Abstract
Background and aims
Sodium tissue content by 23Na magnetic resonance imaging (Na-MRI) has been validated in experimental and human studies. SGLT-2 inhibition blocks the reabsorption of glucose and of sodium in the proximal tubular cells in a 1:1 fashion. We hypothesized that SGLT-2 inhibition in patients with type 2 diabetes characterized by sodium retention leads to decreased tissue sodium content due to its pharmacological action.
Materials and methods
In a prospective double blind, placebo controlled, cross-over trial 59 patients (61 ± 7.6 years) with type 2 diabetes were randomized to either dapagliflozin 10 mg or placebo once daily for 6 weeks each. In addition to metabolic parameters and ambulatory blood pressure (BP) we analysed the sodium content in the skin and muscles of the lower leg by Na-MRI.
Results
Compared to baseline 6 weeks treatment with the SGLT-2 inhibitor dapagliflozin decreased fasting (132 ± 28 vs. 114 ± 19 mg/dl, p < 0.001), postprandial blood glucose (178 ± 66 mg/dl vs. 153 ± 46 mg/dl, p < 0.001), body weight (87.6 vs. 86.6 kg, p < 0.001) and systolic (129 ± 12 vs. 126 ± 11 mmHg, p = 0.010), and diastolic (77.4 ± 9 vs. 75.6 ± 8 mmHg, p = 0.024), 24-h ambulatory BP. Tissue sodium content in the skin was reduced after 6 weeks treatment with dapagliflozin compared to baseline [24.1 ± 6.6 vs. 22.7 ± 6.4 A.U.(arbitrary unit) p = 0.013]. No significant reduction of tissue sodium content was observed in the muscle (M. triceps surae: 20.5 ± 3.5 vs. 20.4 ± 3.7 A.U. p = 0.801). No clear significant difference in tissue water content of muscle and skin was observed after 6 weeks of treatment with dapagliflozin, compared to baseline.
Conclusion
SGLT-2 inhibition with dapagliflozin resulted in a significant decrease in tissue sodium content of the skin after 6 weeks. This observation point to a decrease of total sodium content in patients with type 2 diabetes prone to cardiovascular complications, that might be mitigated by SGLT-2 inhibition.
Trial registration The study was registered at http://www.clinicaltrials.gov (NCT02383238) retrospectively registered
Keywords: Magnetic resonance imaging, Type 2 diabetes, SGLT-2 inhibition, Tissue sodium content
Background
Abnormal renal sodium handling is one of the key mechanisms that lead to hypertension and volume overload in diabetic patients, increasing cardiovascular and all-cause mortality. Increased dietary salt intake has been repeatedly found to be associated with cardiovascular damage [1] and, most recently, increased tissue sodium content in the skin was linked to left ventricular hypertrophy [2], independent of blood pressure and other confounders. First evidence has been found that patients with diabetes have increased tissue sodium content in the skin and muscles [3]. Hence, reduction in total sodium content emerged as a therapeutic goal in treating diabetic patients.
Sodium glucose cotransporter 2 (SGLT-2) inhibitors are now widely used for antihyperglycemic therapy. SGLT-2 inhibitors decrease reabsorption of glucose in the renal tubular system. Their unique insulin-independent glycosuric mechanism is characterized by inhibition of glucose reabsorption in the proximal tubular cells, in parallel to blockade of sodium reabsorption. These effects cause osmotic diuresis and natriuresis and decrease systolic and diastolic blood pressures (BP), [4–6] as well as weight loss [7]. Reduction in BP might be related to body weight loss or/and decrease of total body sodium content. Correction of the increased total body sodium content may directly affect the myocardium [2, 8], and potentially lead to reduction of left ventricular hypertrophy. Compensatory sodium reabsorption by other transporter and in the distal tubular system may counteract sodium loss. Indeed, with means of 24-h sodium excretion, exaggerated natriuresis after initiating SGLT-2 inhibition attenuates after 1–2 weeks [6, 9, 10]. However, 24-h sodium excretion must be considered to be highly variable from day to day and is therefore insufficient to reflect true sodium excretion over time [11].
In the last 10 years we and others have developed 23Na magnetic resonance imaging (23Na-MRI) as a new technique to allow investigations of the role of tissue sodium content in human subjects [12–15]. First studies with 23Na-MRI have shown that skin sodium is related to the level of blood pressure in patients with resistant hypertension, confirming experimental findings, that sodium retention and high tissue sodium content have been linked with arterial hypertension in animal models [16, 17]. Furthermore in patients with chronic renal failure and type 2 diabetes both known to retain sodium we observed that tissue sodium content is increased [2, 3].
Dapagliflozin is a SGLT-2 inhibitor and its anti-hyperglycaemic and anti-hypertensive effects have been demonstrated in a number of trials [18]. So far, there are no available data regarding change of tissue sodium content after initiating SGLT-2 inhibition.
The current exploratory study was performed to evaluate the changes of tissue sodium content in patients with type 2 diabetes mellitus after 6 weeks of treatment with dapagliflozin.
