Abstract
The present study analyzed the predictive value of combined analysis of collapsin response mediator protein 4 (CRMP4) methylation levels and the Cancer of the Prostate Risk Assessment (CAPRA-S) Postsurgical score of patients who required adjuvant hormone therapy (AHT) after radical prostatectomy (RP). We retrospectively analyzed 305 patients with prostate cancer (PCa) who received RP and subsequent androgen deprivation therapy (ADT). Two hundred and thirty patients with clinically high-risk PCa underwent immediate ADT, and 75 patients with intermediate risk PCa underwent deferred ADT. CRMP4 methylation levels in biopsies were determined, and CAPRA-S scores were calculated. In the deferred ADT group, the values of the hazard ratios for tumor progression and cancer-specific mortality (CSM) in patients with ≥15% CRMP4 methylation were 6.81 (95% CI: 2.34–19.80) and 12.83 (95% CI: 2.16–26.10), respectively. Receiver-operating characteristic curve analysis indicated that CRMP4 methylation levels ≥15% served as a significant prognostic marker of tumor progression and CSM. In the immediate ADT group, CAPRA-S scores ≥6 and CRMP4 methylation levels ≥15% were independent predictors of these outcomes (uni- and multi-variable Cox regression analyses). The differences in the 5-year progression-free survival between each combination were statistically significant. Combining CAPRA-S score and CRMP4 methylation levels improved the area under the curve compared with the CRMP4 or CAPRA-S model. Therefore, CRMP4 methylation levels ≥15% were significantly associated with a poor prognosis and their combination with CAPRA-S score accurately predicted tumor progression and metastasis for patients requiring AHT after RP.
Keywords: adjuvant hormone therapy, CAPRA-S, CRMP4, methylation levels, prostatic neoplasms
INTRODUCTION
Prostate cancer (PCa), which is the most frequently diagnosed cancer of the male urinary system, poses a great threat to the health of older adults in China.1 Radical prostatectomy (RP) is the standard surgical treatment for localized PCa,2,3 and locally advanced PCa is treated using multimodal therapy.4 The available method used to assess PCa is unable to distinguish micrometastasis from residual, localized regional disease, and medical centers differ in their selection of treatment.5 However, the major challenges in developing an effective treatment for PCa are the selection of the optimum timing and regimen for treatment as well as the reduction of possible over-treatment. Therefore, an accurate cancer risk assessment method is essential to benefit patients with PCa.6
We previously found that locus-specific demethylation of CpG dinucleotides within the collapsin response mediator protein 4 (CRMP4) promoter in metastatic prostate cancer cells suppresses metastasis, while locus-specific methylation of CpG dinucleotides of the CRMP4 promoter in nonmetastatic prostate cancer cells promotes metastasis.7,8 Therefore, quantitation of CRMP4 methylation levels accurately predicts lymph node (LN) metastasis.7,8 The Cancer of the Prostate Risk Assessment (CAPRA-S) Postsurgical score was initially established using the clinical data of 439 patients who underwent RP.9,10 The CAPRA-S score provides an easy and convenient preoperative model to predict the 5-year biochemical recurrence (BCR)-free survival rate and its efficacy is validated by other studies.9,10 Further investigations of the CAPRA-S score combined the serum prostate-specific antigen (PSA) level with postoperative pathology indexes for predicting prognosis after RP.11,12 Here, we retrospectively analyzed the clinical data and CRMP4 methylation levels associated with the outcomes of 305 patients who underwent RP and subsequent androgen deprivation therapy (ADT). Data were acquired from the database of the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China, from October 2000 to December 2015. Further, we analyzed the predictive value of CRMP4 methylation levels and CAPRA-S score for evaluating metastatic potential and prognosis of patients with PCa before surgery.
