Abstract
We performed this meta-analysis to evaluate the predictive value of different parameters in the sperm retrieval rate (SRR) of microdissection testicular sperm extraction (TESE) in patients with nonobstructive azoospermia (NOA). All relevant studies were searched in PubMed, Web of Science, EMBASE, Cochrane Library, and EBSCO. We chose three parameters to perform the meta-analysis: follicle-stimulating hormone (FSH), testicular volume, and testicular histopathological findings which included three patterns: hypospermatogenesis (HS), maturation arrest (MA), and Sertoli-cell-only syndrome (SCOS). If there was a threshold effect, only the area under the summary receiver operating characteristic curve (AUSROC) was calculated. Otherwise, the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and the diagnostic odds ratio (DOR) were also calculated. Twenty-one articles were included in our study finally. There was a threshold effect among studies investigating FSH and SCOS. The AUSROCs of FSH, testicular volume, HS, MA, and SCOS were 0.6119, 0.6389, 0.6758, 0.5535, and 0.2763, respectively. The DORs of testicular volume, HS, and MA were 1.98, 16.49, and 1.26, respectively. The sensitivities of them were 0.80, 0.30, and 0.27, while the specificities of them were 0.35, 0.98, and 0.76, respectively. The PLRs of them were 1.49, 10.63, and 1.15, respectively. And NLRs were 0.73, 0.72, and 0.95, respectively. All the investigated factors in our study had limited predictive value. However, the histopathological findings were helpful to some extent. Most patients with HS could get sperm by microdissection TESE.
Keywords: microdissection TESE, nonobstructive azoospermia, prediction, sperm retrieval rate
INTRODUCTION
Nonobstructive azoospermia (NOA) is a major cause of male infertility, with a prevalence of about 1% of male population.1 Patients with NOA have no spermatozoa in their semen because of impaired spermatogenesis in the testes.2 However, sperm production can reportedly be detected in the testes of nearly 60% of men with NOA.3 Novel techniques have recently been used to obtain sperm from these patients, such as fine needle aspiration (FNA), testicular sperm extraction (TESE), and microdissection TESE; these techniques could effectively treat male infertility combined with intracytoplasmic sperm injection (ICSI).4
Microdissection TESE is the most effective sperm retrieval technology to date; it was first reported by Schlegel in 1999 and achieved excellent outcomes.5 In contrast to conventional TESE, microdissection TESE is performed under the operating microscope, which allows easier detection of sperm-containing tubules in testes. Studies have shown that microdissection TESE had a higher sperm retrieval rate (SRR) and fewer complications compared with conventional TESE.6,7,8,9 However, it is not possible to obtain sperm from all patients with NOA even using microdissection TESE. As failed sperm retrieval might cause financial and mental stress to patients and their partners, it is necessary to determine reliable factors that can be used to predict the SRR of microdissection TESE.
Spermatogenesis is a complex process that is regulated mainly by the hypothalamic-pituitary-testicle axis. Hence, normal spermatogenesis may be affected by any factor related to this axis, such as follicle-stimulating hormone (FSH), testosterone, and the function of spermatocytes. A number of studies have attempted to determine the predictive factors for successful sperm retrieval, but there is no consensus so far. The aim of our study was to assess the accuracy of different factors in predicting the SRR of microdissection TESE in patients with NOA. We chose three widely investigated predictive factors to conduct meta-analysis, including plasma FSH level, testicular volume, and testicular histopathological findings. Other potential factors, such as age and body mass index (BMI), have not been widely researched and were therefore not included in the meta-analysis.10,11
MATERIALS AND METHODS
Search methods
We searched for relevant clinical studies published before March 1, 2016, in PubMed, Web of Science, EMBASE, Cochrane Library, and EBSCO. The following search strategy was used: ([microdissection testicular sperm extraction] AND [sperm retrieval rate OR sperm recovery]) AND (nonobstructive azoospermia OR nonobstructive azoospermia). The reference lists of the included studies were also manually searched and reviewed. Studies were included without language restriction. Reviews and conference papers were excluded from our study, as they did not provide adequate data. Studies retrieved from different databases were carefully examined to avoid duplications. Two authors performed the literature search independently.
Selection criteria
Our meta-analysis included studies satisfying the following criteria: (1) patients diagnosed with NOA; (2) patients not treated with hormone drugs before the operation; (3) patients who underwent microdissection TESE; (4) studies that investigated predictive factors including at least one of the following parameters: FSH level, testicular volume, or testicular histopathological findings; (5) the primary result was SRR; and (6) 2 × 2 tables could be constructed using study data.
Data extraction
Primary data from all included studies were extracted using a table, including the first author, publication year, study design, number of patients, total SRR, and predictive factors with cutoff values. Data extraction was performed independently by two authors. Discrepancies between the two authors were resolved by consensus.
Quality assessment
Study quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) score,12 which consisted of four main domains: patient selection, index test, reference standard, and flow and timing. It included a total of 14 items, with each item rated as “yes,” “no,” or “unclear” according to the descriptions in the included studies. The quality evaluation process was performed using Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Data analysis
Statistical analysis was performed using the Meta-Disc software 1.4 (Clinical Biostatistics Unit, Ramony Cajal Hospital, Madrid, Spain). We summarized data from primary studies in a 2 × 2 table, including true positive, false positive, true negative, and false negative values, grouped by different predictive factors. When there were multiple cutoff values in one study, the Youden index was adopted to determine the optimal cutoff value. The Youden index was calculated as (sensitivity + specificity − 1) and was used to reflect the diagnostic accuracy of a biomarker.13 We used the Spearman correlation coefficient between sensitivity and specificity to analyze the diagnostic threshold effect. If a threshold effect exists, there will be an inverse correlation between sensitivity and specificity.14 When this occurred, the predictive accuracy was assessed by the area under the summary receiver operating characteristic curve (AUSROC). Otherwise, the predictive accuracy was evaluated by the combination of the summary sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and the diagnostic odds ratio (DOR) with 95% confidence interval (CI), as well as the AUSROC.
