Abstract
Emerging evidence has suggested that cytoreductive prostatectomy (CRP) allows superior oncologic control when compared to current standard of care androgen deprivation therapy alone. However, the safety and benefit of cytoreduction in metastatic prostate cancer (mPCa) has not been proven. Therefore, we evaluated the incidence of complications following CRP in men newly diagnosed with mPCa. A total of 68 patients who underwent CRP from 2006 to 2014 at four tertiary surgical centers were compared to 598 men who underwent radical prostatectomy for clinically localized prostate cancer (PCa). Urinary incontinence was defined as the use of any pad. CRP had longer operative times (200 min vs 140 min, P < 0.0001) and higher estimated blood loss (250 ml vs 125 ml, P < 0.0001) compared to the control group. However, both overall (8.82% vs 5.85%) and major complication rates (4.41% vs 2.17%) were comparable between the two groups. Importantly, urinary incontinence rate at 1-year after surgery was significantly higher in the CRP group (57.4% vs 90.8%, P < 0.0001). Univariate logistic analysis showed that the estimated blood loss was the only independent predictor of perioperative complications both in the unadjusted model (OR: 1.18; 95% CI: 1.02–1.37; P = 0.025) and surgery type-adjusted model (OR: 1.17; 95% CI: 1.01–1.36; P = 0.034). In conclusion, CRP is more challenging than radical prostatectomy and associated with a notably higher incidence of urinary incontinence. Nevertheless, CRP is a technically feasible and safe surgery for selecting PCa patients who present with node-positive or bony metastasis when performed by experienced surgeons. A prospective, multi-institutional clinical trial is currently underway to verify this concept.
Keywords: cytoreductive surgical procedures, metastatic prostate cancer, outcome, radical prostatectomy
INTRODUCTION
Prostate cancer (PCa) is the most common noncutaneous malignancy worldwide.1 In contrast to low-risk or localized disease which may not require aggressive interventions due to its often indolent course, metastatic PCa (mPCa) portends a dismal 5-year survival rate of 28%.1 Unlike the low-volume and localized counterpart, mPCa has been regarded as unfit for local therapy.2 Currently, the standard of care first-line treatment for mPCa is androgen deprivation therapy (ADT).3 However, an overwhelming majority of patients initially treated with medical or surgical castration eventually become resistant to ADT and develop castration-resistant prostate cancer (CRPC).2,4 Despite the advances made with a number of systemic therapies (e.g. abiraterone, docetaxel, enzalutamide, etc.) that have been demonstrated to improve survival in CRPC, a recent analysis has reported that the survival in men who present with metastatic prostate cancer has not changed over the last 20 years.5
Combining cytoreduction with systemic therapies has been shown to improve survival in renal cell carcinoma, colon cancer, and ovarian cancer.6,7,8 However, surgery is not part of the standard treatment armamentarium in men with mPCa. Nevertheless, in 2006, Swanson et al.9 have proposed that cytoreductive prostatectomy (CRP) may be beneficial in patients with mPCa due to tumor debulking, enhanced antitumor immunity, removing the primary site of tumor shedding, blocking paraneoplastic effects, and disrupting tumor production of hormones. Since then, a body of evidence has accumulated to support the hypothesis that treating the primary tumor improves outcomes in PCa patients with clinical evidence of metastasis. First, analysis of the SEER database demonstrated that local tumor control resulted in a better overall survival and cancer-specific mortality in men with newly diagnosed mPCa.10 Second, in patients found to have lymph node metastasis at the time of surgery, completing radical prostatectomy (RP) is now an accepted standard of care because successful surgery is associated with improved cancer-specific and overall survival.11,12 Third, PCa debulking has recently been shown to improve the effectiveness of androgen ablation13 while Gratzke et al.14 have shown that patients who underwent radical prostatectomy had a higher overall survival rate when compared to those who did not undergo surgery (55% vs 21%; P < 0.01). Fourth, we have observed that the overall survival after recurrence is better in men who had RP rather than primary radiation therapy.15 Collectively, these observations suggest that CRP will improve the overall clinical outcome of men with mPCa.
However, to further study the potential clinical benefits of CRP, it is essential to accurately assess the risks associated with CRP before initiating large-scale clinical trials. Therefore, the objective of this study is to determine the complication rates of CRP in men diagnosed with mPCa.
MATERIALS AND METHODS
Study cohort
We conducted a retrospective review of a prospectively maintained prostatectomy database across four tertiary care institutions between the years of 2006 and 2014. The participating institutions were as follows: Rutgers Cancer Institute of New Jersey (New Brunswick, NJ), City of Hope National Medical Center (Duarte, CA), University of California, Irvine (Orange, CA), and Yonsei College of Medicine (Seoul, Korea). Our CRP cohort included men with newly diagnosed clinical T1-T4N1M0 or T1-T4Nx M1a-b who underwent CRP with or without neoadjuvant therapy. A group of men who underwent RP for localized PCa were selected to be a part of the control group from each institution using consecutive sampling between the years of 2011 and 2014 except one institution.
