Abstract
Nanoencapsulation of lipophilic bioactive compounds in food biopolymers is important to functional beverages, but protein-based nanocapsules are unstable around the isoelectric point of protein. The objectives of this work were to study physicochemical properties of self-assembled curcumin-soluble soybean polysaccharide (SSPS) nanoparticles and evaluate the activities against proliferation of human colon HCT116 and mammary adenocarcinoma MCF-7 cancer cells before and after simulated digestions. Capsules with a hydrodynamic diameter of 200–300 nm and an encapsulation efficiency of ~90% were self-assembled after increasing curcumin-SSPS mixture to pH 12.0 and lowering pH to 7.0. The capsule dispersions were stable at pH 2.0–7.0 and after heating at 95 °C for 1 min. No significant difference was observed for the viability of HCT 116 and MCF-7 cells challenged with 0.4, 4.0, and 40 μg/ml nanoencapsulated curcumin before and after simulated gastric and intestinal digestions. These findings may be significant to help develop functional beverages for disease prevention.
Keywords: curcumin, soluble soybean polysaccharide, encapsulation, stability, digestion, anticancer potential
1. Introduction
Lipophilic phytochemicals with anti-oxidant, anti-cancer and anti-inflammatory activities are significant in the prevention of chronic diseases (Scalbert, Andres-Lacueva, Arita, Kroon, Manach, Urpi-Sarda, et al., 2011; Ting, Li, Ho, & Huang, 2013). To develop functional beverages, delivery systems are needed to encapsulate these lipophilic compounds to achieve dispersion stability during processing and shelf-life storage, prevent their degradation during storage and post-ingestion, improve their absorption, and deliver them to the target sites (Pan, Zhong, & Baek, 2013). To enable dispersion stability and sometimes clarity of beverages, nanoscale capsules can be fabricated with food grade ingredients in colloidal structures of biopolymer nanocapsules, nanoemulsions and microemulsions (Pan & Zhong, 2016).
Food biopolymer nanocapsules have advantages such as label-friendliness of “natural” and “fat-free” that may not be the case for nanoemulsions and microemulsions. Food proteins are frequently studied to prepare delivery systems because they are generally recognized as safe, abundant, sustainable and inexpensive (Chen, Remondetto, & Subirade, 2006). Some desirable characteristics of protein-based delivery systems include the improvement in shelf life stability of labile nutraceuticals and the enhancement in the bioavailability and bioactivity (Elzoghby, Samy, & Elgindy, 2012). However, protein-based delivery systems can aggregate at a pH nearby the isoelectric point of proteins and after thermal processing. Their applicability for intestinal delivery is also questionable due to the digestion by proteases.
Anionic polysaccharides can be stable at a wide range of pH and many are dietary fibres, stable against hydrolysis by enzymes in gastric and intestinal juices. Gum arabic and soluble soybean polysaccharides (SSPS) are two possible polysaccharides for functional beverage applications because of their low-viscosity and their ability to bind lipophilic compounds, in addition to their pH stability and their dietary fibre feature (Gomes, Rocha, Pereira, Peres, Moreno, Toca-Herrera, et al., 2010; Nakamura, Yoshida, Maeda, & Corredig, 2006a, 2006b). Gum arabic is a branched polysaccharide with a small proportion of polypeptide chain enabling its amphiphilic properties and its common application for emulsification (Jayme, Dunstan, & Gee, 1999). SSPS is prepared from soybean cotyledons and contains glycoproteins enabling its emulsification activity (Maeda & Nakamura, 2009; Wu, Lin, & Zhong, 2014). Similar to pectin, SSPS can adsorb on protein nanoparticles to provide steric repulsion to prevent aggregation at an acidity near a protein’s isoelectric point (Maeda & Nakamura, 2009; Nakamura, Furuta, Kato, Maeda, & Nagamatsu, 2003; Nakamura, Yoshida, Maeda, & Corredig, 2006b; Pan, Chen, Davidson, & Zhong, 2014; Zhang & Zhong, 2013). However, there are few studies reporting nanocapsules of phytochemicals fabricated with these two polysaccharides with features for functional beverage applications.
The first objective of the present study was therefore to prepare curcumin-SSPS nanocapsules and characterize physicochemical properties of the nanodispersions. Curcumin is a commonly studied lipophilic phytochemical with a water solubility of only about 11 ng/ml (Kaminaga, Nagatsu, Akiyama, Sugimoto, Yamazaki, Maitani, et al., 2003) but with excellent cytotoxic and anti-carcinogenic activities against various cell lines (Babu, Shylesh, & Padikkala, 2002; Ruby, Kuttan, Dinesh Babu, Rajasekharan, & Kuttan, 1995; Soudamini & Kuttan, 1988). Instead of using the commonly studied anti-solvent precipitation method that requires the removal of alcohol (Chen, Ou, Chen, & Tang, 2017), a pH-cycle method from our previous study (Pan, Luo, Gan, Baek, & Zhong, 2014) was used to prepare self-assembled curcumin-SSPS nanocapsules. The principle is based on the pH-dependent solubility of curcumin, which is insoluble at neutral pH but is soluble at alkaline pH with its three hydroxyl groups being deprotonated. Therefore, sequential steps of increasing pH to 12.0 to dissolve curcumin and acidification to neutral pH enabled the in situ encapsulation of precipitated curcumin by sodium caseinate in nanocapsules with a dimension of 20–40 nm based on transmission electron microscopy. Gum arabic was not chosen because of a low encapsulation efficiency in preliminary studies using the pH-cycle method. The second objective of this study was to assess the activity of curcumin nanoencapsualted in SSPS against the proliferation of human colon and breast cancer cells before and after simulated digestions.
2. Materials and methods
2.1. Chemicals
Curcumin with a reported purity of over 90% was procured from Sigma-Aldrich Corp. (St Louis, MO, USA). SSPS (SOYAFIBE-S Serie) was purchased from Fuji Oil Corp. (Osaka, Japan). Other chemicals were products obtained from either Sigma-Aldrich or Thermo Fisher Scientific (Pittsburgh, PA, USA).