The AUSROC represents a summary of the predictive performance of a certain factor.15 The predictive value of a factor will be relatively high if the AUSROC is more than 0.9, while the AUSROC between 0.7 and 0.9 indicates a moderate predictive value. An AUSROC near 0.5 indicates that the predictive value of the parameter is very low. PLR = sensitivity/(1 − specificity), while NLR = (1 − sensitivity)/specificity. PLR >5 and NLR <0.2 indicates a high diagnostic accuracy.16 Another index of the test accuracy is the DOR, with a value ranging from 0 to infinity. Higher values of DOR indicate higher accuracy levels.17 In our study, DOR, sensitivity, specificity, PLR, and NLR with 95% CI of individual studies were presented in the form of forest plots.
The heterogeneity across studies was assessed by the Chi-square test and the inconsistency index I2. P < 0.1 or I2 >50% was taken to indicate considerable heterogeneity among studies. To reduce the influence of heterogeneity, we used random effects model to conduct our meta-analysis. Differences were considered statistically significant when P < 0.05.
As mentioned above, we selected three predictive factors to perform meta-analysis: FSH level, testicular volume, and testicular histopathological findings. The first two parameters were expressed in quantitative values with cutoff values, while the latter was presented as various histopathological patterns. Diverse histopathological classification methods were used in different studies, but most studies separated the histopathological findings into three patterns: hypospermatogenesis (HS), Maturation arrest (MA), and Sertoli-cell-only syndrome (SCOS). Hence, we chose these three patterns as predictive factors and analyzed each pattern separately. When a histopathological pattern was investigated as a positive result, all other patterns were defined as negative results.
RESULTS
Search results and characteristics of included studies
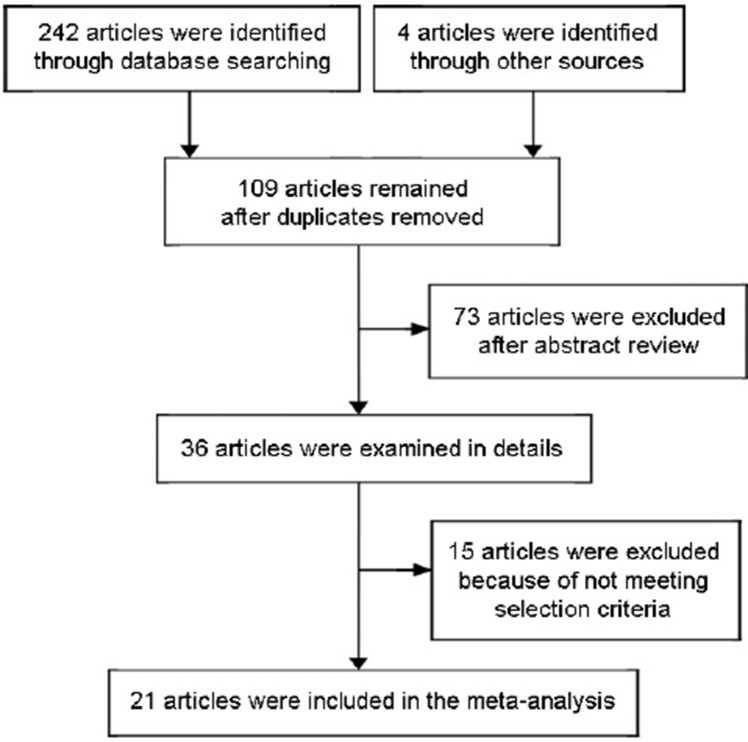
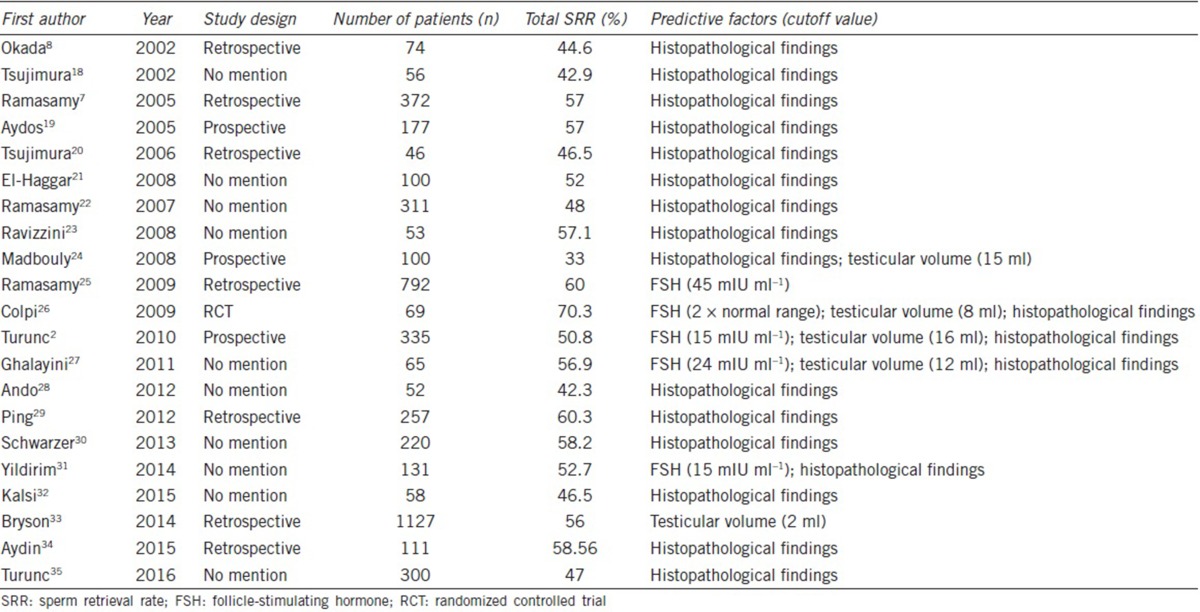
A total of 246 papers were preliminarily identified. After screening, 21 of them met our inclusion criteria and were finally enrolled in our study.2,7,8,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 The flow diagram of our selection process is shown in Figure 1. There were a total of 4364 patients with NOA in these included studies. The characteristics of each study are shown in Table 1. Several studies investigated more than one predictive factor. The correlation of SRR with FSH level was investigated in five studies, and the same number of studies investigated testicular volume. Nineteen studies analyzed the outcomes of SRR in patients with different testicular histopathological patterns.
