et al., 2015) it remains to be demonstrated whether one or all of these cell types are multipotent. Nevertheless, since there are no skeletal structures in the dermis and yet cells from the dermis can contribute to regenerated bone and cartilage, the evidence clearly indicates the existence of a multipotent cell type within the dermis. Unfortunately, the heterogeneity and plasticity of blastemal cells from tissue-specific lineages does not aid in the task of defining the blastema generally, rather, these cellular properties are a function of the specific tissue injured. Instead, a useful definition relies on the fact that some fraction of blastemal cells is stimulated to re-enter the cell cycle and undergo cell cycle progression and division (Globus et al., 1980a; Hay and Fischman, 1961; Tomlinson and Barger, 1987). While the persistence of active cycling cells can be used to identify the blastema, this alone hardly differentiates a blastema from a tumor. Therefore, the presence of proliferating cells must be used in combination with other factors. Given that uncontrolled growth is a characteristic of tumor cells and controlled growth is a characteristic of blastemal cells, comparative profiling of cycling tumor cells and blastemal cells may identify a panel of markers specific to cycling blastemal cells. Until these markers are found, however, one must look to other factors to uniquely define a blastema.
It has long been recognized that a wound epidermis forms atop the blastema after re-epithelialization and is required to direct growth of the blastema, maintain cell proliferation, and prevent premature differentiation (Globus et al., 1987; Globus et al., 1980b; Thornton, 1960). Studies that denude the blastema of the wound epidermis delay regeneration until re-epithelialization occurs again because the wound epidermis continually reforms after injury (Thornton, 1957b). In this regard, the wound epidermis is an integral part of the blastema and essential for successful regeneration. Once formed, the wound epidermis is further modified by the action of re-growing axons (Endo et al., 2004; Satoh et al., 2008). In salamanders and newts, innervation helps transition the new epidermis into a signaling center called the apical epithelial cap (AEC). This interaction with re-growing nerves is required for blastema formation and distinguishes the AEC from the neoepidermis that forms atop non-blastema-based regenerating skin wounds (Endo et al., 2004; Satoh et al., 2008). Several molecular markers have been identified that demarcate this specialized epidermis from epidermis located proximal to the injury or from neoepidermis covering tissue that does not form a blastema (Gawriluk et al., 2016; Han et al., 2001; Mullen et al., 1996; Satoh et al., 2008). In addition to molecular markers, the wound epidermis is morphologically distinct from the normally stratified epithelium typical of adult skin. As regeneration progresses reformation of the mature basement membrane beneath the wound epidermis is delayed and its absence is a useful indicator of the wound epidermis (Gawriluk et al., 2016; Neufeld and Day, 1996; Neufeld et al., 1996; Seifert et al., 2012). Although further molecular and functional characterization of the wound epidermis is necessary, current markers and morphological features can identify a wound epidermis as a fundamental component of the blastema.
Lastly, there is increasing evidence that the blastemal extracellular matrix is a key component of regeneration, although its role as a facilitator, regulator or passive support structure remains unclear (Calve et al., 2010; Gawriluk et al., 2016; Mailman and Dresden, 1979; Marrero et al., 2017; Onda et al., 1991; Satoh et al., 2012; Seifert et al., 2012; Tassava et al., 1996; Vinarsky et al., 2005). Early studies of salamander and newt blastemas detected increased levels of matrix proteins associated with cell migration and proliferation (e.g., fibronectin and tenascin-C) (Onda et al., 1991; Tassava et al., 1996). In vitro work examining newt myotubes on fibronectin, tenascin-c and hyaluronic acid showed these proteins could directly regulate cell behavior (Calve et al., 2010; Calve and Simon, 2012). More recent work in mammals (see below) suggests that the extracellular composition of the blastema can itself help define this transient structure when compared to non-regenerating tissues where a blastema does not form (Gawriluk et al., 2016; Marrero et al., 2017; Seifert et al., 2012). All told, while we have an incomplete definition of a blastema, the indicators outlined above do provide a conceptual foundation to identify pro-regenerative factors from cross-species and inter-injury comparisons.
Two Mammalian Blastemas – Digits and Ears
As we continue to discover fundamental mechanisms underlying a regenerative response to injury, the importance of studying natural examples of reparative regeneration in mammals is undeniable. Adult regeneration models in mammals, while not as spectacular as limb regeneration in urodeles, do afford researchers a comparative approach to understand basic principles of blastema formation and morphogenesis. To this end, comparing regenerating and non-regenerating injuries should help expand our ability to define the regeneration blastema. Moreover, putting phylogenetic considerations aside, important physiological differences between mammals and traditional regeneration models like salamanders, newts and zebrafish (e.g., homeothermy versus poikilothermy, high versus low metabolic rates, etc.) make it vital to understand how tissues can regenerate against the backdrop of mammalian physiology. As another example, although an innate immune response to injury is an ancient weapon against infection, it seems unlikely that this response is exactly the same in newts as it is in a mouse or human. Below we offer two models for epimorphic regeneration in mammals where steady progress is contributing to our understanding of blastema formation, its definition, and more broadly, to the differences between a regenerative or fibrotic response to injury.
Digit tip regeneration
The regeneration of human fingertips is well documented in the clinical literature (Illingworth, 1974) and parallels between digit tip regeneration in mice and fingertip regeneration in humans have peaked interest in the feasibility of human regeneration (Muneoka et al., 2008). The regenerating mouse digit has become an important experimental model to explore fundamental mechanisms of mammalian regeneration and to test strategies aimed at enhancing regenerative capabilities (Dawson et al., 2016). The mouse digit tip regenerates at all stages of development, including adults, and the process involves the formation of a transient blastema. The regeneration competent region consists of the terminal or third phalangeal element (P3), a structurally unique bone that has the shape of a flattened cone with a wide basal region that contains a bone marrow cavity and a tapered distal tip (Figure 2A–B). The second phalangeal element (P2) articulates with the base of P3 forming the P2/P3 joint (Figure 2B). The P3 element is encased by the nail organ which is required for the regenerative response (Takeo et al., 2013), and a thin layer of loose connective tissue consisting of fibroblasts, vasculature and nerves separate the P3 bone from the nail epidermis. Amputation through the distal half of P3 initiates the regenerative response, while amputation at the base of P3 undergoes a healing response with no evidence of regeneration (Neufeld and Zhao, 1995). After amputation, the continuously growing nail encases the regenerate, initially appearing as a truncated outgrowth before eventually conforming to the contour of the blastema (Figure 2C–E). Internal to the nail the regeneration response involves a series of interconnected and interdependent processes that transforms the differentiated stump tissues into a blastema that undergoes morphogenesis to form replacement tissues.
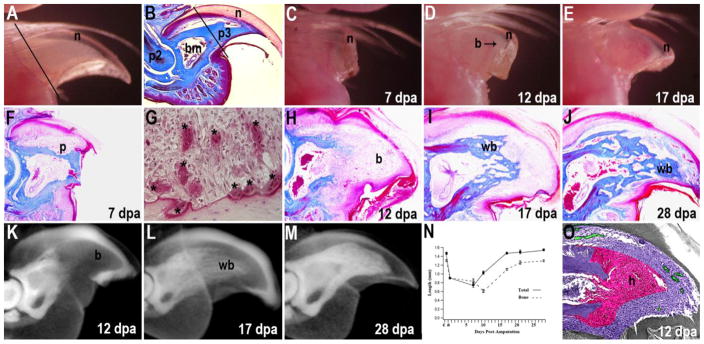
Figure 2. Digit tip regeneration.