Outcome variables
The CRP and control group were compared with respect to perioperative characteristics and rates of overall and major complications. Perioperative parameters included operating room (OR) time, estimated blood loss (EBL), and complication rates. The clinicodemographic parameters analyzed were age, body mass index (BMI), preoperative prostate-specific antigen (PSA), biopsy Gleason score (GS), and clinical stage. Perioperative complications were assessed and graded according to the Clavien-Dindo system.16 Major complications were defined as grade greater than or equal to IIIa.
Cytoreductive prostatectomy
All patients underwent RP with wide resection of neurovascular bundles and extended pelvic lymph node dissection (PLND).
Statistical analysis
If normality was not warranted, nonparametric tests (Wilcoxon rank sum test) were employed; otherwise, two-sample t-tests were used to measure difference in means between CRP and localized surgery. Associations between the type of surgery and categorical variable were tested using Fisher's exact test. Generalized linear mixed models with logit link and random intercept were used to account for the cluster effect of each participating institution. A two-sided P < 0.05 was considered statistically significant. All statistical analyses were performed using both SPSS version 21 (IBM, Armonk, NY, USA) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
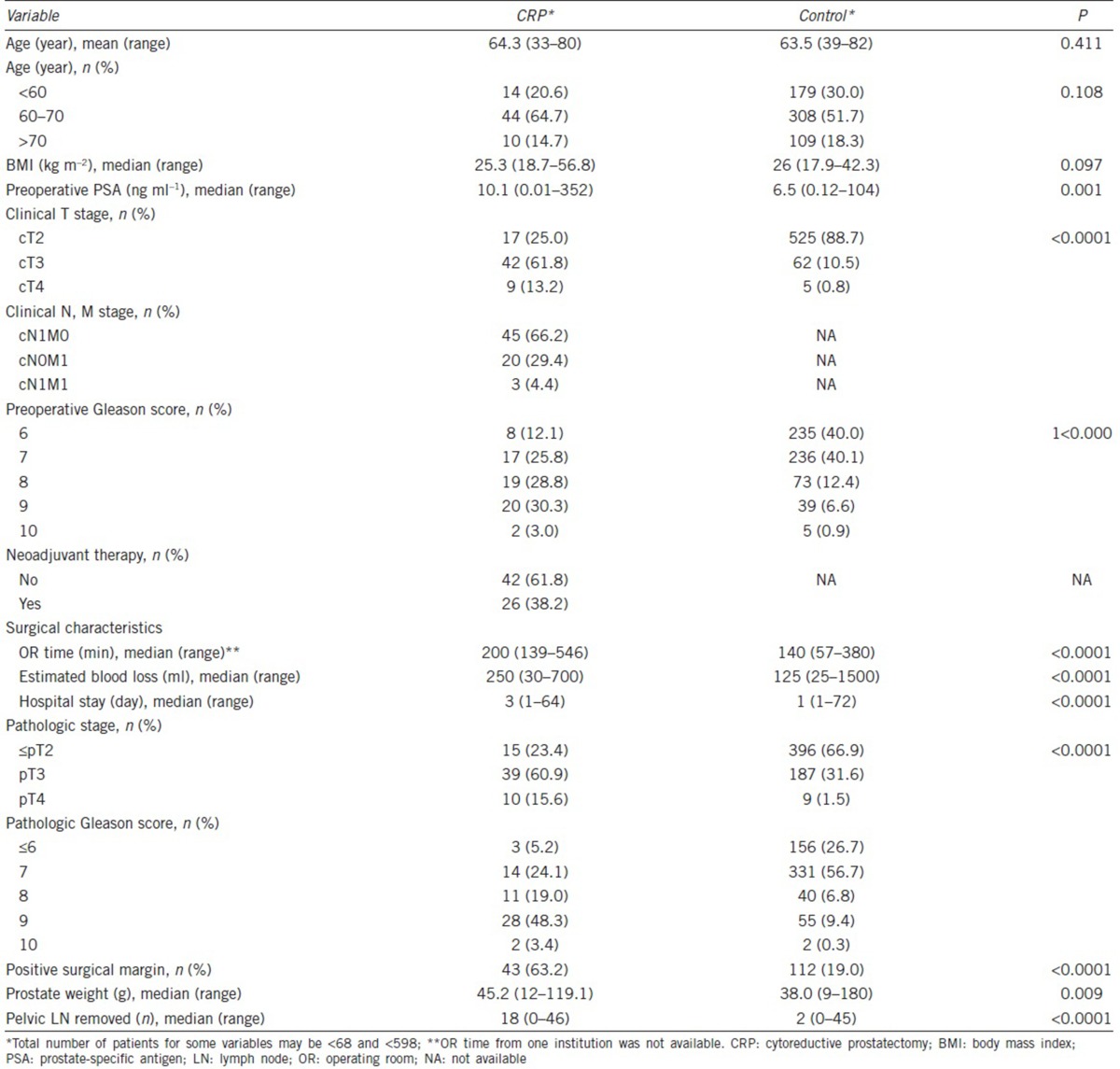
A total of 666 patients across four different institutions were included in the study. Of these, 68 patients underwent CRP while 598 patients with a localized PCa and underwent RP comprised the control group. Summary of patient characteristics and perioperative findings are shown in Table 1. In the CRP group, 23 (33.8%) and 45 (66.2%) patients had clinical M1 and N1 disease, respectively. Neoadjuvant ADT was administered in 26 of the 68 patients (38.2%). In terms of age and body mass index (BMI), the two groups were comparable. As expected, the CRP group had a greater proportion of patients who had clinical stage greater than or equal to T3 (75.0% vs 11.3%; P < 0.0001) and biopsy Gleason score of 8 or above (62.1% vs 19.9%; P < 0.0001). Postoperatively, CRP group exhibited more aggressive forms of PCa again as demonstrated by pathologic staging (≥pT3: 76.5% vs 33.1%; P < 0.0001) and grading (pGS ≥8: 70.8% vs 16.5%; P < 0.0001). With regard to surgical parameters, the CRP group had a significantly longer OR time (240 min vs 140 min; P < 0.0001) and higher EBL (250 ml vs 125 ml; P < 0.0001) compared to the control group.
