Abstract.
Pulmonary metastasectomy for osteosarcoma provides a select group of patients an opportunity for long-term survival and possible cure. Unfortunately, a complete metastasectomy is challenging due an inability to accurately identify lesions that lay below the threshold of preoperative imaging or intraoperative visual and tactile inspection. Growing evidence suggests that osteosarcomas express a number of unique molecular markers, including the folate receptor alpha. In this case report, we describe the application of a folate receptor-targeted, near-infrared optical contrast agent (OTL38) to improve osteosarcoma localization during minimally invasive pulmonary resection. In addition to localizing preoperatively identified lesions, this technology helped identify additional disease that was undetected on preoperative imaging or with traditional intraoperative techniques. This report marks the first successful utilization of a molecular imaging probe useful for osteosarcomas. This technology may provide a unique approach to improve pulmonary metastasectomy of osteosarcomas.
Keywords: osteosarcoma, surgery, intraoperative imaging, folate receptor alpha
1. Background
Osteosarcomas are rare tumors that are typically treated with a combination of surgical resection, chemotherapy, and possibly radiation.1,2 Even with this aggressive treatment protocol, distant metastases are common and ultimately result in patient morbidity or death. For a small group of osteosarcoma patients, the lung represents the single site of metastasis. Within this cohort, pulmonary metastasectomy can eliminate all metastatic disease, thus providing an opportunity for long-term survivorship and possible cure.1,2
To provide complete pulmonary metastasectomy for patients with osteosarcoma, open approaches have traditionally been utilized, which allows the surgeon to assess for additional lesions by visualization and palpation. Although minimally invasive pulmonary resection provides advantages over open approaches, limited visualization and tactile feedback can make localization of metastases challenging.3
Several groups have proposed utilizing targeted, near-infrared (NIR) fluorescent contrast agents for real-time intraoperative detection of pulmonary lesions during minimally invasive pulmonary resection. For example, our group has recently reported phase I results involving a NIR folate receptor- ()-targeted probe, OTL38.4,5 In these initial reports, we have found that of primary lung cancers accumulate OTL38 and generate tumor fluorescence during minimally invasive pulmonary resection. In addition to reproducibly detecting nodules as small as 3 mm, this approach has allowed us to find occult cancers in 12% of patients enrolled.
Interestingly, recent evidence suggests that osteosarcomas express a number of unique molecular markers, one of which is the .6,7 Given previous first-in-human successes with OTL38 and the growing data suggesting expression of in up to 80% of primary osteosarcomas, we hypothesized that molecular imaging with OTL38 could improve lesion localization during pulmonary metastasectomy for osteosarcoma. In this proof-of-principle case report, we describe the application of fluorescence-guided surgery with OTL38 in a 24-year-old male who presented for resection of an osteosarcoma metastasis to the lung.
2. Clinical Summary
A 24-year-old male with history of a left humerus osteosarcoma, 4 years status-post resection followed by chemotherapy (doxorubicin and methotrexate), was seen in our multidisciplinary thoracic oncology clinic for evaluation of left lower lobe (LLL) lesion. Preoperative chest CT demonstrated a 1.4-cm nodule in the LLL that was suspicious for a pulmonary metastasis given his history of osteosarcoma [Fig. 1(a)]. There were no other nodules appreciated. Given concern for malignancy, he was consented for a wedge resection via video-assisted thoracic surgery (VATS).
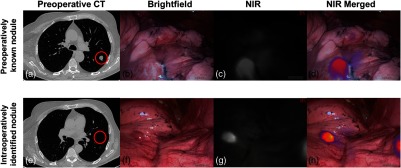
Fig. 1.
Osteosarcoma metastases display real-time fluorescence upon molecular imaging with OTL38. (a) Preoperative CT was suggestive of a 1.4-cm osteosarcoma metastasis of the LLL. Representative real-time images of the LLL nodule upon (b) brightfield imaging, (c) near-infrared imaging, and (d) merged near-infrared imaging. In addition to the known nodule, a 0.4-cm metastasis was identified in the major fissure. (e) This lesion was not identified on preoperative CT. Representative (f) brightfield, (g) near-infrared, and (h) merged images demonstrating high fluorescence of the additional lesion are provided. Areas of pulmonary nodules are gated with a red circle.