Figure 1.
Flowchart of the study selection.
Table 1.
Characteristics of included studies
Quality assessment
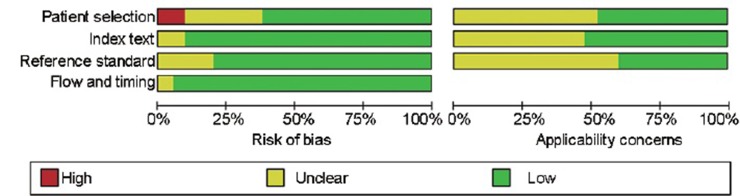
The quality assessment of the included studies is shown in Figure 2. Overall, most of the selected studies were of high quality.
Figure 2.
Methodological quality graph.
FSH
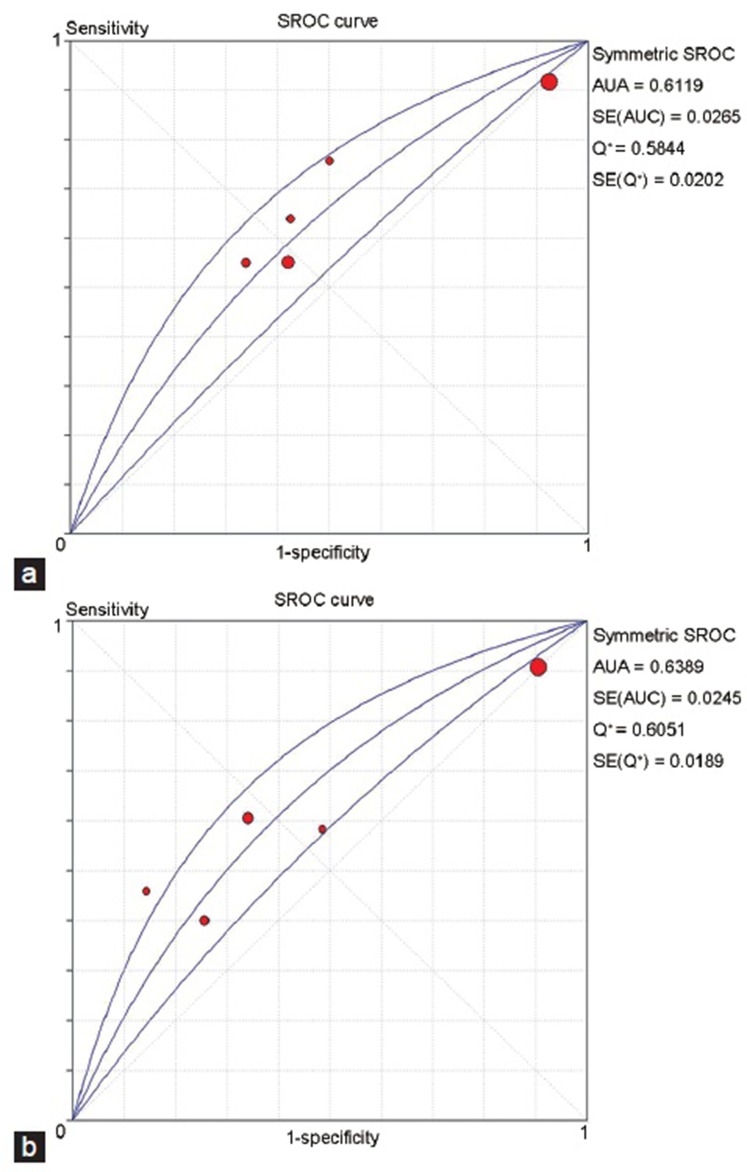
Five studies with a total of 1261 patients were included in the analysis regarding FSH. Some studies used more than one cutoff value, and so we chose the optimal value in each study according to the Youden index. Different cutoff values were identified in different studies, including 15 mIU ml−1, 24 mIU ml−1, and 45 mIU ml−1. However, the study of Colpi et al.26 did not give a definite numerical value, but used a normal range (N) as a threshold without illustrating its size; we chose 2N as the cutoff value for this study according to the Youden index. The Spearman correlation coefficient was 1.0 and P = 0.000, indicating a significant threshold effect among different studies. The AUSROC was 0.6119, indicating a low predictive value for successful sperm retrieval (Figure 3a).