Materials and methods
Study design
The study was a prospective, randomized, double-blind, placebo-controlled, cross-over phase III b trial performed at the Clinical Research Center of Erlangen-Nuremberg, Germany (http://www.crc-erlangen.de) to characterize microvascular changes in type 2 diabetes. The principal results are reported elsewhere [19].
Patients with type 2 diabetes were recruited from the University outpatient clinic, through physician referrals, and through the use of newspaper advertisements and consecutively enrolled if they fulfilled all inclusion and none of the exclusion criteria. After a run-in/washout period [2 weeks for patients not receiving anti-diabetic treatment (N = 13) and 4 weeks for patients treated with anti-diabetic medication (N = 46), respectively], patients were randomised to receive either once daily oral dapagliflozin 10 mg or placebo through the use of computer generated algorithm for this single centre study. After 6 weeks, there was a 1-week washout period, and then the patients crossed over to the other treatment.
The study was approved by the Ethics Committee of the University of Erlangen (IRB/IEC) on the 7th February 2014. Furthermore, it was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent prior to inclusion in the study. The study was registered at http://www.clinicaltrials.gov (NCT02383238).
Study population
Patients were included in the study if they had type 2 diabetes and were between 18 and 70 years of age. Individuals were excluded if they had any other form of diabetes, were treated with insulin or more than one oral anti-diabetic drug, were treated with any medication with loop diuretics, had an HbA1c level ≥ 10% (86 mmol/mol), had a fasting plasma glucose (FPG) level > 240 mg/dl, had BP ≥ 180/110 mmHg, had an eGFR < 60 ml/min/1.73 m2, or had a body mass index (BMI) > 40 kg/m2.
Endpoints
The primary endpoint of this exploratory substudy was to analyse the effect of dapagliflozin from baseline on the sodium content in the skin and muscles of the lower leg, measured by 23Na-MRI. Changes in tissue sodium content in the placebo group from baseline served as a control group. The study was not powered to compare any effect between the groups.
Other clinical characteristics that were measured in parallel were blood glucose levels, HbA1c, office BP and 24-h ambulatory BP.
Clinical parameters
Demographic data were recorded at the first visit. At the randomisation visit, a fasting blood sample was taken in order to measure HbA1c, FPG, lipid levels, and other biochemical safety parameters (e.g. creatinine, liver enzymes).
To estimate dietary salt intake, a 24-h urine collection for sodium excretion, the most reliable analytic standard [20, 21], was performed.
Office BP and heart rate measurements were taken in a seated position after 5 min of rest. Twenty-four hour ambulatory BP was measured in parallel with Spacelab 90207 (Spacelabs Health Care.WA, USA). Measurements were taken every 15 min throughout the day and every 30 min during the night.
All biochemical, microvascular and macrovascular parameters were re-analysed after each of the two 6-week treatment periods [19]. Any adverse events that occurred during the study were recorded.
23Na-MRI measurements
Tissue sodium content was assessed noninvasively with a 3.0T clinical MR system (Magnetom Verio, Siemens Healthineers, Erlangen, Germany). 23Na-MRI was performed with a gradient echo sequence, total acquisition time: 13.7 min, echo time: 2.07 ms, repetition time: 100 ms, flip angle: 90°, 128 averages, resolution: 3 × 3 × 30 mm3) and a frequency-adapted monoresonant transmit/receive birdcage knee coil (32.6 MHz, Stark-Contrast, Erlangen, Germany). 1H-water imaging was performed with the body coil of the scanner using a fat suppressed spin-echo sequence (total acquisition time: 6.5 min, echo time: 12 ms, repetition time: 3000 ms, inversion time: 210 ms, resolution: 1.5 × 1.5 × 5 mm3) Subjects placed one lower leg in the centre of the 23Na knee coil. 23Na-MRI measurements of saline solutions with increasing Na+ concentration (10, 20, 30, and 40 mmol/l) served to calibrate relative tissue Na+. Since our method underestimates the true tissue sodium content, we prefer to use A.U. (arbitrary unit) instead of mmol/l. Additionally, the 10 mmol/l standard defined a relative tissue specific water content of 1 A.U. in the water images. Accuracy of this method has been previously shown [12]. The average coefficient of variation for the analysis of same images between seven different readers was found to be 2.1% for skin sodium, 0.5% for muscle sodium, 7.6% for skin water, and 0.5% for muscle water (inter-reader variability) [2].
Statistics
Data are presented as absolute values and percentages or means with standard deviation (SD). Statistical significance of differences between baseline and changes due to therapy were determined using a paired t-test. Statistical analysis was performed using SPSS release 19.0.
Results
Patients
A total of 67 patients were screened, with 62 undergoing randomisation, 31 to initial dapagliflozin and 31 to initial placebo treatment. Of these, 59 patients completed the study and had all the required MRI data available (full analysis set). The mean age was 60.3 years and 39.0% were female (Table 1). The mean duration of diabetes was 5.54 years and the mean HbA1c level was 6.67% (49 mmol/mol).
