Abstract
The nuclear factor-κB (NF-κB) family of transcription factors is activated by canonical and non-canonical signalling pathways, which differ in both signalling components and biological functions. Recent studies have revealed important roles for the non-canonical NF-κB pathway in regulating different aspects of immune functions. Defects in non-canonical NF-κB signalling are associated with severe immune deficiencies, whereas dysregulated activation of this pathway contributes to the pathogenesis of various autoimmune and inflammatory diseases. Here we review the signalling mechanisms and the biological function of the non-canonical NF-κB pathway. We also discuss recent progress in elucidating the molecular mechanisms regulating non-canonical NF-κB pathway activation, which may provide new opportunities for therapeutic strategies.
The nuclear factor-κB (NF-κB) family of inducible transcription factors includes NF-κB1 p50, NF-κB2 p52, RELA (also called p65), RELB and c-REL1. NF-κB proteins normally exist as components of inactive cytoplasmic complexes bound by members of the inhibitor of κB (IκB) family. This family includes the prototypical member IκBα and several structurally related proteins2. NF-κB1 p50 and NF-κB2 p52 are produced as pre-cursor proteins, p105 and p100, both of which contain a carboxy-terminal IκB-homologous region and function as IκB-like NF-κB inhibitors. Proteasome-mediated selective degradation of the C-terminal region of p105 and p100 (termed p100 processing) yields mature NF-κB1 p50 and NF-κB2 p52 and also interrupts the IκB-like function of these precursor proteins2. The processing of p105 is constitutive and coupled with its translation, although a large proportion of p105 remains unprocessed and functions as an IκB-like molecule2,3. By contrast, the processing of p100 is tightly regulated in a signal-induced manner4.
NF-κB activation occurs via two major signalling pathways: the canonical and the non-canonical NF-κB signalling pathways (FIG. 1). The canonical pathway mediates the activation of NF-κB1 p50, RELA and c-REL (which are also referred to as canonical NF-κB family members), whereas the non-canonical NF-κB pathway selectively activates p100-sequestered NF-κB members, predominantly NF-κB2 p52 and RELB (also referred to as non-canonical NF-κB family members).
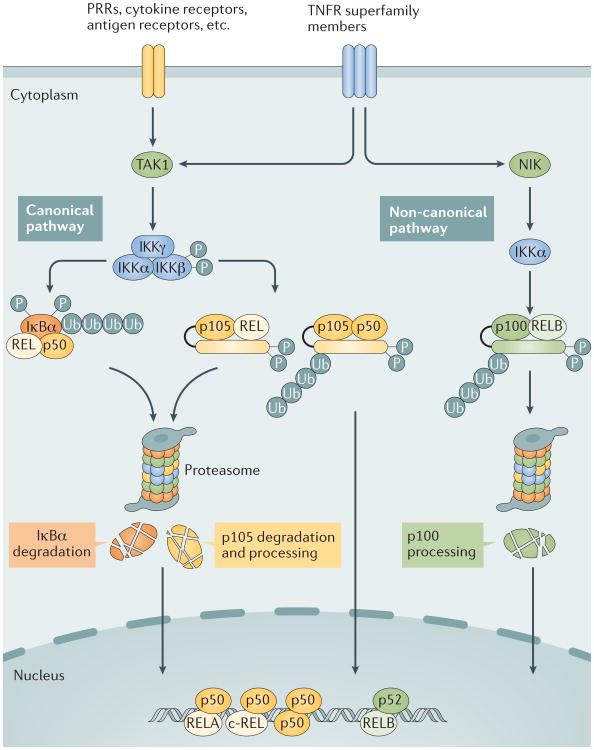
Figure 1. Canonical and non-canonical NF-κB pathways.
The canonical nuclear factor-κB (NF-κB) pathway is triggered by signals from a large variety of immune receptors, which activate the kinase TGFβ -activated kinase 1 (TAK1). TAK1 then activates a trimeric IκB kinase (IKK) complex, composed of catalytic (IKKα and IKKβ) and regulatory (IKKγ) subunits, via phosphorylation of IKKβ. Upon stimulation, the IKK complex, largely through IKKβ, phosphorylates members of the inhibitor of κB (IκB) family, such as the prototypical IκB member IκBα and the I κB-like molecule p105, which sequester NF-κB members in the cytoplasm. IκBα associates with dimers of p50 and members of the REL family (RELA or c-REL), whereas p105 associates with p50 or REL (RELA or c-REL). Upon phosphorylation by IKK, IκBα and p105 are targeted for ubiquitin (Ub)-dependent degradation in the proteasome, resulting in the nuclear translocation of canonical NF-κB family members, which bind to specific DNA elements, termed κB enhancers of target genes, in the form of various dimeric complexes, including RELA–p50, c-REL–p50, and p50–p50 (REF. 1). By contrast, non-canonical NF- κB signalling is based on the processing of p100, an IκB-like molecule that predominantly, although not exclusively, regulates RELB. The non-canonical NF- κB pathway selectively responds to a subset of tumour necrosis factor receptor (TNFR) superfamily members that target the activation of the kinase NFκB-inducing kinase (NIK)4. NIK phosphorylates and activates IKKα, which in turn phosphorylates carboxy-terminal serine residues of p100, triggering selective degradation of the C-terminal IκB-like structure of p100 and leading to the generation of p52 and the nuclear translocation of p52 and RELB4. PRRs, pattern recognition receptors.
The canonical NF-κB pathway responds to stimuli from diverse immune receptors and leads to rapid but transient NF-κB activation1,5,6. A first step in the intracellular signalling cascade is the activation of TGFβ-activated kinase 1 (TAK1; also known as MAP3K7), which, in turn, activates a trimeric IκB kinase (IKK) complex, composed of catalytic (IKKα and IKKβ) and regulatory (IKKγ; also known as NEMO) subunits (FIG. 1). The IKK complex then phosphorylates IκBα or other IκB family members, such as p105. Phosphorylated IκB family members undergo ubiquitylation and proteasomal degradation, resulting in the release and nuclear translocation of the canonical NF-κB family members, predominantly the NF-κB1 p50-RELA and NF-κB1 p50-c-REL dimers. IKK-mediated phosphorylation of p105 targets this IκB-like molecule for complete degradation, although it may also promote p105 processing (that is, the generation of NF-κB1 p50) in some cell types, contributing to the induction of nuclear translocation of p105-sequestered NF-κB members, including NF-κB1 p50, RELA and c-REL2,7,8 (FIG. 1). Genetic evidence suggests that IKKγ and IKKβ are crucial for mediating phosphorylation-dependent IκBα deg radation and canonical NF-κB nuclear translocation, whereas IKKα appears to play a supporting role in activating the canonical NF-κB pathway9.
By contrast, the activation of the non-canonical NF-κB pathway is based on the processing of p100 (REF 4) (FIG. 1). Unlike the constitutive processing of p105, the processing of p100 is tightly controlled and occurs in a strictly inducible manner10. Signal-induced p100 processing leads to the generation of NF-κB2 p52 as well as nuclear translocation of p100-associated NF-κB members, predominantly RELB and NF-κB2 p52 (REF. 4). The processing of p100 is induced via its phosphorylation at specific C-terminal serine residues (serines 866 and 870), which triggers p100 ubiquitylation via recruitment of the E3 ubiquitin ligase βTrCP10–12. A central signalling component of the non-canonical NF-κB pathway is NF-κB-inducing kinase (NIK; also known as MAP3K14), which induces p100 phosphorylation via the activation of the kinase IKKα10,13. In contrast to the rapid and transient activation of the canonical NF-κB pathway, the activation of the non- canonical NF-κB pathway is characteristically slow and persistent. Typical inducers of the non-canonical NF-κB pathway are ligands of a subset of tumour necrosis factor receptor (TNFR) superfamily members. Most of these receptors also stimulate the canonical NF-κB pathway and mediate biological processes that involve the functional cooperation between the two NF-κB pathways (FIG. 1).
Here, we review the activation, signalling mechanisms and biological function of the non-canonical NF-κB pathway. Initially thought to be mainly required for lymphoid organ development and B cell maturation, the non-canonical NF-κB pathway has recently been shown to also regulate different aspects of innate and adaptive immune responses. Dysregulated non-canonical NF-κB activation also contributes to the pathogenesis of inflammatory diseases.
Non-canonical NF-κB signaling
Signalling components
Genetic evidence has established that NIK is a central and specific component of the non-canonical NF-κB pathway10. Although NIK may also regulate NF-κB-independent cellular processes14–16, its predominant function is to mediate non-canonical NF-κB activation, as suggested by the phenotypic similarities between mice carrying mutations or deficiencies in NIK and mice with mutated or deficient NF-κB2 p52 (REF. 17). Another essential component of the non-canonical NF-κB pathway is IKKα, which, as described above, is activated by NIK and directly phosphorylates p100 (REF. 13). However, unlike NIK, IKKα responds to both canonical and non-canonical NF-κB stimuli and possesses many functions beyond non-canonical NF-κB signalling18. For example, IKKα mediates canonical NF-κB pathway activation by certain inducers, although IKKβ is the predominant IKK catalytic subunit for canonical NF-κB pathway activation. IKKα also directly phosphorylates canonical NF-κB family members and thereby activates their function as transcriptional activators. Moreover, IKKα modulates general gene transcription by phosphorylating chromatin components and transcriptional co-activators18. Another well-recognized function of IKKα is to regulate an epidermal growth factor receptor (EGFR) signalling loop in keratinocytes, thereby affecting skin homeostasis and malignancy19. Moreover, IKKα also has a role in the development of lung squamous cell carcinoma. This seems to involve transcriptional suppression of the oncogenic factors ΔNp63 (an amino-terminally truncated isoform of p63) and tripartite motif-containing protein 29 (TRIM29) in K5+ lung epithelial cells20. In vitro experiments suggest that IKKα exerts this function epi-genetically by associating with the promoter regions of p63 and TRIM29, and mediating the downregulation of the repressive histone mark H3K27me3 and the upregulation of the active histone mark H3K4me3 (REF. 20).
IKKα activation by signals that do not activate NIK is insufficient for triggering non-canonical NF-κB pathway activation, the reasons for which are as yet unclear. A possible explanation is that NIK may both activate IKKα and facilitate the binding of IKKα to its substrate p100 (REF. 21). Another intriguing question is whether IKKα functions in the typical IKKα-IKKβ-IKKγ complex or a distinct complex containing NIK during non-canonical NF-κB signalling. However, it is also possible that NIK induces additional signalling factors that functionally cooperate with IKKα in the induction of p100 processing.
Non-canonical NF-κB signalling predominantly activates NF-κB2 p52 and RELB, although additional NF-κB members, such as RELA, are associated with p100 and, in some cell types, are activated via the induction of p100 processing22–24. NF-κB2 p52 and RELB typically function as a heterodimer to induce target gene transcription, but they may also function independently under some conditions. In T cells, NF-κB2 p52 cooperates with the canonical NF-κB member c-REL to induce the expression of the inflammatory cytokine GM-CSF (granulocyte–macrophage colony-stimulating factor)25. In B cells, the combined deletion of Relb and Nfkb2 results in more severe B cell defects than the single deletions, further supporting the idea that these two NF-κB members have both common and unique functions26. It is important to note, however, that the phenotype of Nfkb2 knockout mice is difficult to interpret, as the Nfkb2 deficiency eliminates both NF-κB2 p52 and the IκB-family molecule p100. Caution should also prevail when interpreting the phenotypes of RELB-deficient mice, as RELB activation is not always dependent on non-canonical NF-κB signalling. For example, in dendritic cells (DCs), a large proportion of RELB is regulated by IκBα and activated via the canonical NF-κB pathway27. Moreover, RELB activation by the T cell receptor (TCR) is only partially dependent on the non-canonical NF-κB pathway25. Mouse models with defective p100 processing, such as NF-κB2Lym1 mice (which express a processing-defective p100 mutant)23, are more appropriate than RELB-deficient mice for functional studies of the non-canonical NF-κB pathway.