(A) The distal region of the adult digit tip is comprised of the distal tip of the P3 skeletal element surrounded by a layer of connective tissue and the nail plate. The plane of amputation is indicated by the solid line. (B) Sagittal section through an unamputated P3 element shows the triangle shaped bone (p3) that contains a proximal bone marrow cavity (bm) and is enclosed by the nail (n). The P3 element articulates with the second phalangeal element (p2) at the P2/P3 joint. The plane of amputation is indicated by the solid line. (C) External view of a regenerating digit tip at 7 days post-amputation (dpa) showing distal elongation of the nail plate (n) with no remarkable change of the stump bone. (D) External view of a regenerating digit tip at 12 dpa showing outgrowth of a prominent digit blastema (b) surrounded by the elongating nail (n). (E) External view of a regenerating digit tip at 17 dpa showing a regenerate that has the general shape of the terminal phalanx. Vasculature associated with ossification (v) is apparent proximal to the distal blastema. (F) Mallory’s triple-stained section of a regenerating digit at 7 dpa showing the absence of wound closure and epidermal attachment to the lateral bone surface. Pitting of the stump bone surface (p) is observed at this stage. (G) TRAP staining of a 7 dpa regenerate identifies multinucleated osteoclasts (*) associated with the amputated stump bone. (H) Malory’s triple-stained section of a 12 dpa digit showing the distal undifferentiated blastema (b). (I) Mallory’s triple-stained regenerate at 17 dpa showing the distal stump with newly differentiated woven bone (wb) that is capped with undifferentiated blastema cells (b). (J) At 28 dpa the distal P3 element is regenerated with an interlacing network of new woven bone (wb) that is histologically distinct from the cortical bone of the stump. (K–M) μCT imaging of regenerating digits stained with a soft-tissue contrast agent (alcoholic iodine) to make the epidermis radio-opaque and enable analyses of soft tissue versus bone. (K) At 10 dpa the distal cortical bone is eroded and the bone marrow cavity is contiguous with the distal digit blastema (b). (L) At 17 dpa the regenerated woven bone (wb) appears as a loose network of bony tissue. (M) At 28 dpa the regenerated woven bone (wb) has a mottled appearance and is distinct from the cortical bone of the stump. (N) Graph showing the normalized longitudinal lengths of the digit tip (solid boxes) and the P3 bone (open boxes) during the regeneration process. The arrow highlights the transition between bone degradation, blastema formation and osteogenic differentiation of new bone. Both total digit and P3 length regenerates to normal levels within 3–4 weeks. (O) Hypoxic character of the blastema is shown in a colorimetric overlay of hypoxyprobe staining (pink, O2<1.3%) and oxygen-stabilized FBXL5 immunostaining (green, O2>6.0%) as compared to normoxic (purple) areas at 12 dpa. (P) Cartoon summarizing the dynamics of blastema formation in digit tip regeneration. The digit consists of the P3 bone (blue) with a hypocellular proximal marrow (white) surrounded by connective tissue (green) and epidermis (black). Following amputation of the distal bone the epidermis heals onto the periosteum of the stump. The distal bone stump contains dead bone (red stars). The bone stump is degraded by multinucleated osteoclasts (orange) creating a secondary amputation plane that removes the dead stump bone. This phase is associated with increased cell numbers in the connective tissue and bone marrow (red). Once the secondary amputation is complete, the wound epidermis closes through the region of degraded bone and a blastema forms. Cells from the bone marrow and surrounding connective tissue participate in blastema formation. The P3 level that regenerates is proximal to the original level of digit amputation. A, C–N from Fernando et al., 2011; B, from Han et al., 2018; O, from Sammarco et al., 2014.
Unlike some other regeneration models, the digit amputation wound does not undergo a rapid re-epithelialization response, instead the epidermis initially heals onto the lateral regions of the amputated stump bone and initiates a histolytic phase (Figure 1F) that is dominated by the recruitment of osteoclasts and the progressive degradation of the stump bone (Fernando et al., 2011; Simkin et al., 2015b). This histolytic phase correlates with the acute inflammatory response, and the recruitment of the monocyte/macrophage lineage cells that fuse to form large multinucleated osteoclasts (Lampiasi et al., 2016) that degrade the stump bone (Figure 2G). Once the stump bone is degraded, the epidermis migrates through the region of degraded bone that defines a new amputation level, and the blastema forms distal to the stump (Figure 2H). Wound closure over the stump tissue can be induced by use of a cyanoacrylic wound dressing, and in this case the bone degradation phase is inhibited and a small blastema forms distal to the original amputation (Simkin et al., 2015b). Alternatively, experimentally enhancing the period of bone degradation causes greater stump bone degradation and results in blastemas of a larger size (Sammarco et al., 2015). Thus, there appears to be a relationship between the extent of bone degradation and blastema size, suggestive of a relationship between tissue histolysis and progenitor cell availability. Blastema maturation is characterized by the onset of skeletal differentiation and progresses in a proximal to distal sequence (Figure 2H–J). The regenerating new bone forms by direct ossification with osteoblasts secreting osteoid in a pericellular manner to rapidly form woven bone. The use of μCT imaging has become an important tool to study the regeneration of bone during digit tip regeneration. Bone length and volume can be tracked and quantitated in vivo and soft tissue changes can be quantitated using contrasting reagents (Figure 2K–N).
The question of how the digit blastema forms is key to understanding both regenerative success and regenerative failure, and is an important step for addressing the question of why most mammals studied to date have limited regenerative capabilities. Since a blastema can form following digit tip amputation, it is reasonable to ask why a blastema does not form at other amputation levels. The digit blastema can be defined based on transiently expressed characteristics associated with its formation, and for a more stringent definition, based on a subset of characteristics that are experimentally shown to be functionally linked to successful regeneration. An approach of this kind reflects the general criteria outlined above while also potentially expanding our definition of the blastema. One example is the composition of the extracellular matrix (ECM) produced by blastema cells. Collagen 3 is a minor component of the digit prior to amputation, but becomes a prominent component of the blastema ECM before returning to baseline levels after differentiation (Marrero et al., 2017; Simkin et al., 2015b). The cells that produce collagen 3 have been identified as a subset of digit fibroblasts, called fibroblast reticular cells, and they display an enhanced proliferative response during blastema formation (Marrero et al., 2017). Collagen 3 production by digit fibroblasts is induced in vitro by multi-TNF receptor activation (Katakai et al., 2008; Marrero et al., 2017) suggesting that digit fibroblasts specifically react to inflammation by producing a provisional matrix that supports a regenerative response. Although functional studies on the role of collagen 3 in digit blastema formation are currently lacking, the data are consistent with the conclusion that a collagen 3 rich matrix supports mammalian regeneration. A more comprehensive analysis of the extracellular environment in regenerative-competent P3 amputations compared to regeneration-incompetent P2 amputations will help resolve how matrix proteins contribute to blastema formation.