Table 1.
Patient characteristics: CRP versus control
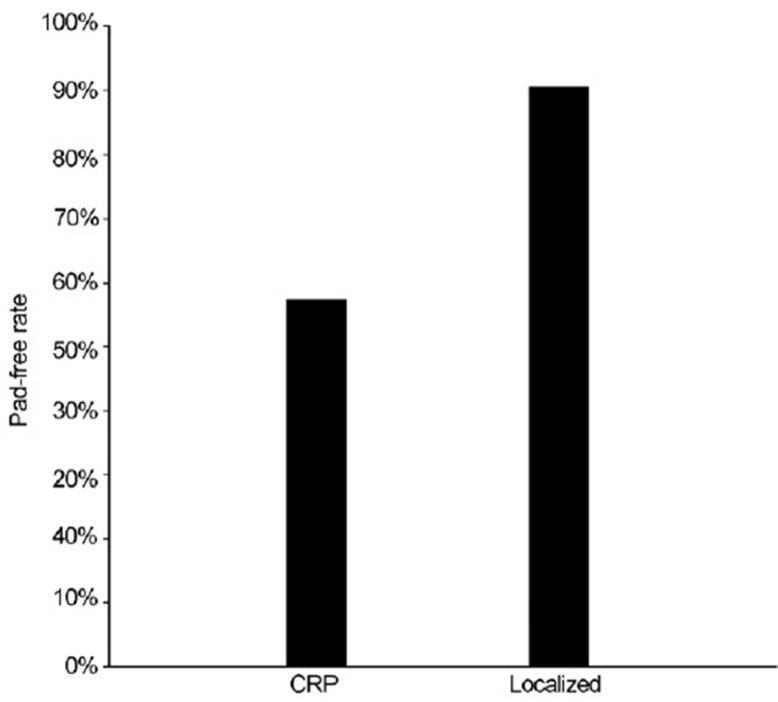
Altogether, 41 patients experienced perioperative complications: six in the CRP group and 35 in the control group (8. 82% vs 5.85%). More importantly, three and 13 men in the CRP and control group, respectively, experienced major surgical complication (4.41% vs 2.17%) (Table 2). Univariate mixed effects logistic regression analysis revealed that the estimated complication rates for the CRP showed a trend for increased risk when compared to the control group (P = 0.43): 0.084 (95% CI: 0.037–0.179) and 0.06 (95% CI: 0.041–0.086), respectively. Similarly, the estimated major complication rates also demonstrated a trend but no statistical difference between the CRP and the control group (6.5% vs 2.0%; P = 0.093). When urinary continence was assessed, the pad-free rate in the CRP group was significantly lower (57.4% vs 90.8%, P < 0.0001) (Figure 1).
