Abstract
Sperm DNA damage is recognized as an important biomarker of male infertility. To investigate this, sperm DNA damage was assessed by the sperm chromatin dispersion (SCD) test in semen and motile spermatozoa harvested by combined density gradient centrifugation (DGC) and swim-up in 161 couples undergoing in vitro fertilization (IVF). Semen analysis and sperm DNA damage results were compared between couples who did or did not achieve pregnancy. The sperm DNA damage level was significantly different between the two groups (P < 0.05) and was negatively correlated with IVF outcomes. Logistic regression analysis confirmed that it was an independent predictor for achieving clinical pregnancy. The effects of different levels of sperm DNA damage on IVF outcomes were also compared. There were significant differences in day 3 embryo quality, blastocyst formation rate, and implantation and pregnancy rates (P < 0.05), but not in the basic fertilization rate between the two groups. Thus, sperm DNA damage as measured by the SCD appears useful for predicting the clinical pregnancy rate following IVF.
Keywords: DNA damage, in vitro fertilization, pregnancy outcome, sperm
INTRODUCTION
Approximately 10%–15% of couples of reproductive age are unable to conceive within 12 consecutive months of unprotected intercourse and are characterized clinically as infertile. Male factors are implicated in almost 50% of cases, either solely (20%) or in combination with female factors (30%−40%).1,2 Assessment of male infertility has traditionally been based on semen analysis classified according to the World Health Organization (WHO) standards,3 which include semen volume, and sperm concentration, motility, and morphology. However, approximately 15% of men with normal basic semen parameters have been diagnosed as infertile.4,5
Obviously, we need more sophisticated testings to determine the functional etiology of male infertility and its relation to reproductive outcomes. Sperm DNA damage is being recognized as a new parameter of semen quality and plays a crucial role in fertilization, implantation, and transmission of paternal genetic information to the offspring.5,6 Many studies have found an adverse effect of sperm DNA damage on the outcomes of in vitro fertilization (IVF).7,8,9,10 However, the clinical value of tests for sperm DNA damage is inconclusive.
The packaging of DNA within the sperm head is the result of a complicated process requiring extensive compaction and remodeling of the chromatin during the final stages of spermatogenesis. Normal mature sperm DNA is highly resistant to physical or chemical denaturation. At least three important mechanisms could explain sperm DNA damage. First, defective chromatin condensation during spermiogenesis might induce DNA damage.11 Second, apoptosis might lead to the functional elimination of possibly defective germ cells from the gene pool.12 Oxidative stress is thought to be another potential cause of sperm DNA damage.13
Currently, there are four widely used methods for the measurement of sperm DNA damage, including the comet assay, terminal deoxyuridine nick end labeling (TUNEL) assay of apoptosis, the sperm chromatin structure assay (SCSA), and the sperm chromatic dispersal (SCD) assay.14,15,16,17 Despite differences in the principle and methodology of these assays, the levels of DNA damage measured by these assays show some degree of correlation.18 The SCD assay is based on the principle that spermatozoa with damaged DNA will fail to produce a characteristic “halo” of dispersed DNA following acid denaturation and removal of nuclear proteins.18
The SCD assay has been applied to assess the extent of sperm DNA damage and to predict the outcome of assisted reproductive technology (ART). Some studies have shown negative effects of sperm DNA damage on ART outcomes and provided a clinical indication for the evaluation of sperm DNA damage that has not been identified using conventional semen parameters before couples are subjected to ART.19,20
The aims of this study were as follows: first, to compare difference in sperm DNA damage between couples who did or did not achieve pregnancy following IVF; second, to test whether the sperm DNA damage was an independent predictor of clinical pregnancy in IVF; and finally, to evaluate the relationship between sperm DNA damage and outcomes after IVF.
PATIENTS AND METHODS
Patients
A total of 161 couples undergoing IVF treatments at the Family Planning Hospital of Guangdong province were included in this study (from March 2013 to September 2015). Informed consent was received from all participants. All experimental procedures and sample procurements were approved by the Ethics Committee of the hospital.