Figure 3.
SROC curve for predictive value of FSH and testicular volume. (a) SROC curve for predictive value of FSH. (b) SROC curve for predictive value of testicular volume. SROC: summary receiver operating characteristic curve; FSH: follicle-stimulating hormone.
Testicular volume
Five studies with a total of 1764 cases involving testicular volume were included in our analysis. Four of these studies chose more than one cutoff value, and so we determined the optimal value using the Youden index. Five different thresholds were used separately in the five studies, including 2 ml, 8 ml, 12 ml, 15 ml, and 16 ml. The Spearman correlation coefficient was 0.8 and P = 0.104, indicating no significant threshold effect. The AUSROC was 0.6389, indicating a low predictive value (Figure 3b). The results of the other indices were as follows (data not shown): pooled DOR 1.98 (95%CI: 1.11–3.53), sensitivity 0.8 (95%CI: 0.78–0.83), specificity 0.35 (95%CI: 0.32–0.39), PLR 1.49 (95%CI: 0.94–2.36), and NLR 0.73 (95%CI: 0.60–0.88). These results showed that testicular volume had limited value in predicting positive sperm retrieval in patients with NOA.
Histopathological findings
There were nineteen articles investigating the predictive value of histopathological findings for SRR. Patients were divided into at least three groups according to the histopathological patterns in each study: HS, MA, and SCOS. Some other patterns, such as tubular sclerosis, were investigated in only a few studies, and were not included in our analysis because of limited numbers. Analysis results for each histopathological pattern are listed as follows.
Hypospermatogenesis
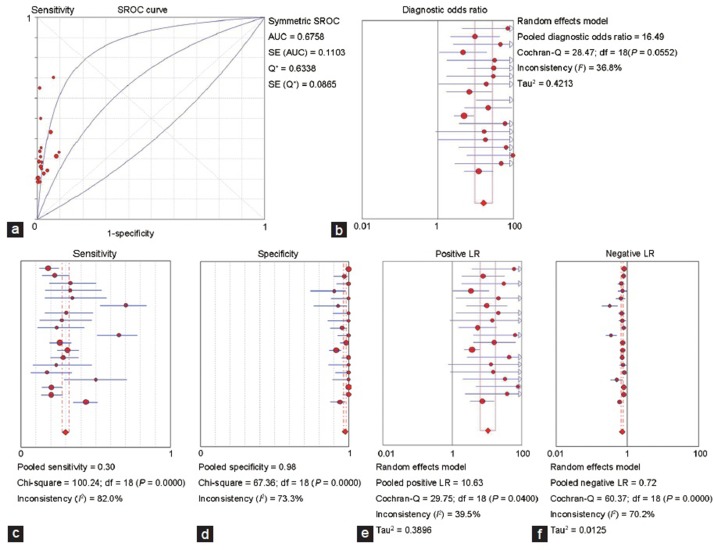
The Spearman correlation coefficient was 0.398 and P = 0.091, indicating no threshold effect. The AUSROC was 0.6758 (Figure 4a). The pooled DOR was 16.49 (95%CI: 9.63–28.23; Figure 4b), indicating a high predictive accuracy. Sensitivity was 0.30 (95%CI: 0.28–0.32; Figure 4c), and specificity was 0.98 (95%CI: 0.97–0.98; Figure 4d). PLR was 10.63 (95%CI: 6.39–17.67; Figure 4e) and NLR was 0.72 (95%CI: 0.67–0.77; Figure 4f), which indicated that sperm was successfully retrieved from most NOA patients with HS. However, there was a high probability that sperm could also be obtained from NOA patients without HS.
Figure 4.
Predictive value of hypospermatogenesis. (a) SROC curve for predictive value of hypospermatogenesis. (b) Forest plot of DOR for predictive value of hypospermatogenesis. (c) Forest plot of sensitivity for predictive value of hypospermatogenesis. (d) Forest plot of specificity for predictive value of hypospermatogenesis. (e) Forest plot of PLR for predictive value of hypospermatogenesis. (f) Forest plot of NLR for predictive value of hypospermatogenesis. SROC: summary receiver operating characteristic curve; DOR: diagnostic odds ratio; PLR: positive likelihood ratio; NLR: negative likelihood ratio.
