Abstract
Purpose
The purpose of this study was to develop and validate a new comprehensive patient-reported measure of treatment burden – the Patient Experience with Treatment and Self-Management (PETS).
Methods
A conceptual framework was used to derive the PETS with items reviewed and cognitively tested with patients. A survey battery, including a pilot version of the PETS, was mailed to 838 multi-morbid patients from two healthcare institutions for validation.
Results
A total of 332 multi-morbid patients returned completed surveys. Diagnostics supported deletion and consolidation of some items and domains. Confirmatory factor analysis supported a domain model for scaling comprised of 9 factors: medical information, medications, medical appointments, monitoring health, interpersonal challenges, medical/healthcare expenses, difficulty with healthcare services, role/social activity limitations, and physical/mental exhaustion. Scales showed good internal consistency (alpha range: 0.79 – 0.95). Higher PETS scores, indicative of greater treatment burden, were correlated with more distress, less satisfaction with medications, lower self-efficacy, worse physical and mental health, and lower convenience of healthcare (Ps<.001). Patients with lower health literacy, less adherence to medications, and more financial difficulties reported higher PETS scores (Ps<.01).
Conclusion
A comprehensive patient-reported measure of treatment burden can help to better characterize the impact of treatment and self-management burden on patient well-being and guide care toward minimally disruptive medicine.
Keywords: treatment burden, adherence, questionnaire, self-management, multi-morbidity, validation
Introduction
Treatment burden refers to the personal workload of healthcare, including treatment and self-management of chronic health conditions, and the impact of this workload on patient functioning and well-being [1-3]. Workload identifies the activities that patients are asked or required to do in order to care for their health (e.g., taking medications, maintaining medical appointments, monitoring health status, engaging in physical therapy). Impact refers to a patient's perception of the effect of the workload on role, social, physical, and psychological functioning. Financial challenges, confusion about medical information, and challenges with the healthcare system may add to the weight of the burden that is felt by the patient [2-6]. High treatment burden is an important clinical issue as evidence suggests that it may negatively affect behavioral and clinical outcomes such as adherence [7-11]. Non-adherence to medical regimens can lead to more hospitalization and readmissions, higher overall healthcare costs, and lower survival rates [12-15]. Clinicians' response to poor patient outcomes is often to intensify treatment, which ostensibly reinforces and adds to the existing burden [16]. Not surprisingly, burdened patients also report poor quality of life [17-20].
People with multiple chronic conditions may be especially vulnerable to treatment burden. One in four American adults have multiple chronic conditions, with the prevalence expected to rise by >1% per year until 2030 [21-24]. Many are required to engage in a complex variety of medical and self-management activities just to maintain their health status at a given level [16,25]. They are often required to seek care from a variety of providers which can lead to care that is fragmented, poorly coordinated [26-28], complex, and straining on personal resources [29,16]. To date, there are limited means of assessing patients' ability to integrate complex care into their lives. It is against this backdrop that we develop a comprehensive measure of treatment burden.
In this report we describe the development and initial validation of the Patient Experience with Treatment and Self-Management (PETS), a comprehensive, patient-reported measure of treatment burden. The measure is informed by a previously generated conceptual measurement framework based largely on qualitative data from patients with multiple chronic conditions (see Eton et al. 2015) [3].
Drafting the Measure
The PETS measure was drafted from the previously articulated conceptual framework [3] then reviewed by a stakeholder panel consisting of physicians, health services researchers, patient advocates and a nurse practitioner. After revision based on feedback from the panel, a new draft was submitted to cognitive pre-testing with patients with multiple chronic conditions. A complete description of this developmental process can be found in a supplement to this report (see Online Resource Supplement 1). The resulting 78-item PETS measure submitted to validation consisted of the following fifteen content domains (and number of items within each): learning about health conditions and care (3), medications (5), difficulty with taking medications (6), medical appointments (6), monitoring health (2), exercise or physical therapy (5), diet (4), medical equipment (3), interpersonal challenges (4), medical/healthcare expenses (8), confusion/concern about medical information (6), healthcare providers (7), difficulty with healthcare services (7), role and social activity limitations (6), and physical/mental exhaustion (6). Items use either a 4- or 5-point ordered, categorical response scale depending on content domain (e.g., very easy to very difficult, not at all to very much, strongly agree to strongly disagree, never to always).
Validation of the PETS Measure
Sample
The study sample was obtained from two clinical sites: the Hennepin County Medical Center (HCMC: Minneapolis, MN) and the Mayo Clinic (Rochester, MN). HCMC is a public “safety net” hospital located in urban Minneapolis that provides care for low income, uninsured, and vulnerable persons. The Mayo Clinic is a multi-specialty integrated practice in southeastern Minnesota. Recruitment from both sites contributed to increased diversity of study participants. In selecting the sample for initial validation we assumed that patients with (a) more chronic conditions and (b) more recent encounters with their healthcare provider(s) for these conditions were more likely to be experiencing treatment burden. Hence, patients meeting the following criteria were eligible: (1) ≥21 years old, (2) assigned to a primary care provider at either the Mayo Clinic or HCMC, (3) medical record-confirmed diagnoses of two or more chronic conditions (specifically conditions likely to require burdensome treatment and/or self-management strategies [30,27,31,32]) with these diagnoses listed on billing encounters of the past 3 years, and (4) at least one medical record-confirmed encounter with a Mayo Clinic or HCMC provider within the past 18 months for one or more of the selected chronic conditions. Patients lacking English language proficiency were excluded. The sampling frame was stratified based on number of diagnosed conditions (2, 3, or 4+), number of encounters with a provider within the past 18 months (1-8, 9-17, 18+), and age (<65 or ≥65). This resulted in eighteen strata from which patients were equally sampled across the two clinical sites.
Procedure
A self-administered, paper-and-pencil formatted survey battery was produced by the Mayo Clinic Survey Research Center. The battery consisted of the 78-item PETS measure as well as several established scales, including a 5-item chronic condition distress scale adapted from the Diabetes Distress Scale [33] and specified generally for “health problems” (L. Fisher, personal communication), the 5-item Side Effects and 3-item Convenience subscales of the Treatment Satisfaction Questionnaire for Medication (TSQM) [34], the 8-item Perceived Medical Condition Self-Management scale (PMCSM), a general dispositional measure of self-efficacy or perceived competence in managing one's health condition [35], and the Patient-Reported Outcomes Measurement Information System (PROMIS) Global-10 featuring global physical health (GPH) and global mental health (GMH) summary scores [36]. All are reliable and valid measures in patients with chronic illnesses [34,36,37,33]. Demographic characteristics (age, race/ethnicity, education, and occupational status), personal health-related issues (i.e., numbers of prescription medications, perceived economic hardship due to medical care), and several other health-relevant concepts (i.e., health literacy, medication adherence, and convenience of healthcare services) [38-40] were assessed using targeted single items. Gender, number and types of chronic conditions, and number of recent encounters with a provider (last 18 months) were extracted from the electronic medical record.
Mailings prepared by each site included the survey battery, a cover letter, a small gift to encourage participation (designer pen or $3 gift card), and a stamped return envelope. Signed consent and privacy authorization were not required as the study was approved as exempt by both the Mayo and HCMC Institutional Review Boards. A total of 838 surveys were mailed based on the stratified random samples generated and expected minimum return rate of 40-50%. To maximize returns, a second mailing to non-respondents occurred three weeks later.
Analyses
Item diagnostics
Prior to scaling analyses (i.e., factor analysis), item responses were first reviewed for missing data. Items with extensive amounts of missing data (≥25%) were set aside as low sample sizes can create unstable factor structures. Next, the inter-item correlation matrix of all PETS items (78 × 78) was examined to determine possible within domain item redundancies and indicate items that might lack conceptual fit to their initially hypothesized domain. Possible item redundancies within a domain were flagged by an item-to-item correlation magnitude suggestive of content overlap (Spearman rho ≥ 0.80). These items were candidates for exclusion. Items showing equivalent or higher cross-domain than within-domain item correlations were inspected for possible deletion or re-mapping prior to factor analysis.