2.2. Encapsulation protocol
The encapsulation protocol followed the previous work (Pan, Luo, Gan, Baek, & Zhong, 2014), with slight modifications. SSPS was hydrated at 2% w/w in deionized water overnight at room temperature (RT, 21 °C) and was adjusted to pH 12.0 using 4.0 M NaOH. Then curcumin crystals were mixed with the SSPS dispersion at an overall concentration of 0.4 mg/ml. After stirring on a magnetic stirring plate for 30 min, the mixture was adjusted to pH 7.0 using 2.0 M HCl, followed by centrifugation at 2920 g (Sorvall RC-5B plus, Sorvall, Newtown, CT, USA) for 10 min at RT to remove big particulates. Lyophilized samples were additionally prepared (model 12 EL freeze drier, VirTis Company, Inc., Gardiner, NY, USA) for subsequent experiments utilizing powdered samples.
2.3. Determination of encapsulation efficiency (EE)
The above supernatant after neutralization and centrifugation was transferred to determine the amount of encapsulated curcumin based on absorbance at 419 nm (Abs419) by referring to a standard curve established from standard curcumin solutions in chloroform (Pan, Zhong, & Baek, 2013). An appropriate amount of chloroform was mixed with the supernatant to fit the curcumin concentration range of the standard curve. After stirring overnight at RT, the bottom chloroform phase was quantified for Abs419 using a UV-Vis spectrophotometer (Evolution 201, Thermo Scientific, Waltham, MA, USA). The EE was determined based on the percentage of curcumin mass in the supernatant with respect to the total curcumin mass in an encapsulation experiment. The EE was determined from three independent replicates.
2.4. Differential scanning calorimetry (DSC)
A model Q2000 calorimeter (TA Instruments, New Castle, DE, USA) was used to characterize thermal properties of lyophilized powder with comparison to pristine curcumin crystals. In each aluminium pan, 10 mg of powder was contained and hermetically sealed. Samples were heated from 30 to 250 °C at a rate of 5 °C/min. Nitrogen was used as a transfer gas at a flow rate of 50 ml/min.
2.5. UV-Vis absorption spectra of curcumin before and after encapsulation
UV-Vis spectroscopy was used to compare structural properties of curcumin before and after encapsulation. The supernatant after encapsulating curcumin was prepared as above and extracted using chloroform. The pristine curcumin solution was prepared by dissolving curcumin crystals in chloroform directly to the same concentration as that extracted from the encapsulation sample. The absorption spectra from 350 to 500 nm were collected at RT, with chloroform being a blank (Evolution 201, Thermo Scientific, Waltham, MA, USA).
2.6. Dynamic light scattering (DLS)
Particle size distributions of SSPS at pH 7.0 before alkalization, at pH 12.0, and after being neutralized back to pH 7.0, as well as SSPS encapsulated with curcumin at pH 7.0, were recorded with a Malvern Nano ZS instrument (Malvern Instrument Ltd, Worcestershire, UK).
2.7. Atomic force microscopy (AFM)
SSPS and SSPS encapsulated with curcumin were diluted in deionized water to an overall solute concentration of 10 ppm. After dilution, 2.0 μl of each sample was spread evenly onto a freshly cleaved mica sheet mounted on a sample disk and was dried overnight in air at RT (Bruker Corp., Santa Barbara, CA, USA). A rectangular cantilever having an aluminium reflective coating on the backside (ScanAsyst, Bruker Corp.) equipped on a Multimode VIII microscope (Bruker AXS, Billerica, MA, USA) was operated in the tapping mode at a scanning speed of 1 Hz to collect topographical images at a preset scan area of 2.0 × 2.0 μm.
2.8. Heat stability of encapsulated curcumin at pH 2.0–7.0
Dispersions diluted 60 times with deionized water were adjusted to pH 2.0–7.0 using 1.0 M HCl, followed by heating at 95 °C in a water bath for 1 min. Abs419 and Hunter Lab color space (L, a, b) values before and after heating were recorded as the chemical stability of curcumin using the above UV-Vis spectrophotometer and a Hunter colorimeter (Hunter Associates Laboratory Inc., Reston, VA, USA), respectively. Absorbance at 500 nm (Abs500) and mean particle size before and after heating were also measured as physical stability of the dispersions using the above UV-Vis and DLS instruments, respectively. Three independent replicates were evaluated.
2.8. Anti-proliferation activity of encapsulated curcumin before and after in vitro digestions
The anti-proliferation activity of curcumin encapsulated in SSPS before and after treatment in simulated gastric and intestinal digestive fluids was compared to free curcumin dissolved in dimethyl sulfoxide (DMSO). The simulated gastric and intestinal digestion conditions followed literature methods, with slight modification (Guri, Haratifar, & Corredig, 2014; Luo, Pan, & Zhong, 2015). Pepsin and pancreatin were dispersed separately at 8.0 mg/ml and 80.0 mg/ml in 10 mM phosphate-buffered saline adjusted to pH 2.0 and pH 7.0, respectively. The freeze-dried sample was reconstituted at 2% w/v in distilled water and stirred overnight at RT. For the simulated gastric digestion, 4 ml of the capsule dispersion was adjusted to pH 2.0 using 1.0 N HCl and added to 100 μl of the pepsin stock solution to get a final pepsin concentration of 0.2 mg/ml. Samples were incubated in a shaking water bath operating at 100 rpm (New Brunswick Scientific Co., Edison, NJ, USA) for 2 h. The reaction was terminated by adjusting pH to 7.0 with 1.0 N NaOH. The mixture was then added to 80 μl of the pancreatin stock solution and subsequently incubated at 37 °C in the above shaking water bath for another 4 h to simulate intestinal digestion. The pancreatic digestion was terminated by heating at 95 °C for 5 min. Samples obtained from each step were centrifuged at 6700 g for 2 min, and the supernatant after extraction with chloroform was measured for Abs419 to estimate curcumin concentration.
