Abstract
Laboratory stress tasks such as the Trier Social Stress Test (TSST) have provided a key piece to the puzzle for how psychosocial stress impacts the hypothalamic-pituitary-adrenal axis, other stress-responsive biomarkers, and ultimately wellbeing. These tasks are thought to work through biopsychosocial processes, specifically social evaluative threat and the uncontrollability heighten situational demands. The present study integrated an experimental modification to the design of the TSST to probe whether additional social evaluative threat, via negative verbal feedback about speech performance, can further alter stress reactivity in 63 men and women. This TSST study confirmed previous findings related to stress reactivity and stress recovery but extended this literature in several ways. First, we showed that additional social evaluative threat components, mid-task following the speech portion of the TSST, were still capable of enhancing the psychosocial stressor. Second, we considered stress-reactive hormones beyond cortisol to include dehydroepiandrosterone (DHEA) and testosterone, and found these hormones were also stress-responsive, and their release was coupled with one another. Third, we explored whether gain- and loss-framing incentive instructions, meant to influence performance motivation by enhancing the personal relevance of task performance, impacted hormonal reactivity. Results showed that each hormone was stress reactive and further had different responses to the modified TSST compared to the original TSST. Beyond the utility of showing how the TSST can be modified with heightened social evaluative threat and incentive-framing instructions, this study informs about how these three stress-responsive hormones have differential responses to the demands of a challenge and a stressor.
Keywords: TSST, Social Evaluative Threat, testosterone, DHEA, cortisol
Introduction
When encountering a stressor, an individual engages in physiological preparedness, which starts with the perception of a threat or a challenge to the organism. A biopsychosocial model is useful for understanding how and why a stressor impacts biological measures (Blascovich, 2008). Often in the laboratory, a stressor’s efficacy is inferred according to whether the context elicits neuroendocrine acute stress response, typically cortisol release (Dickerson & Kemeny, 2004). The Trier Social Stress Test (TSST) developed by Kirschbaum, Pirke, and Hellhammer (1993), involves delivering a speech and mental arithmetic in front of live, white-coated judges and a video camera (Campbell & Ehlert, 2012; Kudielka et al., 2007) and is putatively the most common laboratory stressor. While effective, it was several years after its design that researchers systematically recognized that the TSST’s efficacy relied on social evaluative threat and uncontrollability (Dickerson & Kemeny, 2004). Social evaluative threat occurs when an interchange of social interactions is perceived as a threat or social judgment, and the organism must engage the stressor to protect the self. Uncontrollability also enhances reactivity by increasing the stressor’s demands on the organism. This paradigm has allowed for a burgeoning of our understanding of the timing and mechanisms of the human stress response system and most recently, has extended to systematic TSST alterations to better understand uncontrollability and social evaluative threat. To our knowledge, it is relatively novel that our study explored whether an experimental manipulation of the TSST mid-way through the TSST alters the physiological stress response.
Biopsychosocial Stress Responsive Biomarkers
Changes in cortisol can indicate that the individual is experiencing a stressor at a physiological level. Central reward pathways (Fuchs & Flugge, 2003; Herman et al., 2007; Esch & Stefano, 2010) as well as limbic neurocircuitry (e.g., amygdala, orbitofrontal cortex) are activated (Dedovic et al., 2009) by stress. The stressor begins the hormone cascade when corticotrophin-releasing hormone (CRH) is released from the hypothalamus (see details in Sapolsky, Romero, & Munck, 2000) and ends when steroid hormones are released from target organs, such as the adrenal gland, including the glucocorticoid cortisol within 15–25 minutes following stress exposure (Dickerson & Kemeny, 2004). Cortisol alters lipid and glucose metabolism and influences neural functioning by binding to glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs) at differential binding affinity (Groeneweg, Karst, de Kloet, & Joels, 2012). Cortisol binding to GRs in the hypothalamus largely explains negative feedback as occupied GRs suppress subsequent HPA axis activation. Consequently, different neural preparative and reactive processes are involved in accordance to the level and timecourse of a stressor (Sapolsky, Romero, & Munck, 2000; de Kloet, Karst, & Joels, 2008; Kinner et al., 2016).
Although cortisol is the quintessential stress hormone, it is not the only stress reactive hormone. Dehydroepiandrosterone (DHEA) is also released from the adrenal gland (as well as other glands such as the gonads), in response to adrenocorticotropic hormone (ACTH) and in response to stressors (Shirtcliff, Zahn-Waxler, Klimes-Dougan, & Slattery, 2007), including the TSST (Lennartsson et al., 2012; Shirotsuki et al., 2009). DHEA remains largely understudied as a stress-responsive hormone (Starka, Duskova, & Hill, 2015), despite this abundant hormone’s neuroprotective and anti-glucocorticoid activities within emotion-related neurocircuitry (Maninger et al., 2009). DHEA serves a role as a biosynthetic precursor to neurosteroids and androgens like testosterone and thus this hormone connects both glucocorticoid and androgen hormones (Carlstrom et al., 1988).
Like DHEA, testosterone is underappreciated as a stress-responsive hormone and more often is examined as an end-product of the hypothalamic-pituitary-gonadal (HPG) axis. Testosterone is involved in development of male secondary sexual characteristics, such as increased muscle mass (Mazur & Booth, 1998). Testosterone also has been shown to play an important role in adult social behavior (Booth, Granger, Mazur, & Kivlighan, 2006; Bos, Panksepp, Bluthe, & van Honk, 2012), such as competitive drive (Archer, 2006; Casto & Edwards, 2016). Both genders produce testosterone, yet there are gender differences. Compared to males, females release less testosterone (Booth, Granger, Mazur, & Kivlighan, 2006). Gonadal testosterone follows somewhat different metabolic pathways (Handa & Weiser, 2014), and testosterone in females is largely of adrenal origin, which further bolsters the idea of testosterone can be a stress-responsive hormone (Drury et al., 2014). Few studies have examined testosterone reactivity to the TSST (see Schoofs & Wolf, 2011 for an exception), but a parallel literature illustrates that testosterone acutely rises when an individual faces a challenge or competition (see Archer, 2006 for a review). If testosterone changes during a stressor, it is unknown if testosterone is reactive through enhanced responsiveness to social evaluative threat (like cortisol or, presumably, DHEA) or through a more testosterone-relevant mechanism, such as competition, challenge, or reward.
In addition to examining whether testosterone and DHEA are reactive under social evaluative threat, an emerging literature is demonstrating dual-axis activation within the individual as evidence of crosstalk between the HPA and HPG axes (Shirtcliff et al., 2015; Viau, 2002). Prior theories suggested inhibitory cross-talk (Viau et al., 2002), yet a series of studies find consistent positive within-individual associations, known as ‘coupling,’ of androgens and cortisol (Dismukes et al., 2014; Han et al., 2015), including during stressful contexts (Marceau et al., 2014). This dual-axis view is providing important mechanistic insights into when and why these hormones may work together to influence behavior, such as under conditions of challenge (Mehta, Jones, & Josephs, 2008; Mehta & Josephs, 2010). Initially, ‘coupling’ was theorized to be observed primarily in adolescents who may need to maintain capacity to activate androgens even under stress (Ruttle et al., 2015; Susman et al., 2017), yet positive coupling has also been observed in adults (Bobadilla, Asberg, Johnson, & Shirtcliff, 2014, Harden et al., 2016). Marceau and colleagues (2014) examined coupling in response to three stressors and other research has examined multiple stress biomarkers (Bedgood, Boggiano, & Turan, 2014; Chatterton, Vogelsong, Hudgens, & 1997; Eatough et al, 2009; Knight & Mehta, 2017, Turan, Tackett, Lechtreck, & Browning, 2015). Yet to our knowledge, this is the first study to examine coupling of cortisol, testosterone, and DHEA during the TSST.