Factor structure
After reviewing the item diagnostics, a set of competing confirmatory factor analyses (CFAs) were performed iteratively. The CFAs were conducted using MPLUS version 7.2 [41] with the implementation of polychoric correlation matrices and weighted least squares with adjustments for mean and variance (WLSMV) estimation, which is appropriate for the evaluation of ordered categorical data. The initially hypothesized factor structure was the domain conceptual framework slightly modified by the results of the item diagnostics review. Subsequent models tested more parsimonious solutions (i.e., fewer factors). Several indices were used to establish the fit of the models to the data, including the Comparative Fit Index (CFI: > 0.9 indicating good fit), Tucker-Lewis Index (TLI: > 0.95 indicating good fit), and the Root-Mean-Square Error of Approximation (RMSEA: < 0.10 desirable) [42,43]. Results were used to test whether domain scoring by the conceptual framework could be justified or whether a simpler model is more appropriate.
Scoring of the PETS
All PETS scales were scored such that a higher score indicates greater treatment burden; thus, positively-worded items were reverse-coded before scoring. Following this, raw scale scores were generated by summing the unweighted items within each factor supported by CFA. Given that some items will query issues that may not be applicable to all respondents, aggregated scale scores were prorated for missing item data, provided that at least 50% of the total number of items in a scale were non-missing (e.g., 2 of 3 items, 3 of 5 items, etc.). Prorating in this manner has been used with other patient-reported measures [44]. To further facilitate interpretation, each raw scale score was transformed to a standardized 0 to 100 scale using the following formula: [((raw score - lowest possible raw score) / possible raw score range) × 100].
Reliability and validity
Internal consistency reliability was computed for all PETS scales using Cronbach's alpha, with alphas ≥ 0.70 indicating adequate reliability [45]. Construct validity was determined by correlating PETS domain scores with scores from established measures of conceptually-related constructs (i.e., convergence). For these analyses, we hypothesized that higher PETS scores would be associated with greater chronic condition distress (Chronic Condition Distress scale), greater bother of medication side effects (TSQM side effects), less medication convenience (TSQM convenience), lower self-efficacy for managing chronic illness (PMCSM), poorer overall physical and mental health (PROMIS-10), and lower convenience of healthcare services. A correlation magnitude of at least moderate-level (Spearman rho ≥ 0.30) and in the hypothesized direction would represent evidence of construct validity [46]. Validity was also determined by correlating PETS scores with data extracted from the medical record including, number of chronic conditions and number of recent encounters with healthcare providers. We hypothesized that higher PETS scores would be associated with having more diagnosed conditions and more recent encounters with medical providers. Finally, known-groups validity was evaluated by comparing group means using independent samples t-tests. In comparing known groups, we hypothesized that patients who self-reported lower health literacy, less adherence to prescribed medications, and more financial difficulties due to their physical condition/medical care would have higher PETS scores. Given the large number of analyses, a relatively conservative alpha level of 0.01 was set for all correlations and group comparisons. Analyses were conducted in IBM SPSS Statistics version 20®.
Results
Characteristics of the sample
Of 838 mailed surveys, 332 were completed and returned (40% response). Demographic, medical, and other health-related characteristics of the sample are identified in Table 1. There were slightly more female (56%) than male participants, with a mean age of 66 years (range: 26-90). The majority of respondents identified themselves as white (73%), were married or living with a partner (58%), and had at least some formal college or university education (70%). Among variables extracted from the electronic medical record, the median number of diagnosed conditions was 3 and the median number of encounters with a medical provider in the past 18 months was 13.5. The most common diagnoses were hypertension (77%), lipid metabolic disorders (76%), diabetes (36%), osteoarthritis (36%), and coronary artery disease (19%).
Table 1. Demographic, medical, and other health-related characteristics of survey respondents (N=332).
| Mean age in years (SD) | 65.9 (11.0) |
|
| |
| Range | 26 - 90 |
|
| |
| Gender (N, %) | |
| Female | 185 (56%) |
| Male | 147 (44%) |
|
| |
| Race (N, %) | |
| White | 241 (73%) |
| Black / African-American | 51 (15%) |
| Mixed | 10 (3%) |
| Asian | 8 (2%) |
| Native American / American Indian | 6 (2%) |
| Missing | 16 (5%) |
|
| |
| Hispanic / Latino ethnicity (N, %) | 11 (3%) |
|
| |
| Marital status (N, %) | |
| Married or living with partner | 191 (58%) |
| Not married | 128 (39%) |
| Missing | 13 (4%) |
|
| |
| Education level (N, %) | |
| Less than High school | 22 (7%) |
| High school graduate | 67 (20%) |
| Some college / Associate's degree | 119 (36%) |
| College graduate (B.A., B.S.) | 70 (21%) |
| Graduate school / Advanced degree | 43 (13%) |
| Missing | 11 (3%) |
|
| |
| Work status (N, %) | |
| Not working | 217 (65%) |
| Working full or part-time | 99 (30%) |
| Missing | 16 (5%) |
|
| |
| No. of chronic conditions (N, %) | |
| 2 | 103 (31%) |
| 3 | 112 (34%) |
| 4 or more | 117 (35%) |
| Median no. of conditions | 3.0 |
|
| |
| Diagnosed chronic conditions (N, %) | |
| Hypertension | 254 (77%) |
| Disorders of lipid metabolism (incl. hypercholesterolemia) | 252 (76%) |
| Diabetes mellitus (Type 1 or 2) | 120 (36%) |
| Osteoarthritis | 119 (36%) |
| Coronary artery disease | 63 (19%) |
| Asthma | 47 (14%) |
| Chronic kidney disease | 44 (13%) |
| Depression | 44 (13%) |
| Glaucoma | 41 (12%) |
| Chronic obstructive pulmonary disorder | 34 (10%) |
| Hepatitis (B and C) | 28 (9%) |
| Coronary Heart Failure | 22 (7%) |
| Rheumatoid Arthritis | 15 (5%) |
|
| |
| No. of provider encounters in last 18 months (N, %) | |
| 1-8 | 106 (32%) |
| 9-17 | 99 (30%) |
| 18+ | 127 (38%) |
| Median no. of encounters | 13.5 |
|
| |
| Taking prescription medications? (N, %) | |
| Yes | 310 (93%) |
| No | 3 (1%) |
| Missing | 19 (6%) |
|
| |
| No. of prescription medications (N, %) | |
| 1 | 11 (3%) |
| 2-3 | 80 (24%) |
| 4-5 | 97 (29%) |
| 6 or more | 111 (34%) |
| Missing | 33 (10%) |
|
| |
| Adherence to medications? (N, %) | |
| Always take all medications | 260 (78%) |
| Take all medications ≤80% of the time | 51 (15%) |
| Missing | 21 (6%) |
|
| |
| Any health insurance? (N, %) | |
| Yes | 291 (88%) |
| No | 22 (7%) |
| Not sure | 3 (1%) |
| Missing | 16 (5%) |
|
| |
| Medical treatment causing financial difficulties? (N, %) | |
| Not at all | 158 (48%) |
| A little | 80 (24%) |
| Somewhat or more | 78 (24%) |
| Missing | 16 (5%) |
|
| |
| Difficulty reading and understanding written medical information? (health literacy) (N, %) | |
| Never or rarely | 267 (80%) |
| Sometimes, often, or always | 50 (15%) |
| Missing | 15 (5%) |
To test for possible responder bias, we assessed whether survey respondents were different from non-respondents by comparing frequency distributions of number of conditions (2, 3, 4+), number of encounters (1-8, 9-17, 18+), gender (female, male) and age (<65, ≥65), variables that were all obtained from the medical record. There were no significant differences between responders and non-responders in the number of diagnosed conditions (χ2(2) = 1.94, P=0.47). However, the percentage of responders who had only 1-8 encounters with a provider in the last 18 months (32%) was lower than the percentage of non-responders (42%) with this number of encounters (χ2(2) = 9.34, P=0.009). Furthermore, the percentage of responders who were female (56%) was greater than the percentage of non-responders who were female (46%) (χ2(1) = 7.51, P=0.006), and the percentage of responders who were ≥65 years of age (57%) was greater than the percentage of non-responders who were ≥65 years of age (46%) (χ2(1) = 9.66, P=0.002).