Table 2.
Complication grades and descriptions: CRP versus control
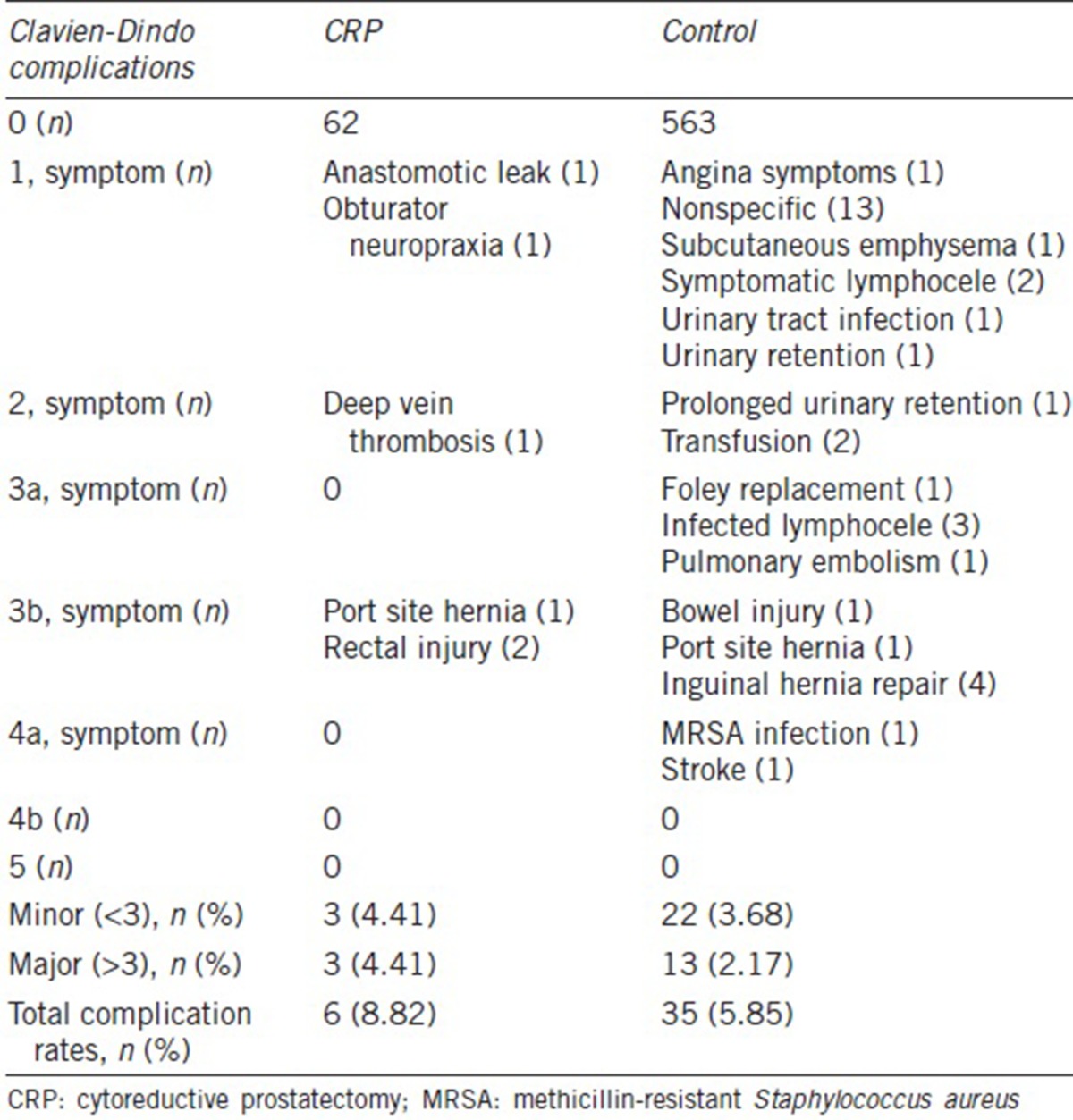
Figure 1.
Urinary continence rate following cytoreductive radical prostatectomy (CRP). Urinary continence was defined as being pad free. Following CRP, urinary continence rate was 57.4%. In contrast, the rate was 90.8% in men who underwent radical prostatectomy for a localized disease (P < 0.0001).
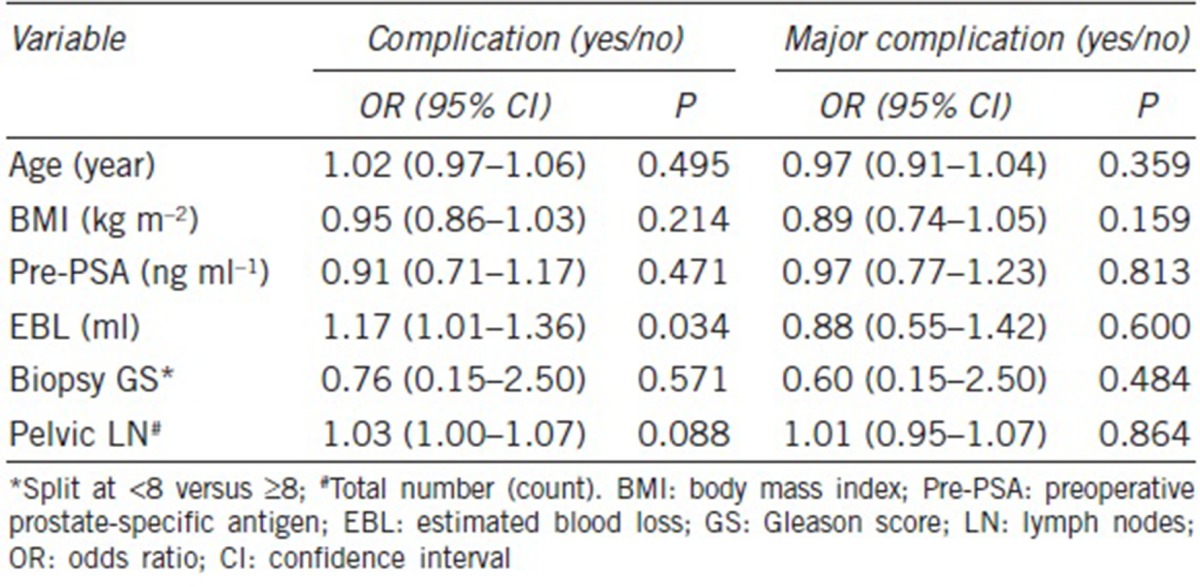
In the unadjusted model, univariate analyses indicated that the patients with larger volume of EBL (OR: 1.18; 95% CI: 1.02–1.37; P = 0.025) were more likely to have complications (Table 3). The number of dissected pelvic LN and the risk of having overall complications showed a marginal significance (OR: 1.03; 95% CI: 1.00–1.06; P = 0.051). When adjusted for surgery type (CRP vs control), only EBL became a significant predictor of complications (OR: 1.17; 95% CI: 1.01–1.36; P = 0.034) (Table 4).