MATERIALS AND METHODS
Clinical data
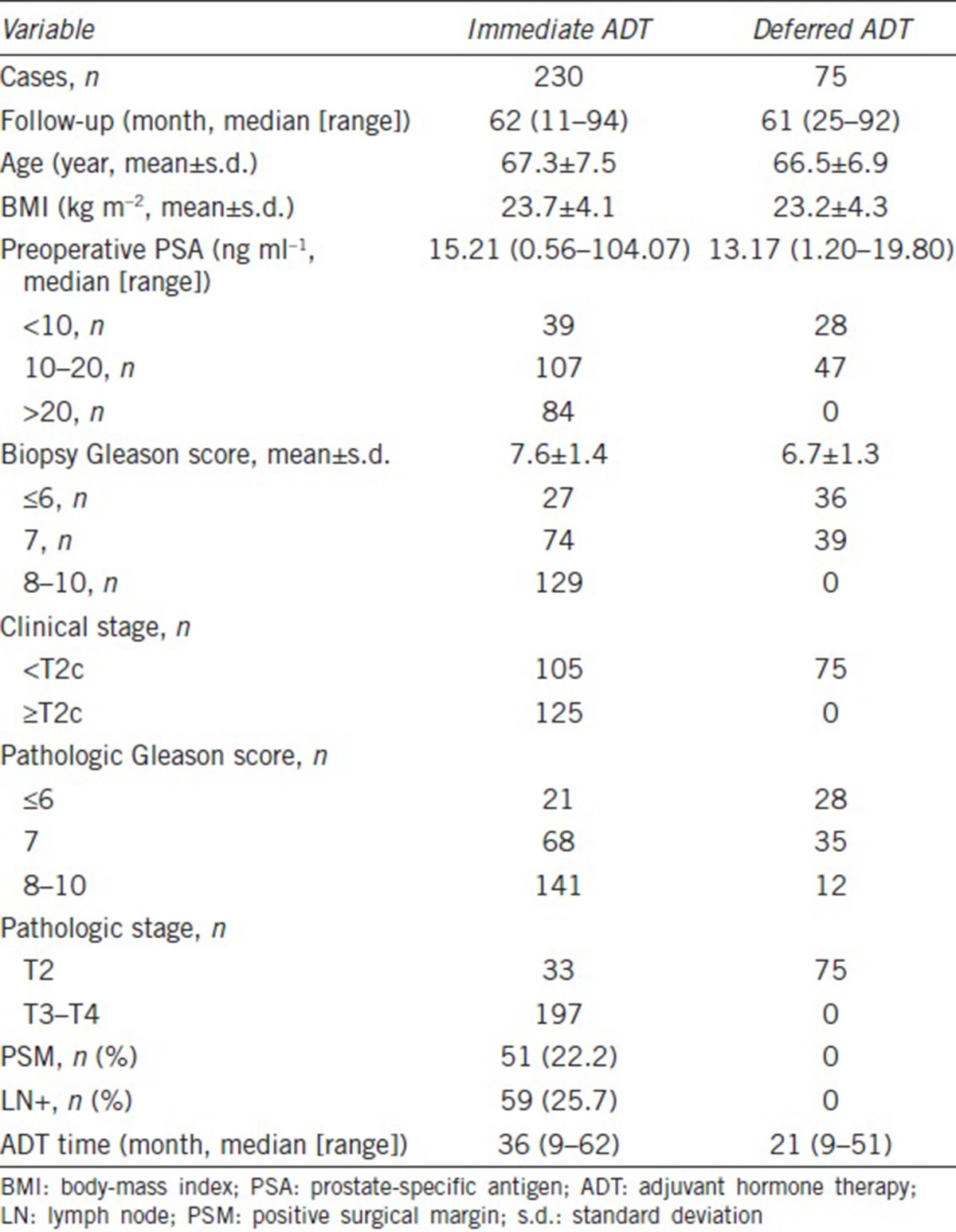
We conducted an analysis of 305 patients with PCa who underwent laparoscopic radical prostatectomy (LRP) plus pelvic LN dissection and ADT from October 2000 to December 2015. The relevant characteristics of the 305 patients are as follows: 230 patients with high-risk PCa, according to the D’Amico risk stratification scheme (clinical stage ≥T2c or Gleason score ≥8 or PSA >20 ng ml−1), had positive surgical margins or PT3-4N0-1M0.13,14 These patients underwent immediate ADT (within 3 months after surgery regardless of postoperative serum PSA levels). Seventy-five patients with intermediate risk (PSA <20 ng ml−1, clinical stage <T2c, PT2N0M0, and negative surgical margin)15,16 PCa underwent deferred ADT (after two consecutive serum PSA values = 0.2 μg l−1, or those whose disease progression was confirmed by radiological examination or core biopsy after LRP) (Table 1).
Table 1.
Baseline demographics of patients
Surgical techniques
RP was performed by a senior urological surgeon and team who had performed more than 1000 LRP procedures. The details of the surgical techniques were previously described.17,18
Pathologic evaluation, tumor grading, and disease staging
The 1997 system that staged PCa according to tumor, node, and metastasis (TNM) was used to evaluate patients treated before 2002. The American Joint Committee on Cancer and 2002 TNM staging systems were used to evaluate patients treated after 2002. A positive surgical margin was defined as the presence of tumor tissue on the inked surface of the specimen, accompanied by the patients’ outcomes noted in the original pathology reports.19
Follow-up
Follow-up examinations were performed every 3 months during the 1st year after surgery, every 6 months during the 2nd year, and annually thereafter. Follow-up examinations included a consultation, digital rectum examination, serum PSA level, cancer progression status, and survival. BCR was defined as two consecutive serum PSA values ≥0.2 μg l−1 after LRP.20 Clinical progression was defined as localized recurrence or systemic metastatic lesion verified using biopsy tissue or imaging techniques. Recurrence in the deferred ADT group was defined by the presence of BCR or clinical progression. cancer-specifc mortality (CSM) was defined as death caused by prostate cancer or cancer-related death.21
Bisulfite pyrosequencing
Genomic DNA extracted from formalin-fixed paraffin- embedded samples was quantified using a ND-2000 spectrophotometer (Nano-Drop Technologies, Thermo Fisher Scientific Inc., Waltham, MA, USA) and modified using an EpiTect Bisulfite Kit (Cat. No. 59104, Qiagen, Hilden, Germany). Bisulfite conversion was performed using thermal cycling conditions. After DNA was converted and depurinated, PCR was performed using a PyroMark PCR kit (Cat. No. 978703, Qiagen, Hilden, Germany) at the recommended cycling conditions, and the amplified DNA was subsequently confirmed. A standard pyrosequencing sample preparation protocol was applied.22 We used a Biotage Q96 Vacuum Workstation to separate, denature, and wash the amplicons, which were then mixed with the pyrosequencing primers in annealing buffer. PyroGold reagents were used for the pyrosequencing reaction at room temperature after primer annealing, and the signal was analyzed using a PSQ 96MA system (Biotage, Uppsala, Sweden). Target CGs were evaluated using software (PSQ96MA 2.1) included with the instrument, which converted the programs into the numerical values of the peak heights and calculated the proportion of methylation at each base, represented as the C/T ratio. The average C/T ratio of the two CpG sites (upstream target start sites −848 and −841) was defined as the final CRMP4 methylation score of a sample. Our previous study suggests that CRMP4 promoter hypermethylation (≥15.0% methylated) is significantly associated with the development of LN metastasis (P < 0.001).23 The primers used in this study are listed in Table 2.