All women were aged ≤35 years and had menstrual cycle day 3 follicle-stimulating hormone (FSH) levels <10 IU−1 and a normal body mass index (BMI) range of 18–25 kg m−2. Cases with factors adversely affecting implantation, including ovarian hyperstimulation syndrome, hydrosalpinx, uterine synechiae, adenomyosis, myomas (≥4 cm in diameter or the submucous type), and uterine abnormalities were excluded from the study. Only freshly ejaculated semen samples prepared for IVF procedures were included in the study.
Preparation of semen samples
Semen samples were collected by masturbation from 161 men after 2–7 days of abstinence on the day of oocyte retrieval for IVF. Semen analyses were performed according to the fifth edition of the WHO guidelines.3 Each ejaculate was split into two parts: one (100 μl) was used directly to prepare sperm smears for SCD tests, and the other was used to harvest a motile sperm fraction by density gradient centrifugation (DGC; SpermGrad, Vitrolife Sweden AB, Göteborg, Sweden) at 300 g for 20 min using two layers of 1 ml 45% and 1 ml 90% SpermGrad, respectively. The pellet of motile sperm obtained using DGC was washed once with 2 ml IVF medium (G-IVF PLUS; Vitrolife Sweden AB, Göteborg, Sweden). Spermatozoa from the sperm pellet were allowed to swim up for 30 min in 0.5 ml IVF medium before IVF. After the swim-up, motile spermatozoa were collected with the IVF medium, adjusted to a concentration of 1 × 106 ml−1, and then left in an incubator with 5% CO2 and 37°C for 2 h to allow for equilibration from the preparation procedures. Motile spermatozoa remaining after being used for IVF were then used to make sperm smears for further SCD tests.
SCD test of sperm nuclear DNA integrity
Sperm DNA damage was assessed by SCD using sperm nuclear DNA integrity kits (Shenzhen Huakang Biomed Co., Ltd., Shenzhen, China). In brief, an aliquot of each semen sample was diluted to 5 × 106–10 × 106 ml−1 in phosphate-buffered saline. Gelled aliquots of low-melting point agarose in Eppendorf tubes were provided in the kits: each one to process a single semen sample. The tubes were placed in a water bath at 90°C–100°C for 5 min to melt the agarose, and then in a water bath at 37°C. After 5 min of equilibration at 37°C, 60 μl aliquots of the diluted semen samples were added to the Eppendorf tubes and mixed with the melted agarose. Of the semen–agarose mix, 30 μl aliquots were pipetted onto slides precoated with agarose (provided in the kits) and covered with a 22 mm × 22 mm coverslip. The slides were placed on a cold plate at 4°C for 4 min to allow the agarose to set into a microgel with sperm cells embedded. The coverslips were removed gently, and the slides were immediately immersed horizontally in an acid solution, previously prepared by mixing 0.8 ml of HCl from an Eppendorf tube in the kit with 100 ml of distilled water, and incubated for 7 min. The slides were immersed horizontally in 10 ml of the lysing solution for 20 min. After washing for 3 min in a tray with abundant distilled water, the slides were dehydrated in increasing concentrations of ethanol (70%, 90%, and 100%) for 2 min each and then air-dried. Then, the slides were covered with a mix of Wright's staining solutions A and B (1:1) for 5 min with continuous airflow. They were briefly washed 10–15 times in tap water and allowed to dry. A minimum set of 400 spermatozoa per sample was scored under a ×40 microscope objective lens. Five SCD patterns were observed: (1) sperm cells with large halos: those whose halo width is similar to or higher than the minor diameter of the core; (2) sperm cells with medium-sized halos: their halo size is between those with high or very small halo; (3) sperm cells with very small-sized halo: the halo width is similar to or smaller than one-third of the minor diameter of the core; (4) sperm cells without a halo; and (5) sperm cells without a halo and degraded: similar to (4) but weakly or irregularly stained. Calculate the percentage of sperm cells with very small-sized halo, sperm cells without a halo and degraded in total spermatozoa as sperm DNA damage.17
IVF procedure
All patients received a midluteal phase downregulation regimen (long protocol). Pituitary function was suppressed with the gonadotropin-releasing hormone agonist Triptorelin (Ferring GmbH, Kiel, Germany) until withdrawal menstrual bleeding and suppressed estradiol (E2) concentrations were achieved. Concomitant stimulation was performed with recombinant FSH (Gonal F, Merck Serono, Geneva, Switzerland). Each ovarian stimulation cycle was monitored by serial vaginal ultrasound examinations and measurement of serum E2 levels, and the dosage of gonadotropin was adjusted accordingly. Ovulation was induced with an injection of 250 μg human chorionic gonadotropin (hCG; Ovidrel, Merck Serono, Geneva, Switzerland) when at least two follicles had a diameter of at least 18 mm. Oocytes were retrieved at 36 h after hCG injection under vaginal ultrasound guidance. The culture and insemination of oocytes were performed in IVF medium (G-IVF PLUS; Vitrolife Sweden AB, Göteborg, Sweden) in an atmosphere of 6% CO2 in air at 37°C, at 39–40 h after the injection of hCG.