A number of studies have identified major developmental pathways important for digit regeneration through functional analysis. These include multiple secreted factor signaling pathways including BMP (Yu et al., 2010), WNT (Lehoczky and Tabin, 2015; Takeo et al., 2013; Takeo et al., 2016), PDGF (Johnston et al., 2016), Oncostatin M (Johnston et al., 2016), CXCL12 (Lee et al., 2013), and VEGF (Yu et al., 2014). Some of these signaling factors are regulated in the blastema microenvironment and control cell proliferation, cell migration or cell differentiation (Lehoczky, 2016; Simkin et al., 2015a). A series of recent studies have identified interactions linking the inflammatory response, angiogenesis, and re-epithelialization to the control of oxygen availability during transformation from stump to blastema. Initial studies identifying the central region of the digit blastema as avascular echoed similar accounts from descriptive studies of the salamander limb blastema (Fernando et al., 2011; Mescher, 1996; Peadon and Singer, 1966; Said et al., 2004). The avascular character of the digit blastema correlates with reduced expression of Vegfa and up-regulated expression of the potent anti-angiogenic factor Pedf, during early regeneration stages in neonates (Muneoka et al., 2008; Yu et al., 2014). Experimental treatment of digit amputations with VEGF induces precocious angiogenesis and inhibits skeletal regeneration without inhibiting the formation of a blastema structure, indicating that the avascular nature of the blastema is a characteristic essential for regeneration (Yu et al., 2014). The region of digit blastema avascularity displays a low oxygen tension (<1.3%) indicating a hypoxic microenvironment (Figure 1O) and suggestive of a relationship between avascularity and hypoxia (Sammarco et al., 2014). When oxygen levels are experimentally elevated by hyperbaric oxygen treatment, the hypoxic state of the blastema is eliminated, and this was found to alter the regeneration response by extending the period of osteoclast-mediated bone degradation (Sammarco et al., 2015; Sammarco et al., 2014). While oxygen treatment does not enhance osteoclastogenesis, it does inhibit the termination of the osteoclast response, thus extending the period and extent of the bone histolytic phase. In classic models of bone degradation, osteoclastogenesis is inhibited by production of osteoprotegrin, a secreted decoy receptor that competes with and inhibits RANKL-RANK signaling that stimulates osteoclastogenesis (Chen et al., 2017). In osteoblasts, osteoprotegrin expression appears directly downstream of Hif2b activity which is regulated via the oxygen sensing activity of prolyl hydroxylase enzymes (Wu et al., 2015). These studies identify a dynamic process that involves regulating angiogenesis to establish a transient hypoxic blastema microenvironment that functions as a regulatory switch to transition the histolytic phase prior to blastema formation to the growth phase once the blastema has formed. It is interesting that the pro-angiogenic microenvironment of granulation tissue in full-thickness skin wounds causes excessive re-vascularization that is linked to scar formation (DiPietro, 2016; Wietecha et al., 2015). Thus, the evidence suggests that the regulation of this angiogenesis/oxygen/hypoxia cascade is a potential evolutionary target responsible for restricted regenerative capabilities in mammals, and also identifies a target pathway to enhance regenerative ability of regeneration incompetent wounds. The extent to which oxygen availability controls blastema formation in other examples of epimorphic regeneration awaits more general investigation.
One of the fundamental questions surrounding epimorphic regeneration responses concerns the source and potency of progenitor cells that form the blastema and contribute to the regenerate. As indicated above, while the identity of these cells may not help define a blastema per se, the source and plasticity of blastemal cells can reveal fundamental aspects of how cellular phenotypes are modified during regeneration. Lineage tracing studies in mice utilizing cell type specific promoter-driven reporter expression are a powerful test for multipotency during regeneration. Not surprisingly, the epidermis has been shown to be lineage restricted during digit tip regeneration as it is during salamander limb regeneration (Kragl et al., 2009; Rinkevich et al., 2011), and cell labeling studies show that cells derived from hematopoietic stem cells do not contribute to the major structural tissues of the regenerated digit (Rinkevich et al., 2011). Endothelial cells and osteoblasts contribute to the blastema and regenerated digit, and both are lineage-restricted with regard to their potency (Lehoczky et al., 2011; Rinkevich et al., 2011). The use of promoter-specific Cre expression coupled with a constitutively active reporter gene to track cell lineage has also been useful in determining whether specific cell types change phenotype during regeneration. Such studies show that Sox9-expressing skeletal cells, Scx-expressing tendon cells, and Tie2-expressing endothelial cells do not undergo transdifferentiation during regeneration (Rinkevich et al., 2011). While lineage studies have led to the general conclusion that the digit blastema is composed of only lineage restricted cells (Lehoczky et al., 2011; Rinkevich et al., 2011), this generalized conclusion must be weighed against the limited number of cell types that have been studied, and that the cell types contributing to the regenerated digit (epidermis, endothelial cells, osteoblasts) are lineage restricted during development. Indeed, with the exception of hematopoietic stem cells that do not contribute to regenerated structures, the contribution of more phenotypically labile cells such as mesenchymal stem cells or tissue-specific stem cells remain unknown. Current studies show that a panel of positive and negative markers is required to identify certain multipotent progenitor cells making it difficult to locate and trace these cells in vivo (Caplan and Correa, 2011; Gökçinar-Yagci et al., 2015). Nevertheless, currently available evidence indicates that a number of cell types participating in digit regeneration are lineage restricted.
Ear pinna regeneration
The regeneration of holes made through the ear pinna has long been recognized as another example of epimorphic regeneration in mammals (Goss and Grimes, 1972; Vorontsova, 1960). The external ear pinna is a complex organ comprised of skin, adipose tissue, skeletal muscle, vasculature, nerves, and elastic cartilage (Figure 2A–B). In some species, a complete hole punched through the pinna removes portions of all of these structures and initiates a regenerative response (Figure 2C–E). The phenomenon was first reported in 1953 with the observation that 1 cm holes made in rabbit ears completely closed with new tissue including new hair follicles, skin and cartilage (Markelova cited by Vorontsova and Liosner 1960). Ensuing investigations in the rabbit ear extended these observations and provided evidence that the type of epidermis (i.e., abdominal vs. ear) and presence of auricular cartilage were required for successful regeneration (Goss and Grimes, 1972; Grimes and Goss, 1972; Joseph and Dyson, 1966; Williams-Boyce and Daniel, 1980). Interestingly, semicircular wounds or notches made at the edges of the ear were reported to initiate, but not complete regeneration (Williams-Boyce and Daniel, 1980). More recently, work with two species of wild African spiny mouse (Acomys kempi and A. percivali) led to the discovery that these rodents could regenerate 4 mm holes in the ear pinna (Seifert et al., 2012) and subsequent research extended these findings to include another species, A. cahirinus (Gawriluk et al., 2016; Matias et al., 2015). Comprehensive studies in spiny mice and rabbits have detailed the regeneration of full-thickness skin, hair follicles, glands, adipose tissue, elastic cartilage, and to a limited degree, muscle fibers (Figure 2E) (Gawriluk et al., 2016; Joseph and Dyson, 1966; Matias et al., 2015). That some species can regenerate ear holes while others cannot make this model of epimorphic regeneration attractive to uncover mechanisms that stimulate blastema formation and differentiate a regenerative or fibrotic response to injury.