Table 1.
Patients characteristics
| Mean ± SD or n/N (%) | |
|---|---|
| Age (years) | 60.3 ± 7.6 |
| Female gender | 23/58 (39.0) |
| Body weight (kg) | 87.6 ± 13 |
| Height (cm) | 171 ± 11 |
| BMI (kg/m2) | 29.8 ± 4.3 |
| Mean duration of diabetes (years) | 5.54 ± 4.9 |
| HbA1c (%) | 6.67 ± 0.7% |
| Blood glucose concentration | |
| Fasting (mg/dl) | 132 ± 28 |
| Postprandiala (mg/dl) | 178 ± 66 |
| Office blood pressure | |
| Systolic (mmHg) | 130 ± 13 |
| Diastolic (mmHg) | 80 ± 9.4 |
| Heart rate (bpm) | 69 ± 11 |
| 24 h ambulatory blood pressure | |
| Systolic (mmHg) | 129 ± 12 |
| Diastolic (mmHg) | 77 ± 9 |
| Heart rate (bpm) | 74 ± 11 |
| Haematocrit (%) | 40.1 ± 2.7 |
| Serum sodium concentration (mmol/l) | 138.6 ± 2.0 |
| Urinary sodium excretion over 24 h (mmol/day) | 216 ± 81 |
N = 59. BMI body mass index, HbA1c glycated haemoglobin
aA standardised breakfast was given
After 6 weeks of treatment, HbA1c concentration had not changed significantly from baseline for neither the dapagliflozin nor placebo, related to the short duration of treatment (Table 2). FPG decreased after dapagliflozin treatment by 18 mg/dl (p < 0.001), while FPG after placebo did not change from baseline. Similar finding was observed for postprandial glucose concentrations. Body weight was significantly lower in the dapagliflozin group after 6 weeks of treatment (87.6 vs. 86.6 kg, p < 0.001).
Table 2.
Clinical characteristics after 6 weeks of dapagliflozin and placebo treatment, respectively
| Placebo Mean ± SD (change from baseline) |
p-value vs. baseline | Dapagliflozin Mean ± SD (change from baseline) |
p-value vs. baseline | |
|---|---|---|---|---|
| BMI (kg/m2) | 29.9 ± 4.2 (+ 0.1) | 0.846 | 29.5 ± 4.1 (−0.3) | < 0.001 |
| HbA1c (%) | 6.79 ± 0.8 (+ 0.12) | 0.064 | 6.62 ± 0.7 (− 0.05) | 0.224 |
| Glucose | ||||
| Fasting (mg/dl) | 135 ± 32 (+ 2.0) | 0.325 | 114 ± 19 (−18) | < 0.001 |
| Postprandial† (mg/dl) | 180 ± 67 (+ 1.0) | 0.766 | 154 ± 46 (− 24) | < 0.001 |
| Office blood pressure | ||||
| Systolic (mmHg) | 129 ± 13 (− 0.1) | 0.340 | 126 ± 12 (− 4.0) | 0.015 |
| Diastolic (mmHg) | 79 ± 8.7 (− 1.0) | 0.827 | 78 ± 8.8 (− 2.0) | 0.058 |
| Heart rate (bpm) | 67.8 ± 9.6 (− 1.3) | 0.123 | 68.2 ± 10.6 (− 0.9) | 0.332 |
| 24-h ambulatory blood pressure | ||||
| Systolic (mmHg) | 129 ± 10.8 (− 0.5) | 0.172 | 126 ± 10.8 (−3.0) | 0.010 |
| Diastolic (mmHg) | 77.1 ± 7.3 (0.0) | 0.765 | 75.4 ± 7.7 (−2.0) | 0.024 |
| Heart rate (bpm) | 75.7 ± 9.5 (+ 1.4) | 0.997 | 74.1 ± 7.6 (− 0.8) | 0.849 |
| Haematocrit (%) | 40.3 ± 3.1 (+ 0.2) | 0.389 | 41.1 ± 2.9 (+ 1.0) | < 0.001 |
| Serum sodium conc. (mmol/l) | 138.1 ± 1.6 (− 0.5) | 0.034 | 138.3 ± 1.6 (− 0.3) | 0.308 |
| Urinary sodium excretion over 24 h (mmol/day) | 222.5 ± 103.6 (− 6.0) | 0.660 | 210.1 ± 71.2 (+ 6.5) | 0.586 |
N = 59. BMI body mass index, HbA1c glycated haemoglobin
†A standardised breakfast was given
Office systolic BP decreased by 4 mmHg from baseline after treatment with dapagliflozin (p = 0.015), but only 1 mmHg after placebo (p = 0.340). Diastolic BP was also lower after treatment with dapagliflozin by 2 mmHg (p = 0.058). Twenty-four hour ambulatory BP was reduced, both systolic and diastolic, (p = 0.021 and p = 0.027, respectively), whereas no change was observed in the placebo group.