Item diagnostics
Of the 78 items in the draft PETS measure, three items were removed due to extensive missing data (i.e., at least 25% missing), including one item each from the difficulty with taking medications, medical/healthcare expenses, and confusion/concern about medical information domains. The majority of inter-item correlations (60%) in the full 78 × 78 item correlation matrix were rho ≥ 0.30 (a medium-sized correlation) [46]. Four more items were removed from the draft measure due to high inter-correlation(s) (rho ≥ 0.80) with at least one other item and a subsequent judgment of content overlap amongst those items, including one item each from the difficulty with taking medications, medical/healthcare expenses, confusion/concern about medical information, and physical/mental exhaustion domains. We also removed the seven items of the healthcare provider domain due to lack of conceptual fit with the treatment burden construct. Positive interpersonal qualities of one's provider are better construed as features that might lessen treatment burden, rather than contribute to it (see [47]). Finally, one other item was removed due to equally high inter-item correlations with two different domains (medical/healthcare expenses and confusion about medical information); hence, it could not easily be mapped to a single domain. The excluded items and full results can be found in Online Resource Supplement 2.
All items in the exercise/physical therapy, diet, and medical equipment domains had substantial amounts of missing data (46% to 61%) due to skip patterns based on preceding yes/no screening questions and thus were excluded from the CFA. Furthermore, after deliberation, three items addressing barriers to medical appointments with low correlations with other items within the medical appointments domain were also excluded from CFA due to lack of conceptual fit. Thus, 48 items were retained from the PETS measure for subsequent testing. See Online Resource Supplement 2 for a record of all item diagnostics results.
Factor analysis and scaling of the PETS
The first CFA model tested featured 11 content domains and was based on the previously outlined conceptual framework [3] with some modification after review of item diagnostics (see above). Domains included learning about health conditions and care, medications, difficulty with taking medications, medical appointments, monitoring health, interpersonal challenges, medical/healthcare expenses, confusion/concern about medical information, difficulty with healthcare services, role/social activity limitations due to self-care, and physical/mental exhaustion due to self-care. This model assumed correlations of all factors, an assumption justified by the large degree of inter-correlation amongst all items (see above). The 11-factor correlated model provided a satisfactory fit to the data (CFI=0.98, TLI=0.97, RMSEA=0.043, 90% CI [0.039, 0.046]). Estimated correlations between the factors appear in Table 2.
Table 2. Confirmatory factor analytic-generated correlations of all 11 initially proposed content domains.
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Learning about health conditions | -- | 0.52 | 0.60 | 0.64 | 0.55 | 0.54 | 0.59 | 0.77 | 0.55 | 0.40 | 0.49 |
| (2) Medications | -- | 0.70 | 0.64 | 0.59 | 0.55 | 0.47 | 0.45 | 0.37 | 0.39 | 0.47 | |
| (3) Difficulty with taking medications | -- | 0.67 | 0.64 | 0.67 | 0.61 | 0.57 | 0.45 | 0.56 | 0.60 | ||
| (4) Medical appointments | -- | 0.64 | 0.67 | 0.61 | 0.62 | 0.60 | 0.57 | 0.66 | |||
| (5) Monitoring health | -- | 0.65 | 0.57 | 0.52 | 0.39 | 0.45 | 0.54 | ||||
| (6) Interpersonal challenges | -- | 0.64 | 0.62 | 0.49 | 0.77 | 0.77 | |||||
| (7) Medical and healthcare expenses | -- | 0.79 | 0.55 | 0.48 | 0.60 | ||||||
| (8) Confusion / concern about medical info. | -- | 0.66 | 0.44 | 0.52 | |||||||
| (9) Difficulty with healthcare services | -- | 0.39 | 0.46 | ||||||||
| (10) Role / social activity limitations | -- | 0.71 | |||||||||
| (11) Physical / mental exhaustion | -- |
After review of the domain inter-correlations as well as the item content of each domain, a slightly modified factor structure was proposed. To obtain greater parsimony, we combined domains that were highly-correlated (rs ≥ 0.70) and deemed by us to have similar item content. Based on this rationale the “learning about health conditions/care” and “confusion/concern about medical information” domains were combined into a single domain labeled, “medical information.” Further, the “medications” and “difficulty with taking medications” domains were consolidated into a single “medications” domain. Two items indicative of distress with taking medications – bother due to reliance on medicines and bother due to side effects of medicines – were analyzed separately. These two items assessing psychological bother appear to be conceptually distinct from the other seven items within the medications domain that assess the behavioral ease with which medications are taken and integrated into everyday life. The resulting 9-factor model featuring 46 items (less the two bother items) was subjected to CFA, which provided a satisfactory fit to the data (CFI=0.98, TLI=0.97, RMSEA=0.045, 90% CI [0.041, 0.049]). For comparative purposes, a one-factor model was also tested (all items loading on a single domain). This model provided a sub-optimal fit to the data (CFI=0.84, TLI=0.84, RMSEA=0.106, 90% CI [0.103, 0.108]). Hence, nine multi-item scales (46 items total) were generated using the pro-rated, standardized scoring as defined above. The two medication bother items were retained with scores standardized to the 0-100 scale. Both were analyzed separately as single-item indicators in the subsequent validation analyses. The multi-item scales represented the following domains: medical information, medications, medical appointments, monitoring health, interpersonal challenges, medical/healthcare expenses, difficulty with healthcare services, role/social activity limitations, and physical/mental exhaustion. Mean scores, score ranges obtained, percentage scoring at floor (lowest possible burden score) and ceiling (highest possible burden score), and the skewness statistics for the total and site-specific samples are shown in Table 3. Mean burden scores were generally higher in the HCMC patients than the Mayo Clinic patients. Overall, scale scores were positively skewed toward lower burden, with the medications, medical appointments, interpersonal challenges, and role/social activity limitations scales showing greatest skewness and the most floor effects. The 48-item version of the PETS can be found in the appendix.
Table 3. Descriptive statistics of nine PETSa domain scale scores and two single-item indicators.