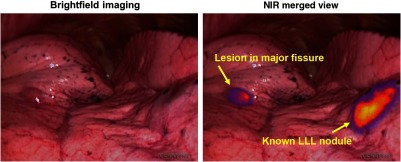
After providing informed consent to participate in a study approved by the University of Pennsylvania Institutional Review Board, the subject received intravenous OTL38 () 4 h before undergoing VATS resection. During surgery, the known LLL lesion displayed high levels of fluorescence upon in situ NIR imaging with an optimized NIR thoracoscope (Iridium; Visionsense, Philadelphia, Pennsylvania), [Figs. 1(b)–1(d) and 2 (Video 1)]. In addition to the known LLL nodule, an additional 0.4-cm nodule was also visualized within the major fissure [Figs. 1(e)–1(h) and 2 (Video 1)]. Both nodules were wedge resected using real-time fluorescence feedback. Of note, in situ tumor-to-background fluorescence ratios of the known LLL lesion and the major fissure lesion were 2.9 and 3.0, respectively.
Fig. 2.
Osteosarcoma metastases display in situ fluorescence during intraoperative molecular imaging. Four hours after delivery of OTL38, osteosarcoma metastases display fluorescence during real-time molecular imaging. This technology may help surgeons localize nodules found by preoperative imaging or identify additional small disease deposits which are undetectable using standard imaging/intraoperative modalities. (Video 1, 18.1 MB, mp4 [URL: http://dx.doi.org/10.1117/1.JBO.23.1.016005.1]).
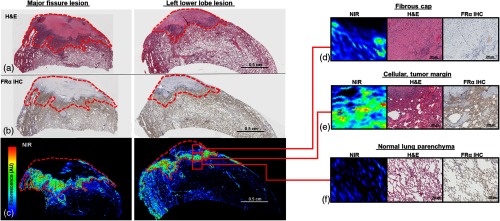
After histopathologic review, both nodules were confirmed to be osteosarcoma metastases. Upon immunohistochemistry, we appreciated moderate levels of expression within acellular portions of the tumor; however, we observed high levels within the highly cellular portions of the tumor near the margin (Fig. 3). Using an NIR microscopic scanner (Odyssey, Li-Cor), we confirmed that areas of high expression correlated with highest levels of contrast agent accumulation and fluorescence.
Fig. 3.
Osteosarcomas accumulate OTL38 in areas of high expression: the major fissure and LLL lesions were evaluated (a) by hematoxylin and eosin staining, (b) by immunohistochemistry, and (c) by microscopic near-infrared scanning. (d) Moderate dye accumulation was appreciated in acellular portions of tumor where expression was limited; (e) however, strong signal was appreciated in the cellular tumor margin, where was highly expressed. Although was present in the normal lung parenchyma, expression was primarily on the apical cell surface resulting in low dye accumulation (f). Tumors outlined in red.
The subject recovered uneventfully without signs of drug toxicity and remains disease free after 1 year of follow-up.
3. Discussion
In this report, we demonstrate feasibility and utility of NIR molecular imaging using the -targeted agent, OTL38, in a patient undergoing pulmonary metastasectomy for osteosarcoma. In addition to localizing the preoperatively identified nodule, this technology assisted in the identification of an additional 0.4-cm metastasis during minimally invasive pulmonary resection. Similar approaches have been implemented for soft tissue sarcomas;8,9 however, this proof-of-principle report supports additional studies exploring this approach for osteosarcomas and potentially other -expressing malignancies.
OTL38 is an optical contrast agent consisting of a modified folate molecule (ligand to the ), which is linked to an NIR fluorophore; OTL38 excites and emits in the NIR range ( 774 and 795).10 In our previous work, we have demonstrated that OTL38 binds the folate receptor with a high affinity, thus allowing for identification of synchronous disease in of lung cancer patients and reproducible detection of subcentimeter nodules.4,5 Based on our initial successes, we have hypothesized that this approach would be applicable to other pulmonary malignancies that express the ; however, the presented data herein are the first data to support this principle.
Osteosarcomas metastases to the lung are an excellent disease to explore additional indications for -targeted molecular imaging for several reasons. Most obviously is a lack of alternative intraoperative adjuncts to allow for localization of known lesions and identification of additional disease deposits. As demonstrated in this case, this technology assisted in localization of the known LLL nodule and highlighted a second nodule which was radiographically occult. Next, despite many limitations of thoracotomy (open surgery), many surgeons advocate for thoracotomy during metastasectomy as it theoretically allows for improved visualization and ability to palpate the lung. As we demonstrate here, utilization of OTL38 may provide supplemental information that allows for detection of small or hard to locate nodules during minimally invasive resection.
Interestingly, upon histopathologic review we noted that expression was heterogenous in the fluorescent areas of the tumor. This expression pattern is similar to our observations involving molecular imaging with OTL38 for pulmonary adenocarcinomas, and we have previously noted that tumor fluorescence is present even when as little as 20% to 30% of tumor expresses .5,11 As we continue enrollment of patients with osteosarcoma metastases in clinical trials, we will better understand how this expression pattern impacts the reproducibility of in vivo tumor fluorescence during pulmonary metastasectomy.