Enhanced Social Evaluative Threat through Verbal Performance Feedback and Incentivized Performance
Modified versions of the TSST are increasingly frequent (Campbell & Ehlert, 2012; Wadiwalla et al., 2010). For example, the TSST has been modified in order to fit the constraints of experimental protocols for use in children (Buske-Kirschbaum et al., 1997), groups (von Dawans, Kirschbaum, & Heinrichs, 2011), for neuroimaging (Kern et al., 2008), and with virtual audiences (Kelly et al., 2007). Other studies have modified the TSST in order to better understand the psychological and social components that make the TSST work as a biological stressor (Andrews et al., 2007). These studies can be framed in terms of a biopsychosocial model (Seery, 2011; Tomaka et al., 1997; Blascovich & Tomaka, 1996) in which a motivated performance situation, like the TSST, relies on psychological processes within the individual (task engagement, evaluation of resources and situational demands).
Situational demands shift according to the level of social evaluative threat or uncontrollability. For instance, the confederate audience changes social evaluative threat (Dickerson, Mycek, & Zaldivar, 2008; Wadiwalla et al., 2010), such that speech tasks without social judgment or with positive social cues from confederates do not stimulate neuroendocrine responsivity (Het et al., 2009; Taylor et al., 2010; Wiemers, Schoofs, & Wolf, 2013; Gruenewald et al., 2004). The original TSST provides no direct verbal feedback about speech performance, but Dedovic et al. (2005) found negative verbal and nonverbal feedback about math performance enhanced cortisol reactivity during the Montreal Imaging Stress Task (Dedovic et al., 2005).
The biopsychosocial model (Seery 2011) also postulates that motivated performance is necessary to elicit increased stress responsivity across multiple physiological systems (see Campbell & Ehlert, 2011 for a comprehensive review). Some studies have altered performance motivation by changing the speech topic to be personal, based on the idea that greater ego involvement and self-referential components should enhance the TSST (Wadiwalla et al., 2010; Andrews et al., 2007). It is possible that social stimuli may enhance performance motivation as individuals are motivated to help or impress others. Dedovic and colleagues (2005) told participants that data would not be used if performance did not improve. Unfortunately, it is unclear whether the usefulness of the data for the researcher is motivating to the participant. A more direct method of motivating performance may be through use of incentives (Seery, Weisbuch, & Blascovich, 2009), given the role of cortisol in punishment and reward sensitivity (Van Honk, Shutter, Hermans, & Putnam, 2003) and the emerging literature that androgens like testosterone are sensitive to reward or challenge (Mehta, Jones, & Josephs, 2008; Mehta & Josephs, 2006; Bos, Hermans, Ramsey, & van Honk, 2012). Several studies attached the descriptor “motivated performance” to the TSST without describing whether (and how) incentives were delivered and performance during the TSST is not typically tied to compensation. Thus, incentives may motivate participants to be in a research study, but not necessarily to perform well during the stressor. Lastly, there is some evidence that multiple TSST modifications best impact reactivity. Wadiwalla and colleagues (2010) found that the effect of ego involvement or divided attention were only observed under conditions of enhanced social evaluative threat, suggesting that the biopsychosocial processes of motivated performance and situational demands are not mutually exclusive.
The Present Study Aims and Hypotheses
We used a multi-pronged experimental modification to the TSST that targeted both social evaluative threat and performance motivation. This was accomplished through Verbal Evaluation of Speech Performance (VESP). Verbal evaluation from the confederates was negatively-valenced so as to enhance social evaluate threat and was delivered immediately after the speech (and before the math) to increase salience of feedback. Such an experimental manipulation is initiated mid-way through the stressor and within the confines of an already well-validated stressor, when the impact of VESP can be exerted on already activated axes. We also sought to enhance performance motivation through use of incentives, explicitly tying information about performance feedback to amount of study compensation (gain or loss framing). We tentatively explored whether the delivery of the incentive was impacted by whether the incentive was framed as a gain or framed as a loss (Seery, Weisbuch, & Blascovich, 2009) in order to understand what type of incentive created the most impactful changes in hormone responses. The gain framing condition provided negative feedback about the speech performance and offered additional compensation if performance improved during math. The loss framing condition provided parallel negative verbal evaluation of speech performance, and then framed incentives as a potential loss of compensation if performance failed to improve.
We hypothesized that cortisol, DHEA and testosterone would be stress reactive to the TSST as compared to time-matched samples collected on a non-stress day. Our primary hypothesis of interest was that these stress-responsive hormones will be more reactive to an enhancement of social evaluative threat via VESP as compared to the original TSST. A secondary (exploratory) hypothesis was that hormonal reactivity would be greater compared to the original TSST if participant performance was tied to study payment using a gain framing or a loss framing incentives. We speculate that the loss framing VESP condition would best enhance hormone reactivity given that it has the greatest social evaluative threat and personal salience about performance. Given the dearth of prior literature, this speculation is ultimately agnostic as to whether the gain framing or loss framing condition would be most effective. Beyond these primary study aims and hypotheses, we present two additional analyses. We examine sex differences in hormone levels and responsivity to the TSST and hormone coupling across these three hormones following the analytic strategy described by Marceau and colleagues (2015).
Methods
Participants
Individuals ages 18 to 30 (M = 21.95, SD = 2.21) were invited to participate via announcements and fliers posted on university campus. A total of 63 individuals completed the study (see Table 1 for participant demographics). Exclusionary criteria were implemented due to influence on hormone levels, which included current use of oral steroids, current use of psychoactive medications, pregnancy, currently breastfeeding, or reporting any current mental illness. At the conclusion of the study, all participants received a $15 compensation for their time and debriefing methods were applied.
Table 1.
Demographic Characteristics of Sample
| Sample (N = 63) |
Original TSST (N = 39) |
VESP-TSST Loss Framing (N = 11) |
VESP-TSST Gain Framing (N = 13) |
|
|---|---|---|---|---|
| Age (M, SD) | 21.95 (2.21) | 21.63 (2.02) | 22.35 (2.59) | 22.55 (2.43) |
| Sex | ||||
| Male | 33 (52 %) | 15 (38%) | 7 (64%) | 8 (62%) |
| Female | 30 (48%) | 24 (62%) | 4 (36%) | 5 (38%) |
| BMI (M, SD) | 24.44 (5.77) | 25.32 (6.15) | 20.70 (4.54) | 24.99 (4.37) |
| Basal box return | 36 (57%) | 21 (54%) | 8 (73%) | 7 (54%) |
Note: There were no group differences in age, sex ratio, BMI, or basal box return.
Procedures
All participants signed informed consent forms, and all study procedures were approved by the University of New Orleans Institutional Review Board. Testing occurred in the afternoon to account for the circadian rhythm of steroid hormones (Dickerson & Kemeny, 2004). Participants arrived at the laboratory at M = 14:02 hour (SD = 0:07). Participants were asked to not to eat, drink, or smoke for 1 hour prior to the laboratory visit. The total test time for each participant was between 2.5 and 3.0 hours. The stress task began 30–60 minutes after arrival to reduce the influence of the “arrival effect” on hormone levels (Ruttle et al., 2011; Hastings et al., 2011). Participants were randomly assigned to the original TSST (N = 39) or the VESP version of the TSST (N = 24) at a ratio of 3-to-1 so that the lab protocol did not ‘drift’ toward the more challenging modifications as confederate response can impact reactivity (Wiemers, Schoofs, & Wolf, 2013; Bosch et al., 2009; Dickerson, Mycek, & Zaldivar, 2008).
Original TSST (n = 39)
Ten minutes before beginning the stress task, a researcher provided the speech topic for participants (a simulation of an interview for their dream career). During the 10-minute “anticipation period,” participants were instructed to prepare their speech. Next, participants stood on a spot-lighted stage in front of three attentive, white-coated judges, while being video and audio recorded. Participants then delivered a 5-minute speech followed by 5 minutes of mental arithmetic in front of the judges. Throughout the original TSST condition, participants were given no verbal feedback regarding their performance beyond the still-faced expression of the confederate judges. The only verbal feedback provided was if participants incorrectly performed mental arithmetic in which confederates only responded, “That’s incorrect, please continue.” For all conditions, judges included both male and female confederate judges.