| PETS domainb | N | Mean (SD)c | Score range | % Floor | % Ceiling | Skewness |
|---|---|---|---|---|---|---|
|
| ||||||
| Medical information (total) | 294 | 25.6 (19.2) | 0-100 | 17 | <1 | 0.67 |
| HCMCd | 133 | 29.3 (21.5) | 0-100 | 17 | <1 | 0.63 |
| Mayoe | 161 | 22.5 (16.5) | 0-83.3 | 17 | 0 | 0.42 |
|
| ||||||
| Medications (total) | 307 | 10.2 (16.4) | 0-83.3 | 50 | 0 | 2.18 |
| HCMC | 137 | 15.9 (20.4) | 0-83.3 | 38 | 0 | 1.48 |
| Mayo | 170 | 5.5 (10.1) | 0-57.1 | 59 | 0 | 2.76 |
|
| ||||||
| Medical appointments (total) | 310 | 11.0 (20.1) | 0-100 | 66 | <1 | 2.11 |
| HCMC | 142 | 17.5 (25.4) | 0-100 | 55 | <1 | 1.37 |
| Mayo | 168 | 5.4 (11.6) | 0-75 | 74 | 0 | 2.76 |
|
| ||||||
| Monitoring health (total) | 305 | 16.8 (22.9) | 0-100 | 52 | 1 | 1.39 |
| HCMC | 140 | 21.9 (25.8) | 0-100 | 44 | 1 | 1.01 |
| Mayo | 165 | 12.4 (19.2) | 0-100 | 59 | <1 | 1.78 |
|
| ||||||
| Interpersonal challenges (total) | 317 | 12.5 (18.9) | 0-87.5 | 52 | 0 | 1.77 |
| HCMC | 144 | 19.4 (22.0) | 0-87.5 | 37 | 0 | 1.12 |
| Mayo | 173 | 6.7 (13.4) | 0-81.3 | 65 | 0 | 2.83 |
|
| ||||||
| Medical & healthcare expenses (total) | 287 | 36.5 (25.7) | 0-100 | 15 | 1 | 0.29 |
| HCMC | 123 | 44.7 (28.0) | 0-100 | 11 | 3 | 0.05 |
| Mayo | 164 | 30.3 (22.0) | 0-85 | 18 | 0 | 0.21 |
|
| ||||||
| Difficulty with healthcare services (total) | 289 | 22.5 (14.6) | 0-75 | 14 | 0 | 0.31 |
| HCMC | 132 | 24.7 (14.6) | 0-66.7 | 14 | 0 | -0.02 |
| Mayo | 157 | 20.6 (14.4) | 0-75 | 15 | 0 | 0.61 |
|
| ||||||
| Role / social activity limitations (total) | 314 | 16.5 (23.5) | 0-100 | 48 | 1 | 1.55 |
| HCMC | 143 | 22.9 (27.1) | 0-100 | 39 | 2 | 1.13 |
| Mayo | 171 | 11.2 (18.5) | 0-83.3 | 56 | 0 | 1.93 |
|
| ||||||
| Physical / mental exhaustion (total) | 315 | 24.1 (24.8) | 0-100 | 31 | 1 | 0.87 |
| HCMC | 144 | 33.1 (26.6) | 0-100 | 20 | 2 | 0.50 |
| Mayo | 171 | 16.4 (20.3) | 0-75 | 41 | 0 | 1.17 |
|
| ||||||
| Bother due to reliance on medicine – single item (total) | 311 | 21.4 (30.4) | 0-100 | 57 | 6 | 1.35 |
| HCMC | 141 | 30.9 (36.1) | 0-100 | 46 | 14 | 0.84 |
| Mayo | 170 | 13.5 (21.9) | 0-100 | 65 | 1 | 1.68 |
|
| ||||||
| Bother due to side effects of medicine – single item (total) | 309 | 16.7 (25.3) | 0-100 | 63 | 2 | 1.43 |
| HCMC | 141 | 21.5 (27.3) | 0-100 | 53 | 3 | 1.12 |
| Mayo | 168 | 12.6 (22.7) | 0-100 | 71 | 1 | 1.77 |
Notes.
PETS = Patient Experience with Treatment and Self-Management.
Higher PETS scale score = more burden.
SD = standard deviation.
HCMC = Hennepin County Medical Center (Minneapolis, Minnesota USA).
Mayo = Mayo Clinic (Rochester, Minnesota USA).
Reliability
As shown in Table 4, all nine multi-item scales showed good internal consistency reliability. Cronbach's alpha coefficients were well above threshold for adequate reliability (alpha ≥ 0.70) [45].
Table 4. Reliability (Cronbach's α) and correlations (Spearman's rho) of PETSa domain scales with other measures.
|
PETS scaleb |
Chronic Condition Distress |
TSQMc side effects |
TSQM convenience |
PMCSMd | PROMISe Physical Health |
PROMIS Mental Health |
H-C services ratingf |
# of chronic conditions |
# of provider visits |
|---|---|---|---|---|---|---|---|---|---|
| Medical information (7 items, α = 0.91) | 0.51 *** | -0.23 *** | -0.51 *** | -0.50 *** | -0.43 *** | -0.49 *** | -0.45 *** | 0.05 NS | 0.09 NS |
| Medications (7 items, α = 0.87) | 0.47 *** | -0.31 *** | -0.51 *** | -0.38 *** | -0.41 *** | -0.38 *** | -0.24 *** | 0.09 NS | 0.25 *** |
| Medical appointments (3 items, α = 0.85) | 0.45 *** | -0.27 *** | -0.28 *** | -0.36 *** | -0.45 *** | -0.37 *** | -0.35 *** | 0.05 NS | 0.11 NS |
| Monitoring health (2 items, α = 0.81) | 0.50 *** | -0.24 *** | -0.41 *** | -0.43 *** | -0.39 *** | -0.37 *** | -0.27 *** | 0.05 NS | 0.09 NS |
| Interpersonal Challenges (4 items, α = 0.79) | 0.65 *** | -0.28 *** | -0.38 *** | -0.47 *** | -0.53 *** | -0.50 *** | -0.27 *** | 0.04 NS | 0.12 NS |
| Medical & healthcare expenses (5 items, α = 0.91) | 0.50 *** | -0.28 *** | -0.42 *** | -0.44 *** | -0.48 *** | -0.44 *** | -0.37 *** | 0.15 * | 0.08 NS |
| Difficulty with healthcare services (7 items, α = 0.85) | 0.40 *** | -0.22 *** | -0.32 *** | -0.39 *** | -0.40 *** | -0.42 *** | -0.58 *** | 0.03 NS | 0.05 NS |
| Role / social activity limitations (6 items, α = 0.95) | 0.62 *** | -0.33 *** | -0.35 *** | -0.40 *** | -0.55 *** | -0.38 *** | -0.25 *** | 0.08 NS | 0.15 * |
| Physical / mental exhaustion (5 items, α = 0.93) | 0.64 *** | -0.39 *** | -0.43 *** | -0.50 *** | -0.58 *** | -0.57 *** | -0.30 *** | 0.12 NS | 0.19 *** |
Notes.
PETS = Patient Experience with Treatment and Self-Management.
Higher PETS scale score = more burden.
TSQM: Treatment Satisfaction Questionnaire for Medication.
PMCSM: Perceived Medical Condition Self-Management scale.
PROMIS: Patient-Reported Outcomes Measurement Information System.
Healthcare (H-C) services rating: “How would you rate the convenience of healthcare services that you seek for your health problems?” (0 = not at all TO 10 = extremely).
P < .001 (1-tailed);
P < .01 (1-tailed); NS = not significant.
Construct and known-groups validity
As indicated in Table 4, all nine PETS scales were significantly correlated with scores of conceptually-related constructs assessed in established measures. Higher PETS scores (i.e., more burden) were associated with greater chronic condition distress (Chronic Condition Distress scale), more bother due to medication side effects (TSQM side effects), less medication convenience (TSQM convenience), lower self-efficacy for managing chronic illness (PMCSM), poorer physical and mental health (PROMIS-10), and lower patient-rated convenience of healthcare services. Magnitudes of most of these correlations (81%) were medium-size or greater (rho ≥ 0.30) [46] supporting construct validity of the PETS scales. PETS scores were largely uncorrelated with patient data extracted from the medical record. While having more diagnosed conditions was associated with more problems with medical/healthcare expenses (rho=0.15) and having more documented encounters with providers was associated with more medication problems (rho=0.25), more role/social activity limitations (rho=0.15), and more physical/mental exhaustion due to self-care (rho=0.19), these correlations were relatively small in magnitude. No other correlations between these medical record variables and PETS scale scores were significant.