Table 2.
Primers used in this study

CAPRA-S score
The CAPRA-S score was determined according to the preoperative PSA level, pathological Gleason score (pGS), surgical margin (SM), presence or absence of extracapsular extension, seminal vesicle invasion (SVI), and LN involvement (LNI). Patients who did not undergo lymphadenectomy were assumed not to have LNI. CAPRA-S scores were dichotomized according to the cutoff values (≥6 and <6) of a previously reported high-risk group.11
Statistical analysis
The data were analyzed using Statistical Product and Service Solutions 19.0 software (SPSS Inc., Chicago, IL, USA). The data are presented as the mean ± standard deviation. The t-test was employed to compare groups, χ2 test was employed to compare data, and Fisher's exact test was employed when the minimum frequency = 0. Progression-free survival (PFS) and cancer-specific survival (CSS) were the primary indexes used for this analysis. The association between CRMP4 methylation levels and the CAPRA-S score with PFS and CSS was assessed using uni- and multi-variable Cox proportional hazards models after adjusting for high-risk clinical parameters as follows: preoperative PSA, biopsy Gleason score, LN status, and seminal vesicle (SV) status.12 Survival was analyzed using Kaplan-Meier method. Differences between groups were assessed using the log-rank test. Receiver operating characteristic (ROC) curves were generated to evaluate the performance of each risk model, and the area under the curve (AUC) was calculated to estimate the power of each model to predict tumor progression and CSM. All P values are two sided and those <0.05 were considered statistically significant.
RESULTS
After the surgery, 230 (75.4%) patients received immediate ADT and 75 (24.6%) patients first received deferred ADT after a median follow-up of 2.1 years. The average ages of the immediate and deferred ADT groups were 67.3 years (range: 57.8–74.8 years) and 66.5 years (range: 59.6–73.4 years), respectively. The median follow-up time for the immediate and deferred ADT groups was 62 months (range: 11–94 months) and 61 months (range: 25–92 months), respectively. The median exposure time of the immediate and deferred ADT groups to ADT was 36 months and 21 months, respectively. Other clinical data are presented in Table 1.
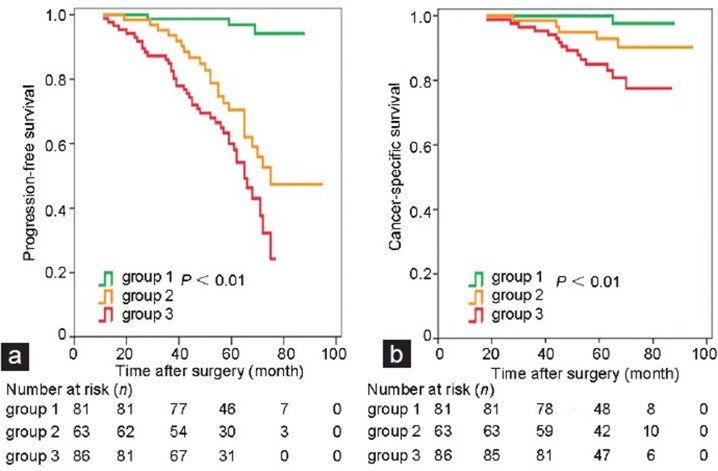
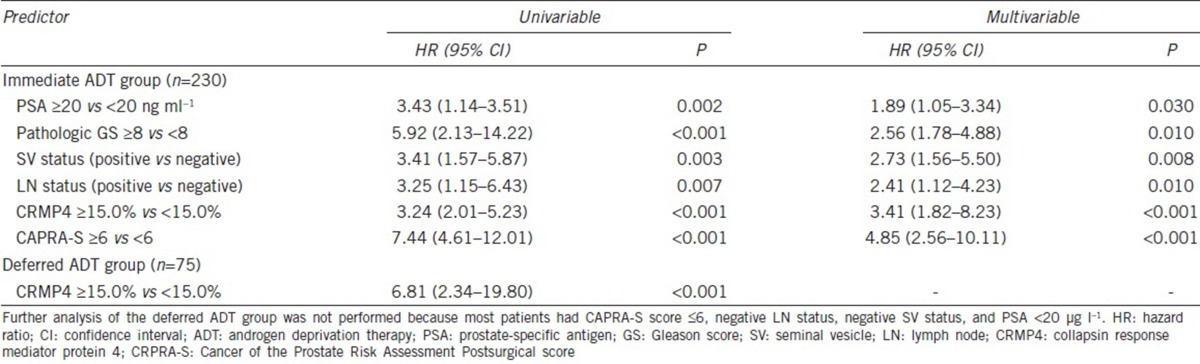
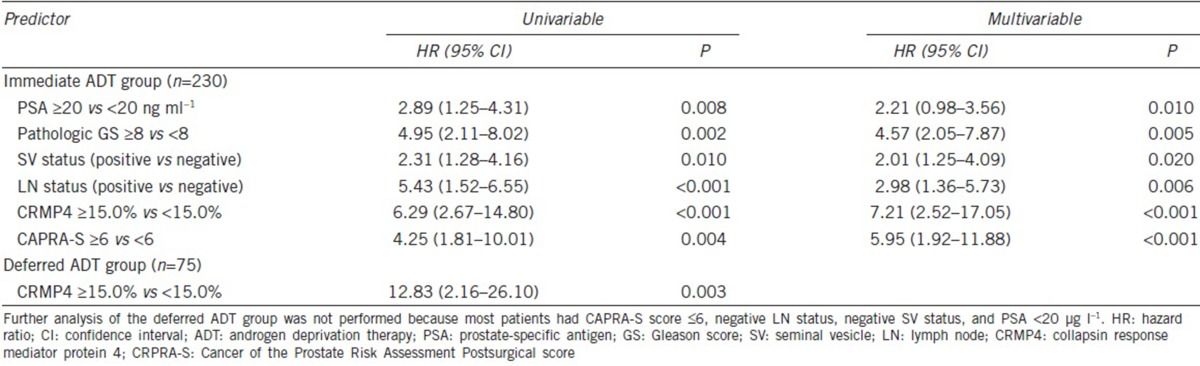
Uni- and multi-variable analyses revealed that CRMP4 methylation levels, CAPRA-S, preoperative PSA, biopsy Gleason score, LN status, and SV status were significant independent predictors of PFS and CSS (Figure 1a and 1b). For CRMP4 methylation levels ≥15%, the hazard ratios (HRs) for tumor progression and CSM were 3.24 (95% CI: 2.01–5.23, P < 0.001) and 6.29 (95% CI: 2.67–14.80, P < 0.001), respectively. For CAPRA-S score ≥6, the HRs for tumor progression and CSM were 7.44 (95% CI: 4.61–12.01, P < 0.001) and 