The growth inhibitory activity of curcumin dissolved in DMSO or encapsulated in SSPS (before and after the above digestions) against the human colon cancer cell line HCT116 and the human mammary adenocarcinoma cell line MCF-7 was determined with the CellTiter 96® AQueous One Solution Cell Proliferation Assay System (Promega Corp., Madison, WI, USA). HCT116 and MCF-7 cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and were grown in McCoy’s 5A and MEM/EBSS medium, respectively. Both media were supplemented with 10% (v/v) fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were cultured at 37 °C in a humid atmosphere of 5% CO2. Once the cells reached confluence, 5 × 103 cells/well for HCT-116 and 1 × 104 cells/well for MCF-7 (100 μl/well) were seeded in a 96-well microtiter plate and incubated at 37 °C in a 5% CO2 environment. After 24 h incubation, cells were treated with curcumin dissolved in DMSO or encapsulated in SSPS, which were previously diluted with the corresponding medium with 5% fetal bovine serum to the desired concentration range (n = 4–6). After another 48 h incubation, the medium was removed, and 100 μl of the fresh medium with 5% fetal bovine serum and 20 μl of the CellTiter 96 Aqueous One Solution were added to each well. After incubation for 1 h at 37 °C, the absorbance at 490 nm was measured with a microplate reader (Bio-Tek Instruments, Winooski, VT, USA). The absorbance of 100 μl medium with 5% fetal bovine serum and 20 μl CellTiter 96 Aqueous One Solution without any cells was used as a blank control. The cell viability was calculated based on the absorbance calibrated by that of the control cells treated with the same amount of DMSO or SSPS as in corresponding curcumin treatments (equation 1).
| (1) |
where A is the absorbance of the wells with cells treated with curcumin for 48 h, A is the absorbance of treatments with DMSO or SSPS but without curcumin for 48 h, and Ablank is the absorbance of the wells including 100 μl medium with 5% fetal bovine serum and 20 μl CellTiter 96 Aqueous One Solution without any cells.
2.9. Statistical analysis
Statistical analyses were performed using the SAS program (version 9.3, SAS Institute, Cary, NC, USA). One-way analysis of variance (ANOVA) was carried out. Differences between pairs of means were compared using a Tukey’s test. The significance level was set at 0.05.
3. Results and discussion
3.1. Encapsulation properties
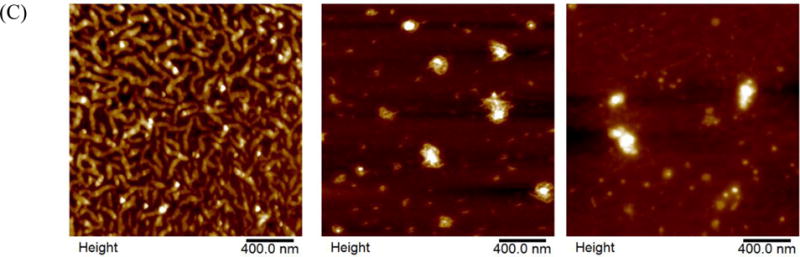
The dispersion was translucent after mixing 0.4 mg/ml curcumin in 2.0% SSPS solution at pH 12.0 for 30 min followed by neutralization and centrifugation and turned transparent after being diluted 10 times with distilled water (Figure 1A). The centrifuged and stable dispersion (before dilution) had a final curcumin concentration of 0.36 mg/ml, corresponding to an EE of 90%. The incubation time of 30 min was selected based on our previous study to obtain a sufficient deprotonation degree of curcumin at pH 12.0 and 21 °C to enable the dissolution and subsequent encapsulation in sodium caseinate during neutralization (Pan, Luo, Gan, Baek, & Zhong, 2014). The centrifuged dispersion was freeze-dried and was analyzed using DSC to understand the thermal behaviour of the capsules. The pristine curcumin crystals showed a characteristic endothermic peak corresponding to the melting of curcumin crystals (Jasim & Talib, 1992; Pan, Zhong, & Baek, 2013), while the lyophilized powder with encapsulated curcumin showed a smooth thermal curve and therefore amorphous structures (Figure 2), indicating the loss of crystalline structure and encapsulation of curcumin in SSPS matrix.
Figure 1.
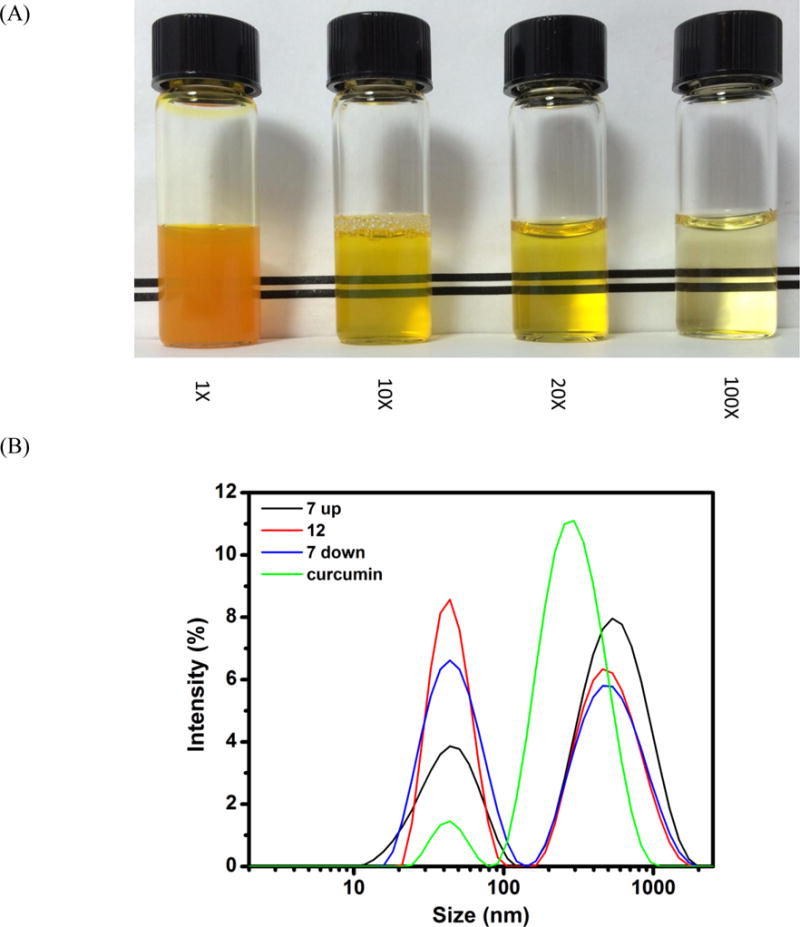
(A) Visual appearance of the dispersion with 0.36 mg/ml encapsulated curcumin at pH 7 before and after 10, 20 and 100 fold dilutions with distilled water; (B) Particle size distributions of SSPS at pH 7.0 (7 up), after adjusting to pH 12.0 (12), and after subsequent pH adjustment to 7.0 (7 down), in comparison to the pH 7.0 dispersion with encapsulated curcumin (curcumin); (C) AFM images of SSPS (left) and SSPS encapsulated with curcumin at pH 7.0 before (middle) and after (right) heating at 95 °C for 1 min.