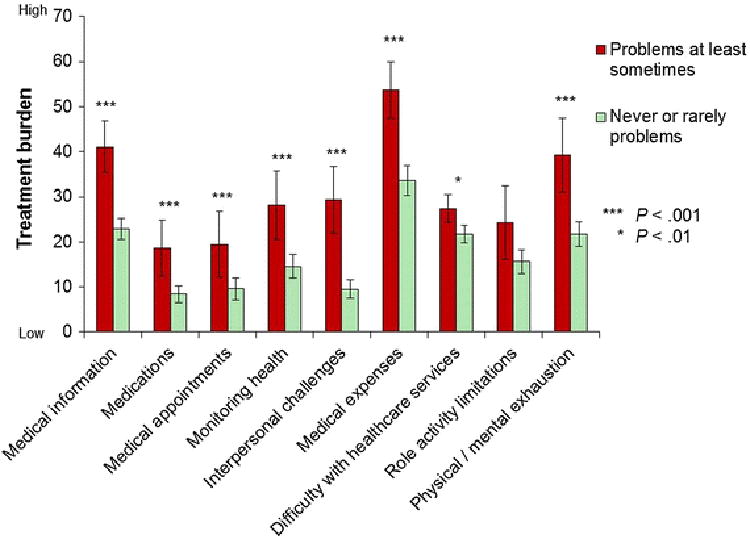
Mean PETS scale scores were also compared across distinct patient groups (i.e., known-groups validity). As shown in Figure 1, patients who have difficulty reading and understanding written medical information (i.e., lower health literacy) reported significantly more treatment burden in 8 of 9 PETS domains compared to those without such difficulty.
Fig. 1. Mean PETS domain score comparisons by health literacy with 95% confidence intervals.
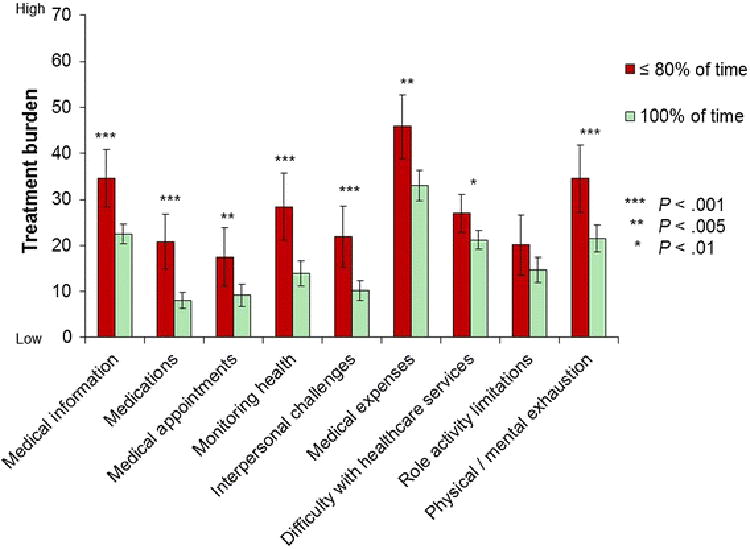
Furthermore, compared to those who reported 100% adherence to medications prescribed by their doctor(s), those adhering to medications ≤ 80% of the time reported significantly more treatment burden in 8 of 9 PETS domains (see Figure 2).
Fig. 2. Mean PETS domain score comparisons by medication adherence with 95% confidence intervals.
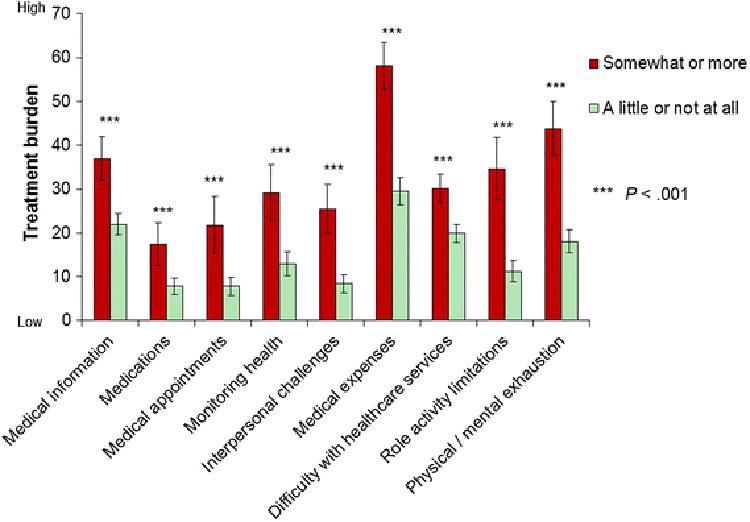
Finally, compared to patients indicating no or only a “a little” financial difficulty due to their medical treatment or physical condition, those indicating financial difficulty “somewhat” or more reported significantly greater treatment burden in all PETS domains (see Figure 3).
Fig. 3. Mean PETS domain score comparisons by self-reported financial difficulties with 95% confidence intervals.
Validation analyses were also conducted on the two single-item indicators of medication bother – bother due to reliance on medicines and bother due to side effects of medicines. Greater bother due to reliance on medicines was significantly correlated with more chronic condition distress (rho=0.42), less convenience of medication (rho=-0.31), lower self-efficacy (rho=-0.32), and poorer overall physical (rho=-0.40) and mental health (rho=-0.37) (all Ps<.001). Greater bother due to medication side effects was also significantly correlated with more chronic condition distress (rho=0.40), less convenience of medication (rho=-0.33), lower self-efficacy (rho=-0.32), and poorer overall physical (rho=-0.27) and mental health (rho=-0.28) (all Ps<.001). Both bother items were correlated with the number of prescription medications being taken (rhos=0.16, Ps<.005); however, neither item was correlated with the number of diagnosed conditions (rhos≤0.02).
Discussion
Substantial burden arises from the complexity of treatment regimens, including the self-management that is often required of patients to maintain health and avoid disease progression. This is especially true for those who must manage multiple chronic health conditions. With its potential to negatively impact clinical outcomes like adherence, treatment burden would seem an important issue to understand and assess. In this study, we describe the development and validation of the Patient Experience with Treatment and Self-Management (PETS), a comprehensive measure of treatment burden that operationalizes issues previously outlined in a patient-informed conceptual framework of general treatment burden (i.e., non-condition specific). Content and face validity of the PETS were ascertained through a review by key stakeholders and patient cognitive interviews. A cross-sectional survey of 332 people with multiple chronic conditions provides initial evidence of the measure's reliability, construct validity, and ability to detect differences in meaningful groups of patients. Furthermore, the PETS appears to provide information about the patient that cannot be gleaned from a review of the medical record.
The PETS measure validated in this study contains 48 items, most of which (46) are organized into 9 content domains (see appendix). Factor analyses supported the scoring of these domains into 9 multi-item scales. Two items indicative of medication bother (reliance and side effects) are scored as single-item indicators. For this initial validation, we chose to err on the side of having more domains (i.e., scores) that are clinically meaningful and easy to interpret. Future studies will evaluate the replicability of the current model and investigate the viability of additional scoring algorithms that consolidate the domains into aggregated dimensions. Dimensional or “component” scoring can be a useful means of summarizing the health or well-being status of a population and is utilized in other well-established measures like the SF-36 and PROMIS Global-10 [36,48]. The inter-correlations of the domains (see Table 2) would appear to justify further investigation of dimensional scoring.
We chose to set aside several items and domains for later analysis and scrutiny. Items in the exercise/physical therapy, diet, and medical equipment content domains were each preceded by a screening question that invoked a skip pattern. Hence, there was a considerable amount of data missing for these items. We also set aside three items addressing barriers to getting to medical appointments. These items were less well correlated with the other items in the medical appointments domain. One or more of these items may be retained in future tests.
Clinical utility of the PETS could be considerable, and remains to be assessed in future trials. Clinicians tend to know little about the burden that their recommendations place upon their patients, and how this burden might result in lesser adherence, attendance at visits, and poorer outcomes. We believe that a clinically-oriented scale of treatment burden would be an impressive adjunct to clinical care, and could guide clinicians and patients into joint discussions about the usefulness of recommendations and prioritizing of best next steps to take together. The lack of correlation with objective information from the medical record (i.e., numbers of conditions and provider visits) would seem to indicate that treatment burden is a subjective experience ideally assessed by patient self-report.