Maturation arrest
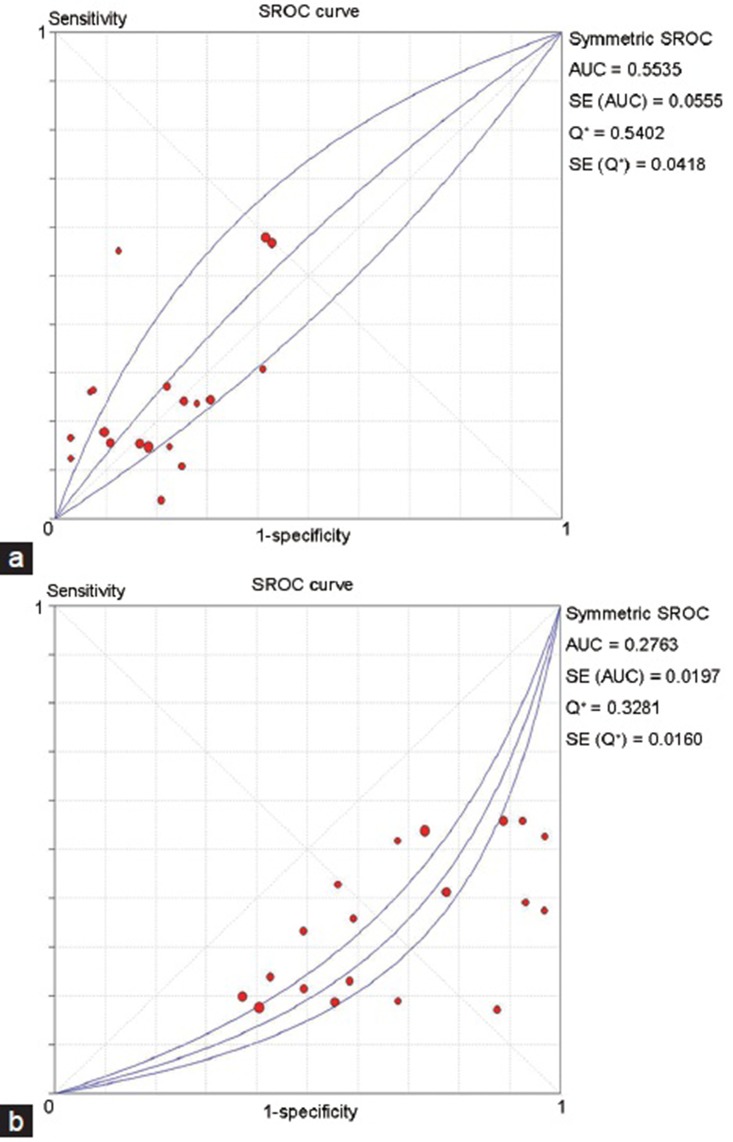
The Spearman correlation coefficient was 0.367 and P = 0.123, indicating no threshold effect. The AUSROC was only 0.5535, indicating a poor predictive accuracy (Figure 5a). The other indices also showed a low predictive value (data not shown). The pooled DOR was 1.26 (95%CI: 0.91–1.75). The sensitivity and specificity were 0.27 (95%CI: 0.24–0.29) and 0.76 (95%CI: 0.74–0.78), respectively. The PLR was 1.15 (95%CI: 0.92–1.44) and the NLR was 0.95 (95%CI: 0.89–1.02).
Figure 5.
SROC curve for predictive value of maturation arrest and SCOS. (a) SROC curve for predictive value of maturation arrest. (b) SROC curve for SCOS. SROC: summary receiver operating characteristic curve; SROS: sertoli-cell-only syndrome; SCOS: Sertoli cell only syndrome.
Sertoli-cell-only syndrome
The Spearman correlation coefficient was 0.565 and P = 0.012, indicating the existence of a threshold effect. The AUSROC was 0.2763, indicating a reverse predictive value (Figure 5b). That is, microdissection TESE was less likely to obtain sperm from NOA patients with SCOS, while NOA patients without SCOS might have higher SRR.
DISCUSSION
Our meta-analysis investigated the predictive value of three different factors for successful sperm retrieval by microdissection TESE in patients with NOA. The results showed that FSH and testicular volume possessed low predictive values, while histopathological findings might be a useful predictor of SRR. Among the three different histopathological patterns, HS usually predicted high SRR, SCOS predicted a poor sperm retrieval outcome, and MA had no predictive value.
Serum FSH was investigated as a predictor of the SRR in TESE. It was reported that the serum FSH could predict the existence of sperm that could be retrieved by conventional TESE.36 However, some other studies showed that FSH had a poor predictive value for sperm retrieval by TESE.37,38 This discrepancy in the study results may be due to the different demographic characteristics in each study. A study from Silber et al.39 indicated that FSH concentration was inversely related to the number of germ cells in the testicle, but it had no correlation with more advanced stages of spermatogenesis. Furthermore, FSH can only reflect the global spermatogenesis function, but cannot judge the function of an isolated area in a testis. As a micromanipulation, microdissection TESE allows careful examination of each part of the testis to find the spermatogenic area. Microdissection TESE might therefore be able to retrieve sperm even if the global spermatogenesis function of the testis is very low. This may be the reason that FSH could not precisely predict the SRR of microdissection TESE. Although two included studies showed that FSH value was related to SRR, they did not conclude that FSH was a predictive factor for successful sperm retrieval.
Testicular volume is another parameter that was widely investigated for predicting sperm retrieval. The testicular volumes of patients with NOA were reportedly usually less than those of patients with obstructive azoospermia.40 In addition, the study of Ziaee et al.41 showed that in patients with NOA, the average testicular volume was 17.5 ml in men with positive sperm retrieval and 5.7 ml in men without sperm retrieval. This might indicate that smaller testicular volume was related to more severe spermatogenesis impairment. However, there might still be areas with normal spermatogenesis, even in a small testis. Bryson et al.33 suggested that small testes should not be a contraindication for microdissection TESE in patients with NOA. Our meta-analysis also found that testicular volume had low predictive value for sperm retrieval.
In contrast to FSH and testicular volume, our meta-analysis showed that histopathological findings might hold some predictive values for SRR in microdissection TESE, especially HS and SCOS. Although the AUSROC of HS was not high, its specificity was up to 0.98. This meant that microdissection TESE could obtain sperm from almost all NOA patients with HS. HS might represent minimal spermatogenesis damage in patients with NOA. In contrast, there was only little likelihood of obtaining sperm from NOA patients with SCOS and therefore these patients should consider carefully before deciding to undergo microdissection TESE. MA could not be used as a predictive tool for sperm retrieval, as its AUSROC was near 0.5.