VESP Modified TSST (n = 24)
All procedures were identical to the original TSST in the VESP-TSST with the following exception. After the speech, the confederates inconspicuously whispered to each other. The speaking confederate then informed participants that the speech performance was not sufficient. Within the gain framing VESP (N = 13), participants were told that their performance was not sufficient, and they needed to improve. If they improved their performance in the mental arithmetic task, monetary compensation would be increased to $15. Within the loss framing VESP (N = 11), the speaking confederate informed participants that their performance was not sufficient, and they needed to improve performance in the math task. If they did not improve performance, monetary compensation of $15 (previously delivered) would be lost. The mental arithmetic task then began. Our study was best powered to examine the modifications with N = 24, but secondary analyses examine the smaller subgroups, commensurate with the statistical power of prior studies (Wadiwalla et al., 2010).
Measures
Hormone assessments
Six saliva samples were collected via passive drool into microvials during the laboratory visit. The first sample was provided by the participant immediately upon arrival (M = 14:04h, SD = 0:07). The second sample was collected right before the start of the TSST (M = 14:59h, SD = 0:18). The third sample was collected immediately after the TSST (M = 15:02h, SD = 0:09). The fourth sample was collected ~30 minutes after the TSST (M = 15:27h, SD = 0:11), the fifth sample ~60 minutes after (M = 15:53h, SD= 0:12), and the last sample was collected ~90 minutes after the TSST (M = 16:46h, SD = 0:13). Saliva samples were frozen at −80° Celsius immediately until thawed at room temperature for hormone assays.
Time-Matched Non-TSST Basal Day
Participants were asked to collect six saliva time-matched to laboratory samples on a non-TSST day (up to 12 samples per individual) in order to estimate each individual’s diurnal rhythm and rule out that changes on the TSST day were an artifact of overall hormone elevations on the TSST day. Participants were given necessary supplies with detailed instructions for their saliva collection. Saliva collection matched times with the TSST day saliva collection for that individual, typically beginning around 2:00 pm and terminating after 6 samples were collected around 4:30 pm. Participants were instructed to keep samples frozen in home freezers until transported on ice via courier to the laboratory. A total of 38 participants (60% of total sample) returned the basal day samples frozen; however, two participants’ basal samples were excluded from analysis for not following saliva collection time instructions. Subsequent analyses included individuals who did and did not return the basal samples so as to maximize the statistical power; this was feasible given our analytic strategy (see below) and because primary analyses focused on reactivity within the laboratory day. Examining the TSST-day hormones, there were no systematic differences between individuals who did and did not return basal day samples in terms of hormone levels [cortisol F(1,54) = 0.31, p = 0.582; DHEA F(1,54) = 0.14, p = 0.711; testosterone F(1,54) = 0.08, p = 0.782], hormone reactivity [cortisol F(5,270) = 1.09, p = 0.371; DHEA F(5,270) = 0.23, p = 0.948; testosterone F(5,270) = 1.52, p = 0.180], or group (see Table 1).
Stressor Validation
To confirm that the stressor was indeed stressful, we compared self-reported emotion ratings at the time of saliva collection on the TSST-day compared to time-matched emotion ratings on the basal days using a 2 (day) × 6 (sample) repeated measures ANOVA separate for four emotions (anxious, angry, happy, sad). As expected, participants reported greater anxiousness on TSST-days than basal days [F(1,170) = 13.30, p = 0.001, Cohen’s d = 0.83; M anxiousness = 34.61 on basal and M anxiousness = 55.12 on TSST-day]. Anxiousness levels changed across each day [F(5,34) = 14.25, p < 0.001] and showed a day*anxiousness interaction [F(5,170) = 6.45, p < 0.001]. Compared to the basal day time-matched emotion ratings, participants reported greater peaks in anxiousness scores upon lab arrival [sample 1: t(40) = 2.87, p = 0.007; M diff = 13.83, SD = 20.48], in anticipation of the stressor [sample 2: t(39) = 4.54, p < 0.001; M diff = 33.8, SD = 47.12], immediately after the stressor [sample 3: t(39) = 4.46, p < 0.001; M diff = 26.9, SD = 49.1], and at trend-level by 30 min after the stressor [sample 4: t(39) = 1.89, p = 0.066], but no longer by sample 5 [t(38) = 0.36, p = 0.720; M diff = 1.69, SD = 28.85] or sample 6 [t(35) = 0.85, p = 0.400; M diff = 4.67, SD = 32.67]. Participants were equivalent in other self-reported emotions on the TSST- vs. basal-day [angry: F(1,170) = 0.01, p = 0.949, Cohen’s d = 0.33; happy: F(1,170) = 1.72, p = 0.198, Cohen’s d = 0.25; sad: F(1,170) = 0.14, p = 0.713, Cohen’s d = 0.23] with the exception of an angry*day interaction [F(5,170) = 3.62, p = 0.004] driven by higher angry ratings (compared to time-matched ratings on the basal day) immediately after the stressor [sample 3: t(39) = 2.00, p = 0.053; M diff = 15.2, SD = 48.09] and 30 min after the stressor [sample 4: t(39) = 2.42, p = 0.020; M diff = 8.87, SD = 23.15] but not any other times (ps > 0.165). These self-report emotions suggested that the TSST was successful in stimulating feelings of anxiousness throughout the stressful lab day and possibly angry during the stressor as well as compared to those same participants’ time-matched emotion ratings on a different day.
Control measures
Participants filled out daily diaries that pertained demographical information, such as body mass index, age, sex, medications, drug use, health status, female menstrual calendar, emotional state, and sleep habits.
Hormone assays
Samples were thawed and then assayed using commercially available enzyme-immunoassay kits for cortisol, DHEA, and testosterone (www.salimetrics.com) following manufacturer recommendations. Included in the assay kits were supplies to test efficacy of ligand binding of cortisol, DHEA, and testosterone antigens, respectively, in saliva to the antibody-coated wells of the microtitre plates. Samples were assayed for all three hormones on the same day to minimize freeze-thaw cycle influences. Each hormone was assayed in duplicate, and the sample was re-assayed if duplicates varied by more than 7% (intra-assay coefficient of variance). Average intra-assay CVs for cortisol was 1.84%; DHEA was 1.63%; and testosterone was 2.35%, which showed good correspondence between duplicates. Inter-assay coefficient of variance on average were for cortisol 7.36%, for DHEA 8.34%, and for testosterone 7.46%, respectively. Using a 4-parameter nonlinear regression curve fit, a standard curve was created, and an average R2 = .999 was calculated.
Data Preparation
All outliers in the hormone data were winsorized (Tukey, 1997). Due to positive skew, all hormone values were natural log-transformed; for cortisol, an added constant to the natural log-transformed values moved the data to above-zero. In HLM, it is important to center predictor variables so that the intercept is interpretable – in our case, as the individual’s peak hormone value. To do so, we identified the sample (and its collection time) at which each individual showed a peak for each hormone rather than assume that each participant achieved a peak at a particular sample. Across six time points of hormone data, researchers coded each individual’s peak hormone value (inter-rater reliability kappa = 0.91), identified as the sample collection time when participants showed either the highest hormone value (across all six time points) or when hormone values significantly rose from the second time point to the third or fourth time point—the second time point was one hour after arrival and considered a marker of baseline levels. This ensured reactivity was to the TSST and not driven by high hormones due to the arrival effect in which participants arrived to the lab already with high hormone levels (Ruttle et al., 2011). The first and second time points (or samples) were only coded as peak when these time points showed highest hormone value or other time points did not show any rise in hormone values (Sample 1: peak N = 2, 1, 1 for cortisol, DHEA, and testosterone, respectively; Sample 2: peak N = 7, 4, 5 for cortisol, DHEA, and testosterone, respectively). The sample collection time in which each individual showed their peak hormone value was then recorded as “time of peak” and was used to generate time variables that would capture reactivity and recovery once regressed in HLM on the hormone outcomes.
Rate of change in hormone levels involved creating the variables time-to-peak, and time-after-peak, separately for lab day and basal day, based on the individual sample collection times and the “time of peak” variable. A time-to-peak [XTTP (X denotes predicted hormone)] variable indexed reactivity as hormone rise to peak (i.e., stress reactivity). In brief, time-to-peak subtracted sample measurement time from the participant’s “time of peak;” this created negative values that decreased as time neared closer to peak and allowed positive beta-weights to indicate greater hormone reactivity. A time-after-peak (XTAP) variable was created to index hormone fall after peak (i.e., stress recovery) by subtracting sample measurement time from the participant’s peak time; this created positive values that increased as time distanced from peak and allowed positive beta-weights to indicate failure to recover from the stressor and negative beta-weights to indicate faster stress recovery. These two time variables (hormone stress reactivity and hormone stress recovery) are predictors of cortisol levels, DHEA levels, and testosterone levels created as separate variables, respectively. Time-matched basal hormone data replicated the same data preparation procedure as lab hormone data, although neither reactivity rises nor recovery declines in hormone levels were anticipated across the basal day sample collections. Instead, the time-matched basal samples allowed statistical test of whether an individual’s responsivity on the lab day was different from what that individual’s hormones were across those same time points on a different day.