4.25 (95% CI: 1.81–10.01, P = 0.004), respectively (Table 3 and 4). The combination of CAPRA-S score ≥6 and CRMP4 methylation levels ≥15% predicted rates as 84.9%, 60.0%, and 85.0% for 3-year PFS, 5-year PFS, and 5-year CSS, respectively. The combination of CAPRA-S score ≥6 and CRMP4 methylation levels <15% or CAPRA-S score <6 and CRMP4 methylation levels ≥15% predicted rates as 93.6%, 70.4%, and 92.9% for 3-year PFS, 5-year PFS, and 5-year CSS, respectively. Further, CAPRA-S score <6 and CRMP4 methylation levels <15% predicted rates as 100%, 96.9%, and 100% for 3-year PFS, 5-year PFS, and 5-year CSS rates, respectively. The differences in PFS rates between each combination were statistically significant (P < 0.05). The differences in the CSS rates between the combination of CRMP4 methylation levels ≥15% with CAPRA-S score ≥6 and the combination of CAPRA-S score <6 with CRMP4 methylation levels <15% were statistically significant (P < 0.001). In contrast, the differences between the other two comparisons were not statistically significant (Figure 2).
Figure 1.
Survival analysis of the immediate androgen deprivation therapy group. Subgroups 1, 2, and 3 had CAPRA-S score <6 and CRMP4 methylation levels <15%; CAPRA-S score ≥6 or CRMP4 methylation levels ≥15%, and CAPRA-S score ≥6 and CRMP4 methylation levels ≥15%, respectively. (a) The difference in the PFS rate between each combination was statistically significant (P < 0.05). (b) The differences in CSS rates between Groups 1 and 3 were statistically significant (P < 0.001). The differences between Groups 2 and 1 and between Groups 2 and 3 were not significant. CI: confidence interval; CRMP4: collapsin response mediator protein 4; CRPRA-S: Cancer of the Prostate Risk Assessment Postsurgical score; PFS: progression-free survival; CSS: cancer-specific survival.
Table 3.
Uni- and multi-variable Cox regression analyses of tumor progression in the immediate and deferred androgen deprivation therapy groups
Table 4.
Uni- and multi-variable Cox regression analyses of cancer-specific mortality of the immediate and deferred androgen deprivation therapy groups
Figure 2.
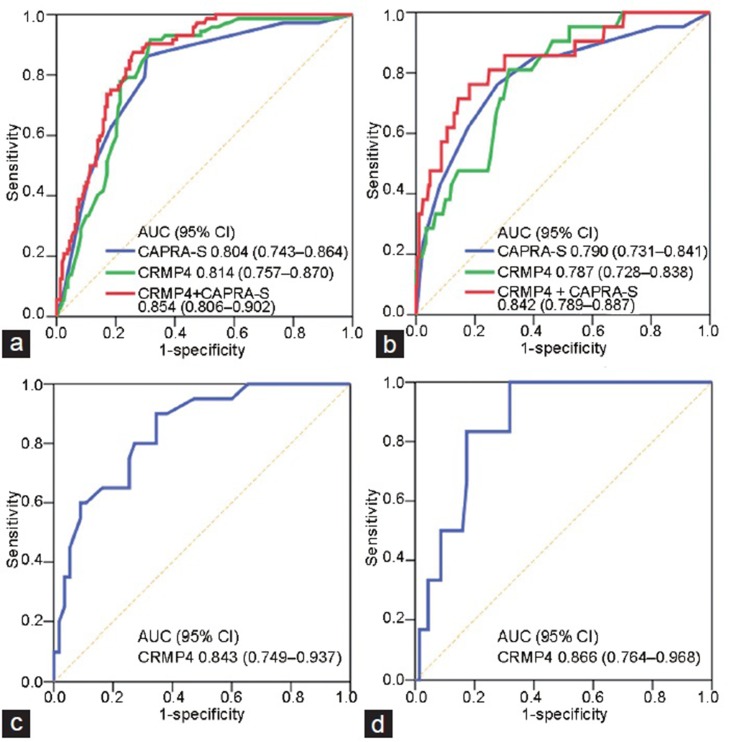
ROC curves for (a) tumor progression and (b) CSM compared with CRPRA-S score alone and combined with the CRMP4 methylation levels model of the immediate ADT group. A score combining the CAPRA-S score and CRMP4 methylation levels increased the AUC compared with the model of CRMP4 methylation levels (P = 0.045) or CAPRA-S score (P = 0.012). CAPRA-S score had the highest AUCs: 0.843 (95% CI: 0.749–0.937) for (c) tumor progression and 0.866 (95% CI: 0.764–0.968) for (d) CSM in the deferred ADT group. CI: confidence interval; CRMP4: collapsin response mediator protein 4; CRPRA-S: Cancer of the Prostate Risk Assessment Postsurgical score; ROC: receiver operating characteristic; CSM: cancer-specific mortality; ADT: androgen deprivation therapy; AUC: area under the curve.