Superficially, the regenerative response follows the stereotypical pattern observed during other examples of vertebrate appendage regeneration. The initial trauma stimulates an injury response that recruits neutrophils and monocytes to the injury site, while the hemostatic response contributes to scab formation (Simkin et al., 2017). Shortly thereafter, wound edge keratinocytes are stimulated to migrate beneath the scab and re-epithelialize the injury (Gawriluk et al., 2016; Joseph and Dyson, 1966; Seifert et al., 2012). Once the epidermal barrier is restored, cells begin to accumulate centripetally beneath the neoepidermis and as this accumulation continues a regeneration blastema forms (Gawriluk et al., 2016; Joseph and Dyson, 1966). Cell cycle re-entry, progression and proliferation are observed in blastemal cells and a small group of epidermal cells at the proximal wound margins (Gawriluk et al., 2016; Seifert et al., 2012). As the ear hole is progressively filled, new tissue growth preferentially occurs in the proximal portion of the hole (Figure 2C–C′) and new tissue growth is coincident with axon regeneration (Gawriluk et al., 2016) and angiogenesis (Joseph and Dyson, 1966; Matias et al., 2015). Re-differentiation of new tissues occurs outside to inside such that new hair follicles and cartilage form nearer to the wound margin before they appear in the center (Figure 2C–D′) (Joseph and Dyson, 1966; Seifert et al., 2012). Hair follicle regeneration in spiny mice is visible twenty days post injury. As new tissue forms to close spiny mouse ear holes, elastic cartilage regeneration occurs at two positions. Similar to fracture healing, a callous forms at the cut end of the cartilage sheet and this appears to contribute some portion of the new cartilage. However, the majority of the new cartilage appears to emerge de novo from mesenchymal condensations within the new tissue (Seifert et al., 2012). Eventually, new tissue completely fills the hole and cellular differentiation restores the excised tissue. These interconnected and overlapping processes together constitute regeneration of a complex musculoskeletal structure. Importantly, provided holes larger than 2mm are made through the pinna, epimorphic regeneration of ear holes can be differentiated from fibrosis-driven partial ear hole closure observed in other rodents and laboratory mouse strains (Gawriluk et al., 2016).
Whereas the digit tip provides a model to study regenerative success and failure in the same organ (e.g., successful regeneration in P3 versus regenerative failure in P2), the ear hole model of epimorphic regeneration exploits interspecies variation in regenerative ability. In contrast to regeneration observed in rabbits and spiny mice, broad phylogenetic sampling shows that the ability to regenerate ear holes is restricted among mammals (Gawriluk et al., 2016; Goss, 1980; Williams-Boyce and Daniel, 1986). Ear hole regeneration may extend to chinchillas, pikas and cats, but other rodents so far studied are unable to regenerate ear holes. This raises the question as to how regeneration occurs in some mammalian species? With the discovery of regeneration in spiny mice, one approach has been to compare the regenerative response of the ear pinna to fibrotic repair of the same injury in other rodents. Comparing spiny mice to outbred Mus musculus showed that formation and persistence of a blastema during healing distinguishes the regenerative response from a scarring response (Gawriluk et al., 2016; Seifert et al., 2012). This distinction provides an opportunity to characterize a mammalian blastema and tease apart cellular and molecular processes that operate during these differential responses to injury.
Careful analysis of tissue regeneration in spiny mice has begun to help define cellular and molecular features of the ear blastema (Gawriluk et al., 2016; Matias et al., 2015; Seifert et al., 2012; Simkin et al., 2017). As indicated above, a key characteristic of the blastema is its epidermal compartment, the wound epidermis, which forms following re-epithelialization. As mesenchymal cells accumulate beneath the epidermis distal to the cut cartilage, epidermal cell proliferation is excluded from the wound epidermis and maintained in a small group of wound edge keratinocytes (Gawriluk et al., 2016). Basal keratinocytes in this distal epidermis maintain a circular morphology rather than assume the columnar morphology typical of normal stratified epidermis and the distal epidermis observed in mice during scarring. Importantly, keratin 17 is upregulated in the wound epidermis and expressed until the ear hole is filled with new tissue, a situation that does not occur during scarring where keratin 17 expression disappears by D15 post injury (Gawriluk et al., 2016). These features are coincident with a failure to reconstitute the lamina densa beneath the distal epidermis until hole closure is complete (Gawriluk et al., 2016; Seifert et al., 2012). Together, these features characterize the unique epidermal compartment of the blastema which persists until hole closure is completed and the circular blastema disappears. Further molecular characterization of the wound epidermis in spiny mice and other vertebrates will help refine our understanding of how this compartment regulates blastema formation and regeneration.
Cells accumulating beneath the wound epidermis constitute the mesenchymal compartment of the blastema, and another salient feature of the blastema is the unique composition of the extracellular matrix (ECM) that supports specific activities of blastemal cells during morphogenesis. Fibrotic ECM is dominated by collagen with a ratio favoring collagen 1 > collagen 3 (Gawriluk et al., 2016; Lo et al., 2012; Marrero et al., 2017; Seifert and Maden, 2014; Simkin et al., 2015b). Analysis of blastema ECM in comparison to matrix composition during fibrotic healing revealed a profound difference in the extracellular environment (Gawriluk et al., 2016; Seifert et al., 2012). While collagen 1 was produced during mammalian regeneration, it comprised only a small fraction of the blastemal ECM, which was instead dominated by fibronectin and tenascin-C among other matrix proteins (Gawriluk et al., 2016). Fibronectin and tenascin-C promote cell migration and proliferation, two cellular attributes required for morphogenesis during regeneration. Beyond the capacity of ECM proteins to physically interact with cells and stimulate behavior, the ECM also acts as a reservoir for growth factors, cytokines and signaling molecules. Although more work is required to fully characterize the blastemal ECM in spiny mice, work across regenerating vertebrates strongly supports ECM composition as a key marker of a regeneration blastema (Calve et al., 2010; Gawriluk et al., 2016; Marrero et al., 2017; Onda et al., 1991; Satoh et al., 2012; Seifert et al., 2012; Tassava et al., 1996). While comparative transcriptomics may suggest that the blastema extracellular environment has a unique molecular signature (Gawriluk et al., 2016), the extent to which known and novel matrix molecules are functionally required for blastema formation and morphogenesis awaits testing.
Epimorphic regeneration in an adult occurs in the context of a fully differentiated and operational immune system. Prior to blastema formation, local injury elicits an immune response. Although innate and adaptive immune defenses occur in both scarring and regenerating systems, recent work from mammals and other vertebrates suggests that aspects of these responses may positively regulate a regenerative response (Gauron et al., 2013; Godwin et al., 2013; Lai et al., 2017; Love et al., 2013; Petrie et al., 2014; Simkin et al., 2017). Comparing the inflammatory response during regeneration and scarring in spiny mice revealed that reactive oxygen species produced by NAPDH-oxidase at the injury site were significantly elevated during regeneration and these molecules persisted in elevated amounts during blastema formation (Simkin et al., 2017). This mirrors a proposed role for reactive oxygen species during tail regeneration in Xenopus and zebrafish (Gauron et al., 2013; Love et al., 2013) and brain regeneration in newts (Hameed et al., 2015). Macrophages are a key regulator of the local immune response and vertebrate studies suggests that they control inflammation, fibrosis and blastema formation during regeneration (Godwin et al., 2013; Petrie et al., 2014; Simkin et al., 2017). Depleting macrophages during normal fibrotic wound healing demonstrated they control the rate of re-epithelialization, fibrosis and cell proliferation (Leibovich and Ross, 1975; Mirza et al., 2009). Similarly, macrophages infiltrate the ear pinna and are present during regeneration and scarring. Depleting macrophages in the ear pinna prior to and during injury demonstrated a functional requirement for these cells during ear hole regeneration (Simkin et al., 2017). Interestingly, depleting macrophages delayed re-epithelialization and inhibited blastema formation, and when macrophages were allowed to infiltrate the ear pinna a blastema formed and regeneration ensued. Beyond the assessment that macrophages are generally required for epimorphic regeneration, some evidence suggests specific macrophage subtypes may differentially regulate this response to injury (Mescher, 2017; Simkin et al., 2017). Interestingly, when macrophage subtypes were analyzed during spiny mouse ear hole regeneration, classically activated (M1) macrophages were largely absent from the blastema (Simkin et al., 2017). This study left open whether the blastema triggered macrophage phenotype switching or instead certain subtypes were restricted from infiltrating the blastema. Further studies will need to determine the importance of particular macrophage subtypes and the extent to which these subtypes may regulate blastema formation either by directly stimulating local fibroblasts or indirectly by polarizing the immune response.