Figure 2.
DSC thermograms of SSPS, curcumin crystals and lyophilized powder with curcumin encapsulated in SSPS (blue).
3.2. Physical structural changes related to encapsulation
To understand the supramolecular structural changes of SSPS during the encapsulation processes, hydrodynamic diameters (Dh) of SSPS at pH 7.0, after adjusting to pH 12.0, and subsequent acidification back to pH 7.0 were studied using DLS (Figure 1B). With pH increasing from 7.0 to 12.0, the binomial particle size distribution of SSPS showed the bigger particle peak shifted to smaller Dh with a reduced proportion, while the smaller particle peak was narrowed, with an undetectable peak center change and an increased proportion. When pH was subsequently acidified to pH 7.0, the centers of both peaks remained unchanged, but the smaller particle peak was broadened. When curcumin was mixed with the SSPS dispersion at pH 12.0 and subsequently neutralized, the Dh distribution also showed two peaks, with a dominant peak centered on 295 nm (mean Dh = 370 nm) that was much smaller than the bigger particle peak of SSPS centering at 531 nm (mean Dh = 485 nm).
AFM was used to further understand physical structure changes during encapsulation of curcumin. As presented in Figure 1C, SSPS showed either as individual molecules or loosely associated structures. After encapsulating curcumin, individual particles were easily identified before and after heating at 95 °C for 1 min. Compared with SSPS, capsule particles had a more compact structure.
SSPS is a branched polysaccharide based on indirect structural analysis after enzymatic digestions (Maeda & Nakamura, 2009; Nakamura, Furuta, Kato, Maeda, & Nagamatsu, 2003). Ikeda, Funnami and Zhang (2005) directly observed the star or comb polymer like structures of single SSPS molecules dispersed at 1–5 μg/ml with Tween 20 in moist samples (air-drying for 10–20 min) using AFM. The branched structures were not observed in the present study (Figure 1C), possibly because of the absence of Tween 20 that can be used to effectively separate associated SSPS molecules and the much longer (overnight) drying time. Based on gel filtration chromatography, SSPS has molecules of 550, 25 and 5 kDa, with the smallest one representing a very small portion, and has an average molecular weight of several hundred kDa (Maeda & Nakamura, 2009). The 550 kDa molecules are more abundant than the 25 kDa ones (Maeda & Nakamura, 2009), which agrees with the DLS data in the present study (Figure 1B). The branched structures of SSPS may be responsible for the measured Dh bigger than 100 nm (Figure 1B). The reduced Dh after adjusting pH to 12.0 can result from the dissociation of loosely flocculated SSPS molecules due to increased charges of anionic groups, which was mostly irreversible after the pH cycle (Figure 1B).
After centrifugation at 10,000 g, SSPS was easily separated into high and low molecular weight fractions (Li, Matsumoto, Nakamura, Maeda, & Matsumura, 2009). The high molecular weight fraction was determined to be composed mostly of glycoproteins contributing to the properties of SSPS emulsifying oils and stabilizing acidified milk proteins, while the low molecular weight fraction contained free peptides and smaller saccharides (Li, Matsumoto, Nakamura, Maeda, & Matsumura, 2009). The adsorption of the polypeptide moiety on oil droplets results in the polysaccharide branches protruding to the continuous aqueous phase to prevent aggregation with electrostatic and steric repulsion mechanisms, similar to the Wattle Blossom Model proposed for gum arabic (Maeda & Nakamura, 2009).
The DLS and AFM results and the literature information of SSPS structures can provide the physical principles of encapsulating curcumin in SSPS. At pH 7.0 before any treatment, SSPS is dispersed in water as single molecules and some are loosely flocculated, while curcumin is present as crystals. After adjusting to pH 12.0, curcumin is solubilized and SSPS molecules are fully dissociated, enabling molecular complexation due to hydrophobic interaction between curcumin and the polypeptides of SSPS. The hydrophobic binding at pH 12.0 was previously demonstrated for sodium caseinate and curcumin based on fluorescence spectroscopy (Pan, Luo, Gan, Baek, & Zhong, 2014). During the acidification back to pH 7.0, curcumin is gradually protonated to lose solubility and aggregates with adsorbed SSPS. The branches of SSPS limit the size of self-assembled SSPS-curcumin complexes, forming a curcumin core-SSPS shell structure that is evident in AFM (Figure 1C). The limited fluidity of curcumin determines the non-spherical structures of capsules (Figure 1C), and the compact structure of capsules resulted in the smaller Dh than SSPS (Figure 1B).
3.3. UV-Vis absorption spectra of curcumin before and after encapsulation
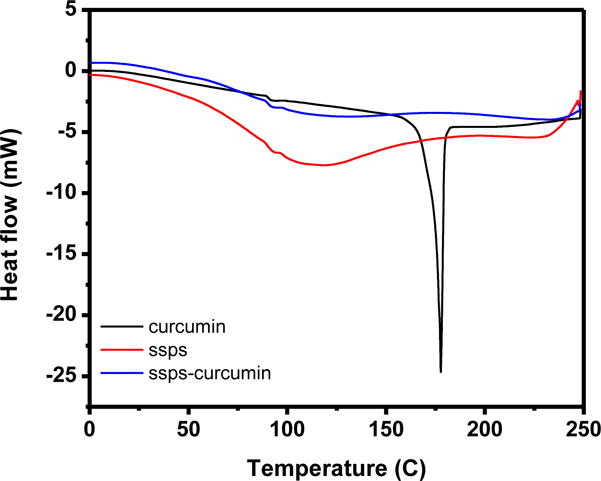
The structural changes of curcumin in the present study, due to exposure at pH 12.0 for up to 30 min at 21 °C, were characterized using UV-Vis spectroscopy. As shown in Figure 3, the absorption peak did not shift and the intensity did not change before and after the pH cycle treatment, indicating the minimum structural changes at the studied encapsulation conditions, and the results agree with our previous study on self-encapsulation of curcumin in sodium caseinate (Pan, Luo, Gan, Baek, & Zhong, 2014).