Finally, it is noteworthy that the term “burden” does not appear anywhere within the measure itself (including the name). During our initial qualitative exploration of the concept, some patients felt that this term had too negative a connotation, implying that a respondent is somehow failing or does not have the requisite skill to accomplish certain critical tasks. Others identified self-management simply as “the work I have to do” [47]. Hence, we felt it appropriate to remove any references to “burden” in the measure. To further diminish any perception of negative tone, for some content domains we opted to use balanced “agree/disagree” or “easy/difficult” response scales, in lieu of always assessing the frequency of “a problem.”
Limitations
Validation of a self-report measure does not occur in a single study, rather it is a process that unfolds over time as psychometric evidence accumulates. While this study provides initial evidence of the PETS' reliability and validity in a diverse patient sample, its cross-sectional design is a limitation. We are currently undertaking a large-scale, prospective validation study of the PETS to determine among other things its responsiveness to change, predictive validity, test-retest reliability, replicability of its factor structure, and viability of alternative scoring algorithms. This will be particularly important to determine as some of the PETS domain scales yielded scores that were somewhat positively skewed toward the favorable end (i.e., less burden) with substantial numbers scoring at the floor. While it is not unusual to observe such skewness in scores of patient-reported measures of health concepts [49], it will be important to determine in future tests whether any skewness of PETS domain scores compromises the ability to detect change in patient status. Finally, as this study was conducted in the United States, it is possible that some issues represented in the PETS may be less relevant to patients of other global health care systems. For instance, medical and healthcare expenses may be of less concern to people residing in countries with government-subsidized health care systems.
Conclusion
While self-report measures of treatment burden exist, current measures target specific diseases [50,19], treatment modes [17,51], circumscribed aspects of burden like perceived task difficulty [18], or are lacking in comprehensiveness [52]. Developing measurement that comprehensively assesses the many domains of treatment burden that can be applied across chronic conditions or with multiple chronic conditions, is still in its infancy. We believe that such a measure would have great value. First, it could provide a novel, patient-centered outcome for comparative effectiveness studies [53,54] and epidemiologic and health services research designed to identify subgroups of patients at risk for poor outcomes [55]. Second, it could be used as a performance measure for healthcare entities or help evaluate innovative healthcare delivery models like patient-centered medical homes, nurse-coordinated care, or self-management support [56-59]. Third, healthcare providers could use information on treatment burden to identify patients who may have problems with adherence to medical regimens or who are overwhelmed and may benefit from a more minimally disruptive medicine [16]. We are currently undertaking work with the PETS in all three of these areas, including construction of shorter versions of the measure. The breadth and richness of the measure will allow for comparisons across a wide variety of healthcare settings and patient conditions. As there is no calculated total score, users may choose to use only those PETS scales that are suited to their study or clinical setting.
Supplementary Material
Supplement 1 [Online resource]. Drafting, reviewing, and pre-testing the Patient Experience with Treatment and Self-Management (PETS) measure
Supplement 2 [Online resource]. Item diagnostic results
Acknowledgments
The research reported in this manuscript was supported by the National Institute of Nursing Research of the National Institutes of Health (USA) under award number R21NR012984. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Ms. Ann Harris and personnel in the Mayo Clinic Survey Research Center for formatting, distribution, and receipt of the survey battery.
Funding: The study was funded by the National Institute of Nursing Research of the National Institutes of Health (USA) under award number R21NR012984, D. Eton (Principal Investigator). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix: The 48-item version of the Patient Experience with Treatment and Self-management (PETS): a measure of perceived treatment burden
| Domain / Item |
|---|
| Medical information: How easy/difficult has it been to … (Responses: very easy, easy, neither easy nor difficulty, difficult, very difficult, not applicable) |
| learn about your health problem(s)? |
| learn what foods you should eat to stay healthy? |
| find information on the medications that you have to take? |
| understand changes to your treatment plan? |
| understand the reasons why you are taking some medicines? |
| find sources of medical information that you trust? |
| understand advice from different healthcare providers? |
| Medications: How much of a problem has it been for you to … (Responses: not at all, a little, somewhat, quite a bit, very much) |
| organize your medicines? |
| take more than one medicine every day? |
| take your medicines several times each day? |
| refill your medicines? |
| adjust your medicines (including the amount, type, or time when you take it)? |
| take your medicines as directed? |
| plan your daily activities around your medicine schedule? |
| Single-item indicators of medication bother: How bothered have you been by … (Responses: not at all, a little, somewhat, quite a bit, very much) |
| how much you have to rely on your medicine(s)? |
| side effects of your medicine(s)? |
| Medical appointments: How much of a problem has it been for you to … (Responses: not at all, a little, somewhat, quite a bit, very much) |
| make or keep your medical appointments? |
| schedule and keep track of your medical appointments? |
| make or keep appointments with different healthcare providers? |
| Monitoring health: How much of a problem has it been for you to … (Responses: not at all, a little, somewhat, quite a bit, very much) |
| monitor your health behaviors, e.g., tracking exercise, foods you eat, or medicines you take? |
| monitor your health condition, e.g., weighing yourself, checking blood pressure, or checking blood sugar? |
| Interpersonal challenges: How bothered have you been by … (Responses: not at all, a little, somewhat, quite a bit, very much) |
| feeling dependent on others for your healthcare needs? |
| others reminding you to do things for your health like take your medicine, watch what you eat, or schedule medical appointments? |
| your healthcare needs creating tension in your relationships with others |
| others not understanding your health situation |
| Medical & healthcare expenses: How easy/difficult has it been for you to … (Responses: very easy, easy, neither easy nor difficulty, difficult, very difficult, not applicable) |
| plan for the future because of your medical expenses? |
| pay for healthy foods? |
| pay for all of your medical expenses? |
| pay for your medicines? |
| understand what is and what is not covered by your health insurance? |
| Difficulty with healthcare services: How much do you agree/disagree with the following? (Responses: strongly agree, agree, disagree, strongly disagree, not applicable) |
| Have problems with different healthcare providers not communicating with each other about my medical care |
| Have to see too many different specialists for my health problem(s) or illness(es) |
| Have problems filling out forms related to my healthcare |
| Have problems getting appointments at times that are convenient for me |
| Have problems getting appointments with a specialist |
| Have to wait too long at my medical appointments |
| Have to wait too long at the pharmacy for my medicine |
| Role and social activity limitations: How much has your self-care interfered with your … (Responses: not at all, a little, somewhat, quite a bit, very much) |
| work (include work at home)? |
| family responsibilities? |
| daily activities? |
| hobbies and leisure activities? |
| ability to spend time with family and friends? |
| ability to travel for work or vacation? |
| Physical and mental exhaustion: How often did your self-care make you feel … (Responses: never, rarely, sometimes, often, always) |
| angry? |
| preoccupied? |
| depressed? |
| worn out? |
| frustrated? |
Note. With the exception of items in the “difficulty with healthcare services” domain, all items reference a recall time period of the past 4 weeks. No recall time period is used for the difficulty with healthcare services items.
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest.
Compliance with Ethical Standards: Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Signed informed consent was not required as the study was approved as exempt by both the Mayo and Hennepin County Medical Center Institutional Review Boards.