The identity and plasticity of progenitor cells that form the ear blastema is currently unknown. The mesenchymal compartment of the ear pinna develops entirely from Hoxa2-expressing cranial neural crest of the second pharyngeal arch, whereas the epidermal compartment is derived from ectoderm (Minoux et al., 2013). Whereas subsequent differentiation of these neural crest progenitors during external ear development is less well understood, Hoxa2 regulates BMP signaling, specifically Bmp5 and Bmp4, during cartilage development in the pinna (Minoux et al., 2013). While the prevailing view of the cellular contribution to the blastema is that lineage restricted cells will supply progenitors to a heterogeneous pool that will expand and differentiate into specific tissue subsets, what remains unknown is the degree to which lineage restriction applies to local cells that contribute to the ear blastema. For instance, if the shared cranial crest cell origin of all non-epidermal tissue structures in the ear pinna sets the plasticity limit during regeneration, then in theory, the pool of available cells for regeneration would extend to all mesenchymal cells of the ear. Similarly, if regeneration in the ear is dependent on reserves of adult stem cells rather than some form of de-differentiation then fewer populations would be necessary to replace all the missing tissues in the ear. A different question is whether the appropriate cells are present for regeneration, but they are simply unable to form a blastema. In this scenario, heterogeneous blastemal cells that normally contribute to regeneration in spiny mice are resistant to cell cycle re-entry, progression and division in non-regenerating mammals. Evidence to support the latter comes from research showing that while the number of Ki67+ cells in the ear during regeneration and scarring is similar, cell cycle progression and division is rarely observed during scarring (Gawriluk et al., 2016). This result was partly attributed to the observation that p21 and p27 were found throughout cell nuclei during scarring, but were absent from the blastema during regeneration. Importantly, the blastema in spiny mice was significantly enriched with markers for the G1/S transition (pRb), DNA replication (EdU) and mitosis (PHH3) which differentiated blastema cells from cells present during fibrotic healing in lab mice (Gawriluk et al., 2016). Understanding whether or not cell proliferation occurs in response to specific paracrine signals or results from an intrinsic ability to undergo cell cycle progression will be vital in discovering how spiny mice form an ear blastema and undergo regeneration.
Conclusions: Expanding the limits of regeneration
The continued study of epimorphic regeneration in mammals has much to add to our understanding of vertebrate regeneration biology. The ear hole model discussed above provides an experimental system to uncover key mechanisms that explain differences in regenerative ability across mammalian species. When applied more broadly, it may also help identify additional mammalian species that possess enhanced regenerative ability. As a comparative system between regenerating and non-regenerating species, the ear punch assay will reveal whether specific molecules or pathways can serve as early indicators for blastema formation and which factors alone or in combination are required to maintain blastema morphogenesis. This will be necessary if we hope to uncover potential targets that might be used to stimulate regeneration. In this light, both the ear hole and digit tip models offer an added translational perspective to the biology of regeneration. In digit studies, amputation of the mouse P2 digit has become a useful regeneration incompetent model to test strategies for inducing a regeneration response (Dawson et al., 2016). BMP signaling has been shown to be important for digit tip regeneration (Yu et al., 2010) and digit amputation at a P2 level is stimulated to regenerate a patterned response by targeted BMP2 treatment in both neonates and adult mice (Dawson et al., 2017; Yu et al., 2012). The BMP2 response indirectly induces the recruitment of cells to the amputation wound by activating CXCL12 production (Lee et al., 2013) and BMP2 functions as a mitogen for chondrocytes that establish an endochondral ossification center at the amputation wound (Dawson et al., 2017; Yu et al., 2012). Once formed, the induced endochondral ossification center organizes the morphogenesis of new distal skeletal tissue that replaces the amputated P2 bone. These studies provide proof of concept that a regenerative response can be stimulated at a regeneration incompetent amputation wound, and when combined with results from the ear leads to two important conclusions. First, progenitor cell availability is not a limiting factor at a mammalian amputation injury. Indeed, it seems unlikely that different progenitor cell populations would be present in the second and third phalanx of the digit or in the ear pinna from different rodent species. Second, limitation of the wound microenvironment to activate progenitor cells is in part responsible for regenerative failure. How exactly this activation is controlled remains the focus of current and future investigations. As studies using mammalian regeneration models progress they will continue providing insight into how injured tissue can be transformed into a regeneration blastema and how blastemal morphogenesis is controlled to appropriately replace injured tissue in situ. This will require dissecting components of the blastema to uncover new characteristics or markers that can indicate when these two transformations take place and using these markers to test approaches at successfully stimulating regeneration where it does not normally occur.
Figure 3. 4mm ear punch assay and epimorphic regeneration in the spiny mouse Acomys cahirinus.
(A) Position of a normal ear punch in an adult spiny mouse relative to the ear pinna along the proximodistal (p/d) axis. (B) Masson’s Trichrome stained tissue section of an uninjured spiny mouse ear pinna indicating: epidermis (e), dermis (d), adipose tissue (ad), skeletal muscle (m), and elastic cartilage (c). Hair follicles and sebaceous glands are visible in the dorsal and ventral skin. Scale bar = 50μm. (C–D) Whole mount images of regenerating ear holes at D30 (C) and D40 (D) post injury along the proximodistal (p/d) axis. In regenerating ears growth is biased to the proximal part of the hole. White circles indicate original 4 mm punch. (C′-D′) Sections through C and D showing the regeneration blastema (C′) and ear hole closure (D′). New hair follicles, dermis, adipose tissue and cartilage are visible at D40. Cartoon at right in C′ shows how tissue sections are collected. (E) A regenerated ear hole in Acomys cahirinus at D85 showing complete regeneration along the proximodistal and dorsoventral axes. New muscle fibers are visible past the proximal amputation plane. Dotted black lines indicate the injury place (C′-E). Image in E modified slightly from Gawriluk et al., 2016.
Highlights.
A unified definition of the vertebrate blastema is proposed
Epimorphic regeneration in the mammalian digit tip and ear pinna are reviewed
Outlines the importance of mammalian models for regenerative biology
Acknowledgments
We would like to thank Jennifer Simkin and two anonymous reviewers for comments and critical reading of the manuscript and Ricardo Zayas and Karen Echeverri for soliciting our review. A.W. Seifert is supported by the National Science Foundation (NSF) and the Office for International Science and Engineering (OISE) (IOS -1353713) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS – NIH) (R01AR070313). K. Muneoka is supported by Texas A&M University.