Figure 3.
UV-Vis absorption spectra of the same concentrations of free curcumin (directly dissolved in chloroform, black) and that extracted from SSPS nanocapsules using chloroform (red).
3.4. Thermal stability of dispersions
For beverage applications, the ability of dispersions to remain dispersed at a wide range of pH conditions after thermal pasteurization or sterilization is important. The dispersions at pH 2.0–7.0 after heating at 95 °C for 1 min showed insignificant changes in turbidity (Figure 4A) without visible precipitation (not shown). Insignificant changes of Dh at pH 4.0–7.0 (p > 0.05) and significant (p < 0.05) increases at pH 2.0 and 3.0 after heating (Figure 4B) further indicated the excellent physical stability of curcumin-SSPS nanoparticles at a wide pH range, resulting from SSPS branches on particle surface, as discussed previously. The significant increase in Dh at pH 2.0 and pH 3.0 after heating can be caused by minor aggregation due to the weakened electrostatic repulsion at an acidity nearby pKa (≈3.5) of carboxyl groups (Cho, Decker, & McClements, 2009). The Abs419 also showed insignificant changes at pH 2.0–7.0 after heating (Figure 4C), indicating the good chemical stability of curcumin after heating at the studied conditions, which was further supported by no significant changes of L, a and b values (Figure 5). The ability to maintain colour after thermal processing is an important property for beverage applications.
Figure 4.
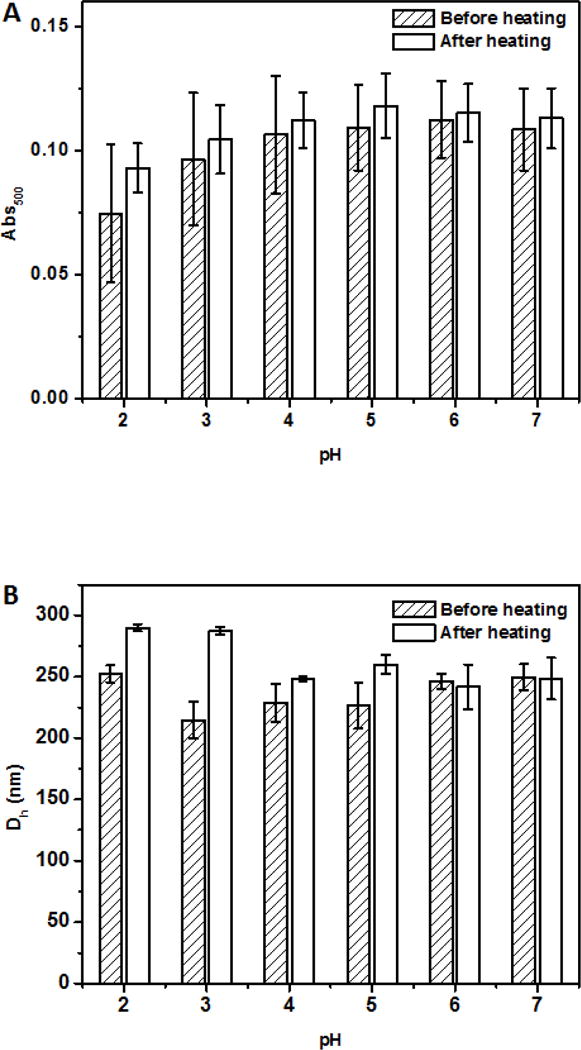
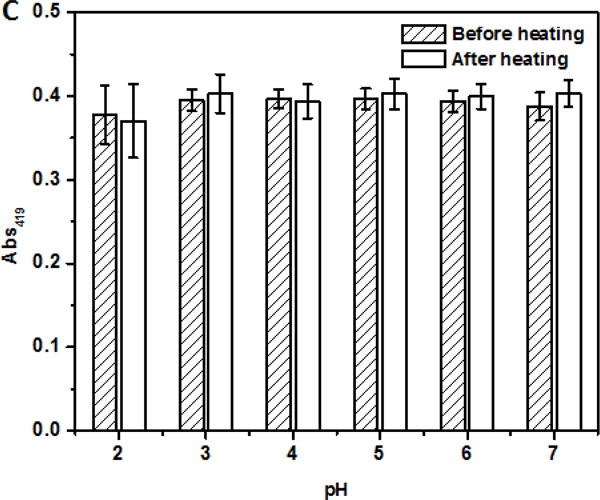
Absorbance at 500 nm (Abs500, A), mean hydrodynamic diameter (Dh, B), and absorbance at 419 nm (Abs419, C) of dispersions with 6 μg/ml encapsulated curcumin adjusted to pH 2.0–7.0, before and after heating at 95 °C for 1 min.
Figure 5.