Table 3.
Univariate model estimating complication and major complication
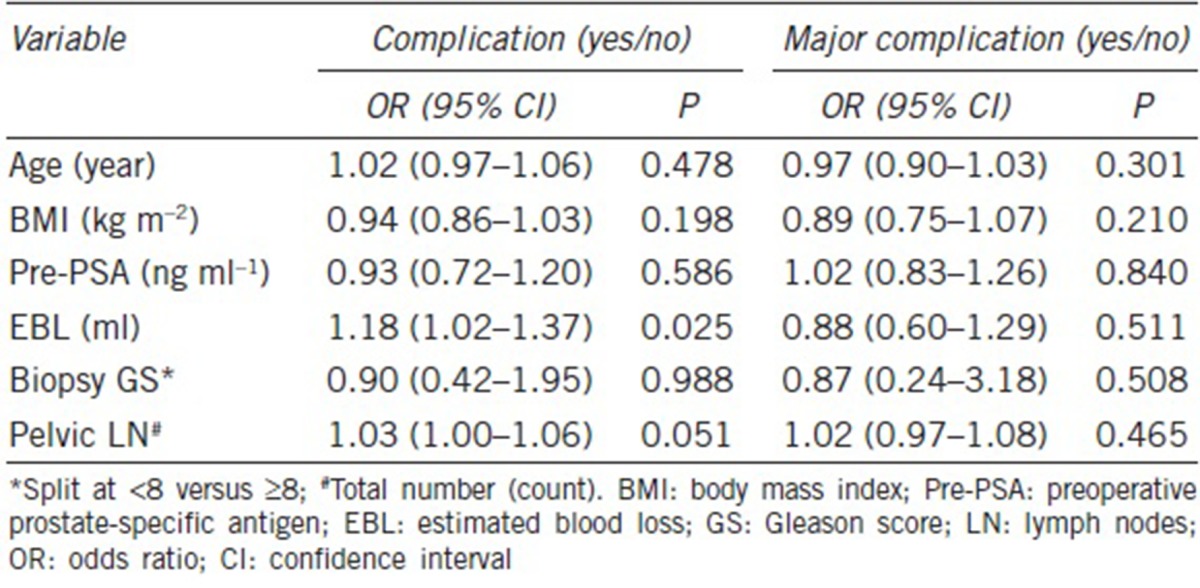
Table 4.
Surgery type-adjusted model estimating complication and major complication
DISCUSSION
As suspected, our study results indicated that CRP is a more difficult operation because it took longer (200 min vs 140 min) and had higher blood loss (250 ml vs 125 ml) than the control group. Although this higher surgical complexity and differences in intraoperative outcomes did not translate into increased overall and major complication rates (8.82% vs 5.85%, P = 0.43 and 4.41% vs 2.17%, P = 0.093, respectively), urinary incontinence was significantly lower in the CRP group (P < 0.0001). These results suggest that CRP should not be performed off clinical trials until a therapeutic benefit is clearly established.
The assessment of surgical feasibility in metastatic PCa is challenging as CRP is only a recent phenomenon. Despite the limited literature on this topic, we may draw some inferences about the safety of CRP from the results of locally advanced cancers because a majority of men undergoing CRP has T3 disease locally. Specifically, Gandaglia et al.17 reported that the 30-day perioperative overall complications were 30.0% and 28.3% for open prostatectomy (OP) and RARP, respectively (P = 0.6), in high-risk PCa patients who met the criteria of clinical stage ≥T2c, biopsy Gleason score 8–10, or PSA levels >20 ng ml−1. Similarly, Ou et al.18 demonstrated that 11 of 148 men in high-risk D′Amico group had overall complication rate of 7.4%. The observed safety of CRP in this study, therefore, compares favorably to surgery in high-risk prostate cancer (T3–4).17,18,19 Moreover, several feasibility studies on salvage radical prostatectomy (SRP), a technically formidable operation in irradiated patients, also suggest that CRP may be done safely in a selective group of patients.20 Hence, the aforementioned studies on high-risk PCa patients along with the current findings provide a strong theoretical framework for safely performing CRP.
A recently published study by Heidenreich et al.21 demonstrated that CRP in 23 patients showed acceptable perioperative complication rates. There were only three patients (12.0%) who were affected by major complications without grade IV or above complications. Our CRP findings were consistent with the above, with a slightly lower overall complication rate of 8.70%. These findings suggest that CRP can be executed safely. In terms of the common complications presented in the present study, four of nine patients (44.4%) developed lymphocele, a condition often caused by the disruption of efferent lymphatics during PLND.22
Although the perioperative complication rates following CRP in the present study confirms the safety and feasibility of the operation, the relatively high urinary incontinence rate is potentially a concern. Specifically, urinary continence defined as being pad free was only 57.4%. Although Heidenreich et al.21 had reported that the continence rate following CRP was 91.3%, this number included men who wore 1 pad per day (ppd). A closer examination of the data revealed that only 13 of 23 (56.5%) men who underwent CRP were pad free. More recently, a retrospective study on 59 patients who underwent CRP reported the continence rate of 64.4%.23 Unfortunately, this study again defined men who were wearing 1 ppd as being continent. Therefore, continence rate, if defined as the percentage of men who are pad free after surgery, will be in the 40%–50% range in the latter study. Taken together, these results suggest that urinary incontinence rate following CRP is significantly higher.