Cellular and pathogenic stimulators
Initial clues as to which receptors stimulate the non-canonical NF-κB pathway came from studies of alymphoplasia (Aly) mice, which carry a loss-of-function mutation in NIK28. Most notably, the Aly/Aly homo zygous mice resemble mice lacking the TNFR superfamily member lymphotoxin-β receptor (LTβR) with regard to impaired development of secondary lymphoid organs. Overexpression of LTβR stimulates NIK-dependent p100 processing10, and crosslinking of LTβR with an agonistic antibody stimulates p100 processing in a NIK- and IKKα-dependent manner29. This demonstrates that LTβR acts as a non-canonical NF-κB pathway stimulating receptor29. Around the same time that these observations were made, two other TNFR members — B cell activating factor receptor (BAFFR) and CD40 — were identified as non-canonical NF-κB pathway stimulating receptors in B cells30–32. It is now clear that several additional TNFR members mediate non-canonical NF-κB pathway activation (TABLE 1); these include receptor activator for NF-κB (RANK), TNFR2, fibroblast growth factor-inducible factor 14 (FN14), CD27, CD30 and OX40 (also known as CD134)17,33–39. A common feature of these TNFRs is the presence of cytoplasmic motifs that bind TNFR associated factor 2 (TRAF2), TRAF3 or both TRAF family members. As discussed in the following section, TRAF2 and TRAF3 facilitate the ubiquitylation of NIK and thereby act as negative regulators, and their degradation serves as a major mechanism of non-canonical NF-κB pathway activation.
Table 1. Immune-stimuli that induce the non-canonical NF-κB pathway.
| Ligands | Receptors | Refs |
|---|---|---|
| TNF superfamily members | ||
| BAFF | BAFFR | 30,32 |
| CD40L | CD40 | 31 |
| CD30L | CD30 | 35,36 |
| CD70 | CD27 | 34 |
| LTα1β2 and LIGHT | LTβR | 29 |
| OX40L | OX40 | 38 |
| RANKL | RANK | 37 |
| TNF (membrane) | TNFR2 | 39 |
| TWEAK | FN14 | 33 |
| Other immune stimuli | ||
| MCSF | MCSFR | 40 |
| MACs | NA | 75 |
| Antibodies directed against CD3 and CD28 | TCR and CD28 | 25 |
BAFF, B cell activating factor; FN14, fibroblast growth factor-inducible factor 14; L, ligand; LIGHT, also known as TNFSF14; LT, lymphotoxin; MACs, membrane attack complexes; MCSF, macrophage colony-stimulating factor; NA, not applicable; R, receptor; RANK, receptor activator for NF-κB; TCR, T cell receptor; TNF, tumour necrosis factor; TWEAK, TNF-like weak inducer of apoptosis.
Some non-TNFR receptors have also been shown to mediate non-canonical NF-κB pathway activation (TABLE 1). One example is macrophage colony-stimulating factor (MCSF) receptor (MCSFR), a growth factor receptor that mediates the differentiation and proliferation of macrophages40. MCSF-induced macrophage differentiation from bone marrow cells is associated with the induction of p100 processing, and like TNFRs, MCSFR is capable of associating with TRAF2 and TRAF3 (REF 40). In T cells, ligation of the TCR and the co-stimulatory receptor CD28 stimulates p100 processing and nuclear translocation of p52 and RELB, which is dependent on NIK and the presence of the C-terminal phosphorylation site of p100 (REF 25). However, as T cell activation is associated with the expression of several TNFR members and their ligands41, it seems likely that the TCR–CD28 signals activate the non- canonical NF-κB pathway indirectly through the induction of TNFRs and their ligands. In support of this hypothesis, ligation of the TNFR family member OX40 promotes TCR–CD28-induced activation of the non-canonical NF-κB pathway38,42. Future studies will examine whether TCR–CD28 signalling acts synergistically with TNFR signalling in the induction of the non-canonical NF-κB pathway.
The non-canonical NF-κB pathway is also a target of some pathogens (TABLE 2). These include several RNA viruses40,43–45, which seem to induce non- canonical NF-κB pathway activation by stimulating the cytoplasmic RNA sensor retinoic acid inducible gene I (RIG- I)45. Some viral oncoproteins are capable of directly stimulating non-canonical NF-κB pathway activation; these include the Tax protein of human T cell leukaemia virus type I, Epstein–Barr virus latent membrane protein 1 (LMP1), the Kaposi sarcoma-associated herpesvirus protein vFLIP, Herpesvirus saimiri transforming protein STP-A11 and the Tio protein of Herpesvirus ateles46–50. Tio is a transmembrane protein that resembles TNFRs and mediates NIK-dependent non-canonical NF-κB activation by interacting with, and inducing the degradation of, TRAF3 (REF. 50). LMP1 and STP-A11 are also transmembrane proteins that contain TRAF-binding sequences, which are required for non- canonical NF-κB activation in a NIK-dependent manner47,49. By contrast, non-canonical NF-κB activation by Tax and vFLIP is independent of NIK but requires IKKγ and IKKα46,48. The non-canonical NF-κB pathway is also activated by some bacteria; these include Helicobacter pylori, a Gram-negative bacterium associated with gastric inflammation and cancer51, and Legionella pneumophila, a pathogen that infects lung macrophages and is associated with Legionnaires' disease52.
Table 2. Pathogens as inducers of the non-canonical NF-κB pathway.
| Pathogen | Pathogen component | Refs |
|---|---|---|
| Virus | ||
| Influenza virus | RNA | 45 |
| Vesicular stomatitis virus | RNA | 40 |
| Sendai virus | RNA | 40 |
| HIV1 | RNA | 44 |
| Respiratory syncytial virus 1 | RNA | 43 |
| Epstein–Barr virus | LMP1 protein | 47 |
| Herpesvirus saimiri | STP-A11 protein | 49 |
| Herpesvirus ateles | Tio protein | 50 |
| Human T cell lymphotropic virus 1 | Tax protein | 46 |
| Kaposi sarcoma-associated herpesvirus | vFLIP protein | 48 |
| Bacteria | ||
| Helicobacter pylori | Unknown | 51 |
| Legionella pneumophila | LegK1 | 52 |
LegK1, Legionella kinase 1; LMP1, latent membrane protein 1.
Negative regulators
A yeast two-hybrid screen for NIK-binding proteins identified TRAF3 as a central negative regulator of the non-canonical NF-κB pathway53. In mammalian cells, newly synthesized NIK is rapidly bound by TRAF3 and targeted for ubiquitin-dependent degradation. Under steady-state conditions, this keeps the level of NIK extremely low, whereas signal-induced TRAF3 degradation leads to NIK accumulation and subsequent p100 processing53 (FIG. 2). TRAF3 knockdown or knockout results in NIK stabilization, which is sufficient for the induction of p100 processing53,54. Moreover, a NIK mutant lacking the TRAF3-binding motif was shown to escape from TRAF3-mediated degradation and to turn into a ‘super-stimulator’ of the non-canonical NF-κB pathway53,55. These findings suggest that signal-induced NIK protein accumulation is sufficient for its activation, an idea that was corroborated by subsequent structural studies that suggested NIK is a kinase that is in a constitutively active conformation56,57.
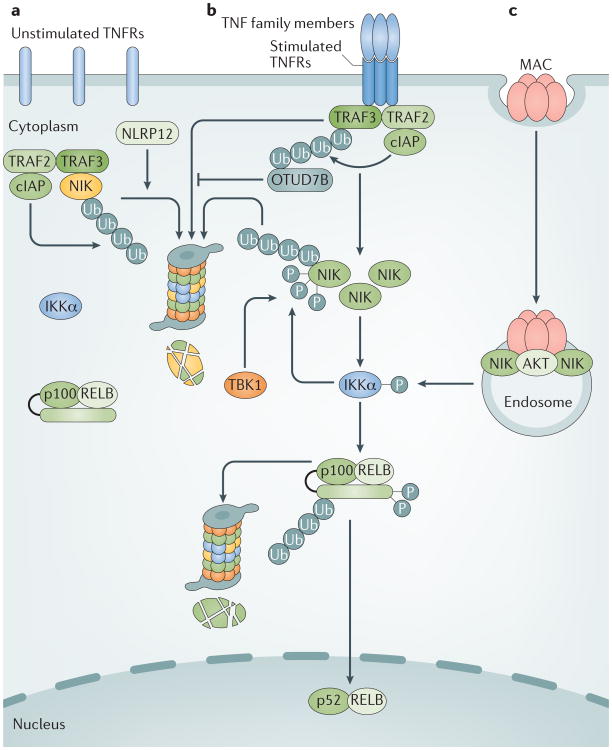
Figure 2. Regulation and activation of the non-canonical NF-κB pathway.
a | Under unstimulated conditions, newly synthesized NF-κB-inducing kinase (NIK) is rapidly bound by TNFR associated factor 3 (TRAF3) and targeted for ubiquitylation (Ub) by the cellular inhibitor of apoptosis (cIAP)–TRAF2–TRAF3 E3 ubiquitin ligase complex4. The continuous degradation of NIK in the proteasome prevents NIK accumulation and non-canonical nuclear factor-κB (NF-κB) pathway activation. b | Ligation of specific TNFR superfamily members by their ligands (TNF family members) stimulates the recruitment of TRAF2, TRAF3, cIAP1 and cIAP2 (shown as cIAP in the figure) to the receptor complex, in which activated cIAP mediates K48 ubiquitylation and proteasomal degradation of TRAF3, resulting in stabilization and accumulation of NIK and the induction of p100 processing, followed by the translocation of NF-κB p52–RELB heterodimers into the nucleus4. Receptor-mediated non-canonical NF-κB activation is negatively regulated via TRAF3 deubiquitylation by OTUD7B and phosphorylation-dependent degradation of NIK is mediated by IκB kinase-α (IKKα) and TANK-binding kinase 1 (TBK1)68–70. c | Complement membrane activation complex (MAC) activates the non-canonical NF-κB pathway via a TRAF-independent mechanism that involves the formation of an endosome-based signalling complex containing MAC, the kinase AKT and NIK, in which activated AKT mediates NIK stabilization75. NLRP12, NACHT, LRR and PYD domain-containing protein 12.
Proteasomal degradation of proteins typically involves their conjugation with lysine (K) 48-linked polyubiquitin chains. TRAF3 lacks K48-specific E3 ligase activity, but instead induces NIK K48 ubiquitylation by recruiting the E3 ligases cellular inhibitor of apoptosis 1 (cIAP1) and cIAP2 (hereafter collectively referred to as cIAP) to NIK, a mechanism that also requires TRAF2, which appears to serve as an adaptor for connecting TRAF3 with cIAP4,58,59. Thus, the deletion of either TRAF2 or TRAF3 or a simultaneous deletion of cIAP causes NIK accumulation and p100 processing53,54,60–63. Similarly, small-molecule antagonists of IAPs, which induce the degradation of cIAP, induce NIK accumulation and p100 processing64,65.
Recent studies suggest that a member of NACHT, LRR and PYD domain-containing protein 12 (NLRP12; also known as Monarch 1) physically interacts with NIK and promotes ubiquitin-dependent NIK degradation in myeloid cells66,67. NLRP12 also binds to TRAF3 and maintains the steady level of TRAF3 stability, and NLRP12 deficiency causes a reduction in the basal level of TRAF3, as shown in DCs66. Thus, NLRP12 may regulate the steady level of non-canonical NF-κB signalling by targeting both TRAF3 and NIK, although the underlying mechanism has not been well defined (FIG. 2).
Signal-induced non-canonical NF-κB pathway activation is also subject to negative regulation. OTUD7B (also known as Cezanne) is a deubiquitinase that interacts with TRAF3 in response to CD40 and LTβR signals and inhibits signal-induced TRAF3 degradation by deconjugating K48-linked polyubiquitin chains from TRAF3 (REF. 68) (FIG. 2). The N-terminal ubiquitin-association domain of OTUD7B is required for its inducible binding to TRAF3, suggesting that TRAF3 ubiquitylation may facilitate the recruitment of OTUD7B and, thereby, allows OTUD7B to deubiquitylate TRAF3 and inhibit TRAF3 degradation68.
NIK itself is also a target of negative regulators under signal-induced conditions. Upon activation by NIK, IKKα phosphorylates NIK at several C-terminal serine residues and thereby destabilizes it, thus serving as a negative feedback mechanism to prevent the aberrant accumulation of NIK following its liberation from TRAF3 (REF. 69). Similar to IKKα, the IKK-related kinase TANK-binding kinase 1 (TBK1) phosphorylates NIK to promote NIK degradation, which occurs in B cells in response to BAFFR and CD40 signalling70.