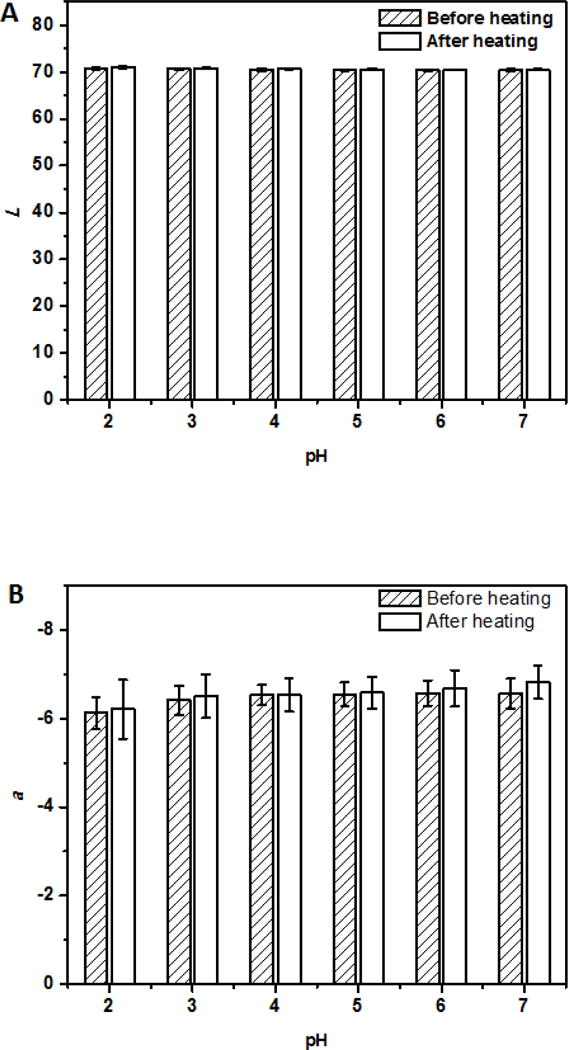
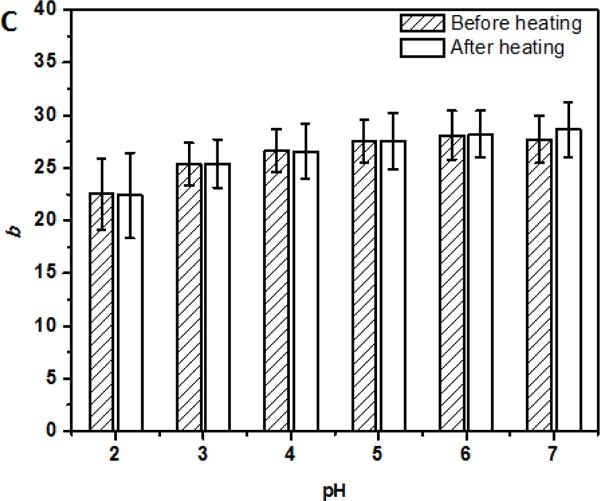
L (A), a (B), and b (C) values of dispersions with 6 μg/ml encapsulated curcumin adjusted to pH 2.0–7.0, before and after heating at 95 °C for 1 min.
3.5. Anti-proliferative activity of encapsulated curcumin
The anti-proliferative activity of nanoencapsulated curcumin tested with HCT-116 and MCF-7 cells is shown in Figure 6. Compared to free curcumin dissolved in DMSO, the activity of curcumin encapsulated in SSPS was marginally improved for both cell types (p > 0.05) that had a lower viability at a higher dose of curcumin. At 4.0 μg/ml, the HCT-116 cells had a lower viability than MCF-7 cells, indicating the higher curcumin susceptibility of the former.
Figure 6.
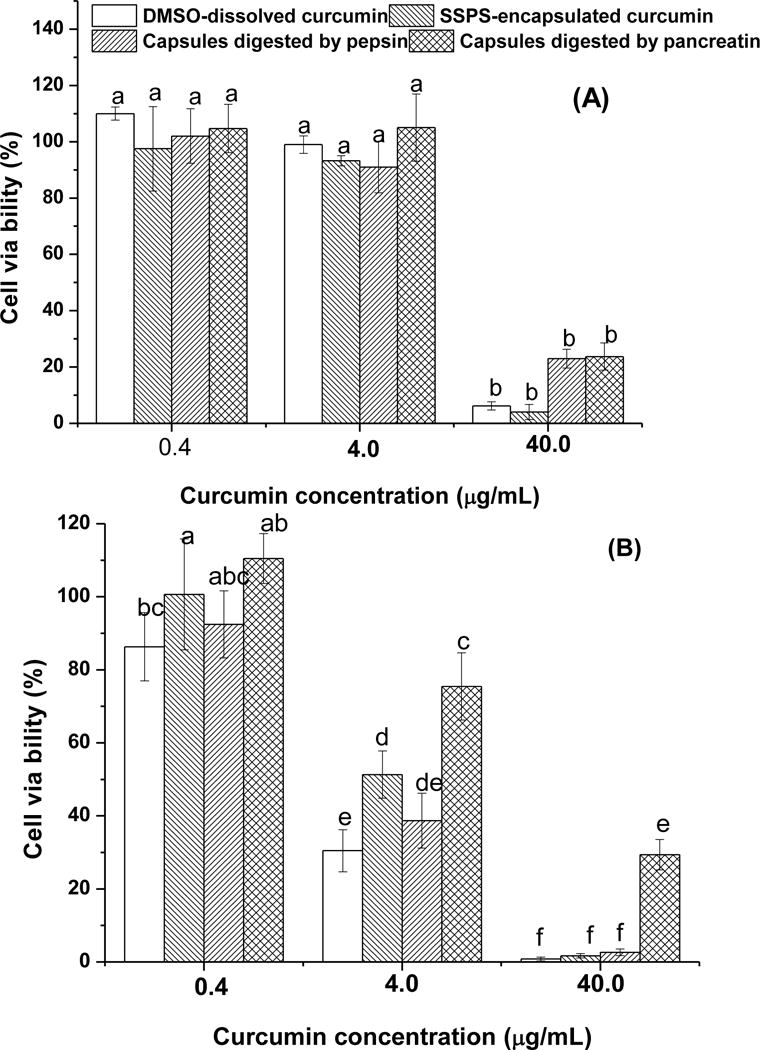
Viability of MCF-7 (A) and HCT-116 (B) cells treated with 0.4, 4.0 and 40.0 μg/ml of curcumin dissolved in DMSO or encapsulated in SSPS, with comparison to same amounts of the encapsulated curcumin after peptic digestion and both peptic and pancreatic digestions. For curcumin dissolved in DMSO, the curcumin concentration in the serum phase after mixing with the simulated gastric juice was below 0.4 μg/ml and therefore was not tested. The error bars represent standard deviation (n ≥ 4). Different letters above bars indicate statistical differences at p < 0.05.