It is well documented that the need for extended PLND (ePLND) and its associated complications are major concerns in utilizing radical prostatectomy in the context of high-risk PCa.24 While no preoperative clinicodemographic variables were predictive of having complications, the number of dissected pelvic LNs demonstrated a borderline association (OR: 1.03; 95% CI: 1.00–1.06; P = 0.051). Despite the benefits of PLND as a diagnostic and therapeutic tool, studies have shown that more ePLND may lead to vascular and neurogenic damages in the neighboring vasculature and obturator nerve.24 However, there was only one patient who experienced obturator neuropraxia (grade I complication) in our CRP group. Furthermore, a greater number of dissected pelvic LNs did not increase the risk of major complications in both crude and surgery type-adjusted model (P = 0.46 and P = 0.86, respectively). While the risk associated with ePLND should carefully be assessed preoperatively, our results have demonstrated that the potentially morbid procedure of ePLND can be safely employed in CRP with only a marginal uptick in overall but not major complication rate. In determining a patient's eligibility for CRP, Heidenreich et al.21 excluded patients with both bulky pelvic lymph node metastasis >3 cm and gross retroperitoneal lymph node metastasis, as these features are associated with significant morbidity. Similarly, our CRP cohort was composed of predominantly N1 diseases (73.8%), demonstrating a safe operative course in this particular population.
In addition to the number of removed LNs, a larger volume of EBL was another factor, predictive of overall complications in both crude model (OR: 1.18; 95% CI: 1.02–1.37; P = 0.025) and surgery type-adjusted model (OR: 1.17; 95% CI: 1.01–1.36; P = 0.034). Nevertheless, none of the patients in the CRP group received blood transfusion. More importantly, EBL was not associated with increased risk of major complications in both unadjusted and surgery type-adjusted model (P = 0.51 and P = 0.60, respectively). The mean EBL of 250 ml in the CRP group was within the range of EBL reported in operative outcomes of high-risk PCa patients.17,18,19,25 Taken together, these findings suggest that CRP can be performed without undue harm or excessive transfusion risk as compared to surgery in locally advanced PCa.
Notwithstanding, a careful preoperative assessment is necessary to prevent complications. Emerging evidence suggests a prominent role for 68Ga-PSMA PET scanning in the detection of metastatic prostate cancer. A recent meta-analysis by Perera and colleagues26 showed a sensitivity of 80% and specificity of 97% for 68Ga-PSMA PET on per-lesion analysis. While its clinical utility remains to be determined, these recent reports demonstrate a substantial improvement in delineating distant metastasis when compared to the traditional imaging techniques such as MRI or CT. Therefore, this may be a potential imaging biomarker that could detect early stages of metastatic dissemination for diagnostic and intraoperative guiding purposes. Reliable imaging modalities may be useful in guiding surgery and precluding patients who are not good surgical candidates. For example, patients with visceral metastasis (M1c) have a limited life-expectancy and will likely have no significant benefit from cytoreduction. Moreover, it is also generally recommended that patients with pathologic stage T4 patients are excluded from surgical considerations due to expected high rate of perioperative complications. In our study, there were ten men (15.4%) with clinical stage T4 in the CRP group. One of these men had rectal injury. Because CRP is still not considered an acceptable treatment for patients with mPCa, enforcing stringent eligibility criteria may be the first step in achieving optimal surgical outcomes.
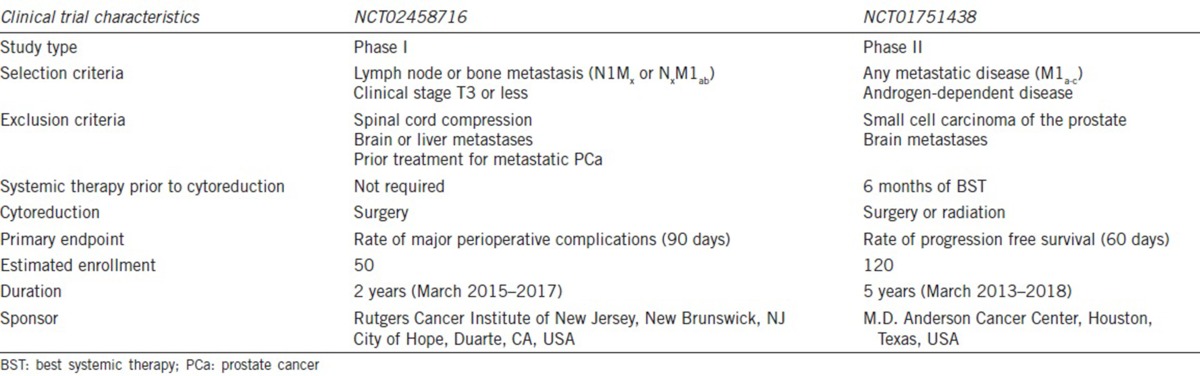
Our study is valuable as this is the first international multi-institutional study that evaluated perioperative outcomes of CRP. Because surgery is never justified when the potential perioperative risks outweigh the benefit of enhanced oncologic control and symptomatic relief, our analysis of surgical outcomes is important in evaluating the safety and technical practicability of CRP before a more widespread implementation. Our results have demonstrated that radical prostatectomy is becoming more encompassing, extending its scope into metastatic diseases for which no surgical procedures were thought to have a therapeutic role previously. Nevertheless, urinary incontinence rate is dramatically higher following CRP. Further analyses and well-designed clinical trials are needed to better define the risk–benefit ratio of CRP. Currently, a few clinical trials are underway, including a phase I multi-institutional study (NCT02458716) designed to further assess complications and continence following CRP (Table 5). Ultimately, a randomized prospective clinical trial will be necessary to determine whether there is a therapeutic benefit of cytoreductive surgery that justifies the 40%–50% urinary incontinence risk. In the meantime, urologists are urged to perform CRP only under clinical trials.