Serum sodium concentration did not change after dapagliflozin and placebo therapy. Likewise, sodium excretion in 24 h urine as an estimate of daily salt intake did not decrease significantly during dapagliflozin treatment compared to baseline (p = 0.586). The same was found for placebo treatment (p = 0.660). Lipid levels remained stable during the study (data not shown).
Skin and muscle sodium content after 6 weeks of treatment (Table 3)
Table 3.
Skin and muscle sodium and water content at baseline and after 6 weeks of treatment
| Baseline | Dapagliflozin vs. baseline | Placebo vs. baseline | |||
|---|---|---|---|---|---|
| X ± SD | X ± SD | p-value | X ± SD | p-value | |
| Skin Na + (A.U.) | 24.5 ± 7.2 | 22.7 ± 6.4 | 0.013 | 23.8 ± 8.3 | 0.314 |
| Skin H2O (A.U.) | 0.153 ± 0.06 | 0.144 ± 0.06 | 0.073 | 0.15 ± 0.07 | 0.763 |
| Na + M. triceps surae (A.U.) | 20.6 ± 3.5 | 20.4 ± 3.7 | 0.801 | 20.3 ± 3.6 | 0.514 |
| H2O M. triceps surae (A.U.) | 0.513 ± 0.03 | 0.513 ± 0.03 | 0.952 | 0.505 ± 0.03 | 0.014 |
| Body weight (kg) | 87.6 ± 13 | 86.6 ± 13 | 0.001 | 87.6 ± 13 | 0.930 |
| Urin Na + 24 h (mmol/day) | 216 ± 81 | 210 ± 17 | 0.586 | 222 ± 104 | 0.660 |
Tissue sodium content in the skin was reduced after 6 weeks treatment with dapagliflozin compared to baseline (24.1 ± 6.6 vs. 22.7 ± 6.4 A.U.; p = 0.013) (Fig. 1). No significant reduction of tissue sodium content was observed after 6 weeks of treatment with dapagliflozin in the muscle (M. triceps surae: 20.6 ± 3.5 vs. 20.4 ± 3.7 A.U.; p = 0.801). No significant difference in tissue water content of muscle and skin was observed after 6 weeks of treatment with dapagliflozin, compared to baseline (H2O skin: 0.153 ± 0.06 vs. 0.144 ± 0.6 A.U. p = 0.073).
Fig. 1.
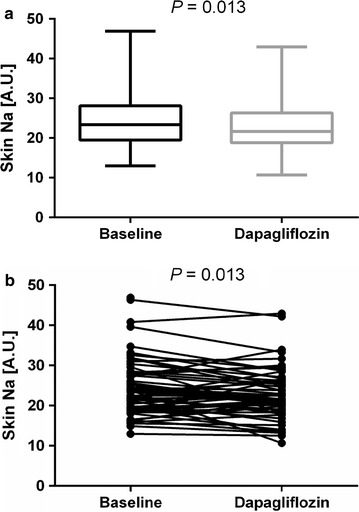
Box and whisker plot (a) and linear graphic (b) of changes in skin sodium content after 6 weeks treatment with dapagliflozin
In contrast, in the placebo group no significant change of tissue sodium content in skin and muscle was observed after 6 weeks of therapy compared to baseline.
Analysing male and female patients separately reduction of skin sodium content in women was numerically higher but without any significant differences (p = 0.400) between the 2 groups (2.00 ± 4.80 A.U.; p = 0.110 vs. baseline in females and 1.01 ± 3.72; A.U.; p = 0.814 vs. baseline in males). Dividing the study cohort on the basis of median age (60.3 years), there was a significant difference in sodium reduction between the two groups; older patients had a lower decrease of skin sodium content than younger patients after 6 weeks therapy with dapagliflozin (0.30 ± 3.75 A.U. vs. 2.50 ± 4.34 A.U.; p = 0.044).
We did not observe any significant changes of muscle sodium content after dapagliflozin therapy when analysing the subgroups categorized by gender or age.
Discussion
Six weeks of treatment with dapagliflozin improved metabolic control, body weight and reduced office and 24-h ambulatory BP in comparison to baseline in our patients with type 2 diabetes, whereas no change to baseline occurred in the placebo group. In parallel, we found in our exploratory analysis that dapagliflozin was capable of reducing skin sodium content significantly after 6 weeks of treatment, an observation which was not noted in the placebo group.
There was little change in HbA1c on treatment with either placebo or dapagliflozin, which is likely due to the short period of time (6 weeks), in contrast to FPG which was significantly lower after the 6 weeks of dapagliflozin treatment. Dapagliflozin treatment suppresses atherogenic small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol [22], [23] and slows the progression of diabetes-associated glomerulosclerosis and liver fibrosis [24]. Furthermore dapagliflozin has previously been shown to reduce BP in patients with type 2 diabetes [25] and improved endothelial function as add-on therapy to metformin to 16 weeks [26], as assessed by flow-mediated dilation in patients with inadequately controlled early stage type 2 diabetes mellitus. This improvement of parameters associated with the early stages of vascular remodelling has been demonstrated repeatedly. Solini et al. showed an acute improvement of endothelial function, reduction of arterial stiffness and renal resistive index in type 2 diabetic patients [27], which is in line with the results of our study group by Ott et al. [19].