Statistical analyses
Each hormone was modeled separately although parallel models were composed to the extent possible. The anticipated limitation of reduced basal day samples was considered in our statistical analysis design in order to make full use of the reactivity data we had while also minimizing missing data. The statistical package hierarchical linear modeling (HLM: Scientific Software International, Skokie IL) was advantageous as it does not exclude cases with partial missing data. Therefore, all 63 cases were included in data analyses even when cases had a few missing data points (most commonly missing basal samples). Given this nested data structure (multiple samples for each participant), a two-level HLM was utilized. Level 1 investigated hormonal changes within an individual (N = 581; 6 samples on the laboratory visit day, 6 samples on basal day when available). Level 2 investigated differences between individuals (N = 63). Hormone natural log-transformed levels were included in Level 1 as the dependent variable (Yij). An intra-class correlation first established whether HLM was necessary using a null model for each hormone, given dependency within the data and provided an overall estimate of the stability for each hormone within each individual.
The intercept (β0j) captured the individual’s peak hormone level following the TSST (i.e., peak cortisol level, peak DHEA level, peak testosterone level). Time-to-peak (β2j * lab_reactivityij) and time-after-peak (β3j * lab_recoveryij) were used to index reactivity and recovery slopes, respectively (i.e., cortisol reactivity, DHEA reactivity, testosterone reactivity). Level 1 equation also included a dummy code that indicated day (β1j * dayij) to allow for differences in hormone levels between lab and basal days. Also, included in Level 1 were two more dummy variables as controls (β4j * basal_reactivityij and β5j * basal_recoveryij). These variables were included to index whether individuals’ hormone profiles differed from the lab day on a non-stressed basal day and accounted for hormone change on basal days at the same collection times. Base models for cortisol, DHEA, and testosterone included random parameters (uij) for β0j, β1j, and β2j because the variance components were significant (ps < 0.001), which indicated that hormone levels between individuals differ more than could be reasonably attributed to chance. Including the random parameters (uij) provided Level 2 residual variance, which was calculated with final estimation of variance components to examine the variance of hormone activities within individuals. A Level-1 HLM model is presented below using cortisol to illustrate variables that were included in the base model:
To examine if TSST-VESP had different effects on hormonal response, the VESP-group membership was included as a Level 2 predictor. This allowed us to investigate the cross-level interaction of VESP by time (e.g., reactivity or recovery) on hormone levels. Exploratory analyses then further divided the VESP-TSST modification group into gain and loss framing categories to examine whether the type of incentive further modulated hormone response beyond negative performance feedback. Control variables (e.g., sex, age, BMI, female follicular menstrual phase) were entered into the models on Level 2. All results reported were from parsimonious models that predicted hormone levels. Beta coefficients reported in the Results section are unstandardized betas. Table 2 included both unstandardized and standardized betas.
Table 2.
Hormone Base Model Comparisons in Hierarchical Linear Modeling and Ordinary Least Squares Regression
| HLM | OLS | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent Variable | WI ICC |
BI ICC |
f2 | L1 N |
R2 | N | ||||||||||||
| Cortisol | Predictors | 80% | 20% | 0.40 | 581 | β | SE | B | t | df | p | 0.24 | 593 | β | SE | B | t | p |
| Intercept | - | 0.04 | 2.81 | 43.02 | 62 | <.001 | - | 0.05 | 3.27 | 69.29 | <.001 | |||||||
| Basal vs. Lab Day | −0.66 | 0.12 | −0.89 | −7.43 | 62 | <.001 | −0.59 | 0.08 | −0.8 | −10.13 | <.001 | |||||||
| Reactivity | 0.31 | 0.05 | 0.30 | 6.41 | 62 | <.001 | 0.33 | 0.05 | 0.31 | 6.63 | <.001 | |||||||
| Recovery | −0.44 | 0.04 | −0.50 | −11.85 | 389 | <.001 | −0.29 | 0.06 | −0.33 | −5.64 | <.001 | |||||||
| Basal Day × Reactivity | −0.33 | 0.10 | −0.48 | −4.93 | 389 | <.001 | −0.25 | 0.08 | −0.36 | −4.42 | <.001 | |||||||
| Basal Day × Recovery | 0.20 | 0.10 | 0.32 | 3.21 | 389 | 0.001 | 0.13 | 0.9 | 0.21 | 2.26 | 0.024 | |||||||
| DHEA | 33% | 67% | 0.16 | 581 | 0.06 | 593 | ||||||||||||
| Intercept | - | 0.09 | 5.13 | 57.42 | 62 | <.001 | − | 0.07 | 5.44 | 78.55 | <.001 | |||||||
| Basal vs. Lab Day | −0.29 | 0.10 | −0.50 | −5.21 | 62 | <.001 | −0.24 | 0.11 | −0.41 | −3.63 | <.001 | |||||||
| Reactivity | 0.18 | 0.04 | 0.20 | 5.79 | 62 | <.001 | 0.15 | 0.06 | 0.16 | 2.65 | 0.008 | |||||||
| Recovery | 0.19 | 0.06 | −0.34 | −6.14 | 389 | <.001 | −0.19 | 0.12 | −0.33 | −2.83 | 0.005 | |||||||
| Basal Day × Reactivity | −0.10 | 0.06 | −0.16 | −2.65 | 389 | 0.008 | −0.14 | 0.1 | −0.09 | −1.37 | 0.172 | |||||||
| Basal Day × Recovery | 0.10 | 0.08 | 0.23 | 2.85 | 389 | 0.005 | 0.05 | 0.16 | 0.11 | 0.67 | 0.505 | |||||||
| Testosterone | 9% | 91% | 0.09 | 581 | 0.01 | 593 | ||||||||||||
| Intercept | − | 0.07 | 4.25 | 56.51 | 62 | <.001 | − | 0.05 | 4.25 | 79.91 | <.001 | |||||||
| Basal vs. Lab Day | −0.21 | 0.06 | −0.27 | −4.42 | 62 | <.001 | −0.14 | 0.08 | −0.19 | −2.22 | 0.027 | |||||||
| Reactivity | 0.10 | 0.02 | 0.08 | 3.47 | 62 | <.001 | 0.12 | 0.05 | 0.10 | 2.03 | 0.043 | |||||||
| Recovery | −0.21 | 0.04 | −0.25 | −5.92 | 389 | <.001 | −0.12 | 0.09 | −0.14 | −1.66 | 0.097 | |||||||
| Basal Day × Reactivity | −0.10 | 0.03 | −0.11 | −3.40 | 389 | <.001 | −0.07 | 0.08 | −0.08 | −1.05 | 0.293 | |||||||
| Basal Day × Recovery | 0.16 | 0.06 | 0.23 | 3.63 | 389 | <.001 | 0.12 | 0.11 | 0.17 | 1.52 | 0.128 | |||||||
Note: The results presented are base models of cortisol, testosterone, and dehydroepiandrosterone. OLS R2's reflected within-individual variance. HLM Cohen's f2 = [R2(AB) - R2(A)] / [1 - R2(AB)]. R2 = (σ2null - σ2random) / σ2null. OLS = Ordinary Least Squares; WI = within-individual; BI = between-individual; ICC = intra-class correlation; f2 = Cohen's f2; L1 = Level 1; β = standardized beta; B = unstandardized beta
Hormone “coupling” analyses were run to test whether a hormone sample was correlated with the other hormones measured at that same time. We replicated Marceau et al.’s (2015) multilevel models in examining potential crosstalk across the HPA and HPG axes within individuals. We described the strategy for cortisol as the outcome, but separate models were run for each hormone as the outcome. Marceau et al. (2015) described the Coupling Model 1 as a basic within-individual test of whether samples that have high testosterone also have high (or low) cortisol, and whether there are individual differences (or variance) in the strength of that bivariate association: LNCORTij = β0j + β1j*(LNTESTOij) + rij. The Coupling Model 2 recapitulates this association using DHEA as a predictor of cortisol: LNCORTij = β0j + β1j*(LNDHEAij) + rij. Next, Marceau et al. (2015) described another model, termed the “trivariate model,” that includes both testosterone and DHEA as predictors of cortisol (Coupling Model 3: LNCORTij = β0j + β1j*(LNDHEAij) + β2j*(LNTESTOij) + rij. This model was included to determine if the androgens were explaining the same variance in cortisol levels, or if they were unique predictors of cortisol. Marceau et al. (2015) lastly considered that coupling could be an artifact of common change patterns. That is, if some individuals were simply more reactive across multiple hormones, they would show positive coupling simply because all three hormones were stress-reactive. Marceau et al. (2015) described a strategy which models the outcome-hormone’s reactivity profile and then, added on the other hormone samples at Level 1. This tested, using cortisol and testosterone as an example, whether a sample with elevated testosterone predicted elevated cortisol above and beyond the cortisol reactivity captured by sample collection time alone. The Coupling Model 3 tested the trivariate associations of cortisol with testosterone and DHEA, respectively, and the same trivariate associations of cortisol with testosterone and DHEA in the same model was tested with the Level-1 HLM base model, which we call the Coupling Model 4: LNCORTij = β0j + β1j*(DAYij) + β2j*(LNDHEAij) + β3j*(LAB_CTTPij) + β4j*(LAB_CTAPij) + β5j*(BASAL_CTTPij) + β6j*(BASAL_CTAPij) + β7j*(LNTESTOij) + rij (see Table 3).