Although additional data are required before drawing final conclusions, we find these results encouraging for several reasons. First, the drug was safely delivered and no toxicity was observed. This is consistent with the low toxicity profile reported with other targeted NIR imaging probes.10,12 Next, the subcentimeter sensitivity observed suggests that this technology may ultimately enhance oncologic resections by identification of small cancer deposits which are often challenging to locate based on preoperative imaging or by visualization/palpation alone. Finally, utilization of this technology was useful during minimally invasive pulmonary resection (VATS) and thus may provide a real-time, intraoperative adjunct to conventional minimally invasive surgery.
In summary, we present the first human application of molecular imaging for identification of pulmonary metastases for patients with osteosarcoma. We found this approach safe, highly sensitive, and useful during minimally invasive pulmonary resection. In the future, this technology may enhance the surgeon’s ability to perform a variety of oncologic procedures, including tumor localization, margin assessment, and intraoperative staging. At this time, we are further evaluating this approach in a phase I clinical trial (NCT02602119).
Supplementary Material
Acknowledgments
J.D.P. was supported by a grant from the American Philosophical Society, the NIH (F32 CA210409) and the Association for Academic Surgery Research Grant. S.S. was supported by the NIH (R01 CA193556).
Biography
Biographies for the authors are not available.
Disclosures
PL is on the Board of Directors at On Target Laboratories, manufacturers of the study drug. None other to report.
References
- 1.Chen F., et al. , “Repeat resection of pulmonary metastasis is beneficial for patients with osteosarcoma of the extremities,” Interact Cardiovasc. Thorac. Surg. 9(4), 649–653 (2009). 10.1510/icvts.2009.212498 [DOI] [PubMed] [Google Scholar]
- 2.Briccoli A., et al. , “Resection of recurrent pulmonary metastases in patients with osteosarcoma,” Cancer 104(8), 1721–1725 (2005). 10.1002/(ISSN)1097-0142 [DOI] [PubMed] [Google Scholar]
- 3.Cerfolio R. J., McCarty T., Bryant A. S., “Non-imaged pulmonary nodules discovered during thoracotomy for metastasectomy by lung palpation,” Eur. J. Cardiothorac. Surg. 35(5), 786–791 (2009). 10.1016/j.ejcts.2009.01.012 [DOI] [PubMed] [Google Scholar]
- 4.Keating J. J., et al. , “Intraoperative near-infrared fluorescence imaging targeting folate receptors identifies lung cancer in a large-animal model,” Cancer 123(6), 1051–1060 (2017). 10.1002/cncr.30419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Predina J. D. K., et al. , “A phase I clinical trial of targeted intraoperative molecular imaging for pulmonary adenocarcinomas,” in STS 53rd Annual Meeting, Houston, Texas: (2017). [Google Scholar]
- 6.Yang R., et al. , “The folate receptor alpha is frequently overexpressed in osteosarcoma samples and plays a role in the uptake of the physiologic substrate 5-methyltetrahydrofolate,” Clin. Cancer Res. 13(9), 2557–2567 (2007). 10.1158/1078-0432.CCR-06-1343 [DOI] [PubMed] [Google Scholar]
- 7.Kansara M., et al. , “Translational biology of osteosarcoma,” Nat. Rev. Cancer 14(11), 722–735 (2014). 10.1038/nrc3838 [DOI] [PubMed] [Google Scholar]
- 8.Hoffman R. M., “Application of GFP imaging in cancer,” Lab. Invest. 95(4), 432–452 (2015). 10.1038/labinvest.2014.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitley M. J., et al. , “A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer,” Sci. Transl. Med. 8(320), 320ra4 (2016). 10.1126/scitranslmed.aad0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoogstins C. E., et al. , “A novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: a translational study in healthy volunteers and patients with ovarian cancer,” Clin. Cancer Res. 23(12), 2929–2938 (2016). 10.1158/1078-0432.CCR-15-2640 [DOI] [PubMed] [Google Scholar]
- 11.Predina J. D., et al. , “Intraoperative molecular imaging combined with positron emission tomography improves surgical management of peripheral malignant pulmonary nodules,” Ann. Surg. 266, 479–488 (2017). 10.1097/SLA.0000000000002382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenthal E. L., et al. , “Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer,” Clin. Cancer Res. 21(16), 3658–3666 (2015). 10.1158/1078-0432.CCR-14-3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.