References
- 1.Eton DT, Elraiyah TA, Yost KJ, Ridgeway JL, Johnson A, Egginton JS, et al. A systematic review of patient-reported measures of burden of treatment in three chronic diseases. Patient Related Outcome Measures. 2013;4:7–20. doi: 10.2147/PROM.S44694. doi: http://dx.doi.org/10.2147/PROM.S44694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eton DT, Ramalho de Oliveira D, Egginton JS, Ridgeway JL, Odell L, May CR, et al. Building a measurement framework of burden of treatment in complex patients with chronic conditions: a qualitative study. Patient Related Outcome Measures. 2012;3:39–49. doi: 10.2147/PROM.S34681. doi: http://dx.doi.org/10.2147/PROM.S34681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eton DT, Ridgeway JL, Egginton JS, Tiedje K, Linzer M, Boehm DH, et al. Finalizing a measurement framework for the burden of treatment in complex patients with chronic conditions. Patient Related Outcome Measures. 2015;6:117–126. doi: 10.2147/PROM.S78955. doi: http://dx.doi.org/10.2147/PROM.S78955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sav A, Kendall E, McMillan SS, Kelly F, Whitty JA, King MA, et al. ‘You say treatment, I say hard work’: treatment burden among people with chronic illness and their carers in Australia. Health & Social Care in the Community. 2013;21(6):665–674. doi: 10.1111/hsc.12052. doi: http://dx.doi.org/10.1111/hsc.12052. [DOI] [PubMed] [Google Scholar]
- 5.Sav A, King MA, Whitty JA, Kendall E, McMillan SS, Kelly F, et al. Burden of treatment for chronic illness: a concept analysis and review of the literature. Health Expectations : an international journal of public participation in health care and health policy. 2013 doi: 10.1111/hex.12046. doi: http://dx.doi.org/10.1111/hex.12046. [DOI] [PMC free article] [PubMed]
- 6.Boehmer KR, Shippee ND, Beebe TJ, Montori VM. Pursuing minimally disruptive medicine: Correlation of patient capacity with disruption from illness and healthcare-related demands. Journal of Clinical Epidemiology. 2016 doi: 10.1016/j.jclinepi.2016.01.006. doi: http://dx.doi.org/10.1016/j.jclinepi.2016.01.006. [DOI] [PubMed]
- 7.Graves MM, Adams CD, Bender JA, Simon S, Portnoy AJ. Volitional nonadherence in pediatric asthma: parental report of motivating factors. Current Allergy & Asthma Reports. 2007;7(6):427–432. doi: 10.1007/s11882-007-0065-4. [DOI] [PubMed] [Google Scholar]
- 8.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. Journal of the American Medical Association. 2002;288(22):2880–2883. doi: 10.1001/jama.288.22.2880. doi: http://dx.doi.org/10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- 9.Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. International Journal of Clinical Practice Suppl. 2009;164:6–10. doi: 10.1111/j.1742-1241.2009.02176.x. doi: http://dx.doi.org/10.1111/j.1742-1241.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 10.Shah S, Akbari M, Vanga R, Kelly CP, Hansen J, Theethira T, et al. Patient perception of treatment burden is high in celiac disease compared with other common conditions. The American Journal of Gastroenterology. 2014;109(9):1304–1311. doi: 10.1038/ajg.2014.29. doi: http://dx.doi.org/10.1038/ajg.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient-centered treatment regimens. Journal of General Internal Medicine. 2005;20(5):479–482. doi: 10.1111/j.1525-1497.2005.0117.x. doi: http://dx.doi.org/10.1111/j.1525-1497.2005.0117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Archives of Internal Medicine. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. doi: http://dx.doi.org/10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 13.Iuga AO, McGuire MJ. Adherence and health care costs. Risk Management and Healthcare Policy. 2014;7:35–44. doi: 10.2147/RMHP.S19801. doi: http://dx.doi.org/10.2147/RMHP.S19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott MM, Schmitt B, Wallner E. Impact of medication nonadherence on coronary heart disease outcomes. A critical review. Archives of Internal Medicine. 1997;157(17):1921–1929. doi: http://dx.doi.org/10.1001/archinte.1997.00440380023002. [PubMed] [Google Scholar]
- 15.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. Journal of the American Medical Association. 2007;297(2):177–186. doi: 10.1001/jama.297.2.177. doi: http://dx.doi.org/10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 16.May C, Montori VM, Mair FS. We need minimally disruptive medicine. British Medical Journal. 2009;339:b2803. doi: 10.1136/bmj.b2803. doi: http://dx.doi.org/10.1136/bmj.b2803. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RT, Skovlund SE, Marrero D, Levine DW, Meadows K, Brod M, et al. Development and validation of the insulin treatment satisfaction questionnaire. Clinical Therapeutics. 2004;26(4):565–578. doi: 10.1016/s0149-2918(04)90059-8. doi: http://dx.doi.org/10.1016/S0149-2918(04)90059-8. [DOI] [PubMed] [Google Scholar]
- 18.Boyd CM, Wolff JL, Giovannetti E, Reider L, Weiss C, Xue QL, et al. Healthcare task difficulty among older adults with multimorbidity. Medical Care. 2014;52 Suppl 3:S118–125. doi: 10.1097/MLR.0b013e3182a977da. doi: http://dx.doi.org/10.1097/MLR.0b013e3182a977da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brod M, Hammer M, Christensen T, Lessard S, Bushnell DM. Understanding and assessing the impact of treatment in diabetes: the Treatment-Related Impact Measures for Diabetes and Devices (TRIM-Diabetes and TRIM-Diabetes Device) Health & Quality of Life Outcomes. 2009;7:83. doi: 10.1186/1477-7525-7-83. doi: http://dx.doi.org/10.1186/1477-7525-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pifferi M, Bush A, Di Cicco M, Pradal U, Ragazzo V, Macchia P, et al. Health-related quality of life and unmet needs in patients with primary ciliary dyskinesia. European Respiratory Journal. 2010;35(4):787–794. doi: 10.1183/09031936.00051509. doi: http://dx.doi.org/10.1183/09031936.00051509. [DOI] [PubMed] [Google Scholar]
- 21.Anderson G. Princeton, NJ: Robert Wood Johnson Foundation; 2010. [Accessed April 7, 2016]. Chronic Care: Making the Case for Ongoing Care. http://www.rwjf.org/pr/product.jsp?id=50968. [Google Scholar]
- 22.Centers for Medicare and Medicaid Services. Baltimore, MD: Center for Medicare and Medicaid Services; 2012. [Accessed April 7, 2016]. Chronic Conditions Among Medicare Beneficiaries, Chartbook, 2012 ed. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/2012ChartBook.html. [Google Scholar]
- 23.Freid VM, Bernstein AB, Bush MA. Multiple chronic conditions among adults aged 45 and over: trends over the past 10 years. NCHS data brief. 2012;100:1–8. doi: http://www.cdc.gov/nchs/data/databriefs/db100.pdf. [PubMed] [Google Scholar]
- 24.Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey, 2010. Preventing Chronic Disease. 2013;10:E65. doi: 10.5888/pcd10.120203. doi: http://dx.doi.org/10.5888/pcd10.120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May CR, Eton DT, Boehmer K, Gallacher K, Hunt K, MacDonald S, et al. Rethinking the patient: using Burden of Treatment Theory to understand the changing dynamics of illness. BMC Health Services Research. 2014;14:281. doi: 10.1186/1472-6963-14-281. doi: http://dx.doi.org/10.1186/1472-6963-14-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamin RM. Multiple chronic conditions: a public health challenge. Public Health Reports. 2010;125(5):626–627. doi: 10.1177/003335491012500502. doi: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2924996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. Journal of General Internal Medicine. 2007;22 Suppl 3:391–395. doi: 10.1007/s11606-007-0322-1. doi: http://dx.doi.org/10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Archives of Internal Medicine. 2002;162(20):2269–2276. doi: 10.1001/archinte.162.20.2269. doi: http://dx.doi.org/10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 29.Bayliss EA, Steiner JF, Fernald DH, Crane LA, Main DS. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Annals of Family Medicine. 2003;1(1):15–21. doi: 10.1370/afm.4. doi: http://dx.doi.org/10.1370/afm.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoenberg NE, Leach C, Edwards W. “It's a toss up between my hearing, my heart, and my hip”: prioritizing and accommodating multiple morbidities by vulnerable older adults. Journal of Health Care of the Poor & Underserved. 2009;20(1):134–151. doi: 10.1353/hpu.0.0115. doi: http://dx.doi.org/10.1353/hpu.0.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee PG, Cigolle C, Blaum C. The co-occurrence of chronic diseases and geriatric syndromes: the health and retirement study. Journal of the American Geriatrics Society. 2009;57(3):511–516. doi: 10.1111/j.1532-5415.2008.02150.x. doi: http://dx.doi.org/10.1111/j.1532-5415.2008.02150.x. [DOI] [PubMed] [Google Scholar]