Table 3.
Trivariate and Base Model Combination Comparisons in Hierarchical Linear Modeling and Ordinary Least Squares Regressions
| HLM | OLS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent Variable |
f2 | R2 | ||||||||||||
| Cortisol | Predictors | 0.55 | β | SE | B | t | df | p | 0.38 | β | SE | B | t | p |
| Intercept | − | 0.08 | 3.10 | 36.71 | 62 | <.001 | − | 0.18 | 1.32 | 7.41 | <.001 | |||
| Testosterone | 0.56 | 0.08 | 0.58 | 7.69 | 62 | <.001 | 0.19 | 0.04 | 0.20 | 4.99 | <.001 | |||
| DHEA | 0.43 | 0.06 | 0.34 | 6.10 | 62 | <.001 | 0.26 | 0.03 | 0.20 | 6.83 | <.001 | |||
| Basal vs. Lab Day | −0.45 | 0.10 | −0.62 | −6.31 | 62 | <.001 | −0.51 | 0.07 | −0.69 | −9.49 | <.001 | |||
| Reactivity | 0.17 | 0.04 | 0.17 | 3.80 | 62 | <.001 | 0.23 | 0.04 | 0.23 | 5.17 | <.001 | |||
| Recovery | −0.33 | 0.04 | −0.37 | −9.19 | 263 | <.001 | −0.22 | 0.05 | −0.25 | −4.69 | <.001 | |||
| Basal Day × Reactivity | −0.24 | 0.08 | −0.35 | −4.29 | 263 | <.001 | −0.23 | 0.07 | −0.33 | −4.53 | <.001 | |||
| Basal Day × Recovery | 0.14 | 0.07 | 0.22 | 3.01 | 263 | 0.003 | 0.10 | 0.09 | 0.16 | 1.90 | 0.058 | |||
| DHEA | 0.51 | 0.31 | ||||||||||||
| Intercept | − | 0.08 | 5.30 | 64.15 | 62 | <.001 | − | 0.22 | 2.31 | 10.47 | <.001 | |||
| Testosterone | 0.40 | 0.06 | 0.52 | 8.23 | 325 | <.001 | 0.37 | 0.05 | 0.49 | 9.99 | <.001 | |||
| Cortisol | 0.18 | 0.04 | 0.23 | 5.44 | 62 | <.001 | 0.27 | 0.05 | 0.34 | 6.43 | <.001 | |||
| Basal vs. Lab Day | −0.11 | 0.07 | −0.18 | −2.56 | 62 | 0.013 | −0.04 | 0.10 | −0.07 | −0.70 | 0.485 | |||
| Reactivity | 0.12 | 0.03 | 0.13 | 5.30 | 62 | <.001 | 0.08 | 0.05 | 0.09 | 1.63 | 0.104 | |||
| Recovery | −0.06 | 0.05 | −0.11 | −2.11 | 325 | 0.035 | −0.09 | 0.10 | −0.16 | −1.61 | 0.109 | |||
| Basal Day × Reactivity | −0.02 | 0.04 | 0.04 | −0.081 | 325 | 0.421 | −0.10 | 0.09 | −0.02 | −0.17 | 0.863 | |||
| Basal Day × Recovery | −0.02 | 0.04 | 0.23 | 5.44 | 62 | <.001 | −0.01 | 0.14 | −0.03 | −0.21 | 0.836 | |||
| Testosterone | 0.60 | 0.27 | ||||||||||||
| Intercept | − | 0.06 | 4.15 | 64.01 | 62 | <.001 | − | 0.17 | 1.91 | 11.24 | <.001 | |||
| Cortisol | 0.18 | 0.03 | 0.18 | 6.01 | 62 | <.001 | 0.25 | 0.04 | 0.24 | 5.60 | <.001 | |||
| DHEA | 0.31 | 0.03 | 0.23 | 6.85 | 62 | <.001 | 0.39 | 0.03 | 0.29 | 9.81 | <.001 | |||
| Basal vs. Lab Day | 0.02 | 0.04 | 0.02 | 0.51 | 62 | 0.613 | 0.09 | 0.08 | 0.11 | 1.40 | 0.163 | |||
| Reactivity | 0.00 | 0.02 | 0.00 | 0.20 | 62 | 0.844 | 0.01 | 0.04 | 0.01 | 0.18 | 0.861 | |||
| Recovery | −0.09 | 0.03 | −0.11 | −3.83 | 263 | <.001 | −0.02 | 0.08 | −0.02 | −0.32 | 0.747 | |||
| Basal Day × Reactivity | 0.00 | 0.03 | 0.00 | 0.14 | 263 | 0.892 | 0.03 | 0.07 | 0.03 | 0.48 | 0.629 | |||
| Basal Day × Recovery | 0.09 | 0.04 | 0.13 | 3.77 | 263 | <.001 | 0.09 | 0.10 | 0.13 | 1.31 | 0.191 | |||
Results
Was cortisol differentially responsive to the TSST and the TSST-VESP condition?
Of the total variance in the initial model for cortisol, 20% of the variance was explained by between-individual differences, and 80% was explained by within-individual cortisol change in levels [χ2(62) = 202.54, p < 0.001). This intra-class correlation is important for justifying the use of HLM as it shows samples collected within an individual were correlated with one another. In testing the base model, we found significant cortisol reactivity (B = 0.30, p < 0.001) and recovery from the TSST (B = −0.51, p < 0.001) on lab day as compared to the individual’s basal day time-matched hormone values. Moreover, peak cortisol levels were significantly higher on the TSST day (B = 0.89, p < 0.001) compared to the basal day’s six time-matched cortisol samples. The results illustrated that cortisol was responsive to the lab stressor.
Focusing on the TSST-day, sex was included in the base model as a Level 2 predictor. Sex differences were found with females showing flatter recovery of cortisol (B = 0.17, p < 0.001) compared to males. Due to the robust sex effect in predicting cortisol levels in recovery, sex was included (a cross-level interaction between sex and cortisol recovery) in all subsequent models to control for sex differences. Other control variables entered in Level 2 had nonsignificant results and did not influence the results of the base model.