In the present study, we observed a decrease in office systolic BP by approximately 4 mmHg and in ambulatory systolic BP by 3 mmHg after 6 weeks of dapagliflozin treatment, which is of a comparable magnitude to those reported in other studies [25]. Body weight was reduced significantly in the dapagliflozin group. Hence, our data indicate an improved cardiovascular risk factor profile as previously described in detail in a real-world primary and diabetologist care setting [28] and also in the CVD-REAL Nordic study, where dapagliflozin treatment was associated with lower risk of cardiovascular events and all-cause mortality in type 2 diabetic patients compared to patients receiving dipeptidyl peptidase-4 inhibitor therapy [29].
The principal finding of this exploratory analysis is that dapagliflozin effected a reduction of sodium tissue content of the skin as measured by 23Na-MRI imaging, Skin water content did not change significantly, although skin water content tended to be lower, in accordance with the decrease in body weight. If any, the slight decrease of water skin content may underestimate the observed decrease of skin sodium content after 6 weeks of treatment with dapagliflozin. The decrease of skin sodium content after treatment with dapagliflozin appeared to be small with a 5.8% reduction compared to baseline, but it is totally unknown whether small or large changes are required to alter cardiovascular endpoints in patients with type-2 diabetes. Previously we observed a reduction in skin sodium content after diuretic treatment in patients with acute heart failure [30]. The precise mechanism of decreased sodium content in the skin due to dapagliflozin treatment is unknown. Whether it is a direct effect of dapagliflozin, or a consequence of prolonged decrease in renal sodium excretion, that remains undetectable in the 24-h urinary sampling (due to low sensitivity) [11], remains to be elucidated.
Kopp et al. already showed that hypertensive patients without diabetes have a higher skin sodium content compared to healthy subjects [31]. This higher sodium storage in skin is due to a perturbed signalling mechanism of sodium deposition in skin in hypertensive patients, and represents predominantly non-osmotic storage of sodium [17, 32]. Our finding is in accordance with previous studies that assessed sodium-retention by analysing the exchangeable total body sodium content [33]. Exchangeable sodium content was found to be increased in diabetic patients, in contrast to non-diabetic hypertensive subjects [34]. Of note, as mentioned above, with our 23Na-MRI methodology we assess tissue sodium content that is primarily bound to proteoglycan and not osmotically active, thereby representing another consequence of sodium-retention.
In our study skin sodium content in males was higher than in females at baseline, which has already been demonstrated in our previous study [31]. Interestingly reduction in skin sodium content was significantly higher in patients younger than the median age. If this is due to differences in skin composition or explained by a direct stronger effect of dapagliflozin on skin sodium content needs to be further explored. We also plan to obtain more specific information on skin composition by MRI. Nevertheless, since every patient was “his own control”, we think that differences in skin composition should not have influenced our results profoundly.
After initiation of dapagliflozin therapy renal excretion of sodium by blocking SGLT-2 cotransporter [35] is observed but this effect is mitigated or vanishes after 2 weeks of SGLT-2 inhibition [2, 4, 10]. Serum sodium concentration remained stable after 6 weeks treatment with dapagliflozin. In this exploratory study we did not focus on the first 2 weeks, when SGLT-2 inhibition produces natriuresis. We analyzed 24-h sodium excretion during the new steady state after 6 weeks of therapy as an estimate of salt intake. We observed that salt intake as assessed by 24-h sodium excretion, with all its limitations [11], was similar after 6 weeks treatment.
In light of the EMPA-REG Outcome study that showed an early separation of the incidence of hospitalization for heart failure and cardiovascular death between the SGLT-2 inhibitor and the placebo group [6, 36] we want to put forward the hypothesis that whereas the diuretic and natriuretic effect of SGLT-2 inhibition observed in the first days after initiating therapy may acutely decrease preload, even a small natriuretic effect per day may be effected and leads to a reduction of the total skin-sodium content in patients with type 2 diabetes over weeks.
A recent study of Schneider et al. in patients with chronic renal failure, also a sodium retention state as type-2 diabetes, showed the association between increased skin sodium content and increased left ventricular mass [2]. It was demonstrated that skin sodium content is a strong predictor of left ventricular mass, independent of blood pressure, total body hydration and other confounders. These data are in accordance with previous clinical studies describing BP-independent effects of high salt intake on vascular remodelling, left ventricular hypertrophy, cardiovascular events and mortality, and experimental data that found increased sodium content to act as pro-fibrotic and pro-hypertrophic stimuli [5, 37, 38].