Non-canonical NF-κB signalling is also regulated at the level of receptor trafficking and degradation, as demonstrated for LTβR71. RNA interference- mediated knockdown of major subunits of ESCRT (endosomal sorting complexes required for transport) causes endosomal accumulation of LTβR, which leads to non-canonical NF-κB pathway activation in a ligand-independent manner. These findings suggest that ESCRT prevents the aberrant sorting of LTβR and constitutive activation of the non-canonical NF-κB pathway71. Whether other non-canonical NF-κB pathway-stimulating receptors are also subject to ESCRT regulation remains to be examined.
Mechanism of activation
The principal mechanism of non-canonical NF-κB pathway activation by inducing receptors involves the disruption of the cIAP–TRAF2–TRAF3 complex, which typically occurs through inducible degradation of TRAF3, although some inducers trigger degradation of TRAF2 or cIAP4 (FIG. 2). Early studies demonstrate that the induction of TRAF3 degradation and p100 processing by BAFFR requires its TRAF3-binding sequence72. Recruitment of TRAF2 and TRAF3, as well as their associated cIAP, which then leads to the disruption of the complex, seems to be a common mechanism mediating non-canonical NF-κB pathway activation by different non-canonical NF-κB inducing receptors17. Recruitment to the receptor is thought to promote the E3 ligase activity of cIAP, a process that may involve K63 ubiquitylation of cIAP by TRAF2, thereby allowing cIAP to mediate K48 ubiquitylation and degradation of TRAF3 (REF. 58). However, for some receptors, such as LTβR, the recruitment of TRAF3 is thought to induce the release of NIK from TRAF3 before TRAF3 degradation73,74. Similarly, HIV is thought to activate NIK by inducing the binding of MYD88 to TRAF3 and, thereby the dissociation of NIK from TRAF3 (REF. 44).
A very unusual mechanism of non-canonical NF-κB activation has recently been proposed for complement membrane attack complexes (MACs), which are structures formed as a result of complement activation in host defences against pathogens or in pathological immune reactions75 (FIG. 2). In contrast to TNFRs, which typically take hours to trigger non-canonical NF-κB signalling, alloantibody-induced MACs have been shown to stimulate NIK stabilization within as little as 30 minutes, and this mechanism does not require TRAF3 degradation but involves MAC-induced phosphorylation of the kinase AKT and the formation of a signalosome complex that includes AKT and NIK on RAB5+ endosomes, whereby activated AKT mediates NIK stabilization75. It remains to be further investigated how AKT mediates NIK stabilization and whether this involves the dissociation of NIK from the cIAP–TRAF2–TRAF3 complex.
Role in immune regulation
Well-characterized functions of the non-canonical NF-κB pathway include the regulation of lymphoid organ development, B cell survival and maturation, and the differentiation of osteoclasts, which are bone-absorbing cells of monocyte–macrophage lineage4,76,77. In addition, recent studies using conditional knockout mice and bone marrow chimeric mice have uncovered several novel functions of the non-canonical NF-κB pathway.
Lymphoid organ development
Primary and secondary lymphoid organs are the sites for lymphocyte development and activation, respectively. A well-characterized function of the non-canonical NF-κB pathway is to mediate the development and architectural organization of secondary lymphoid organs, including the spleen, lymph nodes and mucosal lymphoid tissues4,78. Loss-of-function mutation of NIK in Aly/Aly mice impairs the development of lymph nodes and Peyer's patches and causes disorganized spleen architecture28,79. Similar phenotypes have been detected in mice deficient in the downstream signalling components IKKα and RELB or mice that harbour a mutation in the Nfkb2 gene (NF-κB2Lym1 mice) producing a non-processable p100 (REFS 13,23,80,81). Compared with these mutant mice, the Nfkb2-knockout mice have milder defects in secondary lymphoid organ development82,83. This is probably because the Nfkb2 gene deletion does not block the activation of RELB, which may form a dimer with p50 to partially compensate the function of RELB–p52 dimer, as mice deficient in both Nfkb1 and Nfkb2 have more severe defects in lymphoid organ development83. The predominant NF-κB-inducing receptor that mediates lymphoid organ development is LTβR, which is expressed on stromal organizer cells and responds to stimulation by its ligand, LTα1β2, expressed on haematopoietic lymphoid tissue inducer (LTi) cells84. Through LTβR ligation, the LTi cells stimulate stromal organizer cells to express specific chemokines, including CXC-chemokine ligand 13 (CXCL13; also known as BLC), CC-chemokine ligand 21 (CCL21; also known as SLC) and CCL19 (also known as ELC), as well as cell adhesion molecules that mediate the recruitment and retention of more LTi cells required for the growth and development of lymph nodes84. Induction of these chemokine genes by LTβR requires the non-canonical NF-κB pathway29.
The non-canonical NF-κB pathway also has a role in the development of a primary lymphoid organ, the thymus, where it regulates the development of thymic epithelial cells (TECs)76. The thymus has two different types of TECs: cortical TECs (cTECs) and medullary TECs (mTECs), which mediate the early and late phases of thymocyte development, respectively85. In particular, mTECs have a central role in mediating immune tolerance, as they are required for thymocyte negative selection and the generation of the immunosuppressive regulatory T (Treg) cells85. The development of mTECs involves several members of the TNFR superfamily, including LTβR, CD40 and RANK, all of which are known to mediate the activation of both the canonical and non-canonical NF-κB pathways85. RANK signalling is crucial for mTEC development during embryogenesis, but the different TNFR members seem to have functional redundancies in the postnatal development and maintenance of mTECs86. However, signalling downstream of both the canonical and non-canonical NF-κB pathway is essential for mTEC development. Mice deficient in non- canonical NF-κB signalling components, such as NIK, IKKα or RELB, or the canonical NF-κB signalling component TRAF6, have severe defects in mTEC development87–91. Recent studies suggest that the non-canonical NF-κB pathway is dispensable for initial mTEC lineage specification but is required for the further differentiation of mTEC progenitors into mature mTECs92,93. In addition to mediating mTEC development, non-canonical NF-κB signalling regulates the expression of autoimmune regulator (AIRE), an mTEC-specific transcription factor that is required for the expression of tissue-specific antigens and the induction of central tolerance85. Mice deficient in the non-canonical NF-κB family member NF-κB2 p52 have a defect in Aire expression in mTECs, which is associated with impaired central tolerance94. Although this phenotype may also involve defects in mTEC differentiation, this early study is corroborated by more recent studies demonstrating that the Aire gene contains a conserved non-coding sequence that binds and responds to non-canonical and canonical NF-κB family members and is required for Aire gene expression and immune tolerance95,96.
DC function
DCs serve as the primary antigen-presenting cells (APCs) that connect innate immunity to adaptive immunity by facilitating the activation of antigen-specific naive T cells97. DCs sense infections and tissue damage via pattern recognition receptors (PRRs) and undergo maturation to become competent APCs, and this process requires signalling via the canonical NF-κB pathway. DCs also express TNFR superfamily members, such as CD40, LTβR and RANK, which are capable of stimulating the non-canonical NF-κB pathway98. Early studies using mice deficient in the NF-κB members RELB, c-REL, or both c-REL and NF-κB1 suggested an important role for these NF-κB members in the regulation of DC development and maturation99. In particular, DCs require RELB in order to induce T cell responses via both the conventional antigen presenting pathway and via cross-priming. Elevated levels of RELB expression and nuclear translocation are also associated with DC maturation100. However, as noted above, RELB activation is not solely dependent on the non-canonical NF-κB pathway. More recent studies using Aly/Aly mice and mice with a DC-specific knockout of NIK suggest that non-canonical NF-κB pathway signalling is dispensable for DC development and maturation or for antigen presentation to CD4+ T cells but is required for DC cross-priming of CD8+ T cells101,102.
In addition to priming T cells, DCs have an important role in maintaining immune tolerance103. Non-canonical NF-κB pathway signalling has been implicated in the regulation of immune tolerance by mediating the induction of indoleamine 2,3-dioxygenase 1 (IDO1), an enzyme that catalyses the catabolism of an essential amino acid, tryptophan, to kynurenine and thereby inhibits T cell proliferation and survival44,104. Small interfering RNA (siRNA)-mediated knockdown of NIK or IKKα in human DCs was shown to inhibit IDO1 induction in vitro104,105. It remains to be examined whether NIK and non- canonical NF-κB signalling have an in vivo role in regulating IDO1 expression and the tolerogenic function of DCs.
Antiviral innate immunity
Infection with RNA viruses, such as Sendai virus, vesicular stomatitis virus, HIV1 and respiratory syncytial virus, induces non-canonical NF-κB pathway activation40,43–45,106 (TABLE 2). One study suggests that non-canonical NF-κB signalling negatively regulates virus-stimulated type I interferon (IFN) production40. Macrophages derived from Map3k14-knockout mice (which lack NIK) and Nfkb2Lym1 mice display elevated levels of type I IFN induction by vesicular stomatitis virus and Sendai virus, whereas macrophages expressing a stable form of NIK that lacks the TRAF3-binding sequence (NIKΔT3) have reduced type I IFN induction40. The non-canonical NF-κB family members p52 and RELB seem to suppress the transcription of Ifnb by competing with the canonical NF-κB family member RELA for binding to the Ifnb gene enhancer.
Humoral immunity and germinal centres
A crucial role of the non-canonical NF-κB pathway in humoral immune responses was demonstrated in studies of mouse models60,62,76 and human patients with genetic mutations in genes encoding NIK and NF-κB2 (REFS 107–110). Non-canonical NF-κB signalling is required for the survival, maturation and homeostasis of B cells in peripheral lymphoid organs60,62,76. In addition, there is strong evidence that the non-canonical NF-κB pathway is involved in germinal centre (GC) formation, a hallmark of humoral immune responses against protein antigens111. GCs are sites within the B cell follicles of secondary lymphoid organs where antigen-stimulated B cells undergo clonal expansion and a series of differentiation events, including somatic hypermutation and immunoglobulin class switching, selection of B cells with high-affinity B cell receptors (affinity maturation), and deletion of self-reactive B cells (negative selection)111 (FIG. 3). GC formation requires the activation of the non-canonical NF-κB family members p52 and RELB, as well as the upstream kinases NIK and IKKα, in both lymphoid stromal cells and B cells112–116. In stromal cells, the non-canonical NF-κB pathway responds to LTβR signalling and mediates the induction of specific chemokines, including CXCL13 and CCL21. These are required for the formation of B cell follicles and the follicular DC (FDC) network, which, in turn, are crucial for GC formation114. In B cells, the non-canonical NF-κB pathway not only mediates the survival of naive B cells but also regulates GC formation and maintenance as well as immunoglobulin class switching, as demonstrated by recent studies using GC B cell-specific NIK-deficient mice117,118 (FIG. 3). It has also been shown that simultaneous deletion of Nfkb2 and RelB in GC B cells causes the collapse of established GCs119. Interestingly, NIK is also important for the survival of B cells that have undergone class switching, as well as the survival of fully differentiated plasma cells118.
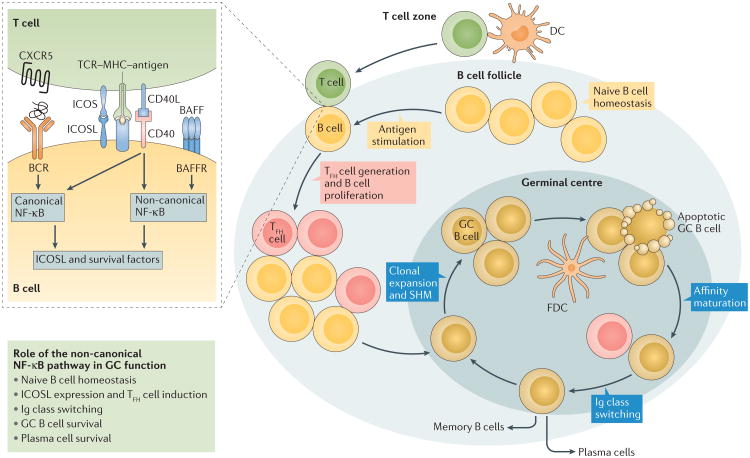
Figure 3. The non-canonical NF-κB pathway regulates GC reactions in multiple steps.