Embryo culture and transfer
Oocytes were assessed to determine whether fertilization had occurred at 16–18 h after insemination. Fertilization was considered normal if two pronuclei and two polar bodies were identified. Oocytes without visible pronuclei were considered to be unfertilized. Oocytes with more than two pronuclei were considered to be abnormally fertilized and were discarded. On day 3 of culture, embryos were scored from Grades 1–4 according to the number, size, and shape of blastomeres and to their degree of fragmentation.21 The grading criteria were as follows: Grade 1, no or <5% fragmentation with equal-sized cells; Grade 2, <20% fragmentation with slightly unequal-sized cells; Grade 3, 20%–50% fragmentation with obviously unequal-sized cells; and Grade 4, >50% damage or severely unequal-sized cells. Embryos classified as Grade 1 or 2 and with ≥6 cells were denoted as “good.” Embryo transfers were performed at 72 h after oocyte retrieval, and only good embryos were selected for transfer. Luteal phase support for at least 16 days after oocyte retrieval consisted of daily intramuscular injections of progesterone in oil and vaginal administration of micronized progesterone (Utrogestan, Besins Manufacturing Belgium SA, Drogenbos, Belgium).
Around noon on day 3, the remaining untransferred Grades 1–3 embryos and those with ≥4 cells were moved to G2 Plus medium (Vitrolife Sweden AB, Göteborg, Sweden) for further culture. The percentages of blastocysts formed were determined and each blastocyst was assigned a score using the system of Gardner et al.22
Pregnancy outcomes
Pregnancy was initially detected 2 weeks after embryo transfer by a positive serum β-hCG level. Ultrasound was performed at 6 weeks of gestation to confirm fetal viability. The clinical pregnancy rate (CPR) was defined from the presence of a gestational sac detected by ultrasound. Women with clinical pregnancies who miscarried before the 12th week were defined as having had a spontaneous abortion.
Statistical analysis
The statistical analysis was performed with SPSS for Windows (version 21.0, SPSS IBM Corp., Armonk, NY, USA). Parameters of ejaculated raw and washed motile spermatozoa were compared for pregnant and nonpregnant couples using independent sample Student's t-tests. The significance of any correlations between sperm DNA damage in both semen and motile spermatozoa and IVF outcomes were examined by Spearman's tests. Any predictive factors for clinical pregnancy after IVF were identified by multivariate logistic regression. Corresponding differences in reproductive outcome (e.g., age, FSH, BMI, number of oocytes, fertilization rate, good embryo formation rate, blastocyst formation rate, CPR, and abortion rates) in different groups were compared using one-way analysis of variance (ANOVA), Chi-squared tests, and Fisher's exact tests. P < 0.05 was considered statistically significant.