Limitations
Our study has strengths and limitations. The measured sodium content of skin by our current MRI technique has been validated [12]. Full detection of fast relaxing sodium compartments (e.g. sodium in tissue) measured by MRI techniques would ideally require the application of ultra-short echo time pulse sequences [39]. In addition, the low spatial resolution of 23Na MRI results in partial volume effects and, thus, may lead to a bias in the 23Na MRI skin measurements. Nevertheless a very close relationship between the MRI measured tissue sodium content and experimentally measured tissue sodium content of the amputated parts (in mmol/kg of wet weight) has been found [12]. In future, 23Na-MRI of skin might be improved by using a dedicated setup at ultra-high magnetic field strength (e.g. 7 Tesla) [40]. As a consequence our findings need interpretation in context to a direct control group and we compared them to our previous data and to the placebo group obtained with the same 23Na-MRI technique [2, 12, 30, 31, 41]. Our results did not show any significant change of tissue sodium content of the muscle. At the moment it is not possible to give a solid explanation for this disparate pattern between the changes of tissue sodium content.
The main limitation of this exploratory study is its short duration of therapy, i.e. we do not know whether the observed effect is maintained over months. This needs to be analysed in future studies (http://www.clinicaltrials.gov; NCT03128528).
Conclusions
Six weeks of treatment with the SGLT-2 inhibitor, dapagliflozin, resulted in improved diabetic control and reduced 24-h ambulatory BP. While these findings have been demonstrated previously, we showed for the first time that SGLT-2 inhibition was capable of reducing skin sodium content in patients with type-2 diabetes.
Future studies are needed to elucidate and investigate in detail whether reduction of skin sodium leads to improvements of cardiovascular and renal outcomes in diabetic patients that might be mitigated by SGLT-2 inhibition.
Authors’ contributions
MVK researched data, contributed to discussion and wrote manuscript. AB and KS researched data, contributed to discussion, reviewed manuscript. CO and MPS reviewed/edited manuscript. DK and FBZ researched data, contributed to discussion. PL, JT and MU contributed to discussion. AMN researched data. RES contributed to discussion, reviewed/edited manuscript. All authors read and approved the final manuscript.
Acknowledgements
We gratefully acknowledge the expert technical assistance of Ortrun Alter, Dorothea Bader-Schmieder, Ingrid Fleischmann, Kerstin Fröhlich-Endreß, Ulrike Heinritz, Susanne Muck, Simone Pejkovic, Sabine Thümmler and Laura Waldmann.
The present work was performed in fulfillment of the requirements for obtaining the degree “Dr. med.” for Franziska Boemke-Zelch.
Competing interests
RES received Speaker fees, Consultancy and Advisory Board fees from Bristol-Myers Squibb/AstraZeneca.
All other authors have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
All authors gave full consent for publication.
Ethics approval and consent to participate
Written informed consent was obtained from each patient before study inclusion. The study protocol of each trial was approved by the Local Ethics Committee (University of Erlangen-Nürnberg), and the studies were conducted in accordance with the Declaration of Helsinki and the principles of good clinical practice guidelines.
Sources of funding
The clinical trial was financially supported by grants from Bristol-Myers Squibb/AstraZeneca.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- A.U.
arbitrary unit
- BMI
body mass index
- BP
blood pressure
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- FPG
fasting plasma glucose
- 23Na-MRI
23Na magnetic resonance imaging
- SD
standard deviation
- SGLT-2
sodium-glucose cotransporter 2
Contributor Information
M. V. Karg, Email: marina.karg@uk-erlangen.de
A. Bosch, Email: agnes.bosch@uk-erlangen.de
D. Kannenkeril, Email: dennis.kannenkeril@uk-erlangen.de
K. Striepe, Email: Kristina.striepe@uk-erlangen.de
C. Ott, Email: christian.ott@uk-erlangen.de
M. P. Schneider, Email: markus.schneider@uk-erlangen.de
F. Boemke-Zelch, Email: franziska.boemke@web.de
P. Linz, Email: peter.linz@uk-erlangen.de
A. M. Nagel, Email: armin.nagel@uk-erlangen.de
J. Titze, Email: jens.m.titze@vanderbilt.edu
M. Uder, Email: michael.uder@uk-erlangen.de
R. E. Schmieder, Phone: +49 9131 85-36245, Email: roland.schmieder@uk-erlangen.de, Email: roland.schmieder@fau.de
References