CRMP4 methylation levels and CAPRA-S score had the highest AUC values for tumor progression as follows: AUC = 0.814 (95% CI: 0.757–0.870) and AUC = 0.804 (95% CI: 0.743–0.864), respectively. The combined model including CRMP4 methylation levels and CAPRA-S score with AUC = 0.854 (95% CI: 0.806–0.902) was an improved predictor of tumor progression compared with a model employing CRMP4 methylation levels (P = 0.0445) or CAPRA-S score (P = 0.0122). The combination of CRMP4 methylation levels and CAPRA-S score was an improved predictor of tumor progression. The AUC value for the combinations (AUC = 0.842 [95% CI: 0.789–0.887]) associated with CSM was higher compared with that of CRMP4 methylation levels (AUC = 0.787 [95% CI: 0.728–0.838]) or CAPRA-S score (AUC = 0.790 [95% CI: 0.731–0.841]) although the difference was not statistically significant (Figure 2a and 2b).
In the deferred ADT group with CRMP4 methylation levels ≥15%, the HRs for tumor progression and with CSM as a continuous variable were 6.81 (95% CI: 2.34–19.80, P < 0.001) and 12.83 (95% CI: 2.16–26.10, P = 0.0026), respectively, indicating that CRMP4 methylation levels ≥15% were a significant independent factor associated with a poor prognosis of patients with intermediate-risk PCa. The AUC values for CAPRA-S score were the highest (AUC = 0.843 [95% CI: 0.749–0.937]) for tumor progression and CSM (AUC = 0.866 [95% CI: 0.764–0.968]), respectively (Figure 2c and 2d). We were unable to conduct an analysis of CAPRA-S score of the deferred ADT group because CAPRA-S score was ≤6 for most patients and CAPRA-S score alone was insufficient to identify patients with a high risk of poor prognosis.
DISCUSSION
The natural history of PCa can differ significantly; for example, these tumors may not progress or cause symptoms, or the cells can acquire a highly invasive phenotype that metastasizes rapidly and causes death.24 Localized PCa responded well to most current treatments although the risk for recurrence increased when locally advanced PCa was treated using monotherapy.25 The choice of treatment for locally advanced PCa is, therefore, a significant clinical issue.
Adjuvant radiotherapy (AT) is considered the optimum treatment for patients with stage-pT3 PCa after undergoing RP.26,27,28,29,30 In our institution, at least 100 patients who received AT experienced disease recurrence. However, investigators at other institutions found that numerous patients with stage-pT3 disease did not receive radiotherapy after RP.29 Slow recovery from urinary incontinence or other long-term urinary complications, including bladder neck contracture and bowel symptoms, occurred during or after AT.30,31,32 The application of radiotherapy to Chinese patients with PCa is not universal and has not been fully developed as the optimum therapy. However, the requirement for adjuvant hormone therapy (AHT) after RP is controversial.
The data published by the Veterans Administration Cooperative Urological Research Group indicated that immediate ADT to treat aggressive cancer may be beneficial for delaying disease progression and increasing the survival rate.33 For patients with PSA relapse as their only sign of early disease recurrence, it is difficult to distinguish between local and distant recurrence, which may complicate the optimal selection of an early treatment regimen.34 An adjuvant ADT applied as early as possible that may benefit patients, which conforms to the guidelines on the treatment options for PSA relapse following local treatment, is described in the updated EAU Guidelines on Prostate Cancer.35 An accurate assessment of the risk of disease progression risk and detection of early, and localized metastatic cancer may confer further comprehensive benefits upon patients who undergo individualized ADT.
The ability of CAPRA-S score to predict disease recurrence and CSM was validated by a multi-institutional study of a diverse sociodemographic clinical population.11,12 Our previous studies found that methylation of CpG sites within the CRMP4 promoter region played a key role in the downregulation of CRMP4 expression in metastatic prostate cancer, strongly indicating that the CRMP4 CpG methylation status can be used as an independent marker for early diagnosis of metastatic prostate cancer.7,8 Two CpG sites primarily contributed to reducing the transcriptional activities of the CRMP4 promoter, which were determined using bisulfite sequencing, were therefore selected for PCR and Pyrosequencing analyses. ROC curve analysis showed that the discriminative ability to determine prognosis was 100% accurate using the cutoff value of 15.0%.19 We conclude therefore that integrating CRMP4 methylation levels into clinical practice for individualized patient risk prediction models can significantly improve the management of patients with highly advanced prostate cancer.