Table 5.
Ongoing clinical trials evaluating cytoreductive prostatectomy
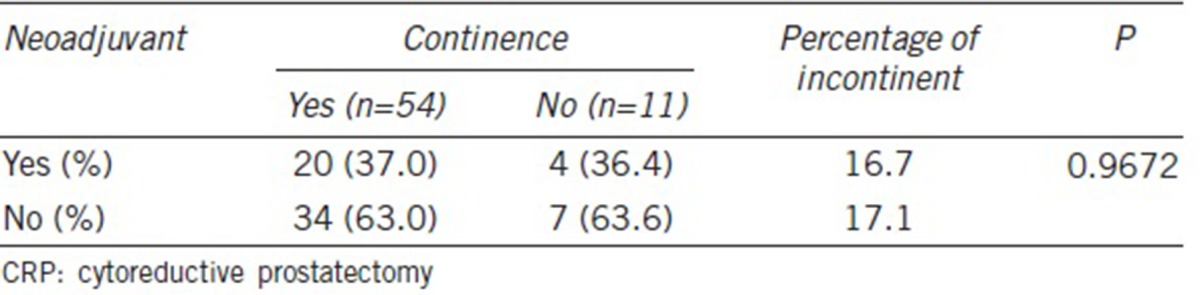
There are a few important limitations in our study. First, the inherent selection bias from a retrospective cohort study may have influenced our study results. Second, the selection of our control group could have been limited to pT3 or greater to allow more focused comparisons with the CRP group. Third, we were not able to fully account for the effect of age and obesity on incontinence rates following RP.27 In addition, our CRP group was heterogeneous in terms of receiving neoadjuvant ADT, and this systemic therapy before surgery can potentially influence the rate of surgical complications. However, a few studies have shown that men with neoadjuvant ADT have similar operative outcomes when compared to those without.28,29 Similarly, ADT given in both neoadjuvant and postoperative setting has been associated with a higher risk of urinary incontinence.30,31 Although the rates of incontinence following CRP may, in part, be influenced by a higher incidence of neoadjuvant therapy, our analysis demonstrated similar continence rates with and without neoadjuvant therapy (Table 6). Fourth, all the participating tertiary surgical care institutions are led by experienced surgeons who are far above their learning curves. Hence, our study findings cannot be generalized. Finally, despite the utilization of statistical methods to account for a center-specific cluster effect, heterogeneities from multiple institutions are sources of confounders and may not be fully adjusted.
Table 6.
Continence rates according to neoadjuvant therapy in the CRP group
CONCLUSIONS
Cytoreductive prostatectomy is a technically feasible surgery for qualified patients with node-positive or bony metastatic prostate cancer when performed by experienced surgeons. Nevertheless, cytoreductive prostatectomy is associated with a prolonged operative time and increased blood loss. More importantly, urinary incontinence rate following cytoreductive prostatectomy occurs in nearly half the patients. An ongoing prospective clinical trial will further assess our study results.