- 1.Frisoli TM, Schmieder RE, Grodzicki T, Messerli FH. Salt and hypertension: is salt dietary reduction worth the effort? Am J Med. 2012;125(5):433–439. doi: 10.1016/j.amjmed.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Schneider MP, Raff U, Kopp C, Scheppach JB, Toncar S, Wanner C, Schlieper G, Saritas T, Floege J, Schmid M, et al. skin sodium concentration correlates with left ventricular hypertrophy in CKD. J Am Soc Nephrol JASN. 2017;28(6):1867–1876. doi: 10.1681/ASN.2016060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannenkeril DKMV, Jumar A, Ott C, Linz P, Titze J, Uder M, Schmieder RE. Tissue sodium content is increased in type 2 diabetes. Diabetologia. 2017;60:1–608. [Google Scholar]
- 4.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G, Cannon CP, Tikkanen I, Zeller C, Ley L, Woerle HJ, Broedl UC, Johansen OE. Impact of empagliflozin on blood pressure in patients with type 2 diabetes mellitus and hypertension by background antihypertensive medication. Hypertension (Dallas, Tex: 1979) 2016;68(6):1355–1364. doi: 10.1161/HYPERTENSIONAHA.116.07703. [DOI] [PubMed] [Google Scholar]
- 6.Reed JW. Impact of sodium-glucose cotransporter 2 inhibitors on blood pressure. Vasc Health Risk Manag. 2016;12:393–405. doi: 10.2147/VHRM.S111991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomlinson B, Hu M, Zhang Y, Chan P, Liu ZM. Evaluation of the pharmacokinetics, pharmacodynamics and clinical efficacy of empagliflozin for the treatment of type 2 diabetes. Exp Opin Drug Metab Toxicol. 2016;13:211–223. doi: 10.1080/17425255.2017.1258401. [DOI] [PubMed] [Google Scholar]
- 8.Marx N, McGuire DK. Sodium-glucose cotransporter-2 inhibition for the reduction of cardiovascular events in high-risk patients with diabetes mellitus. Eur Heart J. 2016;37(42):3192–3200. doi: 10.1093/eurheartj/ehw110. [DOI] [PubMed] [Google Scholar]
- 9.Lovshin JA, Gilbert RE. Are SGLT2 inhibitors reasonable antihypertensive drugs and renoprotective? Curr Hypertens Rep. 2015;17(6):551. doi: 10.1007/s11906-015-0551-3. [DOI] [PubMed] [Google Scholar]
- 10.Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85(5):513–519. doi: 10.1038/clpt.2008.250. [DOI] [PubMed] [Google Scholar]
- 11.Lerchl K, Rakova N, Dahlmann A, Rauh M, Goller U, Basner M, Dinges DF, Beck L, Agureev A, Larina I, et al. Agreement between 24-hour salt ingestion and sodium excretion in a controlled environment. Hypertension (Dallas, Tex: 1979) 2015;66(4):850–857. doi: 10.1161/HYPERTENSIONAHA.115.05851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopp C, Linz P, Wachsmuth L, Dahlmann A, Horbach T, Schofl C, Renz W, Santoro D, Niendorf T, Muller DN, et al. (23)Na magnetic resonance imaging of tissue sodium. Hypertension (Dallas, Tex : 1979) 2012;59(1):167–172. doi: 10.1161/HYPERTENSIONAHA.111.183517. [DOI] [PubMed] [Google Scholar]
- 13.Madelin G, Regatte RR. Biomedical applications of sodium MRI in vivo. J Magn Reson Imaging JMRI. 2013;38(3):511–529. doi: 10.1002/jmri.24168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P, Luft FC, Hilgers KF. Osmotically inactive skin Na + storage in rats. Am J Physiol Renal Physiol. 2003;285(6):F1108–F1117. doi: 10.1152/ajprenal.00200.2003. [DOI] [PubMed] [Google Scholar]
- 15.Hilal SK, Maudsley AA, Ra JB, Simon HE, Roschmann P, Wittekoek S, Cho ZH, Mun SK. In vivo NMR imaging of sodium-23 in the human head. J Comput Assist Tomogr. 1985;9(1):1–7. doi: 10.1097/00004728-198501000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15(5):545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 17.Machnik A, Dahlmann A, Kopp C, Goss J, Wagner H, van Rooijen N, Eckardt KU, Muller DN, Park JK, Luft FC, et al. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension (Dallas, Tex: 1979) 2010;55(3):755–761. doi: 10.1161/HYPERTENSIONAHA.109.143339. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Zhang L, Wu B, Song H, An Z, Li S. Dapagliflozin treatment for type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. DiabetesMetab Res Rev. 2014;30(3):204–221. doi: 10.1002/dmrr.2479. [DOI] [PubMed] [Google Scholar]
- 19.Ott C, Jumar A, Striepe K, Friedrich S, Karg MV, Bramlage P, Schmieder RE. A randomised study of the impact of the SGLT2 inhibitor dapagliflozin on microvascular and macrovascular circulation. Cardiovasc Diabetol. 2017;16(1):26. doi: 10.1186/s12933-017-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Land MA, Webster J, Christoforou A, Praveen D, Jeffery P, Chalmers J, Smith W, Woodward M, Barzi F, Nowson C, et al. Salt intake assessed by 24 h urinary sodium excretion in a random and opportunistic sample in Australia. BMJ Open. 2014;4(1):e003720. doi: 10.1136/bmjopen-2013-003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose G, Stamler J, Stamler R, Elliott P, Marmot M, Pyorala K, Kesteloot H, Joossens J, Hansson L, Mancia G, Dyer A. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ Clin Res ed. 1988;297(6644):319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi T, Fukui T, Nakanishi N, Yamamoto S, Tomoyasu M, Osamura A, Ohara M, Yamamoto T, Ito Y, Hirano T. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetol. 2017;16(1):8. doi: 10.1186/s12933-016-0491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi T, Fukui T, Nakanishi N, Yamamoto S, Tomoyasu M, Osamura A, Ohara M, Yamamoto T, Ito Y, Hirano T. Correction to: dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetol. 2017;16(1):149. doi: 10.1186/s12933-017-0608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang L, Wu Y, Tian M, Sjostrom CD, Johansson U, Peng XR, Smith DM, Huang Y. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am J Physiol Endocrinol Metab. 2017;313(5):E563–E576. doi: 10.1152/ajpendo.00086.2017. [DOI] [PubMed] [Google Scholar]
- 25.Imprialos KP, Sarafidis PA, Karagiannis AI. Sodium-glucose cotransporter-2 inhibitors and blood pressure decrease: a valuable effect of a novel antidiabetic class? J Hypertens. 2015;33(11):2185–2197. doi: 10.1097/HJH.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 26.Shigiyama F, Kumashiro N, Miyagi M, Ikehara K, Kanda E, Uchino H, Hirose T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017;16(1):84. doi: 10.1186/s12933-017-0564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, Bruno RM. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16(1):138. doi: 10.1186/s12933-017-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheerer MF, Rist R, Proske O, Meng A, Kostev K. Changes in HbA1c, body weight, and systolic blood pressure in type 2 diabetes patients initiating dapagliflozin therapy: a primary care database study. Diabetes Metab Syndr Obes Targets Ther. 2016;9:337–345. doi: 10.2147/DMSO.S116243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Persson F, Nystrom T, Jorgensen ME, Carstensen B, Gulseth HL, Thuresson M, Fenici P, Nathanson D, Eriksson JW, Norhammar A, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab. 2017 doi: 10.1111/dom.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammon M, Grossmann S, Linz P, Kopp C, Dahlmann A, Garlichs C, Janka R, Cavallaro A, Luft FC, Uder M, et al. 23Na magnetic resonance imaging of the lower leg of acute heart failure patients during diuretic treatment. PLoS ONE. 2015;10(10):e0141336. doi: 10.1371/journal.pone.0141336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Muller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, et al. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension (Dallas, Tex: 1979) 2013;61(3):635–640. doi: 10.1161/HYPERTENSIONAHA.111.00566. [DOI] [PubMed] [Google Scholar]
- 32.Titze J, Shakibaei M, Schafflhuber M, Schulze-Tanzil G, Porst M, Schwind KH, Dietsch P, Hilgers KF. Glycosaminoglycan polymerization may enable osmotically inactive Na + storage in the skin. Am J Physiol Heart Circ Physiol. 2004;287(1):H203–H208. doi: 10.1152/ajpheart.01237.2003. [DOI] [PubMed] [Google Scholar]
- 33.Weidmann P, Ferrari P. Central role of sodium in hypertension in diabetic subjects. Diabetes Care. 1991;14(3):220–232. doi: 10.2337/diacare.14.3.220. [DOI] [PubMed] [Google Scholar]
- 34.Weidmann P, Beretta-Piccoli C, Trost BN. Pressor factors and responsiveness in hypertension accompanying diabetes mellitus. Hypertension (Dallas, Tex: 1979) 1985;7(6 Pt 2):Ii33–Ii42. doi: 10.1161/01.hyp.7.6_pt_2.ii33. [DOI] [PubMed] [Google Scholar]
- 35.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 37.Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet (London, England) 2007;369(9568):1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 38.Schmieder RE, Messerli FH, Garavaglia GE, Nunez BD. Dietary salt intake. A determinant of cardiac involvement in essential hypertension. Circulation. 1988;78(4):951–956. doi: 10.1161/01.CIR.78.4.951. [DOI] [PubMed] [Google Scholar]
- 39.Konstandin S, Nagel AM. Measurement techniques for magnetic resonance imaging of fast relaxing nuclei. Magma (New York, NY) 2014;27(1):5–19. doi: 10.1007/s10334-013-0394-3. [DOI] [PubMed] [Google Scholar]
- 40.Linz P, Santoro D, Renz W, Rieger J, Ruehle A, Ruff J, Deimling M, Rakova N, Muller DN, Luft FC, et al. Skin sodium measured with (2)(3)Na MRI at 7.0 T. NMR Biomed. 2015;28(1):54–62. doi: 10.1002/nbm.3224. [DOI] [PubMed] [Google Scholar]
- 41.Hammon M, Grossmann S, Linz P, Seuss H, Hammon R, Rosenhauer D, Janka R, Cavallaro A, Luft FC, Titze J, et al. 3 Tesla 23Na Magnetic Resonance Imaging During Acute Kidney Injury. Acad Radiol. 2017;24(9):1086–1093. doi: 10.1016/j.acra.2017.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