Immune responses to protein antigens involve the formation of germinal centres (GCs) within B cell follicles, in which activated B cells undergo clonal expansion and a series of differentiation events, which leads to the generation of antibody-secreting plasma cells or memory B cells. The initial step of GC formation involves interactions between antigen-primed T cells and B cells on the border of the T cell zone and the B cell follicle, which are required for differentiation of the CD4+ T cells into follicular helper T (TFH) cells. The T cell–B cell interaction is initiated through TCR interaction with the MHC–antigen complex and also requires the ICOS–ICOSL interaction. Ligation of CD40 and BAFFR by their ligands, CD40L and BAFF, induces non-canonical NF-κB signalling in B cells, which is important for maintaining high levels of ICOSL expression and, thus, TFH cell differentiation. TFH cells, in turn, promote the clonal expansion and differentiation of B cells in the subsequent steps of GC reactions. Non-canonical nuclear factor-κB (NF-κB) signalling regulates GC reactions through promoting the survival of naive B cells, the differentiation of TFH cells by inducing the expression of inducible co-stimulator ligand (ICOSL) in B cells, GC cell expansion, GC B cell survival, immunoglobulin (Ig) class switching, somatic hypermutation (SHM) and plasma cell survival60,62,76,117–119,121. In addition, non-canonical NF-κB signalling also functions in stromal cells to mediate the induction of chemokines required for the formation of B cell follicles and the follicular dendritic cell network, which in turn are crucial for GC formation114 (not shown in Figure). BAFF, B cell activating factor; BAFFR, BAFF receptor; BCR, B cell receptor; CXCR5, CXC-chemokine ligand 5; DC, dendritic cell; FDC, follicular dendritic cell; TCR, T cell receptor.
GC reactions require a subset of CD4+ effector T cells termed T follicular helper (TFH) cells, which characteristically express the chemokine receptor CXC-chemokine receptor 5 (CXCR5) and can migrate to the GCs in B cell follicles, where they promote the activation, proliferation and differentiation of antigen- stimulated B cells120. TFH cell generation involves cognate interactions of antigen-stimulated T cells (thought to be the precursors of TFH cells) and B cells at the border of the T cell zone and B cell follicles. A crucial event in this T cell–B cell interaction is the engagement of inducible co-stimulator (ICOS) on T cells by ICOS ligand (ICOSL) on B cells120. Non-canonical NF-κB activation by BAFFR and CD40 is important for maintaining high levels of ICOSL expression in B cells and, thus, antigen-stimulated TFH cell differentiation121 (FIG. 3). Patients with loss-of-function mutations in the gene encoding NIK have been found to have impaired ICOSL expression in B cells and impaired TFH cell generation107. Similarly, patients with mutations in NFKB2, producing non-processable p100 mutants lacking C-terminal phosphorylation sites, have reduced numbers of B cells and TFH cells and develop common variable immunodeficiency108,110,122,123. The non-canonical NF-κB pathway is also required to induce ICOSL expression on the CD8- subset of DCs, which contributes to the induction of TFH cells124. Therefore, the non-canonical NF-κB pathway facilitates GC reactions via multiple mechanisms, including the survival of naive and GC B cells and the generation of TFH cells (FIG. 3).
T cell responses
T cells serve as the central component of adaptive immunity and are also responsible for many auto-immune and inflammatory diseases. Upon activation, CD4+ T cells differentiate into several subsets of effector T cells, including T helper 1 (TH1), TH2, TH9, TH17 and TFH cells, each with specialized helper or regulatory functions125. The non-canonical NF-κB pathway is dispensable for the initial activation of naive T cells through TCR signalling but is crucial for the in vivo generation and maintenance of effector and memory T cells25,126,127. As described above, non-canonical NF-κB signalling facilitates the differentiation of TFH cells involved in GC reactions, although this function of non-canonical NF-κB signalling is not T cell-intrinsic. Non-canonical NF-κB s ignalling has a cell-intrinsic role in mediating the induction of TH9 cells stimulated by OX40, a member of the TNFR family, which contributes to the pathogenesis of airway inflammation42.
Recent studies using NIK-deficient mice and NF-κB2Lym1 mice have also demonstrated a crucial role of the non-canonical NF-κB pathway in the induction of TH1 and TH17 effector cells in the neuroinflammation model experimental autoimmune encephalomyelitis (EAE)25,126,127. Compared with the situation in vivo, the non-canonical NF-κB pathway is much less important for TH1 and TH17 cell differentiation in an in vitro system, in which T cells are stimulated using agonistic TCR- and CD28-targeted antibodies in the presence of polarizing cytokines25,100. A likely explanation for this is that in vivo, the non-canonical NF-κB pathway may be activated by TNFR members that are stimulated by ligands expressed on APCs.
In addition to regulating T cell differentiation, the non-canonical NF-κB pathway may also modulate the effector function of differentiated T cells. For example, the non-canonical NF-κB family member p52 promotes the pathological function of TH17 cells in neuroinflammation by mediating the induction of the cytokine GM-CSF25. Based on the current knowledge, a model can be proposed to explain how the non-canonical NF-κB pathway regulates T cell responses (FIG. 4). The initial activation of naive T cells via the TCR and the CD28 co-stimulatory receptor relies on the canonical, but not the non-canonical, NF-κB pathway. Following T cell activation, several TNFRs are induced41, which mediate the activation of the non-canonical NF-κB pathway upon ligation by their ligands on APCs. As activated T cells also express the ligands for TNFRs41, it is likely that non-canonical NF-κB activation also involves T cell–T cell interactions. Upon activation, NF-κB family members induced by the non-canonical pathway regulate the expansion and survival of effector T cells as well as their production of effector cytokines (FIG. 4).
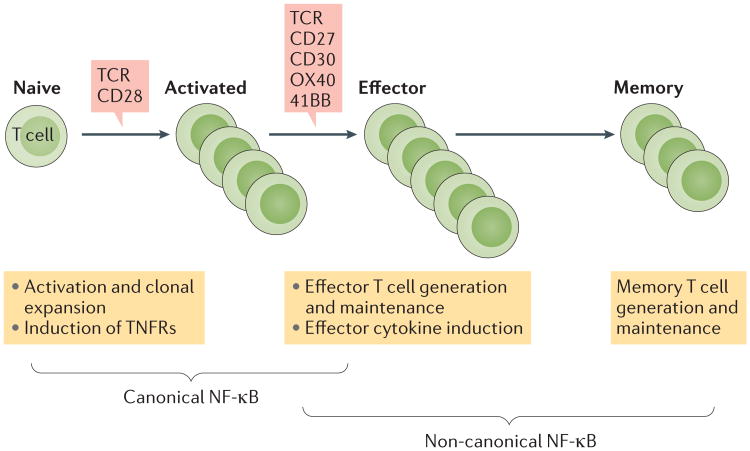
Figure 4. Regulation of T cell responses by canonical and non-canonical NF-κB pathways.
Naive T cell activation via the T cell receptor (TCR) and signalling through the co-receptor CD28 leads to the rapid activation of the canonical nuclear factor-κB (NF-κB) pathway, which contributes to the initial activation and clonal expansion of T cells2. T cell activation is associated with TCR stimulation and the inducible expression of several TNF receptor (TNFR) superfamily members such as CD27, CD30, OX40 and 41BB, which mediate the activation of non-canonical NF-κB signalling upon ligation by their ligands on antigen-presenting cells and activated T cells. Non-canonical NF-κB signalling is crucial for the generation and maintenance of effector and memory T cells and also has a role in mediating the induction of effector cytokines25,126,127. Since TNFRs also activate canonical NF-κB signalling, it is likely that the two pathways function cooperatively during the effector and memory phases of T cell responses.
Non-canonical NF-κB signalling also has a role in regulating the development of FOXP3+ Treg cells. Early studies suggested that non-canonical NF-κB signalling mediates Treg cell development indirectly through maintaining the integrity of the thymic stroma and the function of DCs89,128. However, more recent studies using bone marrow adoptive transfer and mice with a conditional depletion of NIK in T cells have demonstrated a T cell- intrinsic function for the non-canonical NF-κB pathway in regulating the peripheral maintenance of T cells126,129. T cell-specific deletion of NIK reduces the frequency and absolute number of Treg cells in the spleen and lymph nodes, although not in the thymus126. Thus, non-canonical NF-κB signalling has paradoxical roles in regulating T cell responses. The non-canonical NF-κB pathway also has a role in regulating the development of natural killer T (NKT) cells and γδ T cells, and this function is indirectly mediated via the regulation of mTECs130–132.
Role in inflammatory diseases
Inflammation is a protective host response to infections and tissue damage that serves as a mechanism to prevent pathogen spread and to promote pathogen destruction and wound healing133. A typical inflammatory response involves the production of pro-inflammatory cytokines, chemokines and other inflammatory mediators in the affected tissue, thereby causing a series of local reactions, such as vasodilation and recruitment of neutrophils and monocytes. Inflammation also involves T cells, especially the inflammatory TH1 and TH17 cells133. Although inflammation is usually resolved in a timely manner and beneficial to the host, dysregulated inflammation contributes to the pathogenesis of various chronic inflammatory diseases134.
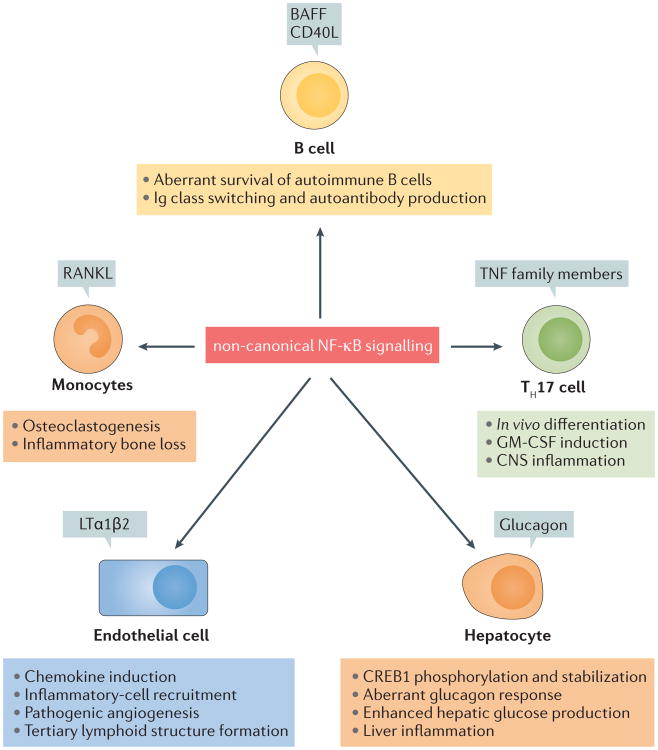
When aberrantly activated, non-canonical NF-κB signalling in different cell types can promote auto-immunity and inflammation (FIG. 5). As discussed below, dysregulated non-canonical NF-κB activation causes aberrant survival of self-reactive B cells, rendering them resistant to negative selection and leading to autoimmune antibody production associated with several inflammatory diseases. Furthermore, dysregulated non- canonical NF-κB signalling in endothelial cells can result in aberrant chemokine production and inflammatory cell recruitment, thereby causing excessive and/or chronic tissue damage and inflammation. The non-canonical NF-κB pathway is also activated in other cell types, such as T cells, monocytes and hepatocytes, whereby it promotes inflammation with different mechanisms (FIG. 5).
Figure 5. The non-canonical NF-κB pathway regulates inflammation in different cell types.