RESULTS
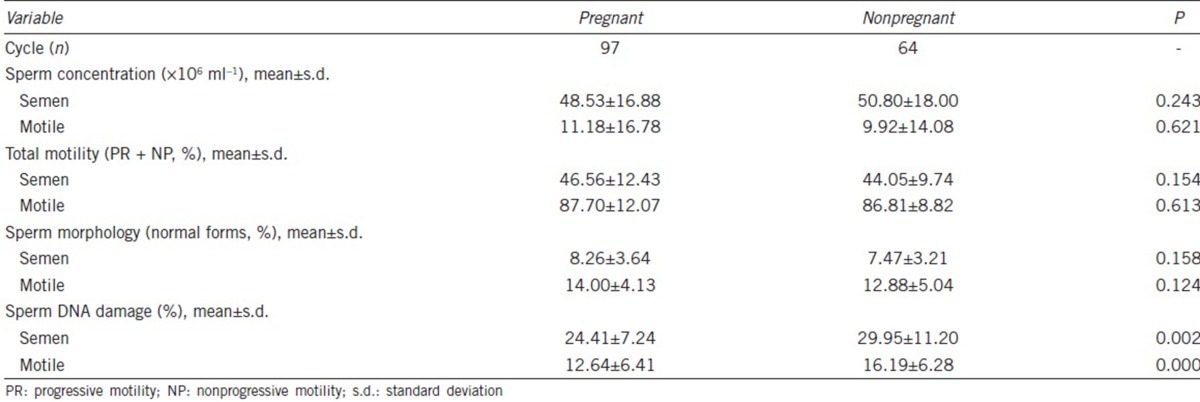
This study included a total of 161 cycles of IVF. According to the outcomes, we divided the cases into pregnant and nonpregnant groups. There was a significant difference in sperm DNA damage between groups (P < 0.05), whether from the ejaculated untreated spermatozoa or washed motile spermatozoa (Table 1).
Table 1.
Comparison of various sperm parameters on in vitro fertilization treatment
Spearman correlation coefficients between sperm tests including sperm DNA damage in both semen and purified motile spermatozoa and IVF outcomes (day 3 embryo quality, blastocyst formation and embryo implantation rates and CPR) were determined. All sperm DNA damage measures including those for semen and motile spermatozoa were significantly correlated with IVF outcomes, with an increase in DNA damage associated with decreases in embryo quality and CPR (Table 2).
Table 2.
Correlations (Spearman test) between sperm DNA damage in semen or motile spermatozoa and in vitro fertilization outcomes
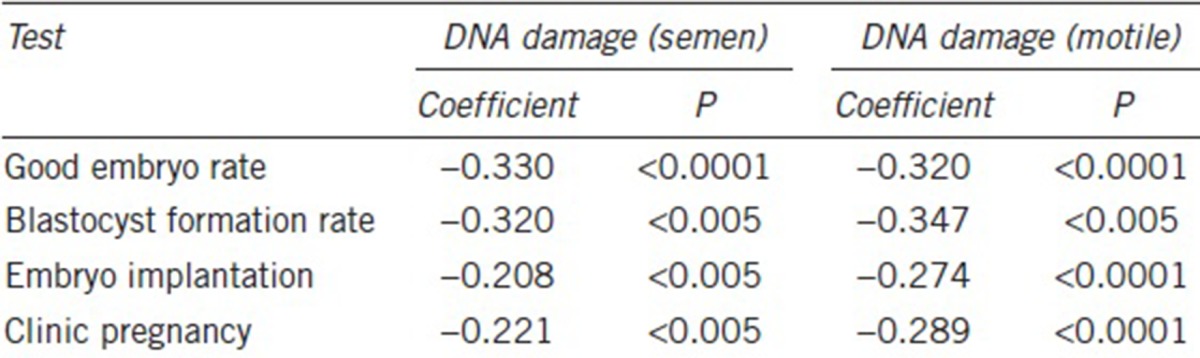
We considered female and male ages, number of oocytes retrieved, oocytes at meiosis (M) stage II, sperm concentration and total motility, sperm morphology, and sperm DNA damage in raw semen as covariates, with the clinical pregnancy rate as the dependent variable for logistic regression analysis. This confirmed that sperm DNA damage was an independent predictor of the CPR in these IVF cycles with an odds ratio of 0.279 (95% confidence interval: 0.086–0.901; Table 3).