In our center, certain high-risk patients with PCa of positive surgical margins or of stage PT3-4N0-1M0, who refused radiotherapy, were treated with ADT within 3 months after surgery and achieved satisfactory overall tumor control. These findings are consistent with those of other studies.36,37,38,39,40 For patients in immediate ADT group with CAPRA-S score <6 and CRMP4 methylation levels <15%, low tumor progression rates, and CSM, we believe it is reasonable to perform only postoperative follow-up examinations to avoid over-treatment. CAPRA-S score ≥6 and CRMP4 methylation levels ≥15% would be expected to be associated with a poor outcome. Therefore, early ADT benefitted patients by delaying cancer progression, improving overall survival (OS), and ensuring improved outcomes of cancer control. CAPRA-S score ≥6 and CRMP4 methylation levels ≥15 are variables significantly associated with a poor prognosis. If CAPRA-S score or CRMP4 methylation levels exceed the threshold, we believe that to select a method that comprehensively discriminates between patients’ outcomes, the timing of adjuvant therapy should be determined using other clinical indicators such as pGS, SVI, and LNI. For example, high pGS, SVI, and LNI are high-risk factors associated with cancer progression and metastasis,12 indicating that this subpopulation of patients may achieve improved long-term cancer control outcomes if they are managed using surgery combined with adjuvant hormone therapy.40,41
In the present study, patients at intermediate risk of PCa did not routinely undergo immediate ADT after LRP until two consecutive serum PSA values = 0.2 μg l−1 or when symptomatic progression occurred. Further, the chance of progression and the rate of CSM for patients with localized PCa are low, and additional ADT may impair the health of patients and their quality of life.39 However, the potential risk of tumor progression and metastasis exists in patients with low- or intermediate-risk PCa.42,43,44 A minimum two-point increase in the CAPRA-S score indicates at least a doubling of the risk of recurrence.12
The establishment of thresholds for assigning CAPRA-S score among low-risk (0–2), intermediate-risk (3–5), and high-risk (6–12) patients should facilitate their use as a risk stratification tool in clinical research.12 However, most patients with intermediate risk have CAPRA-S score ≤6, whereas the CAPRA-S score alone is insufficient to identify patients at a high risk of poor prognosis. In the present study, CRMP4 methylation levels ≥15% were significantly associated with poor prognosis of the deferred ADT group. The ROC curve indicated that CRMP4 methylation levels had high diagnostic efficiency. Therefore, CRMP4 methylation levels can be used to exclude patients with an increased risk of tumor progression and metastasis, while most were classified with low or intermediate risk of PCa and CAPRA-S score <6.
The frequency of detection of locally advanced PCa is high in China because PSA screening is not universal.45 Moreover, early hormone therapy administered to selected patients compared with those who did or did not receive hormone therapy after detection of cancer progression will improve the rates of recurrence-free survival and OS.37,40,41,46 However, the molecular mechanisms of progression and metastasis of locally advanced PCa are unknown. Further, the sensitivities of the conventional methods (e.g., computerized tomography, bone scanning, magnetic resonance imaging, and fluorodeoxyglucose positron emission tomography47,48,49,50) are low for detecting early-stage metastatic cancer. Conventional assessment using the serum PSA level for postoperative BCR requires long-term follow-up. In contrast, CRMP4 methylation levels and CAPRA-S score can be used to quickly assess prognosis after RP. Therefore, the risk assessment model comprising CRMP4 methylation levels and CAPRA-S score makes possible early and convenient assessment of prognosis to guide the selection of optimized timing for adjuvant therapy before disease progression can be detected using standard clinical techniques such as imaging and biochemical analyses.
The limitations of the present study are as follows: first, a relatively small population of patients from a single institution was enrolled, which may introduce selection bias. For example, the CAPRA-S score and CRMP4 methylation levels of patients with intermediate risk in the deferred ADT group were poorly distributed, which may reduce the efficacy of the test. Second, the cutoff value of CRMP4 methylation levels requires further evaluation using larger cohorts from multiple institutions in different countries, which may achieve increased accuracy of evaluating metastatic potential and prognosis. Moreover, longer follow-up will likely improve the efficacy of the assessment of the effects of adjuvant ADT on PFS, CSS, and OS.
CONCLUSIONS
We demonstrated that CRMP4 methylation levels and CAPRA-S score provided improved prognostic value for cancer control in patients who underwent LRP. Thus, combined analysis may be useful for clinicians to design individualized treatment regimens.
AUTHOR CONTRIBUTIONS
QXH and CTX participated in the study design and coordination and drafted the manuscript. ZC and MHL performed quantification of CRMP4 methylation. JP organized the pathological data. JMD and ZHL participated in the follow-up and performed the statistical analyses. XG conceived the study, participated in the study design and structure analysis, and edited the final manuscript for publication. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (81172430, 81372728, 81572503), the Science and Technology Planning Project of Guangdong Province, China (2012A030400009), and the Clinical Medical Research and Transformation Centre Projects of Guangzhou, China (201604020006).