Under physiological conditions, non-canonical nuclear factor-κB (NF-κB) signalling mediates the survival and homeostasis of B cells, the generation and effector function of T helper 17 (TH17) cells, the differentiation of osteoclasts from monocytes, chemokine production in endothelial cells, and glucagon responses in hepatocytes25,60,62,76,77,126,139,161. These cellular events become pathogenic in situations in which the non-canonical NF-κB pathway is aberrantly activated owing to the uncontrolled production of its inducing agents or genetic deficiencies in its negative regulators. Aberrant B cell survival renders self-reactive B cells resistant to negative selection, contributing to the accumulation of autoantibodies associated with inflammatory diseases147. Autoimmune TH17 cells mediate inflammation in the central nervous system (CNS) and other tissues. Excessive and chronic production of chemokines by endothelial cells promotes the recruitment of inflammatory cells and the formation of tertiary lymphoid structures in tissue-specific inflammation138,139. Aberrant osteoclast generation is a pathological mechanism of inflammatory bone loss, and aberrant glucagon responses are associated with metabolic diseases77,161. BAFF, B cell activating factor; CD40L, CD40 ligand; CREB1, cAMP-responsive element-binding protein; GM-CSF, granulocyte–macrophage colony-stimulating factor; Ig, immunoglobulin; LTα 1β2, lymphotoxin α 1 β2; RANKL, RANK, receptor activator for NF- κB ligand; TNF, tumour necrosis factor.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is an autoimmune disease that is characterized by chronic inflammation and hyperplasia of synovial tissue associated with destruction of cartilage and bone135. The pathogenesis of RA involves the recruitment of innate immune cells and inflammatory T cells, particularly TH17 cells, to the synovial tissue, where they release pro-inflammatory cytokines and induce synovitis. Canonical NF-κB signalling has long been recognized for its pro-inflammatory role in RA, but strong evidence suggests that non-canonical NF-κB signalling is also involved in different aspects of RA pathogenesis136. The aetiology of RA is linked to several TNF and TNFR superfamily members known to mediate non-canonical NF-κB activation137. Furthermore, NIK is highly expressed in the synovial endothelial cells of patients with RA and promotes pathogenic angiogenesis and synovial inflammation through the induction of chemokines such as CXCL12 (REFS 138,139). NIK activation in endothelial cells may also promote the formation of tertiary lymphoid structures, which are associated with chronic inflammatory diseases like RA140.
Progressive joint damage in patients with RA involves the aberrant generation and activation of osteoclasts141. Osteoclastogenesis requires RANK, which can stimulate both the canonical and the non-canonical NF-κB pathways in osteoclast precursor cells77. Defects in non-canonical NF-κB signalling lead to an accumulation of p100, which serves as an IκB to prevent the activation of several NF-κB members, resulting in impaired osteoclast generation77. The non-canonical NF-κB pathway is also required for bone erosion in animal models of inflammatory arthritis142,143. Conversely, dysregulated non-canonical NF-κB activation, owing to TRAF3 deletion or the transgenic expression of NIKΔT3 (a stable form of NIK), causes increased osteoclast formation and bone loss144,145.
RA pathogenesis also involves B cells, which are responsible for the production of autoantibodies and also have additional pathological functions, including the regulation of other immune cells, the formation of synovial tertiary lymphoid tissue and the production of cytokines146. The B cell survival factor BAFF has been linked to RA and explored as a therapeutic target in clinical trials146. Under physiological conditions, BAFF mediates the survival and maturation of B cells, which crucially involves the activation of the non-canonical NF-κB pathway. However, overproduction of BAFF, as revealed in BAFF-transgenic mice, promotes the aberrant survival of autoreactive B cells and the development of autoimmunity147. Patients with RA often have elevated levels of BAFF in the serum and synovial fluid, which is associated with disease severity146. These studies suggest a role for the non-canonical NF-κB pathway in regulating RA pathogenesis in different cell types.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an auto immune and chronic inflammatory disease that affects multiple tissues and organs148. The aetiology of SLE is multifactorial, including genetic, environmental, hormonal and immunological factors. A hallmark of the immunopathology of SLE is the accumulation of autoantibodies as immune complexes with nucleic acids, which stimulate PRRs on innate immune cells for the aberrant production of type I IFNs. As the source of auto antibodies, B cells have a central role in the pathogenesis of SLE and have been exploited as a major target in SLE therapy149. As observed in patients with RA, the pathological survival and differentiation of autoantibody-producing B cells in patients with SLE rely on the non-canonical NF-κB pathway, which is activated via TNFR superfamily members, particularly BAFFR and CD40. Patients with SLE frequently have elevated serum levels of BAFF, which is associated with disease activity150,151. Belimumab, a BAFF-neutralizing antibody, has been approved for SLE therapy, and several other BAFF inhibitors have been explored in clinical trials as therapeutic agents for SLE152.
Kidney inflammation and injury
The non-canonical NF-κB pathway has been implicated in the pathogenesis of IgA nephropathy, a leading cause of primary glomerulonephritis kidney diseases, which are characterized by aberrant production of glycosylated IgA and the deposition of IgA-immune complexes in kidney glomeruli153. Studies of mouse models suggest that dysregulated non-canonical NF-κB pathway activation in B cells (owing to the loss of the negative regulator TBK1) is associated with elevated serum levels of IgA and nephropathy-like disease symptoms, including antibody deposition in kidney glomeruli and signs of kidney dysfunction70. Similar pathological phenotypes have been detected in transgenic mice that overexpress BAFF in B cells154. An increasing number of studies also show that non-canonical NF-κB signalling in kidney cells can regulate inflammation when activated by TNF-like weak inducer of apoptosis (TWEAK), a TNF superfamily member implicated in the pathogenesis of kidney diseases153,155. Elevated expression of TWEAK and its receptor, FN14, has been detected in patients with kidney diseases and in animal models of kidney injury, and it has been implicated in the pathogenesis of kidney diseases155. TWEAK engages FN14 on kidney cells, including tubular epithelial cells, mesangial cells and podocytes, and stimulates both the canonical and non-canonical NF-κB pathways, thereby inducing the expression of pro-inflammatory cytokines and chemo kines. The non-canonical NF-κB pathway mediates TWEAK-induced expression of CCL21, a chemokine involved in the recruitment of T cells and the induction of kidney inflammation and fibrosis156–158.
Metabolic inflammation
Metabolic diseases, such as obesity and type 2 diabetes, are associated with dysregulated immune responses and chronic inflammation, and the targeting of the inflammatory mediators is considered an attractive therapeutic approach159. Recent studies have revealed the involvement of the non-canonical NF-κB pathway in metabolic diseases160–162. Upregulated levels of NIK protein are found in skeletal muscle cells of patients with metabolic syndrome160. Furthermore, gastric bypass surgery, leading to weight loss, has been shown to correlate with a decrease in skeletal muscle cell NIK protein levels160. The levels of NIK protein are also upregulated in cortical kidney cells in a mouse model of type 2 diabetes and in pancreatic islets of diet-induced obese mice162,163. In the pancreatic islands of obese mice, NIK is stimulated by RANK ligand (RANKL), a non-canonical NF-κB inducer that has also been shown to mediate the induction of type 2 diabetes in mouse models163,164.
Direct evidence for a functional contribution of NIK to the development of metabolic diseases came from a study showing that NIK is activated in hepatocytes in a mouse model of dietary and genetic obesity161. Moreover, NIK deficiency or the liver-specific expression of a kinase-dead NIK mutant results in reduced glucagon responses and hepatic glucose production, whereas hepatocyte-specific over expression of wild-type NIK increases glucagon responses and hepatic glucose production161. Mechanistically, NIK stabilizes cAMP-responsive element-binding protein (CREB1), a transcription factor that mediates glucagon-stimulated gluconeogenesis by inducing the expression of the gluconeogenesis enzymes phosphoenol pyruvate carboxykinase and glucose 6-phosphatase161. NIK phosphorylates CREB1 and stabilizes CREB1 via both phosphorylation-dependent and independent mechanisms. NIK is also implicated as an inflammatory mediator in liver inflammation and injury induced by hepatitis viruses and alcohol consumption, and NIK over expression in hepatocytes induces fatal liver injury and fibrosis165,166. Inhibition of NIK with a small-molecule inhibitor protects mice from toxin-induced liver inflammation and injury167. Furthermore, non-canonical NF-κB pathway activation in pancreatic β-cells via the conditional deletion of TRAF2 or TRAF3, which act as negative regulators of NIK, reduces insulin secretion and causes exacerbated glucose intolerance in a mouse model of diet-induced obesity163. Consistent with these findings, NIK induction by SMAC mimetics, which induce the degradation of the NIK-inhibitory cIAP proteins, impairs glucose-induced insulin secretion in mouse and human islets163.
Other inflammatory diseases
Largely on the basis of studies of animal models, non-canonical NF-κB signalling has been implicated in the pathogenesis of several other inflammatory diseases. These include EAE, a widely used animal model of multiple sclerosis, which is a neuroinflammatory disease that is thought to involve autoreactive inflammatory TH1 and TH17 cells168. Several studies have demonstrated an essential role for NIK and non-canonical NF-κB signalling in mediating the pathogenesis of EAE14,25,126,169. Moreover, a recent genome-wide association study identified NIK as one of the candidate genes for multiple sclerosis susceptibility170. As described above, the non-canonical NF-κB pathway regulates the pathological effector function of TH17 cells, as well as recall responses of autoreactive T cells25,126. Another study also proposed a DC-specific role for non-canonical NF-κB signalling in mediating the pathogenesis of EAE169; however, this finding was not supported by a more recent study using DC-conditional NIK-deficient mice102.
In contrast to its essential role in EAE induction, NIK acts as a negative regulator in hypereosinophilic syndrome-like disease, an inflammatory disorder characterized by a persistent increase in eosinophil count in the blood and various organs16. NIK-deficient mice develop hypereosinophilic syndrome-like disease involving aberrant TH2 cell responses. However, bone marrow adoptive transfer experiments suggest that the disease development in NIK-deficient mice depends on non-haematopoietic cells16. Furthermore, as mice carrying a mutation in the activation loop of IKKα (the phosphorylation site of NIK) do not develop hypereosinophilic syndrome, this pathological phenotype in NIK-deficient mice may be independent of IKKα and non-canonical NF-κB pathway signalling16.
A recent study using epidermal cell-specific Traf2-knockout (Traf2EKO) mice suggest that TRAF2 deficiency in keratinocytes causes NIK accumulation and constitutive non-canonical NF-κB activation, which is associated with heightened expression of inflammatory cytokines and the development of psoriasis-like skin inflammation171. It seems that TNF-dependent cell death promotes early inflammation, whereas constitutive activation of the non-canonical NF-κB pathway may lead to the subsequent onset of skin inflammation in the Traf2EKO mice. In support of this hypothesis, deletion of both Nfkb2 and Tnf in the Traf2EKO mice completely prevents skin inflammation171. Skin inflammation also involves IL-17-producing γδ T cells172, the development of which requires NIK-mediated signalling in thymic epithelial cells132,173.
A role for the non-canonical NF-κB pathway in colon inflammation was revealed in a study of mice deficient for NLRP12, which negatively regulates non- canonical NF-κB activation66. The NLRP12-deficient mice are hypersensitive to the induction of colitis and colitis-associated cancer, and this anti-inflammatory function of NLRP12 involves the inhibition of non- canonical NF-κB pathway activation. In line with this study, Nfkb2-knockout mice show resistance to dextran sulfate sodium (DSS)-induced colitis and colitis- associated colon cancer174. Non-canonical NF-κB signalling also has an important role in regulating mucosal immunity against infections, which may involve crosstalk with the canonical NF-κB pathway175. Defects in non- canonical NF-κB signalling render mice more sensitive to the intestinal pathogen Citrobacter rodentium, leading to gut inflammation, whereas enhanced activation of the non-canonical NF-κB pathway promotes host defence against infections68,175–177. Thus, physiologically regulated non-canonical NF-κB pathway function is important for maintaining immunity and inducing inflammation in the intestine.
Concluding remarks
The non-canonical NF-κB pathway has been a hot topic of studies in recent years, and substantial progress has been made in our understanding of its signalling mechanism and biological functions. It has become clear that NIK is a central and specific component that integrates various TNFR signals, resulting in the activation of non-canonical NF-κB family members. A paradigm has been established by demonstrating an unusual mechanism of NIK regulation4,53 and by characterizing a NIK-specific E3 ubiquitin ligase complex, cIAP–TRAF2–TRAF3 (REFS 4,58,59,64,65). Recent studies have also demon- strated new functions of this pathway in the immune sys- tem, highlighting its involvement in both normal immune responses and inflammatory diseases, as discussed above.