- 32.Schneider KM, O'Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States' Medicare population. Health & Quality of Life Outcomes. 2009;7:82. doi: 10.1186/1477-7525-7-82. doi: http://dx.doi.org/10.1186/1477-7525-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–631. doi: 10.2337/diacare.28.3.626. doi: http://dx.doi.org/10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 34.Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health & Quality of Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12. doi: http://dx.doi.org/10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallston KA, Osborn CY, Wagner LJ, Hilker KA. The Perceived Medical Condition Self-Management Scale applied to persons with HIV/AIDS. Journal of Health Psychology. 2011;16(1):109–115. doi: 10.1177/1359105310367832. doi: http://dx.doi.org/10.1177/1359105310367832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Quality of Life Research. 2009;18(7):873–880. doi: 10.1007/s11136-009-9496-9. doi: http://dx.doi.org/10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallston KA, Rothman RL, Cherrington A. Psychometric properties of the Perceived Diabetes Self-Management Scale (PDSMS) Journal of Behavioral Medicine. 2007;30(5):395–401. doi: 10.1007/s10865-007-9110-y. doi: http://dx.doi.org/10.1007/s10865-007-9110-y. [DOI] [PubMed] [Google Scholar]
- 38.Agency for Healthcare Research and Quality. CAHPS: Surveys and Tools to Advance Patient-Centered Care. [Accessed April 7, 2016];2013 https://cahps.ahrq.gov/
- 39.Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Family Practice. 2006;7:21. doi: 10.1186/1471-2296-7-21. doi: http://dx.doi.org/10.1186/1471-2296-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoenthaler A, Montague E, Baier Manwell L, Brown R, Schwartz MD, Linzer M. Patient-physician racial/ethnic concordance and blood pressure control: the role of trust and medication adherence. Ethnicity & Health. 2014;19(5):565–578. doi: 10.1080/13557858.2013.857764. doi: http://dx.doi.org/10.1080/13557858.2013.857764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muthen LK, Muthen BO. MPLUS User's Guide. 6th. Los Angeles, CA: Muthen & Muthen; 2010. [Google Scholar]
- 42.Hatcher L. A step-by-step approach to using SAS for factor analysis and structural equation modeling. Cary, NC: SAS Institute Inc; 1994. [Google Scholar]
- 43.Cappelleri JC, Zou KH, Bushmakin AG, Alvir JMJ, Alemayehu D, Symonds T. Patient-Reported Outcomes: Measurement, Implementation, and Interpretation. New York: CRC Press; 2014. Exploratory and confirmatory factor analyses; pp. 75–116. [Google Scholar]
- 44.Cella D. Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System - Version 4. Evanston, IL: Center on Outcomes, Research & Education (CORE), Evanston Northwestern Healthcare and Northwestern University; 1997. [Google Scholar]
- 45.Frost MH, Reeve BB, Liepa AM, Stauffer JW, Hays RD. What is sufficient evidence for the reliability and validity of patient-reported outcome measures? Value in Health, 10 Suppl. 2007;2:S94–S105. doi: 10.1111/j.1524-4733.2007.00272.x. doi: http://dx.doi.org/10.1111/j.1524-4733.2007.00272.x. [DOI] [PubMed] [Google Scholar]
- 46.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 47.Ridgeway JL, Egginton JS, Tiedje K, Linzer M, Boehm D, Poplau S, et al. Factors that lessen the burden of treatment in complex patients with chronic conditions: a qualitative study. Patient Preference & Adherence. 2014;8:339–351. doi: 10.2147/PPA.S58014. doi: http://dx.doi.org/10.2147/PPA.S58014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 49.Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to Their Development and Use. 4th. Oxford, UK: Oxford University Press; 2008. [Google Scholar]
- 50.Bennett SJ, Milgrom LB, Champion V, Huster GA. Beliefs about medication and dietary compliance in people with heart failure: an instrument development study. Heart & Lung : the journal of critical care. 1997;26(4):273–279. doi: 10.1016/s0147-9563(97)90084-4. doi: http://dx.doi.org/10.1016/S0147-9563(97)90084-4. [DOI] [PubMed] [Google Scholar]
- 51.Murphy SP, Powers MJ, Jalowiec A. Psychometric evaluation of the Hemodialysis Stressor Scale. Nursing Research. 1985;34(6):368–371. [PubMed] [Google Scholar]
- 52.Tran VT, Montori VM, Eton DT, Baruch D, Falissard B, Ravaud P. Development and description of measurement properties of an instrument to assess treatment burden among patients with multiple chronic conditions. BMC Medicine. 2012;10:68. doi: 10.1186/1741-7015-10-68. doi: http://dx.doi.org/10.1186/1741-7015-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maciejewski ML, Bayliss EA. Approaches to comparative effectiveness research in multimorbid populations. Medical Care. 2014;52 Suppl 3:S23–30. doi: 10.1097/MLR.0000000000000060. doi: http://dx.doi.org/10.1097/MLR.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 54.Tinetti ME, Studenski SA. Comparative Effectiveness Research and Patients with Multiple Chronic Conditions. New England Journal of Medicine. 2011;364:2478–2481. doi: 10.1056/NEJMp1100535. doi: http://dx.doi.org/10.1056/NEJMp1100535. [DOI] [PubMed] [Google Scholar]
- 55.U.S. Department of Health and Human Services. Washington, DC: 2010. [Accessed April 7, 2016]. Multiple Chronic Conditions -- A Strategic Framework: Optimum health and quality of life for individuals with multiple chronic conditions. http://www.hhs.gov/ash/initiatives/mcc/mcc_framework.pdf. [Google Scholar]
- 56.Boult C, Reider L, Leff B, Frick KD, Boyd CM, Wolff JL, et al. The effect of guided care teams on the use of health services: results from a cluster-randomized controlled trial. Archives of Internal Medicine. 2011;171(5):460–466. doi: 10.1001/archinternmed.2010.540. doi: http://dx.doi.org/10.1001/archinternmed.2010.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Counsell SR, Callahan CM, Clark DO, Tu W, Buttar AB, Stump TE, et al. Geriatric care management for low-income seniors: a randomized controlled trial. Journal of the American Medical Association. 2007;298(22):2623–2633. doi: 10.1001/jama.298.22.2623. doi: http://dx.doi.org/10.1001/jama.298.22.2623. [DOI] [PubMed] [Google Scholar]
- 58.Marek KD, Stetzer F, Ryan PA, Bub LD, Adams SJ, Schlidt A, et al. Nurse care coordination and technology effects on health status of frail older adults via enhanced self-management of medication: randomized clinical trial to test efficacy. Nursing research. 2013;62(4):269–278. doi: 10.1097/NNR.0b013e318298aa55. doi: http://dx.doi.org/10.1097/NNR.0b013e318298aa55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tinetti ME, Basu J. Research on multiple chronic conditions: where we are and where we need to go. Medical Care. 2014;52 Suppl 3:S3–6. doi: 10.1097/MLR.0000000000000093. doi: http://dx.doi.org/10.1097/MLR.0000000000000093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1 [Online resource]. Drafting, reviewing, and pre-testing the Patient Experience with Treatment and Self-Management (PETS) measure
Supplement 2 [Online resource]. Item diagnostic results