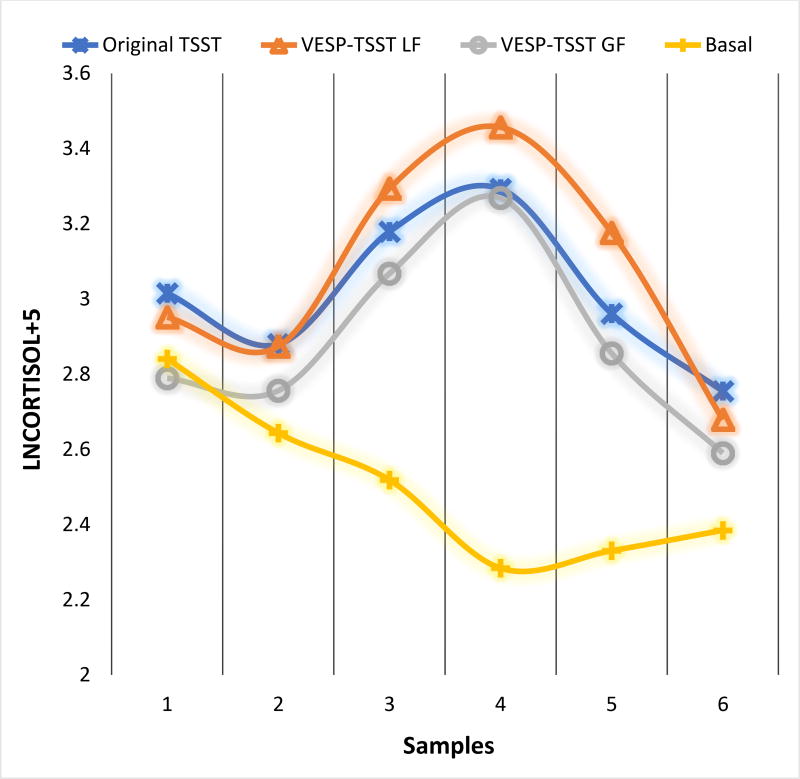
Group differences (original TSST vs. TSST-VESP), as Level 2 predictors, in response to stress are visually demonstrated in Figure 1. Participants who received the TSST-VESP modification showed sharper reactivity rises (B = 0.11, p = 0.048) and subsequently steeper recovery declines (B = −0.12, p = 0.008) as compared to the original TSST. These results supported the hypothesis that participants receiving the negative performance feedback were differentially responsive compared to those in the original TSST condition.
Figure 1.
Original TSST and VESP-TSST group differences in cortisol responses
Note: VESP-TSST GF: Verbal Evaluation of Speech Performance Gain Framing Condition; VESP-TSST LF: Verbal Evaluation of Speech Performance Loss Framing Condition
When we explored the loss- and gain-framing subsets, we found participants in the loss framing group showed steeper recovery (B = −0.14, p = 0.007) in cortisol levels, but no further group differences in reactivity or recovery were found for participants in the gain framing group (cortisol reactivity: B = 0.08, p = 0.221; cortisol recovery: B = −0.04, p = 0.506).
Was DHEA differentially responsive to the TSST and the TSST-VESP modification?
Sixty-seven percent of the total variance in the initial model for DHEA explained between individual differences, and 33% of the variance was explained by moment-to-moment fluctuations within an individual [χ2(62) = 1229.29, p < 0.001]. Using the base model with DHEA as the outcome variable, DHEA response to psychosocial stress showed significant reactivity rises (B = 0.19, p < 0.001) as well as steeper recovery declines (B = −0.32, p < 0.001) on lab day compared to the basal day. Moreover, DHEA levels were significantly higher (B = 0.50, p < 0.001) on the lab day compared to a basal day. In other words, DHEA levels across all six time points were higher on lab day than those six time points on the basal day. Similar to cortisol, DHEA showed stress-reactive levels on lab day when compared to basal day.
Focusing on the TSST-day, no sex differences were found in DHEA overall levels and in DHEA reactivity and recovery. Therefore, sex was not included as a Level 2 control variable in subsequent models. Also, other control variables did not moderate any effects of the base model results, and therefore, not included in subsequent models.
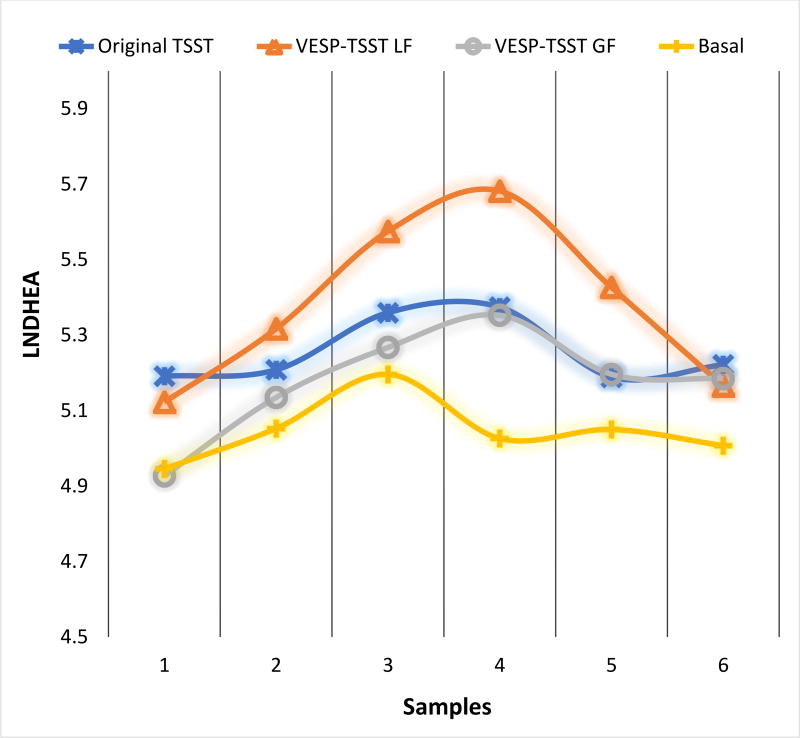
Differences in reactivity or recovery between the original TSST and the TSST-VESP group were not significant for DHEA.
When we explored the loss- and gain-framing subsets of the TSST-VESP, group differences became apparent for the specific TSST-VESP conditions. The loss framing group showed higher DHEA levels (B = 0.48, p = 0.018) on the TSST day compared to the basal day indicating that the loss framing modification was robust in activating DHEA stress response overall on the TSST-VESP day. For the gain framing group, flatter recovery was shown (B = 0.16, p = 0.031) on the TSST-VESP day. These results suggest that the gain-framing and loss-framing incentives may impact DHEA differentially (see Figure 2).
Figure 2.
Original TSST and VESP-TSST group differences in DHEA responses
Was testosterone differentially responsive to the TSST and the modified versions of the TSST?
The initial base model indicated that 91% of the variance in testosterone was due to differences between individuals, and 9% of the variance was accounted for by momentary fluctuations within an individual [χ2(62) = 2694.76, p < 0.001]. We found steeper testosterone reactivity rises (B = 0.08; p < 0.001), higher testosterone peak levels (B = 0.27; p < 0.001), and steeper recovery declines (B = −0.25; p < 0.001) on TSST-day compared to the basal day. This indicated that testosterone was responsive to the social stressor with greater change in testosterone levels on the day of the TSST than on the basal day.
Focusing on the TSST-day, females had lower testosterone peak levels (B = −0.94; p < 0.001) and had steeper recovery (B = −0.12; p = 0.037) compared to males. Sex was included in all subsequent models as a control variable as a cross-level interaction (sex*testosterone peak and sex*testosterone recovery), but not the other control variables due to non-significant effects on base model results.