Despite these advances, several outstanding questions remain to be addressed. For example, the mechanism by which TNFR family members stimulate NIK-dependent p100 processing is only partially understood. Although IKKα has been established as a downstream kinase of NIK that mediates p100 phosphorylation, IKKα can also be activated by NIK- independent signals that do not induce p100 processing18. Does IKKα need to be assembled into a NIK-specific complex for accessing the substrate p100? Does NIK activate additional signalling factors that functionally cooperate with IKKα? Another question concerns the mechanism of NIK activation. Although signal-induced NIK accumulation typically involves the disruption of the cIAP–TRAF2–TRAF3 complex, recent studies suggest that some stimuli activate NIK without involving cIAP-TRAF degradation. Thus, the biochemical mechanism of NIK activation warrants further studies. Furthermore, we are just beginning to understand the cell type-specific functions of the non-canonical NF-κB pathway in the regulation of immune and inflammatory responses. Further studies along this line, with the help of the recently generated conditional knockout mice, will provide new insights into the immunoregulatory roles of this NF-κB pathway. Finally, an exciting area for future studies is to explore the therapeutic efficacies of small-molecule inhibitors of NIK in the treatment of inflammatory diseases.
Acknowledgments
Work in the author's laboratory is supported by grants from the US National Institutes of Health (AI057555, AI064639, GM84459 and AI104519) and the Cancer Prevention Research Institute of Texas (RP140244 and RP150235).
Glossary
- Tumour necrosis factor receptor (TNFR) superfamily
A large family of cytokine receptors that are engaged by members of the TNF superfamily of cytokines and mediate signal transduction
- Epidermal growth factor receptor (EGFR)
A receptor tyrosine kinase that responds to the growth factor EGF and mediates cell growth and survival by triggering several intracellular signalling pathways
- p63
A p53 homologue produced as two main isoforms, TAp63 and ΔNp63, with ΔNp63 lacking the N-terminal typical transactivation domain and functioning as a dominant-negative form to promote oncogenesis
- Tripartite motif-containing protein 29 (TRIM29)
A member of the TRIM family implicated in oncogenesis
- Stromal organizer cells
Matrix cells of mesenchymal origin that characteristically express the cell adhesion molecules VCAM1 and ICAM1 and the TNFR member LTβR and are required for lymphoid organ development
- Cross-priming
A mechanism of CD8+ T cell priming, in which antigen-presenting cells take up extracellular antigens, process and present them with MHC class I molecules to CD8+ T cells
- Small interfering RNA (siRNA)
Short double-stranded RNA molecules, typically 20–25 bp in length, which bind to and induce degradation of mRNAs with complementary sequences, thereby interfering with production of the corresponding proteins
- Common variable immunodeficiency
A frequently diagnosed and heterogeneous type of primary immunodeficiency characterized by low to undetectable levels of antibodies and increased susceptibility to infections
- Experimental autoimmune encephalomyelitis (EAE)
A commonly used animal model of the autoimmune neuroinflammatory disease multiple sclerosis, characterized by infiltration of the central nervous system with T cells and monocytes that cause inflammation and demyelination leading to limb paralysis
- Rheumatoid arthritis(RA)
An autoimmune disease characterized by chronic inflammation in the joints and destruction of cartilage and bone
- Systemic lupus erythematosus (SLE)
An autoimmune disease characterized by chronic inflammation throughout the body, causing tissue damage in multiple organs
- SMAC mimetics
A class of small-molecule compounds that bind to and antagonize cIAP by mimicking the endogenous IAP antagonist SMAC
Footnotes
Competing interests statement: The author declares no competing interests.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Sun SC, Ley SC. New insights into NF-κB regulation and function. Trends Immunol. 2008;29:469–478. doi: 10.1016/j.it.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin L, DeMartino GN, Greene WC. Cotranslational biogenesis of NF-κB p50 by the 26S proteasome. Cell. 1998;92:819–828. doi: 10.1016/s0092-8674(00)81409-9. [DOI] [PubMed] [Google Scholar]
- 4.Sun SC. The noncanonical NF-κB pathway. Immunol Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 6.Hu H, Sun SC. Ubiquitin signaling in immune responses. Cell Res. 2016;26:457–483. doi: 10.1038/cr.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang HT, et al. Coordinate regulation of TPL-2 and NF-κB signaling in macrophages by NF-κB1 p105. Mol Cell Biol. 2012;32:3438–3451. doi: 10.1128/MCB.00564-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sriskantharajah S, et al. Proteolysis of NF-κB1 p105 is essential for T cell antigen receptor-induced proliferation. Nat Immunol. 2009;10:38–47. doi: 10.1038/ni.1685. [DOI] [PubMed] [Google Scholar]
- 9.Israel A. The IKK complex, a central regulator of NF-κB activation. Cold Spring Harb Perspect Biol. 2010;2:a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao G, Harhaj EW, Sun SC. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. This is the first paper to report inducible processing of p100 and to identify NIK as an inducing kinase. [DOI] [PubMed] [Google Scholar]
- 11.Fong A, Sun SC. Genetic evidence for the essential role of beta-transducin repeat-containing protein in the inducible processing of NF-κB2/p100. J Biol Chem. 2002;277:22111–22114. doi: 10.1074/jbc.C200151200. [DOI] [PubMed] [Google Scholar]
- 12.Liang C, Zhang M, Sun SC. β-TrCP binding and processing of NF-κB2/p100 involve its phosphorylation at serines 866 and 870. Cell Signal. 2006;18:1309–1317. doi: 10.1016/j.cellsig.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Senftleben U, et al. Activation of IKKa of a second, evolutionary conserved, NF-kB signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. This paper identifies IKKα as a NIK-target kinase that directly phosphorylates p100 to induce p100 processing. [DOI] [PubMed] [Google Scholar]
- 14.Jin W, Zhou XF, Yu J, Cheng X, Sun SC. Regulation of Th17 cell differentiation and EAE induction by the MAP3K NIK. Blood. 2009;113:6603–6610. doi: 10.1182/blood-2008-12-192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boutaffala L, et al. NIK promotes tissue destruction independently of the alternative NF-κB pathway through TNFR1/RIP1-induced apoptosis. Cell Death Differ. 2015;22:2020–2033. doi: 10.1038/cdd.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hacker H, Chi L, Rehg JE, Redecke V. NIK prevents the development of hypereosinophilic syndrome-like disease in mice independent of IKKalpha activation. J Immunol. 2012;188:4602–4610. doi: 10.4049/jimmunol.1200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun SC. Non-canonical NF-κB signaling pathway. Cell Res. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, Xia Y, Parker AS, Verma IM. IKK biology. Immunol Rev. 2012;246:239–253. doi: 10.1111/j.1600-065X.2012.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B, et al. IKKalpha is required to maintain skin homeostasis and prevent skin cancer. Cancer Cell. 2008;14:212–225. doi: 10.1016/j.ccr.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao Z, et al. The pivotal role of IKKalpha in the development of spontaneous lung squamous cell carcinomas. Cancer Cell. 2013;23:527–540. doi: 10.1016/j.ccr.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao G, Fong A, Sun SC. Induction of p100 processing by NF-κB-inducing kinase involves docking IκB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J Biol Chem. 2004;279:30099–30105. doi: 10.1074/jbc.M401428200. [DOI] [PubMed] [Google Scholar]
- 22.Sun SC, Ganchi PA, Beraud C, Ballard DW, Greene WC. Autoregulation of the NF-kB transactivator Rel A (p65) by multiple cytoplasmic inhibitors containing ankyrin motifs. Proc Natl Acad Sci USA. 1994;91:1346–1350. doi: 10.1073/pnas.91.4.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucker E, et al. A novel mutation in the Nfkb2 gene generates an NF-κ B2 “super repressor”. J Immunol. 2007;179:7514–7522. doi: 10.4049/jimmunol.179.11.7514. [DOI] [PubMed] [Google Scholar]
- 24.Zarnegar B, Yamazaki S, He JQ, Cheng G. Control of canonical NF-κB activation through the NIK-IKK complex pathway. Proc Natl Acad Sci USA. 2008;105:3503–3508. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu J, et al. T cell-intrinsic function of the noncanonical NF-κB pathway in the regulation of GM-CSF expression and experimental autoimmune encephalomyelitis pathogenesis. J Immunol. 2014;193:422–430. doi: 10.4049/jimmunol.1303237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Silva NS, Silva K, Anderson MM, Bhagat G, Klein U. Impairment of mature B cell maintenance upon combined deletion of the alternative NF-κB transcription factors RELB and NF-κB2 in B cells. J Immunol. 2016;196:2591–2601. doi: 10.4049/jimmunol.1501120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih VF, et al. Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-κB pathways. Nat Immunol. 2012;13:1162–1170. doi: 10.1038/ni.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinkura R, et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-κB-inducing kinase. Nat Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 29.Dejardin E, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-κB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 30.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-κB2 in maturing B cells. Nat Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 31.Coope HJ, et al. CD40 regulates the processing of NF-κB2 p100 to p52. EMBO J. 2002;15:5375–5385. doi: 10.1093/emboj/cdf542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kayagaki N, et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-κB2. Immunity. 2002;17:515–524. doi: 10.1016/s1074-7613(02)00425-9. References 29–32 are the first papers to report ligand-induced non-canonical NF-κB activation. [DOI] [PubMed] [Google Scholar]
- 33.Saitoh T, et al. TWEAK induces NF-κB2 p100 processing and long lasting NF-κB activation. J Biol Chem. 2003;278:36005–36012. doi: 10.1074/jbc.M304266200. [DOI] [PubMed] [Google Scholar]
- 34.Ramakrishnan P, Wang W, Wallach D. Receptor-specific signaling for both the alternative and the canonical NF-κB activation pathways by NF-κB-inducing kinase. Immunity. 2004;21:477–489. doi: 10.1016/j.immuni.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Nishikori M, Ohno H, Haga H, Uchiyama T. Stimulation of CD30 in anaplastic large cell lymphoma leads to production of nuclear factor-κB p52, which is associated with hyperphosphorylated Bcl-3. Cancer Sci. 2005;96:487–497. doi: 10.1111/j.1349-7006.2005.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nonaka M, et al. Aberrant NF-κB2/p52 expression in Hodgkin/Reed-Sternberg cells and CD30-transformed rat fibroblasts. Oncogene. 2005;24:3976–3986. doi: 10.1038/sj.onc.1208564. [DOI] [PubMed] [Google Scholar]
- 37.Novack DV, et al. The IκB function of NF-κB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198:771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray SE, et al. NF-κB-inducing kinase plays an essential T cell-intrinsic role in graft-versus-host disease and lethal autoimmunity in mice. J Clin Invest. 2011;121:4775–4786. doi: 10.1172/JCI44943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McPherson AJ, Snell LM, Mak TW, Watts TH. Opposing roles for TRAF1 in the alternative versus classical NF-κB pathway in T cells. J Biol Chem. 2012;287:23010–23019. doi: 10.1074/jbc.M112.350538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin J, et al. Noncanonical NF-κB pathway controls the production of type I interferons in antiviral innate immunity. Immunity. 2014;40:342–354. doi: 10.1016/j.immuni.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao X, et al. OX40 signaling favors the induction of TH9 cells and airway inflammation. Nat Immunol. 2012;13:981–990. doi: 10.1038/ni.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu P, Li K, Garofalo RP, Brasier AR. Respiratory syncytial virus induces RelA release from cytoplasmic 100-kDa NF-κ B2 complexes via a novel retinoic acid-inducible gene-I∙NF- κ B-inducing kinase signaling pathway. J Biol Chem. 2008;283:23169–23178. doi: 10.1074/jbc.M802729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manches O, Fernandez MV, Plumas J, Chaperot L, Bhardwaj N. Activation of the noncanonical NF-κB pathway by HIV controls a dendritic cell immunoregulatory phenotype. Proc Natl Acad Sci USA. 2012;109:14122–14127. doi: 10.1073/pnas.1204032109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruckle A, et al. The NS1 protein of influenza A virus blocks RIG-I-mediated activation of the noncanonical NF-κB pathway and p52/RelB-dependent gene expression in lung epithelial cells. J Virol. 2012;86:10211–10217. doi: 10.1128/JVI.00323-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao G, et al. Retroviral oncoprotein Tax induces processing of NF-κB2/p100 in T cells: evidence for the involvement of IKKalpha. EMBO J. 2001;20:6805–6815. doi: 10.1093/emboj/20.23.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luftig M, et al. Epstein-Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK alpha-dependent noncanonical NF-κB activation. Proc Natl Acad Sci USA. 2004;101:141–146. doi: 10.1073/pnas.2237183100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matta H, Chaudhary PM. Activation of alternative NF-κ B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP) Proc Natl Acad Sci USA. 2004;101:9399–9404. doi: 10.1073/pnas.0308016101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho IR, et al. Activation of non-canonical NF-κB pathway mediated by STP-A11, an oncoprotein of Herpesvirus saimiri. Virology. 2007;359:37–45. doi: 10.1016/j.virol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 50.de Jong SJ, et al. Noncanonical NF-κB activation by the oncoprotein Tio occurs through a nonconserved TRAF3-binding motif. Sci Signal. 2013;6:ra27. doi: 10.1126/scisignal.2003309. [DOI] [PubMed] [Google Scholar]