Footnotes
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Calve S, Odelberg SJ, Simon HG. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev Biol. 2010;344:259–271. doi: 10.1016/j.ydbio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calve S, Simon HG. Biochemical and mechanical environment cooperatively regulate skeletal muscle regeneration. The FASEB Journal. 2012;26:2538–2545. doi: 10.1096/fj.11-200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Correa D. The MSC: an injury drugstore. Cell stem cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BM. Morphogenetic interactions between rotated skin cuffs and underlying stump tissues in regenerating axolotl forelimbs. Developmental biology. 1974;39:263–285. doi: 10.1016/0012-1606(74)90239-5. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2017:1–9. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook AB, Seifert AW. Beryllium nitrate inhibits fibroblast migration to disrupt epimorphic regeneration. Development. 2016;143:3491–3505. doi: 10.1242/dev.134882. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Simkin J, Sauque M, Pela M, Palkowski T, Muneoka K. Analogous cellular contribution and healing mechanisms following digit amputaton and phalangeal fracture in mice. Regeneration. 2016;3:13. doi: 10.1002/reg2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson LA, Yu L, Yan M, Marrero L, Schanes PP, Dolan C, Pela M, Peterson B, Han M, Muneoka K. The periosteal requirement and temporal dynamics of BMP2-induced middle phalanx regeneration in the adult mouse. Regeneration. 2017:4. doi: 10.1002/reg2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro LA. Angiogenesis and wound repair: when enough is enough. J Leukoc Biol. 2016;100:979–984. doi: 10.1189/jlb.4MR0316-102R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, Watt FM. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunis DA, Namenwirth M. The role of grafted skin in the regeneration of x-irradiated axolotl limbs. Dev Biol. 1977;56:97–109. doi: 10.1016/0012-1606(77)90157-9. [DOI] [PubMed] [Google Scholar]
- Endo T, Bryant SV, Gardiner DM. A stepwise model system for limb regeneration. Dev Biol. 2004;270:135–145. doi: 10.1016/j.ydbio.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Farkas JE, Freitas PD, Bryant DM, Whited JL, Monaghan JR. Neuregulin-1 signaling is essential for nerve-dependent axolotl limb regeneration. Development. 2016;143:2724–2731. doi: 10.1242/dev.133363. [DOI] [PubMed] [Google Scholar]
- Fernando WA, Leininger E, Simkin J, Li N, Malcom CA, Sathyamoorthi S, Han M, Muneoka K. Wound healing and blastema formation in regenerating digit tips of adult mice. Dev Biol. 2011;350:301–310. doi: 10.1016/j.ydbio.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauron C, Rampon C, Bouzaffour M, Ipendey E, Teillon J, Volovitch M, Vriz S. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Scientific reports. 2013;3:2084. doi: 10.1038/srep02084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawriluk TR, Simkin J, Thompson KL, Biswas SK, Clare-Salzler Z, Kimani JM, Kiama SG, Smith JJ, Ezenwa VO, Seifert AW. Comparative analysis of ear-hole closure identifies epimorphic regeneration as a discrete trait in mammals. Nat Commun. 2016;7:11164. doi: 10.1038/ncomms11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globus M, Vethamany-Globus S, Kesik A. Control of blastema cell proliferation by possible interplay of calcium and cyclic nucleotides during newt limb regeneration. Differentiation. 1987;35:94–99. doi: 10.1111/j.1432-0436.1987.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Globus M, Vethamany-Globus S, Lee YC. Effect of apical epidermal cap on mitotic cycle and cartilage differentiation in regeneration blastemata in the newt, Notophthalmus viridescens. Dev Biol. 1980a;75:358–372. doi: 10.1016/0012-1606(80)90169-4. [DOI] [PubMed] [Google Scholar]
- Globus M, Vethamany-Globus S, Lee YCI. Effect of apical epidermal cap on mitotic cycle and cartilage differentiation in regeneration blastemata in the newt, Notophthalmus viridescens. Developmental biology. 1980b;75:358–372. doi: 10.1016/0012-1606(80)90169-4. [DOI] [PubMed] [Google Scholar]
- Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gökçinar-Yagci B, Uçkan-Çetinkaya D, Çelebi-Saltik B. Pericytes: properties, functions and applications in tissue engineering. Stem Cell Reviews and Reports. 2015;11:549–559. doi: 10.1007/s12015-015-9590-z. [DOI] [PubMed] [Google Scholar]
- Goss RJ. Principles of regeneration. Academic Press; New York: 1969. [Google Scholar]
- Goss RJ. Prospects for Regeneration in Man. Clinical orthopaedics and related research. 1980;151:270–282. [PubMed] [Google Scholar]
- Goss RJ, Grimes LN. Tissue Interactions in Regeneration of Rabbit Ear Holes. American Zoologist. 1972;12:151–157. [Google Scholar]
- Grimes LN, Goss RJ. Morphogenetic Influence of Cartilage in Regenerating Rabbit Ear Holes. American Zoologist. 1972;12:708–709. [Google Scholar]
- Hameed LS, Berg DA, Belnoue L, Jensen LD, Cao Y, Simon A. Environmental changes in oxygen tension reveal ROS-dependent neurogenesis and regeneration in the adult newt brain. Elife. 2015:4. doi: 10.7554/eLife.08422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MJ, An JY, Kim WS. Expression patterns of Fgf-8 during development and limb regeneration of the axolotl. Dev Dyn. 2001;220:40–48. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1085>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Hay ED, Fischman DA. Origin of the blastema in regenerating limbs of the newt Triturus viridescens. An autoradiographic study using tritiated thymidine to follow cell proliferation and migration. Dev Biol. 1961;3:26–59. doi: 10.1016/0012-1606(61)90009-4. [DOI] [PubMed] [Google Scholar]
- Illingworth CM. Trapped fingers and amputated finger tips in children. J Pediatr Surg. 1974;9:853–858. doi: 10.1016/s0022-3468(74)80220-4. [DOI] [PubMed] [Google Scholar]
- Johnston AP, Yuzwa SA, Carr MJ, Mahmud N, Storer MA, Krause MP, Jones K, Paul S, Kaplan DR, Miller FD. Dedifferentiated Schwann Cell Precursors Secreting Paracrine Factors Are Required for Regeneration of the Mammalian Digit Tip. Cell Stem Cell. 2016;19:433–448. doi: 10.1016/j.stem.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Joseph J, Dyson M. Tissue replacement in the rabbit’s ear. Br J Surg. 1966;53:372–380. doi: 10.1002/bjs.1800530415. [DOI] [PubMed] [Google Scholar]
- Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181:6189–6200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- Kierdorf U, Kierdorf H, Szuwart T. Deer antler regeneration: cells, concepts, and controversies. J Morphol. 2007;268:726–738. doi: 10.1002/jmor.10546. [DOI] [PubMed] [Google Scholar]