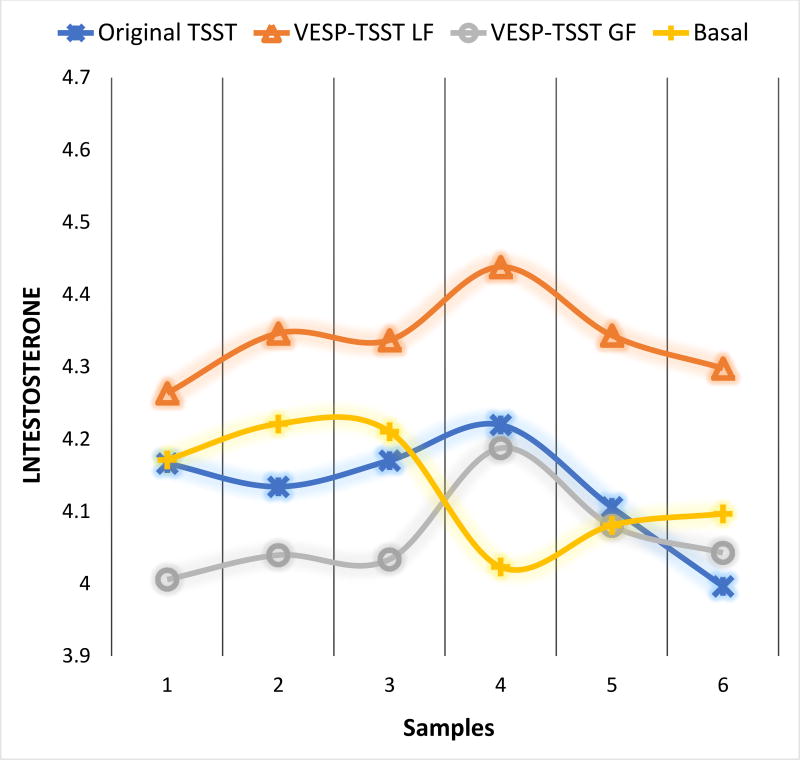
No significant differences in testosterone levels were found between the TSST-VESP compared to the original TSST group.
When we explored the specific incentive types within the TSST-VESP condition, we found differential testosterone responses to the loss framing and gain framing incentive description. Participants in the loss framing TSST-VESP group showed steeper testosterone reactivity rises (B = 0.09; p = 0.017) and steeper recovery declines (B = −0.11; p = 0.005) compared to other participants (see Figure 3). Participants in the gain framing TSST-VESP showed lower peak testosterone levels in response to the stressor (B = −0.33; p = 0.026) and a flatter recovery slope (B = 0.10; p = 0.024) compared to the other participants. Based upon these results, participants who received the VESP versions of the TSST were differentially responsive to the stressors than those in the original TSST.
Figure 3.
Original TSST and VESP-TSST group differences in testosterone responses
Was there dual-axis hormone coupling?
Cortisol as the outcome variable
In Coupling Model 1, cortisol was positively coupled with testosterone (B = 1.21, p < 0.001) and, in Coupling Model 2, with DHEA (B = 0.73, p < 0.001). These models suggested that for samples with higher testosterone or DHEA, respectively, also displayed higher cortisol. Following the “trivariate” model described by Marceau et al. (2015), when both testosterone and DHEA predicted cortisol (Coupling Model 3), both androgens persisted as significant predictors of cortisol levels (DHEA: B = 0.57, p < 0.001; testosterone: B = 0.66, p < 0.001). Cortisol’s reactivity base model was then re-introduced as Level 1 predictors, and we tested whether coupling between hormone samples was still apparent beyond the cortisol levels predicted by sample time alone. In combination of Marceau et al.’s (2015) Model 1 and this study’s base model (see Coupling Model 4 above and Table 3), we found that testosterone continued to be coupled with cortisol levels such that samples with elevated testosterone predicted elevated cortisol beyond the level predicted by reactivity and recovery alone. Similar results were observed for DHEA. The convergence of the two models allowed for us to examine whether testosterone or DHEA were driving the coupling with cortisol. Both DHEA and testosterone effects persisted as predictors of cortisol levels, although testosterone had a larger effect on cortisol (B = 0.58, p < 0.001) than DHEA (B = 0.34, p < 0.001), which indicated hormone coupling of the HPA and HPG axes.
Testosterone as the outcome variable
Switching testosterone as the predicted outcome (Coupling Models 1 and 2), results showed how cortisol (B = 0.28, p < 0.001) and DHEA (B = 0.36, p < 0.001) were coupled with testosterone release. In the trivariate model (or Coupling Model 3), DHEA maintained its strong positive coupling with testosterone level (B = 0.25, p < 0.001) and the testosterone-cortisol coupling was significant, though diminished (B = 0.15, p < 0.001). Results from Coupling Model 4 further showed that coupling persisted after controlling for testosterone stress reactivity in both bivariate and trivariate models, which indicated hormone coupling impacted the total hormone output above and beyond those who were reactive to the TSST or those with overall higher testosterone (see Table 3).
DHEA as the outcome variable
There was also a strong positive DHEA-cortisol association (Coupling Model 1: B = 0.47, p < 0.001) and especially strong coupling between DHEA-testosterone (Coupling Model 2: B = 0.98, p < 0.001). Trivariate correlations were also present with testosterone still showing a stronger association with DHEA (B = 0.54, p < 0.001) even accounting for cortisol effect (B = 0.32, p < 0.001). After accounting for DHEA reactivity, coupling with cortisol and testosterone (Coupling Model 4) in the trivariate model persisted (see Table 3). There was hormone coupling of DHEA with both cortisol and testosterone, and coupling appeared strongest for DHEA with testosterone.
Discussion
The present study adds to the extant literature about hormone response in three primary ways. First, all three hormones showed stress responsivity to the TSST. This was evident when we examined the reactivity rises from pre- to post-TSST, and also when we examined the TSST day compared to the basal samples taken on another day in a subset of participants (see Figures 1–3). Reactivity and recovery were expected for cortisol given the robust stress-reactivity literature and also that only 20% of the variance in cortisol was due to stable levels, and the rest of the variance showed fluctuations from moment-to-moment. Putatively, this study’s larger contribution is that the TSST was a salient stressor for the androgens DHEA and testosterone, despite the high level of stability in androgen levels within each individual (90% and 67% of the variance was stable for testosterone and DHEA, respectively). Second, the added delivery of social evaluative threat via verbal evaluation of speech performance mid-way through the TSST stimulated greater cortisol reactivity compared to the original TSST. Third, for all three hormones, it was the method in which the incentive was tied to speech performance which best altered responsivity. The present study showed that the manner in which the modification influenced reactivity and recovery were hormone-specific, which is suggestive of underlying psychosocial influences on each of these hormones. Below, we explored the influence of experimental modification to social evaluative threat and incentive, respectively, on these stress-reactive hormones.
The current study utilized the TSST to show that social evaluative threat can be enhanced with negative verbal feedback about speech performance and that it can be done mid-TSST. This finding on verbal feedback modifications to social evaluative threat is not without precedence (Dedovic et al., 2005) and, arguably, the TSST provides performance feedback about math errors. Other studies have explored experimental enhancements of social evaluative threat. Bosch et al. (2009) tested speech performance task in front of different sets of confederate judges’ presence: no judges, one judge, or four judges. The location or evaluative tone of the confederate judges completely changed social evaluative threat and concomitant physiological activation (Dickerson, Mycek, & Zaldivar, 2008; Het et al., 2009; Taylor et al., 2010; Wiemers, Schoofs, & Wolf, 2011; Wadiwalla et al., 2010). Findings from Andrews et al. (2007) confirmed that cognitive awareness of social evaluative threat, despite visual presence of confederate judges or in a separate room, still showed significant increases in cortisol above and beyond the TSST stressor. Others have found that the physical presence of the confederates is not required for participants to experience evaluative threat and stimulate cortisol reactivity (Westenberg et al, 2009; Kelly et al, 2007; Wadiwalla et al., 2010). Taken together, our study adds to a growing body of literature that supports the idea that cortisol is attuned to social information, and social evaluation or threat of judgment elicits a robust cortisol response. Our study is novel by showing social evaluative threat is salient enough that it magnifies a cortisol response even when negative verbal feedback is delivered verbally part-way through a challenging task. Findings do not mean to imply that our experiment should replace the TSST, but rather we hope the experimental modification helps the field further understand why the TSST works so effectively.