Table 3.
Logistic regression analysis of prognostic factors and clinical pregnancy
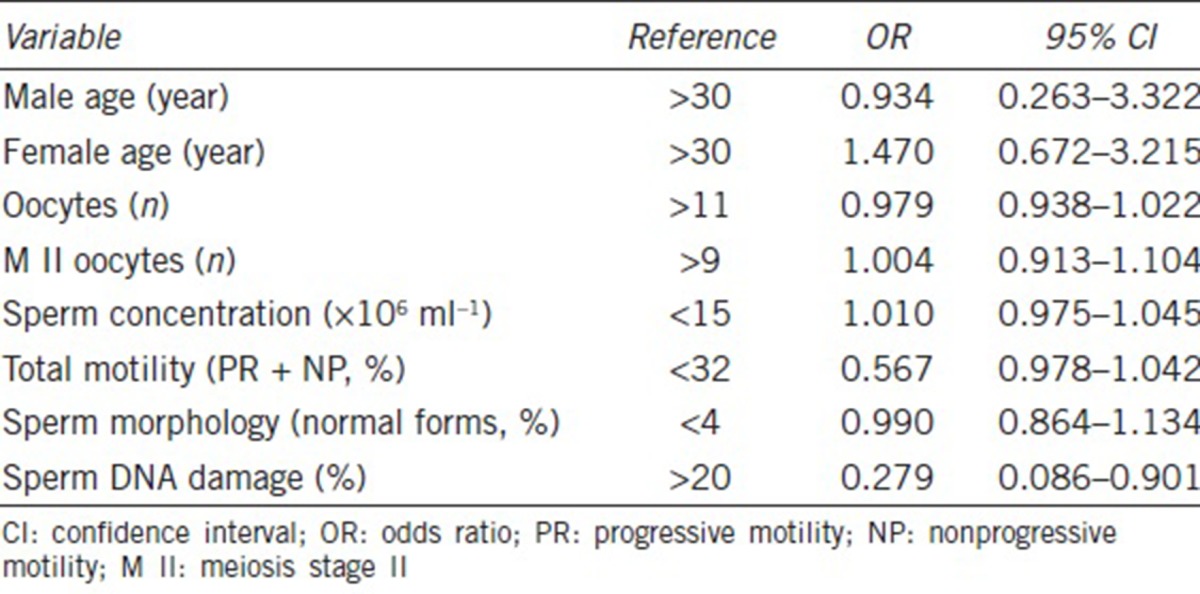
To further examine the relationship between sperm DNA damage and early embryo development, we analyzed the levels of sperm DNA damage and embryonic development after IVF. Descriptive analysis of data for couples receiving IVF is listed in Table 4. We divided cycles into three groups according to the level of DNA damage of motile spermatozoa (≤10%, 11%–20%, and ≥21%) and did not identify any statistically significant differences in the three groups with regard to female or male age, FSH level, female BMI, number of MII oocytes, or fertilization rates, but the day 3 good embryo formation, blastocyst formation, and implantation rates and CPR in the group with sperm DNA damage ≥21% were significantly lower than in the groups with ≤10% and 11%–20% damage (P < 0.05). There was a tendency for an increased spontaneous abortion rate in the three groups with increasing levels of DNA damage (6.4% vs 9.3% vs 15.4%, respectively) although no statistical difference could be detected (Table 4).
Table 4.