REFERENCES
- 1.Pang C, Guan Y, Li H, Chen W, Zhu G. Urologic cancer in China. Jpn J Clin Oncol. 2016;46:497–501. doi: 10.1093/jjco/hyw034. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Dorin RP, Daneshmand S, Lassoff MA, Cai J, Skinner DG, et al. Long-term outcomes of open radical retropubic prostatectomy for clinically localized prostate cancer in the prostate-specific antigen era. Urology. 2012;79:626–31. doi: 10.1016/j.urology.2011.09.051. [DOI] [PubMed] [Google Scholar]
- 4.Attard G, Parker C, Eeles RA, Schroder F, Tomlins SA, et al. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell RE, Katz MH, McKiernan JM, Benson MC. The evaluation and staging of clinically localized prostate cancer. Nat Clin Pract Urol. 2005;2:356–7. doi: 10.1038/ncpuro0260. [DOI] [PubMed] [Google Scholar]
- 6.Conran CA, Brendler CB, Xu J. Personalized prostate cancer care: from screening to treatment. Asian J Androl. 2016;18:505–8. doi: 10.4103/1008-682X.179529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao X, Pang J, Li LY, Liu WP, Di JM, et al. Expression profiling identifies new function of collapsin response mediator protein 4 as a metastasis-suppressor in prostate cancer. Oncogene. 2010;29:4555–66. doi: 10.1038/onc.2010.213. [DOI] [PubMed] [Google Scholar]
- 8.Li K, Pang J, Cheng H, Liu WP, Di JM, et al. Manipulation of prostate cancer metastasis by locus-specific modification of the CRMP4 promoter region using chimeric TALE DNA methyltransferase and demethylase. Oncotarget. 2015;6:10030–44. doi: 10.18632/oncotarget.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooperberg MR, Pasta DJ, Elkin EP, Litwin MS, Latini DM, et al. The University of California, San Francisco cancer of the prostate risk assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–42. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao KH, Hernandez DJ, Han M, Humphreys EB, Mangold LA, et al. External validation of University of California, San Francisco, cancer of the prostate risk assessment score. Urology. 2008;72:396–400. doi: 10.1016/j.urology.2007.11.165. [DOI] [PubMed] [Google Scholar]
- 11.Cooperberg MR, Davicioni E, Crisan A, Jenkins RB, Ghadessi M, et al. Combined value of validated clinical and genomic risk stratification tools for predicting prostate cancer mortality in a high-risk prostatectomy cohort. Eur Urol. 2015;67:326–33. doi: 10.1016/j.eururo.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: a straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011;117:5039–46. doi: 10.1002/cncr.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2016;71:618–29. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Spahn M, Briganti A, Capitanio U, Kneitz B, Gontero P, et al. Outcome predictors of radical prostatectomy followed by adjuvant androgen deprivation in patients with clinical high risk prostate cancer and pT3 surgical margin positive disease. J Urol. 2012;188:84–90. doi: 10.1016/j.juro.2012.02.2572. [DOI] [PubMed] [Google Scholar]
- 15.Beauval JB, Ploussard G, Cabarrou B, Roumiguie M, Ouzzane A, et al. Improved decision making in intermediate-risk prostate cancer: a multicenter study on pathologic and oncologic outcomes after radical prostatectomy. World J Urol. 2016 doi: 10.1007/s00345-016-1979-z. doi: 10.1007/s00345-016-1979-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Meng MV, Elkin EP, Latini DM, Duchane J, Carroll PR. Treatment of patients with high risk localized prostate cancer: results from cancer of the prostate strategic urological research endeavor (CaPSURE) J Urol. 2005;173:1557–61. doi: 10.1097/01.ju.0000154610.81916.81. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Qiu JG, Zhang B, Cai YB, Hong LQ. Re: nerve sparing laparoscopic radical prostatectomy. Asian J Androl. 2003;5:338. [PubMed] [Google Scholar]
- 18.Chen MK, Luo Y, Zhang H, Qiu JG, Wen XQ, et al. Laparoscopic radical prostatectomy plus extended lymph nodes dissection for cases with non-extra node metastatic prostate cancer: 5-year experience in a single Chinese institution. J Cancer Res Clin Oncol. 2013;139:871–8. doi: 10.1007/s00432-013-1395-3. [DOI] [PubMed] [Google Scholar]
- 19.Iczkowski KA, Lucia MS. Frequency of positive surgical margin at prostatectomy and its effect on patient outcome. Prost Cancer. 2011;2011:673021. doi: 10.1155/2011/673021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lughezzani G, Gallina A, Larcher A, Briganti A, Capitanio U, et al. Radical prostatectomy represents an effective treatment in patients with specimen-confined high pathological Gleason score prostate cancer. BJU Int. 2013;111:723–30. doi: 10.1111/j.1464-410X.2012.11114.x. [DOI] [PubMed] [Google Scholar]
- 21.Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162:433–8. [PubMed] [Google Scholar]
- 22.Tost J, Gut IG. Analysis of gene-specific DNA methylation patterns by pyrosequencing technology. Methods Mol Biol. 2007;373:89–102. doi: 10.1385/1-59745-377-3:89. [DOI] [PubMed] [Google Scholar]
- 23.Gao X, Li LY, Rassler J, Pang J, Chen MK, et al. Prospective study of CRMP4 promoter methylation in prostate biopsies as a predictor for lymph node metastases. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw282. pii: djw282. [DOI] [PubMed] [Google Scholar]