HS, MA, and SCOS differ histopathologically. All stages of spermatogenesis exist in HS, but to a reduced degree. Hence, it is reasonable that NOA patients with HS had a higher SRR. In MA, spermatogenesis is arrested at a particular stage and mature sperm cannot be produced. SCOS is a condition with more severe testicular damage, which manifests no seminiferous tubules containing germ cells in the testes.42 However, as histopathological examination was performed clinically only in a part of each testis, it could not represent the changes in the whole testis. Therefore, patients diagnosed with severe histopathological patterns might also possess normal spermatogenic function in other parts of the testis. Hence, we could not consider the presence of severe histopathological patterns as a definitive contraindication for microdissection TESE.
Although histopathological findings possess some predictive values for SRR, the application of this technique is controversial. Testicular biopsy is an invasive examination that can cause inflammation, hematoma, or even parenchymal fibrosis in testes.43 Furthermore, as mentioned above, sperm could still be retrieved from some patients with severe histopathological patterns. This may mean that histopathological findings have little instructional meaning for patients considering whether to undergo microdissection TESE. However, testicular biopsy still has some values, as it can contribute to making a diagnosis. In addition, it could mentally prepare both patients and clinicians for the results of the microdissection TESE if the procedure was performed.
Our results showed that neither FSH nor testicular volume could predict the SRR; however, were there other noninvasive factors possessing predictive values? Ramasamy et al.11 investigated the predictive value of BMI and showed that the SRR was not different among men with different BMI values. Another study found that age had no predictive value for SRR.10 Hence, an appropriate noninvasive predictive factor for SRR has not yet been identified. However, Tsujimura et al.44 developed a formula to predict the SRR of microdissection TESE, which included three preoperative noninvasive parameters: FSH, total testosterone, and inhibin B. Although the association of the predicted probabilities and the real outcomes was only 0.77 in this study, it has stimulated the investigation of combinations of parameters as predictive tools for SRR. More studies are expected to explore more reliable formulas predicting the SRR of microdissection TESE.
Our meta-analysis had some limitations. First, there was only a limited number of relevant studies, and so we analyzed only three parameters as predictors for SRR. Some other factors, such as age, BMI, and inhibin B, might also possess predictive value, but there were very few relevant studies to perform a meta-analysis. Second, there were significant threshold effects in the analyses of FSH and SCOS. Therefore, we could not calculate their pooled sensitivities, specificities, PLRs, NLRs, and DORs. Third, statistically significant heterogeneity existed in some analyses, which might reduce the reliability of our results. Even though we used random effects models, the results should be interpreted with caution. Finally, the measurement methods and reference levels of both FSH and testicular volume varied among different studies, which might affect the results of our analysis.
FSH and testicular volume were the two most commonly investigated noninvasive parameters predicting the SRR of microdissection TESE in patients with NOA, but our results showed that neither had dependable predictive value. However, histopathological findings could partially help clinicians make a decision in practice. Microdissection TESE could obtain sperm from most NOA patients with HS, so it was a good choice for HS patients to undergo the surgery. On the contrary, SCOS is a relatively serious histopathological pattern and its SRR was lower than the other two patterns. However, SCOS was not a definitive contraindication to microdissection TESE. The SRR of MA patients was in between that of HS and SCOS patients, and MA had no predictive value for SRR. Histopathological examination has been the most reliable predictive factor of SRR to date. However, it is not recommended to perform the testicular biopsy just to predict the SRR of microdissection TESE, as it is an invasive examination.
CONCLUSIONS
All three investigated factors had limited predictive values and none could be used as a sole predictive factor in clinical practice. Other factors, such as inhibin B and age, might also be helpful to predict the probability of successful sperm retrieval, but further study is required. The combination of different parameters might be a novel approach for predicting SRR in microdissection TESE, and we expect that more relevant studies will be performed.
AUTHOR CONTRIBUTIONS
HL and LPC drafted the manuscript. TW, JHL, and SGW revised the manuscript. JY and MCL searched and selected relevant studies. RBC and RZL extracted the data. HL analyzed the data. All authors read and approved the manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
The study was supported by a grant from the Natural Science Foundation of China (No. 81471451).