Comparison of various levels of sperm DNA damage in outcomes of in vitro fertilization
DISCUSSION
Some studies have suggested that measuring sperm DNA damage could be a promising tool in determining a man's fertility status; however, the relationship between this measure and ART outcomes remains uncertain.7,10,19,23,24,25,26 Some studies have suggested that this arises because different types of sperm samples were used for research into sperm DNA damage, namely, freshly ejaculated semen samples or washed motile spermatozoa.27 Here, a reduction in DNA fragmentation was seen in all samples after DGC and swim-up, and this was probably because of the removal of immotile, nonviable, and degenerated sperm. However, when we compared semen parameters and sperm DNA damage levels between the pregnant and nonpregnant groups, including freshly ejaculated spermatozoa and washed motile spermatozoa, we found a significant difference in the levels of DNA damage between the two groups, both in fresh ejaculates and washed motile spermatozoa. One reasonable explanation for this is that DGC might assist in selecting motile spermatozoa, but cannot remove the effect of sperm DNA damage on embryo development; moreover, the forces produced during centrifugation might even cause new DNA damage. Sperm DNA damage clearly affected our pregnancy outcomes following IVF regardless of whether we measured it in fresh ejaculates or in washed motile spermatozoa, and this was consistent with some previous reports.19,20
Several studies have attempted to investigate the prognostic value of sperm DNA assessment to predict ART outcomes, but the results are still controversial. Some studies have concluded that the methods for assessing sperm DNA damage are not sufficiently robust to provide any clinical advantage of these assays in evaluating infertile men and ART outcomes.10,25,26 In contrast, other studies have shown a negative effect of sperm DNA damage on outcomes and provided a clinical indication for the evaluation of sperm DNA damage before ART.19,20,28,29 Here, we confirmed that sperm DNA damage was negatively correlated with IVF outcomes (day 3 embryo quality, blastocyst formation, embryo implantation, and clinical pregnancy rates), and that sperm DNA damage was an independent predictor of achieving a clinical pregnancy.
In the present study, we divided cases into three groups according to the level of sperm DNA damage in motile spermatozoa. The analysis of IVF data and sperm DNA damage did not indicate a clear predictive power regarding fertilization rate. In fact, there is some controversy regarding the effect of sperm DNA damage on fertilization rates. Some studies reported that low fertilization rates were linked to high levels of sperm DNA damage.25,30 However, other investigators have shown that sperm DNA damage has minimal effects at the fertilization stage,31 in agreement with our finding that sperm DNA damage correlated poorly with fertilization rate in IVF. Pronuclear formation and early embryo development do not appear to be dependent on sperm DNA integrity, as the embryonic genome is only expressed after the second cell division.32,33 In addition, if the type and extent of DNA damage can be repaired by the oocyte, it is possible to achieve embryo development even in the presence of elevated sperm DNA damage.5,10,34
During early embryogenesis, the paternal genome is activated just after the 4–8-cell stage, so further development of the embryo is potentially affected by the integrity of the sperm DNA.33 In cases with elevated levels of sperm DNA damage, apoptosis and damage can also be present in the embryo, leading to slow or arrested embryo development and difficulty in reaching the blastocyst stage,35 along with low implantation and pregnancy rates.36,37 Interestingly, lower blastocyst formation and implantation rates and the CPR in this study were associated with increased levels of sperm DNA damage, consistent with previous studies.
Correlations between sperm DNA damage and abortion rates have not always been found to be statistically significant, but a tendency for this was confirmed in our study. Other studies have shown that an increased proportion of sperm DNA damage is a deleterious factor for sustaining pregnancies and results in miscarriage.5,7 Moreover, men with abnormal sperm parameters had a higher proportion of sperm sex chromosome aneuploidy than men with normal sperm parameters.38 Thus, paternal genomic abnormalities might be a significant cause of miscarriage.
CONCLUSIONS
Our data showed that sperm DNA damage was negatively correlated with day 3 embryo quality, with the blastocyst formation rate and the CPR. Therefore, addition of sperm DNA damage tests to the conventional semen analysis might improve the clinical prediction of male infertility and ART outcomes. Targeted large-scale studies are necessary to standardize the test methods, sperm variables, and reference values before these could be integrated into routine ART.
AUTHOR CONTRIBUTIONS
WWZ designed the project, conducted the SCD tests, performed embryo culture, reviewed and analyzed the data, and wrote the paper. GS recruited the patients, reviewed and analyzed the data. QLW conducted the statistical analysis. XLZ and AZ performed the andrological clinical examinations. SMD and SWL performed most of the SCD tests. YMT performed embryo culture. YT also helped recruit the patients. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The work was supported by grant from the Guangdong Medical Research Foundation (B2014100).