- 51.Ohmae T, et al. Helicobacter pylori activates NF-κBvia the alternative pathway in B lymphocytes. J Immunol. 2005;175:7162–7169. doi: 10.4049/jimmunol.175.11.7162. [DOI] [PubMed] [Google Scholar]
- 52.Ge J, et al. A Legionella type IV effector activates the NF-κB pathway by phosphorylating the IκB family of inhibitors. Proc Natl Acad Sci USA. 2009;106:13725–13730. doi: 10.1073/pnas.0907200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-κB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. This paper demonstrates that NIK is regulated by TRAF3-dependent proteolysis and activated via signal-induced TRAF3 degradation. [DOI] [PubMed] [Google Scholar]
- 54.He JQ, et al. Rescue of TRAF3-null mice by p100 NF-κ B deficiency. J Exp Med. 2006;203:2413–2418. doi: 10.1084/jem.20061166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasaki Y, et al. NIK overexpression amplifies, whereas ablation of its TRAF3-binding domain replaces BAFF:BAFF-R-mediated survival signals in B cells. Proc Natl Acad Sci USA. 2008;105:10883–10888. doi: 10.1073/pnas.0805186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Leon-Boenig G, et al. The crystal structure of the catalytic domain of the NF-κB inducing kinase reveals a narrow but flexible active site. Structure. 2012;20:1704–1714. doi: 10.1016/j.str.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 57.Liu J, et al. Structure of the nuclear factor κB-inducing kinase (NIK) kinase domain reveals a constitutively active conformation. J Biol Chem. 2012;287:27326–27334. doi: 10.1074/jbc.M112.366658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vallabhapurapu S, et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-κB signaling. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zarnegar BJ, et al. Noncanonical NF-κB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. References 58 and 59 propose a model whereby TRAF3 functions as a cIAP–TRAF2–TRAF3 complex to mediate NIK ubiquitylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 61.Grech AP, et al. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-κB activation in mature B cells. Immunity. 2004;21:629–642. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 62.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gardam S, et al. Deletion of cIAP1 and cIAP2 in murine B lymphocytes constitutively activates cell survival pathways and inactivates the germinal center response. Blood. 2011;117:4041–4051. doi: 10.1182/blood-2010-10-312793. [DOI] [PubMed] [Google Scholar]
- 64.Varfolomeev E, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 65.Vince JE, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. References 64 and 65 demonstrated the involvement of cIAP in the negative regulation of non-canonical NF-κB pathway. [DOI] [PubMed] [Google Scholar]
- 66.Allen IC, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity. 2012;36:742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lich JD, et al. Monarch-1 suppresses non-canonical NF-κB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007;178:1256–1260. doi: 10.4049/jimmunol.178.3.1256. [DOI] [PubMed] [Google Scholar]
- 68.Hu H, et al. OTUD7B controls non-canonical NF-κB activation through deubiquitination of TRAF3. Nature. 2013;494:371–374. doi: 10.1038/nature11831. This study identified the OTUD7B as a deubiquitinase of TRAF3 that controls non-canonical NF-κB signalling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Razani B, et al. Negative feedback in non-canonical NF-κB signaling modulates NIK stability through IKKα-mediated phosphorylation. Sci Signal. 2010;3:ra41. doi: 10.1126/scisignal.2000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin J, et al. The kinase TBK1 controls IgA class switching by negatively regulating noncanonical NF-κB signaling. Nat Immunol. 2012;13:1101–1109. doi: 10.1038/ni.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maminska A, et al. ESCRT proteins restrict constitutive NF-κB signaling by trafficking cytokine receptors. Sci Signal. 2016;9:ra8. doi: 10.1126/scisignal.aad0848. [DOI] [PubMed] [Google Scholar]
- 72.Morrison MD, Reiley W, Zhang M, Sun SC. An atypical tumor necrosis factor (TNF) receptor-associated factor-binding motif of B cell-activating factor belonging to the TNF family (BAFF) receptor mediates induction of the noncanonical NF-κB signaling pathway. J Biol Chem. 2005;280:10018–10024. doi: 10.1074/jbc.M413634200. [DOI] [PubMed] [Google Scholar]
- 73.Sanjo H, Zajonc DM, Braden R, Norris PS, Ware CF. Allosteric regulation of the ubiquitin:NIK and ubiquitin:TRAF3 E3 ligases by the lymphotoxin-beta receptor. J Biol Chem. 2010;285:17148–17155. doi: 10.1074/jbc.M110.105874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ganeff C, et al. Induction of the alternative NF-κB pathway by lymphotoxin alphabeta (LTalphabeta) relies on internalization of LTbeta receptor. Mol Cell Biol. 2011;31:4319–4334. doi: 10.1128/MCB.05033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jane-wit D, et al. Complement membrane attack complexes activate noncanonical NF-κB by forming an Akt+ NIK+ signalosome on Rab5+ endosomes. Proc Natl Acad Sci USA. 2015;112:9686–9691. doi: 10.1073/pnas.1503535112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dejardin E. The alternative NF-κB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem Pharmacol. 2006;72:1161–1179. doi: 10.1016/j.bcp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 77.Novack DV. Role of NF-κB in the skeleton. Cell Res. 2011;21:169–182. doi: 10.1038/cr.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weih F, Caamaño J. Regulation of secondary lymphoid organ development by the nuclear factor-κB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 79.Miyawaki S, et al. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. 1994;24:429–434. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 80.Matsushima A, et al. Essential role of nuclear factor (NF)-κB-inducing kinase and inhibitor of κB (IκB) kinase alpha in NF-κB activation through lymphotoxin beta receptor, but not through tumor necrosis factor receptor I. J Exp Med. 2001;193:631–636. doi: 10.1084/jem.193.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yilmaz ZB, Weih DS, Sivakumar V, Weih F. RelB is required for Peyer's patch development: differential regulation of p52-RelB by lymphotoxin and TNF. EMBO J. 2003;22:121–130. doi: 10.1093/emboj/cdg004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carragher D, et al. A stroma-derived defect in NF-κB2-/- mice causes impaired lymph node development and lymphocyte recruitment. J Immunol. 2004;173:2271–2279. doi: 10.4049/jimmunol.173.4.2271. [DOI] [PubMed] [Google Scholar]
- 83.Lo JC, et al. Coordination between NF-κB family members p50 and p52 is essential for mediating LTbetaR signals in the development and organization of secondary lymphoid tissues. Blood. 2006;107:1048–1055. doi: 10.1182/blood-2005-06-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 85.Abramson J, Anderson G. Thymic epithelial cells. Annu Rev Immunol. 2017;35:85–118. doi: 10.1146/annurev-immunol-051116-052320. [DOI] [PubMed] [Google Scholar]
- 86.Akiyama T, et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 87.Burkly L, et al. Expression of relB is required for the development of thymic medulla and dentritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 88.Weih F, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κ B/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 89.Kajiura F, et al. NF-κ B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol. 2004;172:2067–2075. doi: 10.4049/jimmunol.172.4.2067. [DOI] [PubMed] [Google Scholar]
- 90.Kinoshita D, et al. Essential role of IκB kinase alpha in thymic organogenesis required for the establishment of self-tolerance. J Immunol. 2006;176:3995–4002. doi: 10.4049/jimmunol.176.7.3995. [DOI] [PubMed] [Google Scholar]
- 91.Akiyama T, et al. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science. 2005;308:248–251. doi: 10.1126/science.1105677. [DOI] [PubMed] [Google Scholar]
- 92.Onder L, et al. Alternative NF-κB signaling regulates mTEC differentiation from podoplanin-expressing presursors in the cortico-medullary junction. Eur J Immunol. 2015;45:2218–2231. doi: 10.1002/eji.201545677. [DOI] [PubMed] [Google Scholar]
- 93.Baik S, Sekai M, Hamazaki Y, Jenkinson WE, Anderson G. Relb acts downstream of medullary thymic epithelial stem cells and is essential for the emergence of RANK+ medullary epithelial progenitors. Eur J Immunol. 2016;46:857–862. doi: 10.1002/eji.201546253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu M, et al. NF-κB2 is required for the establishment of central tolerance through an Aire-dependent pathway. J Clin Invest. 2006;116:2964–2971. doi: 10.1172/JCI28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.LaFlam TN, et al. Identification of a novel cis-regulatory element essential for immune tolerance. J Exp Med. 2015;212:1993–2002. doi: 10.1084/jem.20151069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haljasorg U, et al. A highly conserved NF-κB-responsive enhancer is critical for thymic expression of Aire in mice. Eur J Immunol. 2015;45:3246–3256. doi: 10.1002/eji.201545928. [DOI] [PubMed] [Google Scholar]
- 97.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 98.Summers deLuca L, Gommerman JL. Fine-tuning of dendritic cell biology by the TNF superfamily. Nat Rev Immunol. 2012;12:339–351. doi: 10.1038/nri3193. [DOI] [PubMed] [Google Scholar]
- 99.Gerondakis S, et al. Unravelling the complexities of the NF-κB signalling pathway using mouse knockout and transgenic models. Oncogene. 2006;25:6781–6799. doi: 10.1038/sj.onc.1209944. [DOI] [PubMed] [Google Scholar]
- 100.Seki T, et al. Visualization of RelB expression and activation at the single-cell level during dendritic cell maturation in Relb-Venus knock-in mice. J Biochem. 2015;158:485–495. doi: 10.1093/jb/mvv064. [DOI] [PubMed] [Google Scholar]
- 101.Lind EF, et al. Dendritic cells require the NF-κB2 pathway for cross-presentation of soluble antigens. J Immunol. 2008;181:354–363. doi: 10.4049/jimmunol.181.1.354. [DOI] [PubMed] [Google Scholar]
- 102.Katakam AK, et al. Dendritic cells require NIK for CD40-dependent cross-priming of CD8+ T cells. Proc Natl Acad Sci USA. 2015;112:14664–14669. doi: 10.1073/pnas.1520627112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hammer GE, Ma A. Molecular control of steady- state dendritic cell maturation and immune homeostasis. Annu Rev Immunol. 2013;31:743–791. doi: 10.1146/annurev-immunol-020711-074929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tas SW, et al. Noncanonical NF-κB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation. Blood. 2007;110:1540–1549. doi: 10.1182/blood-2006-11-056010. [DOI] [PubMed] [Google Scholar]
- 105.Kim NS, et al. Chimeric vaccine stimulation of human dendritic cell indoleamine 2, 3-dioxygenase occurs via the non-canonical NF-κB pathway. PLoS ONE. 2016;11:e0147509. doi: 10.1371/journal.pone.0147509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choudhary S, Boldogh S, Garofalo R, Jamaluddin M, Brasier AR. Respiratory syncytial virus influences NF-κB-dependent gene expression through a novel pathway involving MAP3K14/NIK expression and nuclear complex formation with NF-κB2. J Virol. 2005;79:8948–8959. doi: 10.1128/JVI.79.14.8948-8959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Willmann KL, et al. Biallelic loss-of-function mutationin NIK causes a primary immunodeficiency with multifaceted aberrant lymphoid immunity. Nat Commun. 2014;5:5360. doi: 10.1038/ncomms6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen K, et al. Germline mutations in NFKB2 implicate the noncanonical NF-κB pathway in the pathogenesis of common variable immunodeficiency. Am J Hum Genet. 2013;93:812–824. doi: 10.1016/j.ajhg.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee CE, et al. Autosomal-dominant B-cell deficiency with alopecia due to a mutation in NFKB2 that results in nonprocessable p100. Blood. 2014;124:2964–2972. doi: 10.1182/blood-2014-06-578542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lindsley AW, et al. Combined immune deficiency in a patient with a novel NFKB2 mutation. J Clin Immunol. 2014;34:910–915. doi: 10.1007/s10875-014-0095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mesin L, Ersching J, Victora GD. Germinal center B cell dynamics. Immunity. 2016;45:471–482. doi: 10.1016/j.immuni.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caamano JH, et al. Nuclear factor (NF)-κ B2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med. 1998;187:185–196. doi: 10.1084/jem.187.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Franzoso G, et al. Mice deficient in nuclear factor (NF)-κ B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med. 1998;187:147–159. doi: 10.1084/jem.187.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weih DS, Yilmaz ZB, Weih F. Essential role of RelB in germinal center and marginal zone formation and proper expression of homing chemokines. J Immunol. 2001;167:1909–1919. doi: 10.4049/jimmunol.167.4.1909. [DOI] [PubMed] [Google Scholar]