The observation in Figure 6 is different from our previous observations of the significant improvement in the anti-proliferative activity of curcumin after nanoencapsulation in caseinate (Pan, Luo, Gan, Baek, & Zhong, 2014; Pan, Zhong, & Baek, 2013). Caseins do not have any anticancer activity (Pan, Luo, Gan, Baek, & Zhong, 2014; Pan, Zhong, & Baek, 2013), while SSPS does (Ko, Jeong, Choi, & Ryu, 2013). The anti-proliferative activity of ~0.2% SSPS, a concentration corresponding to the 40.0 μg/ml curcumin treatments, in Figure 6 was also observed for the two cancer cell types in this study, which was 5.92±0.68% inhibition on MCF-7 and 14.91±3.91% inhibition on HCT-116. Because curcumin is only soluble in water at 11 ng/ml (Kaminaga, et al., 2003), the cytotoxicity is likely enabled after capsules contact cancer cells. Caseins do not have defined three-dimensional structures and are considered as intrinsically disordered proteins (van der Lee, Buljan, Lang, Weatheritt, Daughdrill, Dunker, et al., 2014). The flexibility of caseins enabled the enhanced uptake of nanoencapsulated curcumin by human colorectal HCT-116 and pancreatic BxPC3 cancer cells than that dissolved in DMSO based on flow cytometry (Pan, Luo, Gan, Baek, & Zhong, 2014). In contrast, SSPS is a branched polysaccharide (Maeda & Nakamura, 2009) and its rigid and bulky structure makes it less effective than caseins for cancer cells to uptake curcumin.
Some studies have shown the activity loss of polyphenols due to biotransformation during gastrointestinal digestion (Spencer, 2003; Visioli, Lastra, Andres-Lacueva, Aviram, Calhau, Cassano, et al., 2011), which may be improved by encapsulation. Therefore, the anti-proliferative activity of encapsulated curcumin was also determined after the simulated gastric and intestinal digestions. After mixing the gastric juice with curcumin dissolved in DMSO, the supernatant after centrifugation was below the detection range based on Abs419 due to precipitation and therefore was not tested. For 0.4 and 4.0 μg/ml encapsulated curcumin, the viability was not significantly different (p > 0.05) before and after the simulated digestions, except for the reduced activity against HCT-116 cells after sequential peptic and pancreatic digestions. At 40.0 μg/ml, the peptic and pancreatic digestions had insignificant effects on the anti-proliferative activity of curcumin against MCF-7 cells (p > 0.05), while insignificant (0.931%, p > 0.05) and significant (27.684%, p < 0.05) increases in relative viability were observed for HCT-116 cells after peptic and peptic-pancreatic digestions, respectively. Therefore, encapsulation not only enabled the dispersion of lipophilic curcumin in water but also maintained most of its activity after mixing with digestive juices. These characteristics are critical to develop functional beverages. The data may be more easily translated to the prevention of colon cancers, after considering possible in vivo uptake. As for mammary cancer prevention, further in vivo studies are needed for orally administered curcumin. In any case, well-designed in vivo experiments are needed to further evaluate the biological functions of curcumin nanodispersions.
4. Conclusions
In conclusion, curcumin has been successfully encapsulated in SSPS nanoparticles utilizing the pH-dependent solubility characteristics of curcumin and the ability of SSPS to adsorb on lipophilic molecules. The dispersions were stable at pH 2.0–7.0 and had satisfactory physical and chemical stabilities after heating at 95 °C for 1 min. There was no significant difference observed for DMSO-dissolved and encapsulated curcumin against the studied human colon and mammary cancer cells, and the encapsulated curcumin maintained most of its anti-proliferative activity after the simulated gastric and intestinal digestions. These findings suggest the potential application of the studied encapsulation system in functional beverages to prevent diseases.
Highlights.
Curcumin (Cur) was encapsulated in soluble soybean polysaccharide (SSPS) with 90% efficiency.
Self-assembly was achieved by adjusting pH of Cur-SSPS mixture to 12.0 and back to 7.0.
The encapsulated Cur was stable at pH 2.0–7.0 and after heating at 95 °C for 1 min.
The cytotoxicity of Cur encapsulated in SSPS or dissolved in DMSO was similar.
The cytotoxicity of the encapsulated Cur was maintained after simulated digestions.
Acknowledgments
Research reported in this publication was financially supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number a 1R21EB018937-01A1, the University of Tennessee Institute of Agriculture, and the USDA National Institute of Food and Agriculture Hatch Project 223984. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Babu B, Shylesh B, Padikkala J. Tumour reducing and anticarcinogenic activity of Acanthus ilicifolius in mice. Journal of Ethnopharmacology. 2002;79(1):27–33. doi: 10.1016/s0378-8741(01)00347-6. [DOI] [PubMed] [Google Scholar]
- Chen F, Ou S, Chen Z, Tang C. Soy soluble polysaccharide as a nanocarrier for curcumin. Journal of Agricultural and Food Chemistry. 2017;65(8):1707–1714. doi: 10.1021/acs.jafc.6b05087. [DOI] [PubMed] [Google Scholar]
- Chen L, Remondetto GE, Subirade M. Food protein-based materials as nutraceutical delivery systems. Trends in Food Science & Technology. 2006;17(5):272–283. [Google Scholar]
- Cho YH, Decker EA, McClements DJ. Competitive adsorption of mixed anionic polysaccharides at the surfaces of protein-coated lipid droplets. Langmuir. 2009;25(5):2654–2660. doi: 10.1021/la8033287. [DOI] [PubMed] [Google Scholar]
- Elzoghby AO, Samy WM, Elgindy NA. Protein-based nanocarriers as promising drug and gene delivery systems. Journal of Controlled Release. 2012;161(1):38–49. doi: 10.1016/j.jconrel.2012.04.036. [DOI] [PubMed] [Google Scholar]