REFERENCES
- 1.Saleh RA, Agarwal A, Nelson DR, Nada EA, ElTonsy MH, et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril. 2002;78:313–8. doi: 10.1016/s0015-0282(02)03219-3. [DOI] [PubMed] [Google Scholar]
- 2.Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006;85:629–34. doi: 10.1016/j.fertnstert.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Laboratory Manual for the Examination and Processing for Human Semen. 5th ed. Switzerland: WHO Press; 2010. pp. 223–5. [Google Scholar]
- 4.Agarwal A, Allamaneni SS. Sperm DNA damage assessment: a test whose time has come. Fertil Steril. 2005;84:850–3. doi: 10.1016/j.fertnstert.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 5.Lin MH, Kuo-Kuang Lee R, Li SH, Lu CH, Sun FJ, et al. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril. 2008;90:352–9. doi: 10.1016/j.fertnstert.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Lewis SE, Agbaje I, Alvarez J. Sperm DNA tests as useful adjuncts to semen analysis. Syst Biol Reprod Med. 2008;54:111–25. doi: 10.1080/19396360801957739. [DOI] [PubMed] [Google Scholar]
- 7.Coughlan C, Clarke H, Cutting R, Saxton J, Waite S, et al. Sperm DNA fragmentation, recurrent implantation failure and recurrent miscarriage. Asian J Androl. 2015;17:681–5. doi: 10.4103/1008-682X.144946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelopoulos T, Moshel YA, Lu L, Macanas E, Grifo JA, et al. Simultaneous assessment of sperm chromatin condensation and morphology before and after separation procedures: effect on the clinical outcome after in vitro fertilization. Fertil Steril. 1988;69:740–7. doi: 10.1016/s0015-0282(98)00016-8. [DOI] [PubMed] [Google Scholar]
- 9.Evenson DP, Wixon R. Data analysis of two in vivo fertility studies using sperm chromatin structure assay-derived DNA fragmentation index vs pregnancy outcome. Fertil Steril. 2008;90:1229–31. doi: 10.1016/j.fertnstert.2007.10.066. [DOI] [PubMed] [Google Scholar]
- 10.Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril. 2008;89:823–31. doi: 10.1016/j.fertnstert.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 11.Aitken RJ, Bronson R, Smith TB, De Iuliis GN. The source and significance of DNA damage in human spermatozoa; a commentary on diagnostic strategies and straw man fallacies. Mol Hum Reprod. 2013;19:475–85. doi: 10.1093/molehr/gat025. [DOI] [PubMed] [Google Scholar]
- 12.Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9:331–45. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 14.Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Mol Hum Reprod. 1996;2:613–9. doi: 10.1093/molehr/2.8.613. [DOI] [PubMed] [Google Scholar]
- 15.Gorczyca W, Gong J, Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assay. Cancer Res. 1993;53:1945–51. [PubMed] [Google Scholar]
- 16.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14:1039–49. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 17.Ferandez JL, Muriel L, Goyanes V, Segrelles E, Gosalvez J, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84:833–42. doi: 10.1016/j.fertnstert.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 18.Chohan KR, Griffin JT, Lafromboise M, De Jonge CJ, Carrell DT. Comparison of chromatin assays for DNA fragmentation evaluation in human sperm. J Androl. 2006;27:53–9. doi: 10.2164/jandrol.05068. [DOI] [PubMed] [Google Scholar]
- 19.Simon L, Lutton D, McManus J, Lewis SE. Sperm DNA damage measured by the alkaline comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertil Steril. 2011;95:652–7. doi: 10.1016/j.fertnstert.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Simon L, Zini A, Dyachenko A, Ciampi A, Carrell DT. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl. 2017;19:80–90. doi: 10.4103/1008-682X.182822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang GL. Advanced assisted reproductive techniques. Beijing: People's Medical Publishing House; 2005. pp. 240–1. [Google Scholar]