REFERENCES
- 1.Su LM, Palermo GD, Goldstein M, Veeck LL, Rosenwaks Z, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: testicular histology can predict success of sperm retrieval. J Urol. 1999;161:112–6. [PubMed] [Google Scholar]
- 2.Turunc T, Gul U, Haydardedeoglu B, Bal N, Kuzgunbay B, et al. Conventional testicular sperm extraction combined with the microdissection technique in nonobstructive azoospermic patients: a prospective comparative study. Fertil Steril. 2010;94:2157–60. doi: 10.1016/j.fertnstert.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Schlegel PN. Nonobstructive azoospermia: a revolutionary surgical approach and results. Semin Reprod Med. 2009;27:165–70. doi: 10.1055/s-0029-1202305. [DOI] [PubMed] [Google Scholar]
- 4.Devroey P, Liu J, Nagy Z, Goossens A, Tournaye H, et al. Pregnancies after testicular sperm extraction and intracytoplasmic sperm injection in non-obstructive azoospermia. Hum Reprod. 1995;10:1457–60. doi: 10.1093/humrep/10.6.1457. [DOI] [PubMed] [Google Scholar]
- 5.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131–5. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 6.Amer M, Ateyah A, Hany R, Zohdy W. Prospective comparative study between microsurgical and conventional testicular sperm extraction in non-obstructive azoospermia: follow-up by serial ultrasound examinations. Hum Reprod. 2000;15:653–6. doi: 10.1093/humrep/15.3.653. [DOI] [PubMed] [Google Scholar]
- 7.Ramasamy R, Yagan N, Schlegel PN. Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology. 2005;65:1190–4. doi: 10.1016/j.urology.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 8.Okada H, Dobashi M, Yamazaki T, Hara I, Fujisawa M, et al. Conventional versus microdissection testicular sperm extraction for nonobstructive azoospermia. J Urol. 2002;168:1063–7. doi: 10.1016/S0022-5347(05)64575-2. [DOI] [PubMed] [Google Scholar]
- 9.Bernie AM, Mata DA, Ramasamy R, Schlegel PN. Comparison of microdissection testicular sperm extraction, conventional testicular sperm extraction, and testicular sperm aspiration for nonobstructive azoospermia: a systematic review and meta-analysis. Fertil Steril. 2015;104:1099–103. doi: 10.1016/j.fertnstert.2015.07.1136. [DOI] [PubMed] [Google Scholar]
- 10.Ramasamy R, Trivedi NN, Reifsnyder JE, Palermo GD, Rosenwaks Z, et al. Age does not adversely affect sperm retrieval in men undergoing microdissection testicular sperm extraction. Fertil Steril. 2014;101:653–5. doi: 10.1016/j.fertnstert.2013.11.123. [DOI] [PubMed] [Google Scholar]
- 11.Ramasamy R, Bryson C, Reifsnyder JE, Neri Q, Palermo GD, et al. Overweight men with nonobstructive azoospermia have worse pregnancy outcomes after microdissection testicular sperm extraction. Fertil Steril. 2013;99:372–6. doi: 10.1016/j.fertnstert.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 13.Yin J, Samawi H, Linder D. Improved nonparametric estimation of the optimal diagnostic cut-off point associated with the Youden index under different sampling schemes. Biom J. 2016;58:915–34. doi: 10.1002/bimj.201500036. [DOI] [PubMed] [Google Scholar]
- 14.Devillé WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leeflang MM. Systematic reviews and meta-analysis of diagnostic test accuracy. Clin Microbiol Infect. 2014;20:105–13. doi: 10.1111/1469-0691.12474. [DOI] [PubMed] [Google Scholar]
- 16.Deng H, Qi X, Guo X. Diagnostic accuracy of APRI, AAR, FIB-4, FI, King, Lok, Forns, and FibroIndex scores in predicting the presence of esophageal varices in liver cirrhosis. Medicine. 2015;94:e1795. doi: 10.1097/MD.0000000000001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–35. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 18.Tsujimura A, Matsumiya K, Miyagawa Y, Tohda A, Miura H, et al. Conventional multiple or microdissection testicular sperm extraction: a comparative study. Hum Reprod. 2002;17:2924–9. doi: 10.1093/humrep/17.11.2924. [DOI] [PubMed] [Google Scholar]
- 19.Aydos K, Demirel LC, Baltaci V, Unlu C. Enzymatic digestion plus mechanical searching improves testicular sperm retrieval in non-obstructive azoospermia cases. Eur J Obstet Gynecol Reprod Biol. 2005;120:80–6. doi: 10.1016/j.ejogrb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Tsujimura A, Miyagawa Y, Takao T, Takada S, Koga M, et al. Salvage microdissection testicular sperm extraction after failed conventional testicular sperm extraction in patients with nonobstructive azoospermia. J Urol. 2006;175:1446–9. doi: 10.1016/S0022-5347(05)00678-6. [DOI] [PubMed] [Google Scholar]
- 21.El-Haggar S, Mostafa T, Abdel Nasser T, Hany R, Abdel Hadi A. Fine needle aspiration vs. mTESE in non-obstructive azoospermia. Int J Androl. 2008;31:595–601. doi: 10.1111/j.1365-2605.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramasamy R, Schlegel PN. Microdissection testicular sperm extraction: effect of prior biopsy on success of sperm retrieval. J Urol. 2007;177:1447–9. doi: 10.1016/j.juro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 23.Ravizzini P, Carizza C, Abdelmassih V, Abdelmassih S, Azevedo M, et al. Microdissection testicular sperm extraction and IVF-ICSI outcome in nonobstructive azoospermia. Andrologia. 2008;40:219–26. doi: 10.1111/j.1439-0272.2008.00846.x. [DOI] [PubMed] [Google Scholar]