- 115.Mills DM, Bonizzi G, Karin M, Rickert RC. Regulation of late B cell differentiation by intrinsic IKKalpha-dependent signals. Proc Natl Acad Sci USA. 2007;104:6359–6364. doi: 10.1073/pnas.0700296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamada T, et al. Abnormal Immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-κB-inducing kinase. J Immunol. 2000;165:804–812. doi: 10.4049/jimmunol.165.2.804. [DOI] [PubMed] [Google Scholar]
- 117.Brightbill HD, et al. Conditional deletion of NF-κB-inducing kinase (NIK) in adult mice disrupts mature B cell survival and activation. J Immunol. 2015;195:953–964. doi: 10.4049/jimmunol.1401514. [DOI] [PubMed] [Google Scholar]
- 118.Hahn M, Macht A, Waisman A, Hovelmeyer N. NF-κB-inducing kinase is essential for B-cell maintenance in mice. Eur J Immunol. 2016;46:732–741. doi: 10.1002/eji.201546081. [DOI] [PubMed] [Google Scholar]
- 119.De Silva NS, et al. Transcription factors of the alternative NF-κB pathway are required for germinal center B-cell development. Proc Natl Acad Sci USA. 2016;113:9063–9068. doi: 10.1073/pnas.1602728113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Linterman MA, Vinuesa CG. Signals that influence T follicular helper cell differentiation and function. Semin Immunopathol. 2010;32:183–196. doi: 10.1007/s00281-009-0194-z. [DOI] [PubMed] [Google Scholar]
- 121.Hu H, et al. Noncanonical NF-κB regulates inducible costimulator (ICOS) ligand expression and T follicular helper cell development. Proc Natl Acad Sci USA. 2011;108:12827–12832. doi: 10.1073/pnas.1105774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu Y, et al. Novel NFKB2 mutation in early-onset CVID. J Clin Immunol. 2014;34:686–690. doi: 10.1007/s10875-014-0064-x. [DOI] [PubMed] [Google Scholar]
- 123.Brue T, et al. Mutations in NFKB2 and potential genetic heterogeneity in patients with DAVID syndrome, having variable endocrine and immune deficiencies. BMC Med Genet. 2014;15:139. doi: 10.1186/s12881-014-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shin C, et al. CD8alpha– dendritic cells induce antigen-specific T follicular helper cells generating efficient humoral immune responses. Cell Rep. 2015;11:1929–1940. doi: 10.1016/j.celrep.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 125.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Y, et al. Cell intrinsic role of NF-κB-inducing kinase in regulating T cell-mediated immune and autoimmune responses. Sci Rep. 2016;6:22115. doi: 10.1038/srep22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rowe AM, et al. A cell-intrinsic requirement for NF-κB-inducing kinase in CD4 and CD8 T cell memory. J Immunol. 2013;191:3663–3672. doi: 10.4049/jimmunol.1301328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tamura C, et al. Impaired function of dendritic cells in alymphoplasia (aly/aly) mice for expansion of CD25+CD4+ regulatory T cells. Autoimmunity. 2006;39:445–453. doi: 10.1080/08916930600833390. [DOI] [PubMed] [Google Scholar]
- 129.Murray SE. Cell-intrinsic role for NF-κ B-inducing kinase in peripheral maintenance but not thymic development of Foxp3+ regulatory T cells in mice. PLoS ONE. 2013;8:e76216. doi: 10.1371/journal.pone.0076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Elewaut D, et al. NIK-dependent RelB activation defines a unique signaling pathway for the development of Vα14i NKT Cells. J Exp Med. 2003;16:1623–1633. doi: 10.1084/jem.20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor κB family members in natural killer T cell development. J Exp Med. 2003;197:1613–1621. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mair F, et al. The NFκB-inducing kinase is essential for the developmental programming of skin-resident and IL-17-producing gammadelta T cells. eLife. 2015;4:e10087. doi: 10.7554/eLife.10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ashley NT, Weil ZM, Nelson RJ. Inflammation: mechanisms, costs, and natural variation. Annu Rev Ecol Evol Syst. 2012;43:385–406. [Google Scholar]
- 134.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 135.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 136.Noort AR, Tak PP, Tas SW. Non-canonical NF-κB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde? Arthritis Res Ther. 2015;17:15. doi: 10.1186/s13075-015-0527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vinay DS, Kwon BS. Targeting TNF superfamily members for therapeutic intervention in rheumatoid arthritis. Cytokine. 2012;57:305–312. doi: 10.1016/j.cyto.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 138.Noort AR, et al. NF-κB-inducing kinase is a key regulator of inflammation-induced and tumour-associated angiogenesis. J Pathol. 2014;234:375–385. doi: 10.1002/path.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maijer KI, et al. Nuclear factor-κB-inducing kinase is expressed in synovial endothelial cells in patients with early arthritis and correlates with markers of inflammation: a prospective cohort study. J Rheumatol. 2015;42:1573–1581. doi: 10.3899/jrheum.150245. [DOI] [PubMed] [Google Scholar]
- 140.Noort AR, et al. Tertiary lymphoid structures in rheumatoid arthritis: NF-κB-inducing kinase-positive endothelial cells as central players. Am J Pathol. 2015;185:1935–1943. doi: 10.1016/j.ajpath.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 141.Baum R, Gravallese EM. Bone as a target organ in rheumatic disease: impact on osteoclasts and osteoblasts. Clin Rev Allergy Immunol. 2016;51:1–15. doi: 10.1007/s12016-015-8515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aya K, et al. NF-κB-inducing kinase controls lymphocyte and osteoclast activities in inflammatory arthritis. J Clin Invest. 2005;115:1848–1854. doi: 10.1172/JCI23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yao Z, Xing L, Boyce BF. NF-κB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. J Clin Invest. 2009;119:3024–3034. doi: 10.1172/JCI38716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xiu Y, et al. Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation. J Clin Invest. 2014;124:297–310. doi: 10.1172/JCI66947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang C, et al. NIK stabilization in osteoclasts results in osteoporosis and enhanced inflammatory osteolysis. PLoS ONE. 2010;5:e15383. doi: 10.1371/journal.pone.0015383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wei F, Chang Y, Wei W. The role of BAFF in the progression of rheumatoid arthritis. Cytokine. 2015;76:537–544. doi: 10.1016/j.cyto.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 147.Mackay F, Tangye SG. The role of the BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr Opin Pharmacol. 2004;4:347–354. doi: 10.1016/j.coph.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 148.Kaul A, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2:16039. doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- 149.Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:326–337. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang J, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 151.Petri M, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 2008;58:2453–2459. doi: 10.1002/art.23678. [DOI] [PubMed] [Google Scholar]
- 152.Stohl W. Inhibition of B cell activating factor (BAFF) in the management of systemic lupus erythematosus (SLE) Expert Rev Clin Immunol. 2017 doi: 10.1080/1744666X.2017.1291343. http://dx.doi.org/10.1080/1744666X.2017.1291343. [DOI] [PubMed]
- 153.Zhang H, Sun SC. NF-κB in inflammation and renal diseases. Cell Biosci. 2015;5:63. doi: 10.1186/s13578-015-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McCarthy DD, et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest. 2011;121:3991–4002. doi: 10.1172/JCI45563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Poveda J, et al. TWEAK/Fn14 and non-canonical NF-κB signaling in kidney disease. Front Immunol. 2013;4:447. doi: 10.3389/fimmu.2013.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sakai N, et al. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sanz AB, et al. TWEAK activates the non-canonical NFκB pathway in murine renal tubular cells: modulation of CCL21. PLoS ONE. 2010;5:e8955. doi: 10.1371/journal.pone.0008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Valino-Rivas L, et al. Non-canonical NFκB activation promotes chemokine expression in podocytes. Sci Rep. 2016;6:28857. doi: 10.1038/srep28857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 160.Choudhary S, et al. NF-κB-inducing kinase (NIK) mediates skeletal muscle insulin resistance: blockade by adiponectin. Endocrinology. 2011;152:3622–3627. doi: 10.1210/en.2011-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sheng L, et al. NF-κB-inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat Med. 2012;18:943–949. doi: 10.1038/nm.2756. This is a key report demonstrating the involvement of NIK in metabolic diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Starkey JM, et al. Diabetes-induced activation of canonical and noncanonical nuclear factor-κBpathways in renal cortex. Diabetes. 2006;55:1252–1259. doi: 10.2337/db05-1554. [DOI] [PubMed] [Google Scholar]
- 163.Malle EK, et al. Nuclear factor κB-inducing kinase activation as a mechanism of pancreatic beta cell failure in obesity. J Exp Med. 2015;212:1239–1254. doi: 10.1084/jem.20150218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kiechl S, et al. Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med. 2013;19:358–363. doi: 10.1038/nm.3084. [DOI] [PubMed] [Google Scholar]
- 165.Kim WH, et al. Additive activation of hepatic NF-κB by ethanol and hepatitis B protein X (HBX) or HCV core protein: involvement of TNF-alpha receptor 1-independent and -dependent mechanisms. FASEB J. 2001;15:2551–2553. doi: 10.1096/fj.01-0217. [DOI] [PubMed] [Google Scholar]
- 166.Shen H, et al. Mouse hepatocyte overexpression of NF-κB-inducing kinase (NIK) triggers fatal macrophage-dependent liver injury and fibrosis. Hepatology. 2014;60:2065–2076. doi: 10.1002/hep.27348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ren X, et al. A small-molecule inhibitor of NF-κB-inducing kinase (NIK) protects liver from toxin-induced inflammation, oxidative stress, and injury. FASEB J. 2017;31:711–718. doi: 10.1096/fj.201600840R. [DOI] [PubMed] [Google Scholar]
- 168.Simmons SB, Pierson ER, Lee SY, Goverman JM. Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol. 2013;34:410–422. doi: 10.1016/j.it.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hofmann J, Mair F, Greter M, Schmidt-Supprian M, Becher B. NIK signaling in dendritic cells but not in T cells is required for the development of effector T cells and cell-mediated immune responses. J Exp Med. 2011;208:1917–1929. doi: 10.1084/jem.20110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hussman JP, et al. GWAS analysis implicates NF-κB-mediated induction of inflammatory T cells in multiple sclerosis. Genes Immun. 2016;17:305–312. doi: 10.1038/gene.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Etemadi N, et al. TRAF2 regulates TNF and NF-κB signalling to suppress apoptosis and skin inflammation independently of sphingosine kinase 1. eLife. 2015;4:e10592. doi: 10.7554/eLife.10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cai Y, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Eshima K, et al. Significant involvement of nuclear factor-κB-inducing kinase in proper differentiation of alphabeta and gammadelta T cells. Immunology. 2014;141:222–232. doi: 10.1111/imm.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Burkitt MD, et al. NF-κB1, NF-κB2 and c-Rel differentially regulate susceptibility to colitis-associated adenoma development in C57BL/6 mice. J Pathol. 2015;236:326–336. doi: 10.1002/path.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Banoth B, et al. Stimulus-selective crosstalk via the NF-κB signaling system reinforces innate immune response to alleviate gut infection. eLife. 2015;4:e05648. doi: 10.7554/eLife.05648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang Y, et al. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity. 2010;32:403–413. doi: 10.1016/j.immuni.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Giacomin PR, et al. Epithelial-intrinsic IKKalpha expression regulates group 3 innate lymphoid cell responses and antibacterial immunity. J Exp Med. 2015;212:1513–1528. doi: 10.1084/jem.20141831. [DOI] [PMC free article] [PubMed] [Google Scholar]