- 24.Khoddami M, Khademi Y, Kazemi Aghdam M, Soltanghoraee H. Correlation between Gleason scores in needle biopsy and corresponding radical prostatectomy specimens a twelve-year review. Iran J Pathol. 2016;11:120–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Gakis G, Boorjian SA, Briganti A, Joniau S, Karazanashvili G, et al. The role of radical prostatectomy and lymph node dissection in lymph node-positive prostate cancer: a systematic review of the literature. Eur Urol. 2014;66:191–9. doi: 10.1016/j.eururo.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 26.Thompson IM, Valicenti RK, Albertsen P, Davis BJ, Goldenberg SL, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441–9. doi: 10.1016/j.juro.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Lin T, Zhou Y, Li D, Xu K, et al. Adjuvant and salvage radiotherapy after prostatectomy: a systematic review and meta-analysis. PLoS One. 2014;9:e104918. doi: 10.1371/journal.pone.0104918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daly T, Hickey BE, Lehman M, Francis DP, See AM. Adjuvant radiotherapy following radical prostatectomy for prostate cancer. Cochrane Database Syst Rev. 2011;12:CD007234. doi: 10.1002/14651858.CD007234.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Ghia AJ, Shrieve DC, Tward JD. Adjuvant radiotherapy use and patterns of care analysis for margin-positive prostate adenocarcinoma with extracapsular extension: postprostatectomy adjuvant radiotherapy: a SEER analysis. Urology. 2010;76:1169–74. doi: 10.1016/j.urology.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 30.Suardi N, Gallina A, Lista G, Gandaglia G, Abdollah F, et al. Impact of adjuvant radiation therapy on urinary continence recovery after radical prostatectomy. Eur Urol. 2014;65:546–51. doi: 10.1016/j.eururo.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann T, Gaensheimer S, Buchner A, Rohloff R, Schilling A. An unrandomized prospective comparison of urinary continence, bowel symptoms and the need for further procedures in patients with and with no adjuvant radiation after radical prostatectomy. BJU Int. 2003;92:360–4. doi: 10.1046/j.1464-410x.2003.04365.x. [DOI] [PubMed] [Google Scholar]
- 32.Sowerby RJ, Gani J, Yim H, Radomski SB, Catton C. Long-term complications in men who have early or late radiotherapy after radical prostatectomy. Can Urol Assoc J. 2014;8:253–8. doi: 10.5489/cuaj.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byar DP, Corle DK. Hormone therapy for prostate cancer: results of the veterans administration cooperative urological research group studies. NCI Monogr. 1988;7:165–70. [PubMed] [Google Scholar]
- 34.Mehta SS, Lubeck DP, Sadetsky N, Pasta DJ, Carroll PR. Patterns of secondary cancer treatment for biochemical failure following radical prostatectomy: data from CaPSURE. J Urol. 2004;171:215–9. doi: 10.1097/01.ju.0000100087.83112.23. [DOI] [PubMed] [Google Scholar]
- 35.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–79. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Chang K, Qin XJ, Zhang HL, Dai B, Zhu Y, et al. Comparison of two adjuvant hormone therapy regimens in patients with high-risk localized prostate cancer after radical prostatectomy: primary results of study CU1005. Asian J Androl. 2016;18:452–5. doi: 10.4103/1008-682X.160884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messing EM, Manola J, Yao J, Kiernan M, Crawford D, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–9. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 38.Dorff TB, Flaig TW, Tangen CM, Hussain MH, Swanson GP, et al. Adjuvant androgen deprivation for high-risk prostate cancer after radical prostatectomy: SWOG S9921 study. J Clin Oncol. 2011;29:2040–5. doi: 10.1200/JCO.2010.32.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schubert M, Joniau S, Gontero P, Kneitz S, Scholz CJ, et al. The role of adjuvant hormonal treatment after surgery for localized high-risk prostate cancer: results of a matched multiinstitutional analysis. Adv Urol. 2012;2012:612707. doi: 10.1155/2012/612707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rove KO, Crawford ED. Traditional androgen ablation approaches to advanced prostate cancer: new insights. Can J Urol. 2014;21:14–21. [PubMed] [Google Scholar]
- 41.Tsurumaki Sato Y, Fukuhara H, Suzuki M, Fujimura T, Nakagawa T, et al. Long-term results of radical prostatectomy with immediate adjuvant androgen deprivation therapy for pT3N0 prostate cancer. BMC Urol. 2014;14:13. doi: 10.1186/1471-2490-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu M, Zhang L, Liang C. Is it appropriate to conduct conventional active surveillance for Asian men with low-risk prostate cancer? Int Urol Nephrol. 2016;48:1287–9. doi: 10.1007/s11255-016-1287-y. [DOI] [PubMed] [Google Scholar]
- 43.Hwang I, Lim D, Jeong YB, Park SC, Noh JH, et al. Upgrading and upstaging of low-risk prostate cancer among Korean patients: a multicenter study. Asian J Androl. 2015;17:811–4. doi: 10.4103/1008-682X.143751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoue T, Kinoshita H, Inui H, Komai Y, Nakagawa M, et al. Pathological outcomes of Japanese men eligible for active surveillance after radical prostatectomy. Int J Clin Oncol. 2014;19:379–83. doi: 10.1007/s10147-013-0553-6. [DOI] [PubMed] [Google Scholar]
- 45.Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–9. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 46.Bolla M, Collette L, Blank L, Warde P, Dubois JB, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 47.Bjurlin MA, Rosenkrantz AB, Beltran LS, Raad RA, Taneja SS. Imaging and evaluation of patients with high-risk prostate cancer. Nat Rev Urol. 2015;12:617–28. doi: 10.1038/nrurol.2015.242. [DOI] [PubMed] [Google Scholar]
- 48.Dou S, Bai Y, Shandil A, Ding D, Shi D, et al. Detecting prostate cancer and prostatic calcifications using advanced magnetic resonance imaging. Asian J Androl. 2016 doi: 10.4103/1008-682X.177840. [Doi: 10.4103/1008-682X177840] [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris MJ, Akhurst T, Osman I, Nunez R, Macapinlac H, et al. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology. 2002;59:913–8. doi: 10.1016/s0090-4295(02)01509-1. [DOI] [PubMed] [Google Scholar]
- 50.Priester A, Natarajan S, Khoshnoodi P, Margolis DJ, Raman SS, et al. Magnetic resonance imaging underestimation of prostate cancer geometry: use of patient specific molds to correlate images with whole mount pathology. J Urol. 2016;197:320–6. doi: 10.1016/j.juro.2016.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]