- 24.Madbouly K, Alaskar A, Al Matrafi H. Sensitivity, specificity and accuracy of intraoperative findings in microdissection testicular sperm extraction: a prospective study. Curr Urol. 2008;2:130–4. [Google Scholar]
- 25.Ramasamy R, Lin K, Gosden LV, Rosenwaks Z, Palermo GD, et al. High serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extraction. Fertil Steril. 2009;92:590–3. doi: 10.1016/j.fertnstert.2008.07.1703. [DOI] [PubMed] [Google Scholar]
- 26.Colpi GM, Colpi EM, Piediferro G, Giacchetta D, Gazzano G, et al. Microsurgical TESE versus conventional TESE for ICSI in non-obstructive azoospermia: a randomized controlled study. Reprod Biomed Online. 2009;18:315–9. doi: 10.1016/s1472-6483(10)60087-9. [DOI] [PubMed] [Google Scholar]
- 27.Ghalayini IF, Al-Ghazo MA, Hani OB, Al-Azab R, Bani-Hani I, et al. Clinical comparison of conventional testicular sperm extraction and microdissection techniques for non-obstructive azoospermia. J Clin Med Res. 2011;3:124–31. doi: 10.4021/jocmr542w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ando M, Yamaguchi K, Chiba K, Miyake H, Fujisawa M. Expression of VASA mRNA in testis as a significant predictor of sperm recovery by microdissection testicular sperm extraction in patient with nonobstructive azoospermia. J Androl. 2012;33:711–6. doi: 10.2164/jandrol.110.012278. [DOI] [PubMed] [Google Scholar]
- 29.Ping P, Ma M, Chen X, Sun K, Liu Y, et al. Appliance of microsurgery in the treatment of male infertility. Chin J Urol. 2012;33:843–6. [in Chinese] [Google Scholar]
- 30.Schwarzer JU, Steinfatt H, Schleyer M, Kohn FM, Fiedler K, et al. No relationship between biopsy sites near the main testicular vessels or rete testis and successful sperm retrieval using conventional or microdissection biopsies in 220 non-obstructive azoospermic men. Asian J Androl. 2013;15:795–8. doi: 10.1038/aja.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yildirim ME, Koc A, Kaygusuz IC, Badem H, Karatas OF, et al. The association between serum follicle-stimulating hormone levels and the success of microdissection testicular sperm extraction in patients with azoospermia. Urol J. 2014;11:1825–8. [PubMed] [Google Scholar]
- 32.Kalsi JS, Shah P, Thum Y, Muneer A, Ralph DJ, et al. Salvage micro-dissection testicular sperm extraction; outcome in men with non-obstructive azoospermia with previous failed sperm retrievals. BJU Int. 2015;116:460–5. doi: 10.1111/bju.12932. [DOI] [PubMed] [Google Scholar]
- 33.Bryson CF, Ramasamy R, Sheehan M, Palermo GD, Rosenwaks Z, et al. Severe testicular atrophy does not affect the success of microdissection testicular sperm extraction. J Urol. 2014;191:175–8. doi: 10.1016/j.juro.2013.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aydin T, Sofikerim M, Yucel B, Karadag M, Tokat F. Effects of testicular histopathology on sperm retrieval rates and ICSI results in non-obstructive azoospermia. J Obstet Gynaecol. 2015;35:829–31. doi: 10.3109/01443615.2015.1009879. [DOI] [PubMed] [Google Scholar]
- 35.Turunc T, Kuzgunbay B. Is learning curve short for microTESE operation in nonobstructive azoospermic patients? J Clin Anal Med. 2016;7:231–5. [Google Scholar]
- 36.Ishikawa T. Surgical recovery of sperm in non-obstructive azoospermia. Asian J Androl. 2012;14:109–15. doi: 10.1038/aja.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jezek D, Knuth UA, Schulze W. Successful testicular sperm extraction (TESE) in spite of high serum follicle stimulating hormone and azoospermia: correlation between testicular morphology, TESE results, semen analysis and serum hormone values in 103 infertile men. Hum Reprod. 1998;13:1230–4. doi: 10.1093/humrep/13.5.1230. [DOI] [PubMed] [Google Scholar]
- 38.Ezeh UI, Taub NA, Moore HD, Cooke ID. Establishment of predictive variables associated with testicular sperm retrieval in men with non-obstructive azoospermia. Hum Reprod. 1999;14:1005–12. doi: 10.1093/humrep/14.4.1005. [DOI] [PubMed] [Google Scholar]
- 39.Silber SJ, van Steirteghem A, Nagy Z, Liu J, Tournaye H, et al. Normal pregnancies resulting from testicular sperm extraction and intracytoplasmic sperm injection for azoospermia due to maturation arrest. Fertil Steril. 1996;66:110–7. doi: 10.1016/s0015-0282(16)58396-4. [DOI] [PubMed] [Google Scholar]
- 40.Moon MH, Kim SH, Cho JY, Seo JT, Chun YK. Scrotal US for evaluation of infertile men with azoospermia. Radiology. 2006;239:168–73. doi: 10.1148/radiol.2391050272. [DOI] [PubMed] [Google Scholar]
- 41.Ziaee SA, Ezzatnegad M, Nowroozi M, Jamshidian H, Abdi H, et al. Prediction of successful sperm retrieval in patients with nonobstructive azoospermia. Urol J. 2006;3:92–6. [PubMed] [Google Scholar]
- 42.McLachlan RI, Rajpert-De Meyts E, Hoei-Hansen CE, de Kretser DM, Skakkebaek NE. Histological evaluation of the human testis - Approaches to optimizing the clinical value of the assessment: mini review. Hum Reprod. 2007;22:2–16. doi: 10.1093/humrep/del279. [DOI] [PubMed] [Google Scholar]
- 43.Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod. 1997;12:1688–92. doi: 10.1093/humrep/12.8.1688. [DOI] [PubMed] [Google Scholar]
- 44.Tsujimura A, Matsumiya K, Miyagawa Y, Takao T, Fujita K, et al. Prediction of successful outcome of microdissection testicular sperm extraction in men with idiopathic nonobstructive azoospermia. J Urol. 2004;172:1944–7. doi: 10.1097/01.ju.0000142885.20116.60. [DOI] [PubMed] [Google Scholar]