- Gomes JF, Rocha S, Pereira MDC, Peres I, Moreno S, Toca-Herrera J, Coelho MA. Lipid/particle assemblies based on maltodextrin–gum arabic core as biocarriers. Colloids and Surfaces B: Biointerfaces. 2010;76(2):449–455. doi: 10.1016/j.colsurfb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Guri A, Haratifar S, Corredig M. Bioefficacy of Tea Catechins Associated with Milk Caseins Tested Using Different In Vitro Digestion Models. Food Digestion. 2014;5(1):8–18. [Google Scholar]
- Ikeda S, Funnami T, Zhang Y. Visualizing surface active hydrocolloids by atomic force microscopy. Carbohydrate Polymers. 2005;62:192–196. [Google Scholar]
- Jasim F, Talib T. Some observations on the thermal behaviour of curcumin under air and argon atmospheres. Journal of Thermal Analysis and Calorimetry. 1992;38(44):2549–2552. [Google Scholar]
- Jayme M, Dunstan D, Gee M. Zeta potentials of gum arabic stabilised oil in water emulsions. Food Hydrocolloids. 1999;13(6):459–465. [Google Scholar]
- Kaminaga Y, Nagatsu A, Akiyama T, Sugimoto N, Yamazaki T, Maitani T, Mizukami H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Letters. 2003;555(2):311–316. doi: 10.1016/s0014-5793(03)01265-1. [DOI] [PubMed] [Google Scholar]
- Ko Y, Jeong J, Choi Y, Ryu C. Soy soluble polysaccharide induces apoptosis in HCT116 human colon cancer cells via reactive oxygen species generation. Molecular Medicine Reports. 2013;8(6):1767–1772. doi: 10.3892/mmr.2013.1725. [DOI] [PubMed] [Google Scholar]
- Li J, Matsumoto S, Nakamura A, Maeda H, Matsumura Y. Characterization and functional properties of sub-fractions of soluble soybean polysaccharides. Bioscience, Biotechnology, and Biochemistry. 2009;73(12):2568–2575. doi: 10.1271/bbb.70799. [DOI] [PubMed] [Google Scholar]
- Luo YC, Pan K, Zhong QX. Casein/pectin nanocomplexes as potential oral delivery vehicles. International Journal of Pharmaceutics. 2015;486(1–2):59–68. doi: 10.1016/j.ijpharm.2015.03.043. [DOI] [PubMed] [Google Scholar]
- Maeda H, Nakamura A. Soluble soybean polysaccharide. In: Phillips GO, Williams PA, editors. Handbook of Hydrocolloids. CRC Press; Boca Raton, FL: 2009. pp. 693–709. [Google Scholar]
- Nakamura A, Furuta H, Kato M, Maeda H, Nagamatsu Y. Effect of soybean soluble polysaccharides on the stability of milk protein under acidic conditions. Food Hydrocolloids. 2003;17(3):333–343. [Google Scholar]
- Nakamura A, Yoshida R, Maeda H, Corredig M. Soy soluble polysaccharide stabilization at oil-water interfaces. Food Hydrocolloids. 2006a;20(2–3):277–283. [Google Scholar]
- Nakamura A, Yoshida R, Maeda H, Corredig M. The stabilizing behaviour of soybean soluble polysaccharide and pectin in acidified milk beverages. International Dairy Journal. 2006b;16(4):361–369. [Google Scholar]
- Pan K, Chen H, Davidson PM, Zhong Q. Thymol nanoencapsulated by sodium caseinate: physical and antilisterial properties. Journal of Agricultural and Food Chemistry. 2014;62(7):1649–1657. doi: 10.1021/jf4055402. [DOI] [PubMed] [Google Scholar]
- Pan K, Luo Y, Gan Y, Baek SJ, Zhong Q. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter. 2014;10(35):6820–6830. doi: 10.1039/c4sm00239c. [DOI] [PubMed] [Google Scholar]
- Pan K, Zhong Q. Organic nanoparticles in foods: fabrication, characterization, and utilization. Annual Review of Food Science and Technology. 2016;7:245–266. doi: 10.1146/annurev-food-041715-033215. [DOI] [PubMed] [Google Scholar]
- Pan K, Zhong Q, Baek SJ. Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nanocapsules. Journal of Agricultural and Food Chemistry. 2013;61(25):6036–6043. doi: 10.1021/jf400752a. [DOI] [PubMed] [Google Scholar]
- Ruby A, Kuttan G, Dinesh Babu K, Rajasekharan K, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Letter. 1995;94(1):79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Andres-Lacueva C, Arita M, Kroon P, Manach C, Urpi-Sarda M, Wishart D. Databases on food phytochemicals and their health-promoting effects. Journal of Agricultural and Food Chemistry. 2011;59(9):4331–4348. doi: 10.1021/jf200591d. [DOI] [PubMed] [Google Scholar]
- Soudamini K, Kuttan R. Cytotoxic and tumour reducing properties of curcumin. Indian Journal of Pharmaceutical Sciences. 1988;20(2):95. [Google Scholar]
- Spencer JP. Metabolism of tea flavonoids in the gastrointestinal tract. Journal of Nutrition. 2003;133(10):3255S–3261S. doi: 10.1093/jn/133.10.3255S. [DOI] [PubMed] [Google Scholar]
- Ting Y, Li S, Ho CT, Huang Q. Emulsion in oral delivery of bioactive lipophilic phytochemicals. In: Ho C-T, Mussinan Cynthia, Shahidi Fereidoon, editors. Nutrition, Functional and Sensory Properties of Foods. Cambridge, UK: Royal Society of Chemistry; 2013. [Google Scholar]
- van der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Dunker AK, Fuxreiter M, Gough J, Gsponer J, Jones DT, Kim PM, Kriwacki RW, Oldfield CJ, Pappu RV, Tompa P, Uversky VN, Wright PE, Babu MM. Classification of intrinsically disordered regions and proteins. Chemical Reviews. 2014;114(13):6589–6631. doi: 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visioli F, Lastra CADL, Andres-Lacueva C, Aviram M, Calhau C, Cassano A, D’Archivio M, Faria A, Favé G, Fogliano V. Polyphenols and human health: a prospectus. Critical reviews in food science and nutrition. 2011;51(6):524–546. doi: 10.1080/10408391003698677. [DOI] [PubMed] [Google Scholar]
- Wu J, Lin J, Zhong Q. Physical and antimicrobial characteristics of thyme oil emulsified with soluble soybean polysaccharide. Food Hydrocolloids. 2014;39:144–150. [Google Scholar]
- Zhang Y, Zhong Q. Encapsulation of bixin in sodium caseinate to deliver the colorant in transparent dispersions. Food Hydrocolloids. 2013;33(1):1–9. [Google Scholar]