- 22.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8. doi: 10.1016/s0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 23.Simon L, Proutski I, Stevenson M, Jennings D, McManus J, et al. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod Biomed Online. 2013;26:68–78. doi: 10.1016/j.rbmo.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Tandara M, Bajić A, Tandara L, Bilić-Zulle L, Šunj M, et al. Sperm DNA integrity testing: big halo is a good predictor of embryo quality and pregnancy after conventional IVF. Andrology. 2014;2:678–86. doi: 10.1111/j.2047-2927.2014.00234.x. [DOI] [PubMed] [Google Scholar]
- 25.Pregl Breznik B, Kovačič B, Vlaisavljević V. Are sperm DNA fragmentation, hyperactivation, and hyaluronan-binding ability predictive for fertilization and embryo development in in vitro fertilization and intracytoplasmic sperm injection? Fertil Steril. 2013;99:1233–41. doi: 10.1016/j.fertnstert.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 26.The Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril. 2013;99:673–7. doi: 10.1016/j.fertnstert.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 27.Liu DY, Liu MI. Clinical value of sperm DNA damage should be assessed in motile sperm fraction rather than whole ejaculated sperm. Fertil Steril. 2013;99:367–71. doi: 10.1016/j.fertnstert.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Wang L, Cai J, Huang H. Correlation of sperm DNA damage with IVF and ICSI outcomes: a systematic review and meta-analysis. J Assist Reprod Genet. 2006;23:367–76. doi: 10.1007/s10815-006-9066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;23:2663–8. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 30.Payne JF, Raburn DJ, Couchman GM, Price TM, Jamison MG, et al. Redefining the relationship between sperm deoxyribonucleic acid fragmentation as measured by the sperm chromatin structure assay and outcomes of assisted reproductive techniques. Fertil Steril. 2005;84:356–64. doi: 10.1016/j.fertnstert.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 31.Simon L, Murphy K, Shamsi MB, Liu L, Emery B, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014;29:2402–12. doi: 10.1093/humrep/deu228. [DOI] [PubMed] [Google Scholar]
- 32.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four and eight-cell stages of preimplantation development. Nature. 1988;332:459–61. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 33.Tesarik J, Mendoza C, Greco E. Paternal effects acting during the first cell cycle of human preimplantation development after ICSI. Hum Reprod. 2002;17:184–9. doi: 10.1093/humrep/17.1.184. [DOI] [PubMed] [Google Scholar]
- 34.Muriel L, Segrelles E, Goyanes V, Gosálvez J, Fernández JL. Structure of human sperm DNA and background damage, analysed by in situ enzymatic treatment and digital image analysis. Mol Hum Reprod. 2004;10:203–9. doi: 10.1093/molehr/gah029. [DOI] [PubMed] [Google Scholar]
- 35.Acharyya S, Kanjilal S, Bhattacharyya AK. Does human sperm nuclear DNA integrity affect embryo quality? Indian J Exp Biol. 2005;43:1016–22. [PubMed] [Google Scholar]
- 36.Spano M, Seli E, Bizzaro D, Manicardi GC, Sakkas D. The significance of sperm nuclear DNA strand breaks on reproductive outcome. Curr Opin Obstet Gynecol. 2005;17:255–60. doi: 10.1097/01.gco.0000169102.77504.66. [DOI] [PubMed] [Google Scholar]
- 37.Muriel L, Goyanes V, Segrelles E, Gosalvez J, Alvarez JG, et al. Increased aneuploidy rate in sperm with fragmented DNA as determined by the sperm chromatin dispersion (SCD) test and FISH analysis. J Androl. 2007;28:38–49. doi: 10.2164/jandrol.106.000067. [DOI] [PubMed] [Google Scholar]
- 38.Ramasamy R, Scovell JM, Kovac JR, Cook PJ, Lamb DJ, et al. Fluorescence in situ hybridization detects increased sperm aneuploidy in men with recurrent pregnancy loss. Fertil Steril. 2015;103:906–9. doi: 10.1016/j.fertnstert.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]