- Kierdorf U, Li C, Price JS. Improbable appendages: Deer antler renewal as a unique case of mammalian regeneration. Semin Cell Dev Biol. 2009;20:535–542. doi: 10.1016/j.semcdb.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Knopf F, Hammond C, Chekuru A, Kurth T, Hans S, Weber CW, Mahatma G, Fisher S, Brand M, Schulte-Merker S, Weidinger G. Bone regenerates via dedifferentiation of osteoblasts in the zebrafish fin. Dev Cell. 2011;20:713–724. doi: 10.1016/j.devcel.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–65. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318:772–777. doi: 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Marin-Juez R, Moura PL, Kuenne C, Lai JKH, Tsedeke AT, Guenther S, Looso M, Stainier DY. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. Elife. 2017:6. doi: 10.7554/eLife.25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampiasi N, Russo R, Zito F. The Alternative Faces of Macrophage Generate Osteoclasts. Biomed Res Int. 2016;2016:9089610. doi: 10.1155/2016/9089610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Marrero L, Yu L, Dawson LA, Muneoka K, Han M. SDF-1alpha/CXCR4 signaling mediates digit tip regeneration promoted by BMP-2. Dev Biol. 2013;382:98–109. doi: 10.1016/j.ydbio.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Lehoczky JA. Are fingernails a key to unlocking the puzzle of mammalian limb regeneration? Exp Dermatol. 2016 doi: 10.1111/exd.13246. [DOI] [PubMed] [Google Scholar]
- Lehoczky JA, Robert B, Tabin CJ. Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proc Natl Acad Sci U S A. 2011;108:20609–20614. doi: 10.1073/pnas.1118017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoczky JA, Tabin CJ. Lgr6 marks nail stem cells and is required for digit tip regeneration. Proc Natl Acad Sci U S A. 2015;112:13249–13254. doi: 10.1073/pnas.1518874112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- Lheureux E. Nouvelles donnees sur les roles de la peau et des tissues internes dans la regeneration du membre du triton Pleurodeles waltlii, Michah (amphibian urodele). Wilhelm Roux. Arch. 1975a;776:285–301. doi: 10.1007/BF00575322. [DOI] [PubMed] [Google Scholar]
- Lheureux E. Régénération des membres irradiés dePleurodeles waltlii Michah.(Urodčle). Influence des qualités et orientations des greffons non irradies. Wilhelm Roux’Archiv für Entwicklungsmechanik der Organismen. 1975b;176:303–327. doi: 10.1007/BF00575323. [DOI] [PubMed] [Google Scholar]
- Li C. Deer antler regeneration: a stem cell-based epimorphic process. Birth Defects Res C Embryo Today. 2012;96:51–62. doi: 10.1002/bdrc.21000. [DOI] [PubMed] [Google Scholar]
- Lo DD, Zimmermann AS, Nauta A, Longaker MT, Lorenz HP. Scarless fetal skin wound healing update. Birth Defects Research Part C: Embryo Today: Reviews. 2012;96:237–247. doi: 10.1002/bdrc.21018. [DOI] [PubMed] [Google Scholar]
- Love NR, Chen Y, Ishibashi S, Kritsiligkou P, Lea R, Koh Y, Gallop JL, Dorey K, Amaya E. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nature cell biology. 2013;15:222–228. doi: 10.1038/ncb2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman ML, Dresden MH. Denervation effects on newt limb regeneration: collagen and collagenase. Developmental biology. 1979;71:60–70. doi: 10.1016/0012-1606(79)90082-4. [DOI] [PubMed] [Google Scholar]
- Marrero L, Simkin J, Sammarco MC, Muneoka K. Fibroblast reticular cells engineer a blastema extracellular network during digit tip regeneration in mice. Regeneration. 2017;4:16. doi: 10.1002/reg2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias DS, Martins AR, Casanellas I, Ova AB, Araújo IM, Power D, Tiscornia G. Ear wound regeneration in the African Spiny mouse Acomys cahirinus. Regeneration. 2015 doi: 10.1002/reg2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL. The cellular basis of limb regeneration in urodeles. Int J Dev Biol. 1996;40:785–795. [PubMed] [Google Scholar]
- Mescher AL. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration. 2017 doi: 10.1002/reg2.77. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL, Connell E, Hsu C, Patel C, Overton B. Transferrin is necessary and sufficient for the neural effect on growth in amphibian limb regeneration blastemas. Dev Growth Differ. 1997;39:677–684. doi: 10.1046/j.1440-169x.1997.t01-5-00003.x. [DOI] [PubMed] [Google Scholar]
- Mescher AL, Neff AW. Regenerative capacity and the developing immune system. Adv Biochem Eng Biotechnol. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- Minoux M, Kratochwil CF, Ducret S, Amin S, Kitazawa T, Kurihara H, Bobola N, Vilain N, Rijli FM. Mouse Hoxa2 mutations provide a model for microtia and auricle duplication. Development. 2013;140:4386–4397. doi: 10.1242/dev.098046. [DOI] [PubMed] [Google Scholar]
- Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. The American journal of pathology. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan JR, Maden M. Cellular plasticity during vertebrate appendage regeneration. Curr Top Microbiol Immunol. 2013;367:53–74. doi: 10.1007/82_2012_288. [DOI] [PubMed] [Google Scholar]
- Morgan TH. Regeneration. The Macmillan Company; Macmillan & Co., ltd; New York, London: 1901. [Google Scholar]
- Mullen LM, Bryant SV, Torok MA, Blumberg B, Gardiner DM. Nerve dependency of regeneration: the role of Distal-less and FGF signaling in amphibian limb regeneration. Development. 1996;122:3487–3497. doi: 10.1242/dev.122.11.3487. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Allan CH, Yang X, Lee J, Han M. Mammalian regeneration and regenerative medicine. Birth Defects Res C Embryo Today. 2008;84:265–280. doi: 10.1002/bdrc.20137. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Fox WF, Bryant SV. Cellular contribution from dermis and cartilage to the regenerating limb blastema in axolotls. Dev Biol. 1986;116:256–260. doi: 10.1016/0012-1606(86)90062-x. [DOI] [PubMed] [Google Scholar]
- Neufeld DA, Day FA. Perspective: a suggested role for basement membrane structures during newt limb regeneration. Anat Rec. 1996;246:155–161. doi: 10.1002/(SICI)1097-0185(199610)246:2<155::AID-AR1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Neufeld DA, Day FA, Settles HE. Stabilizing role of the basement membrane and dermal fibers during newt limb regeneration. Anat Rec. 1996;245:122–127. doi: 10.1002/(SICI)1097-0185(199605)245:1<122::AID-AR17>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Neufeld DA, Zhao W. Bone regrowth after digit tip amputation in mice is equivalent in adults and neonates. Wound Repair Regen. 1995;3:461–466. doi: 10.1046/j.1524-475X.1995.30410.x. [DOI] [PubMed] [Google Scholar]
- Onda H, Poulin ML, Tassava RA, Chiu IM. Characterization of a newt tenascin cDNA and localization of tenascin mRNA during newt limb regeneration by in situ hybridization. Dev Biol. 1991;148:219–232. doi: 10.1016/0012-1606(91)90331-v. [DOI] [PubMed] [Google Scholar]
- Peadon AM, Singer M. The blood vessels of the regenerating limb of the adult newt, Triturus. J Morphol. 1966;118:79–89. doi: 10.1002/jmor.1051180106. [DOI] [PubMed] [Google Scholar]
- Petrie TA, Strand NS, Yang CT, Rabinowitz JS, Moon RT. Macrophages modulate adult zebrafish tail fin regeneration. Development. 2014;141:2581–2591. doi: 10.1242/dev.098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature. 2011;476:409–413. doi: 10.1038/nature10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 2015;348:aaa2151. doi: 10.1126/science.aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said S, Parke W, Neufeld DA. Vascular supplies differ in regenerating and nonregenerating amputated rodent digits. Anat Rec A Discov Mol Cell Evol Biol. 2004;278:443–449. doi: 10.1002/ar.a.20034. [DOI] [PubMed] [Google Scholar]