AUTHOR CONTRIBUTIONS
DKK, JSP, and YSK reviewed the pertinent literature, analyzed the results, and drafted and edited the manuscript. YSK and SK performed the statistical analysis. BS and NL captured relevant data and merged multiple databases. IYK was responsible for the entire project. IYK designed the study concept, guided the study design, conducted data acquisition, and revised the manuscript critically for important intellectual content. BS, NL, NF, TA, DS, BY, NR, WJK, and KHR collected data, analyzed data, and revised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 3.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 4.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu JN, Fish KM, Evans CP, Devere White RW, Dall’Era MA. No improvement noted in overall or cause-specific survival for men presenting with metastatic prostate cancer over a 20-year period. Cancer. 2014;120:818–23. doi: 10.1002/cncr.28485. [DOI] [PubMed] [Google Scholar]
- 6.Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219–28. doi: 10.1016/S1470-2045(04)01425-1. [DOI] [PubMed] [Google Scholar]
- 7.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 8.Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–9. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 9.Swanson G, Thompson I, Basler J, Crawford ED. Metastatic prostate cancer-does treatment of the primary tumor matter? J Urol. 2006;176:1292–8. doi: 10.1016/j.juro.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 10.Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65:1058–66. doi: 10.1016/j.eururo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Ghavamian R, Bergstralh EJ, Blute ML, Slezak J, Zincke H. Radical retropubic prostatectomy plus orchiectomy versus orchiectomy alone for pTxN+prostate cancer: a matched comparison. J Urol. 1999;161:1223–7. [PubMed] [Google Scholar]
- 12.Engel J, Bastian PJ, Baur H, Beer V, Chaussy C, et al. Survival benefit of radical prostatectomy in lymph node-positive patients with prostate cancer. Eur Urol. 2010;57:754–61. doi: 10.1016/j.eururo.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Qin XJ, Ma CG, Ye DW, Yao XD, Zhang SL, et al. Tumor cytoreduction results in better response to androgen ablation—a preliminary report of palliative transurethral resection of the prostate in metastatic hormone sensitive prostate cancer. Urol Oncol. 2012;30:145–9. doi: 10.1016/j.urolonc.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Gratzke C, Engel J, Stief CG. Role of radical prostatectomy in metastatic prostate cancer: data from the Munich Cancer Registry. Eur Urol. 2014;66:602–3. doi: 10.1016/j.eururo.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Shao YH, Kim S, Moore DF, Shih W, Lin Y, et al. Cancer-specific survival after metastasis following primary radical prostatectomy compared with radiation therapy in prostate cancer patients: results of a population-based, propensity score-matched analysis. Eur Urol. 2014;65:693–700. doi: 10.1016/j.eururo.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandaglia G, Abdollah F, Hu J, Kim S, Briganti A, et al. Is robot-assisted radical prostatectomy safe in men with high-risk prostate cancer? Assessment of perioperative outcomes, positive surgical margins, and use of additional cancer treatments. J Endourol. 2014;28:784–91. doi: 10.1089/end.2013.0774. [DOI] [PubMed] [Google Scholar]
- 18.Ou YC, Yang CK, Wang J, Hung SW, Cheng CL, et al. The trifecta outcome in 300 consecutive cases of robotic-assisted laparoscopic radical prostatectomy according to D’Amico risk criteria. Eur J Surg Oncol. 2013;39:107–13. doi: 10.1016/j.ejso.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Novara G, Ficarra V, D’Elia C, Secco S, Cavalleri S, et al. Prospective evaluation with standardised criteria for postoperative complications after robotic-assisted laparoscopic radical prostatectomy. Eur Urol. 2010;57:363–70. doi: 10.1016/j.eururo.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 20.Kaffenberger SD, Smith JA. Salvage robotic radical prostatectomy. Indian J Urol. 2014;30:429–33. doi: 10.4103/0970-1591.142074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol. 2015;193:832–8. doi: 10.1016/j.juro.2014.09.089. [DOI] [PubMed] [Google Scholar]
- 22.Sogani PC, Watson RC, Whitmore WF., Jr Lymphocele after pelvic lymphadenectomy for urologic cancer. Urology. 1981;17:39–43. doi: 10.1016/0090-4295(81)90009-1. [DOI] [PubMed] [Google Scholar]
- 23.Sooriakumaran P, Karnes J, Stief C, Copsey B, Montorsi F, et al. A multi-institutional analysis of perioperative outcomes in 106 men who underwent radical prostatectomy for distant metastatic prostate cancer at presentation. Eur Urol. 2016;69:788–94. doi: 10.1016/j.eururo.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Ploussard G, Briganti A, de la Taille A, Haese A, Heidenreich A, et al. Pelvic lymph node dissection during robot-assisted radical prostatectomy: efficacy, limitations, and complications - a systematic review of the literature. Eur Urol. 2014;65:7–16. doi: 10.1016/j.eururo.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 25.Jung JH, Seo JW, Lim MS, Lee JW, Chung BH, et al. Extended pelvic lymph node dissection including internal iliac packet should be performed during robot-assisted laparoscopic radical prostatectomy for high-risk prostate cancer. J Laparoendosc Adv Surg Tech A. 2012;22:785–90. doi: 10.1089/lap.2011.0516. [DOI] [PubMed] [Google Scholar]
- 26.Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, et al. Sensitivity, specificity, and predictors of positive [68] Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–37. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Gacci M, Sebastianelli A, Salvi M, De Nunzio C, Tubaro A, et al. The impact of central obesity on storage luts and urinary incontinence after prostatic surgery. Curr Urol Rep. 2016;17:61. doi: 10.1007/s11934-016-0620-4. [DOI] [PubMed] [Google Scholar]
- 28.Scolieri MJ, Altman A, Resnick MI. Neoadjuvant hormonal ablative therapy before radical prostatectomy: a review. Is it indicated? J Urol. 2000;164:1465–72. [PubMed] [Google Scholar]
- 29.Yang SW, Song KH, Lim JS, Sul CK. Neoadjuvant hormonal therapy preceding radical prostatectomy for clinically localized prostate cancer: early postoperative complications and biochemical recurrence. Korean J Urol. 2011;52:19–23. doi: 10.4111/kju.2011.52.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlsson S, Drevin L, Loeb S, Widmark A, Lissbrant IF, et al. Population-based study of long-term functional outcomes after prostate cancer treatment. BJU Int. 2016;117:E36–45. doi: 10.1111/bju.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gacci M, Corona G, Apolone G, Lanciotti M, Tosi N, et al. Influence of serum testosterone on urinary continence and sexual activity in patients undergoing radical prostatectomy for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:168–72. doi: 10.1038/pcan.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]