- Sammarco MC, Simkin J, Cammack AJ, Fassler D, Gossmann A, Marrero L, Lacey M, Van Meter K, Muneoka K. Hyperbaric Oxygen Promotes Proximal Bone Regeneration and Organized Collagen Composition during Digit Regeneration. PLoS One. 2015;10:e0140156. doi: 10.1371/journal.pone.0140156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammarco MC, Simkin J, Fassler D, Cammack AJ, Wilson A, Van Meter K, Muneoka K. Endogenous Bone Regeneration Is Dependent Upon a Dynamic Oxygen Event. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Graham GM, Bryant SV, Gardiner DM. Neurotrophic regulation of epidermal dedifferentiation during wound healing and limb regeneration in the axolotl (Ambystoma mexicanum) Dev Biol. 2008;319:321–335. doi: 10.1016/j.ydbio.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Satoh A, Hirata A, Makanae A. Collagen reconstitution is inversely correlated with induction of limb regeneration in Ambystoma mexicanum. Zoological science. 2012;29:191–197. doi: 10.2108/zsj.29.191. [DOI] [PubMed] [Google Scholar]
- Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys) Nature. 2012;489:561–565. doi: 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Maden M. New insights into vertebrate skin regeneration. International review of cell and molecular biology. 2014;310:129–169. doi: 10.1016/B978-0-12-800180-6.00004-9. [DOI] [PubMed] [Google Scholar]
- Simkin J, Gawriluk TR, Gensel JC, Seifert AW. Macrophages are necessary for epimorphic regeneration in African spiny mice. elife. 2017;6:e24623. doi: 10.7554/eLife.24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin J, Sammarco MC, Dawson LA, Schanes PP, Yu L, Muneoka K. The mammalian blastema: regeneration at our fingertips. Regeneration. 2015a;2:13. doi: 10.1002/reg2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin J, Sammarco MC, Dawson LA, Tucker C, Taylor LJ, Van Meter K, Muneoka K. Epidermal closure regulates histolysis during mammalian (Mus) digit regeneration. Regeneration. 2015b;2:14. doi: 10.1002/reg2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. The influence of the nerve in regeneration of the amphibian extremity. The Quarterly review of biology. 1952;27:169–200. doi: 10.1086/398873. [DOI] [PubMed] [Google Scholar]
- Singh BN, Doyle MJ, Weaver CV, Koyano-Nakagawa N, Garry DJ. Hedgehog and Wnt coordinate signaling in myogenic progenitors and regulate limb regeneration. Dev Biol. 2012;371:23–34. doi: 10.1016/j.ydbio.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007;21:1292–1315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Wang H, Barsacchi R, Simon A, Tanaka EM. MARCKS-like protein is an initiating molecule in axolotl appendage regeneration. Nature. 2016 doi: 10.1038/nature16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo M, Chou WC, Sun Q, Lee W, Rabbani P, Loomis C, Taketo MM, Ito M. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature. 2013;499:228–232. doi: 10.1038/nature12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo M, Hale CS, Ito M. Epithelium-Derived Wnt Ligands Are Essential for Maintenance of Underlying Digit Bone. J Invest Dermatol. 2016;136:1355–1363. doi: 10.1016/j.jid.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank PW, Holder N. The distribution of cells in the upper forelimb of the axolotl, Ambystoma mexicanum. J Exp Zool. 1979;209:435–442. [Google Scholar]
- Tassava R, Mescher A. The roles of injury, nerves, and the wound epidermis during the initiation of amphibian limb regeneration. Differentiation. 1975;4:23–24. doi: 10.1111/j.1432-0436.1975.tb01439.x. [DOI] [PubMed] [Google Scholar]
- Tassava RA, Nace JD, Wei Y. Extracellular matrix protein turnover during salamander limb regeneration. Wound Repair and Regeneration. 1996;4:75–81. doi: 10.1046/j.1524-475X.1996.40113.x. [DOI] [PubMed] [Google Scholar]
- Thornton CS. The effect of apical cap removal on limb regeneration in Amblystoma larvae. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology. 1957a;134:357–381. doi: 10.1002/jez.1401340209. [DOI] [PubMed] [Google Scholar]
- Thornton CS. The effect of apical cap removal on limb regeneration in Amblystoma larvae. J Exp Zool. 1957b;134:357–381. doi: 10.1002/jez.1401340209. [DOI] [PubMed] [Google Scholar]
- Thornton CS. Influence of an eccentric epidermal cap on limb regeneration in Amblystoma larvae. Developmental biology. 1960;2:551–569. doi: 10.1016/0012-1606(60)90054-3. [DOI] [PubMed] [Google Scholar]
- Thornton CS, Steen TP. Eccentric blastema formation in aneurogenic limbs of Ambystoma larvae following epidermal cap deviation. Dev Biol. 1962;5:328–343. doi: 10.1016/0012-1606(62)90017-9. [DOI] [PubMed] [Google Scholar]
- Thornton CS, Thronton MT. The regeneration of accessory limb parts following epidermal cap transplantation in urodeles. Experientia. 1965;21:146–148. doi: 10.1007/BF02141984. [DOI] [PubMed] [Google Scholar]
- Tomlinson BL, Barger PM. A test of the punctuated-cycling hypothesis in Ambystoma forelimb regenerates: the roles of animal size, limb innervation, and the aneurogenic condition. Differentiation. 1987;35:6–15. doi: 10.1111/j.1432-0436.1987.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Tu S, Johnson SL. Fate restriction in the growing and regenerating zebrafish fin. Dev Cell. 2011;20:725–732. doi: 10.1016/j.devcel.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinarsky V, Atkinson DL, Stevenson TJ, Keating MT, Odelberg SJ. Normal newt limb regeneration requires matrix metalloproteinase function. Developmental biology. 2005;279:86–98. doi: 10.1016/j.ydbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Vorontsova MaL, LD . Asexual propagation and regeneration. Pergamon Press; New York: 1960. [Google Scholar]
- Wallace H. Vertebrate Limb Regeneration. John Wiley and Sons; 1981. [Google Scholar]
- Wietecha MS, Krol MJ, Michalczyk ER, Chen L, Gettins PG, DiPietro LA. Pigment epithelium-derived factor as a multifunctional regulator of wound healing. Am J Physiol Heart Circ Physiol. 2015;309:H812–826. doi: 10.1152/ajpheart.00153.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Boyce PK, Daniel JC., Jr Regeneration of rabbit ear tissue. J Exp Zool. 1980;212:243–253. doi: 10.1002/jez.1402120211. [DOI] [PubMed] [Google Scholar]
- Williams-Boyce PK, Daniel JC., Jr Comparison of ear tissue regeneration in mammals. J Anat. 1986;149:55–63. [PMC free article] [PubMed] [Google Scholar]
- Wu C, Rankin EB, Castellini L, Alcudia JF, LaGory EL, Andersen R, Rhodes SD, Wilson TL, Mohammad KS, Castillo AB, Guise TA, Schipani E, Giaccia AJ. Oxygen-sensing PHDs regulate bone homeostasis through the modulation of osteoprotegerin. Genes Dev. 2015;29:817–831. doi: 10.1101/gad.255000.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Han M, Yan M, Lee EC, Lee J, Muneoka K. BMP signaling induces digit regeneration in neonatal mice. Development. 2010;137:551–559. doi: 10.1242/dev.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Han M, Yan M, Lee J, Muneoka K. BMP2 induces segment-specific skeletal regeneration from digit and limb amputations by establishing a new endochondral ossification center. Dev Biol. 2012;372:263–273. doi: 10.1016/j.ydbio.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Yan M, Simkin J, Ketcham PD, Leininger E, Han M, Muneoka K. Angiogenesis is inhibitory for mammalian digit regeneration. Regeneration (Oxf) 2014;1:33–46. doi: 10.1002/reg2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]