Very few studies have examined testosterone as reactive to the TSST (see Bedgood, Boggiano, & Turan, 2014; Turan et al., 2015 for exceptions). Acute testosterone rises are typically examined in the context of competition and challenge (Carre et al., 2013; Carre et al., 2014; Zilioli & Watson, 2013; Zilioli & Watson, 2014). In our study, the TSST-VESP did not readily appear to impact testosterone reactivity beyond the original TSST. Instead, testosterone reactivity and recovery from the challenge were more pronounced to the loss framing incentive instructions, and testosterone was consistently low within individuals who received the gain framing incentive instructions. We speculate, given the exploratory nature of these analyses, that the stressor must be viewed as an opportunity to receive a salient reward, and a possible threat to that reward may invoke a challenge response as testosterone is mechanistically tied with these social status threats and opportunities (Mehta, Jones, & Josephs, 2008; van Honk, Harmon-Jones, Morgan, & Schutter, 2010). Testosterone administration activates reward-related neurocircuitry, which enhance the individual’s motivation and attention towards potential rewards (Hermans, Ramsey, & van Honk, 2008). Other studies have found testosterone heightens vigilance to potential social status threats, decreases trust, and increases neural activation to signals of untrustworthiness (Bos, Hermans, Ramsey, & van Honk, 2012; Bos, Panksepp, Bluthe, & van Honk, 2012; Bos, Terburg, & van Honk, 2010; van Honk et al., 2000; Boksem et al., 2013). Thus, it seemed appropriate to extend the biopsychosocial model (Seery, 2011) and incorporate the challenge hypothesis (Archer, 2006; Wingfield et al., 1990; Wingfield, 2017) in order to view the TSST as a potential challenge, and to view testosterone as a biomarker that is sensitive to aspects of verbal performance evaluation, specifically when poor performance threatens loss of incentives or rewards. This may alter the individual’s evaluation of intrinsic resources and demands put onto those in the incentive conditions.
In accordance with testosterone responsivity to the TSST, even fewer studies have examined DHEA as stress responsive to the TSST. As with testosterone, we did not find that the TSST-VESP impacted DHEA overall. Instead, our exploration of the differential patterns for the gain framing or loss framing incentive instructions were apparent for DHEA, especially within the recovery phase of the stress response as well as differences on the TSST day compared to the samples collected on a basal day. The biopsychosocial model of challenge and threat may apply to the androgen DHEA in that the gain and loss framing instructions differentially impacted DHEA. We based the speculation that DHEA acted in line with reward cues largely on the literature on DHEA modulation of substance use seeking behaviors (Doron et al., 2006; Maayan et al., 2006), which tied this neurosteroid with reward-and motivation-related processes. Thus, while both DHEA and testosterone appeared stress-reactive, androgenic hormone rises may be driven by the motivation to complete a task and to successfully receive a performance-based incentive with the amount of intrinsic resources available to meet the expected demands. DHEA is both stress-responsive and an androgen (Doom, Cichetti, Rogosch, & Dackis, 2013; Eatough, Shirtcliff, Hanson, & Pollak, 2009; Han et al., 2015), and it may provide an important link between the two axes under conditions characterized as both challenging and stressful/threatening (Dismukes et al., 2014; Dismukes, Shirtcliff, Hanson, & Pollak, 2015). Increasingly, research is acknowledging the linkage between HPA and HPG axes lies in the mechanics of mediating processes within the systems, and DHEA may help shed light on those mechanics. There is substantial overlap of the hormonal cascades of the HPA and HPG from the top (e.g., emotion-related neurocircuitry including limbic system (Chichinadze & Chichinadze, 2008; Wingfield & Sapolsky, 2003; Hermans, Ramsey, & van Honk, 2007), down through the axes (Dismukes et al., 2015), and all the way to the target peripheral organs, such as the gonads and adrenals (Viau, 2002). Reactivity of both axes could indicate shared regulatory functions when the individual confronts a context, which is both stressful and challenging.
Adding to this interpretation, we briefly investigated which hormones jointly shared regulatory functions under social evaluative threat or were “coupled” within the individual. We found that when one hormone was elevated, the other hormones for that sample were also elevated. Positive coupling persisted even after accounting for reactivity. The closest study to ours to examine coupling in hormone reactivity across three stressors (Marceau et al., 2014) also found positive coupling, but differed slightly in that the HPA axis appeared to be driving coupling with androgens. Our study hinted that the HPG axis may have been driving positive coupling, in three ways. First, testosterone’s influence on cortisol release was comparatively stronger compared to cortisol’s influence on testosterone. Coupling even persisted in the “trivariate” model such that, for example, both androgens predicted cortisol levels independently. Second, the coupling between DHEA and testosterone was particularly strong, as though they are both behaving as androgens. Third, as described above, the mere observation of androgen reactivity to the TSST and its modifications demonstrates involvement of the HPG axis in momentary hormone changes. A parsimonious explanation of this coupling is that participants found the TSST (or its modification) to invoke a challenge more than a stressor response. Robust coupling across axes fits with the emerging dual-axis models (Marceau et al., 2015; Susman, Peckins, Bowes, & Dorn, 2017; Harden et al., 2016; Juster, Raymond, Desrochers, Lupien, 2016; Stephens, Mahon, McCaul, & Wand, 2016), and with an interpretation of the TSST as both a stressor and a challenge (Mehta, Jones, & Josephs, 2008; Mehta, Welker, Zilioli, & Carre, 2015; Denson, Mehta, & Tan, 2013; Salvador, 2005). It is more difficult to integrate with models that describe inhibition of one axis by the other (Viau, 2002; Koob & LeMoal, 2001), such as studies which describe androgens as a stress buffer, DHEA as an anti-glucocorticoid (Kalimi et al., 1994), or which find unique high testosterone/cortisol reactivity ratio findings (Glenn et al., 2011; Huovinen et al., 2009; Welker et al., 2014). Given that few studies have explored cortisol, DHEA, and testosterone coupling in acute reactivity settings, we are cautious about the extent to which we would expect the HPG axis to be driving hormone changes. Under prototypical stressful conditions like venipuncture, DHEA may, instead, counteract increases in cortisol to function as a buffer (Marceau et al., 2014).
Limitations and Future Directions
Some limitations are notable in the current study. First, although the TSST-VESP findings were statistically significant and consistent with expectations, sample size was a limitation, particularly when we explored gain and loss framing incentive instructions. Results interpreted from significance tests in HLM need to be used with caution since they may not be robust results, especially tests for random effects or covariance components (Sullivan, Dukes, and Losina, 1999). Nonetheless, Level 2 units were greater than thirty (N > 35) in all analyses that included covariates. A related limitation is that the TSST-VESP group assignment was randomly decided as a 3-to-1 ratio, leading to unequal group sizes. Inherent group differences in hormone responses were shown, despite randomly assigning participants to each condition.
Second, mid-task modification of the TSST through verbal feedback has novel strengths but also some weaknesses. Strengths include (a) being able to parse out reactivity to social evaluative threat compared to performance-based VESP; (b) showing discernable differences between gain or loss framing incentives; (c) showing reactivity to multiple hormones. Mid-task modifications, however, also introduced some weaknesses. Reactivity is statistically defined as a comparison pre-task to peak level, which encompasses a period of time before VESP was implemented; thus a reactivity rise may partially be a statistical artifact because VESP was implemented part-way through pre-to-peak changes. Alternatively, reactivity rises may have been larger if VESP was offered earlier in the reactivity period. It is interesting, then, that overall response patterns were still evident across all hormones when comparing gain and loss framing TSST-VESP groups despite the fact that half of the task was already completed in the speech portion of the TSST. Second, the TSST-VESP experimental manipulation employed mixed underlying mechanisms (i.e., social evaluative threat, incentivized motivated performance, gain framing or loss framing incentives) which can make interpretation challenging. Prior studies have shown that commonly utilized mixed tasks were operating from different underlying mechanisms, such as social versus achievement stress (Stroud et al., 2009; Wadiwalla et al., 2010), or psychosocial versus physical stress (Schwabe, Haddad, & Schachinger, 2008). Nonetheless, multiple manipulations are called for with stress tasks (e.g., uncontrollability, unpredictability, evaluative threat, achievement) in order to overwhelm the individual’s coping resources, and Wadiwalla et al. (2010) found specifically that modifications needed to be mixed in order to observe added impact of TSST manipulations. The TSST itself utilizes mixed underlying mechanisms (Kirschbaum, Pirke, & Hellhammer, 1993; Seery, 2011) and works best when it does so (Dickerson & Kemeny, 2004).
Third, one potential limitation is that VESP was only provided about the speech for a chance to improve performance during the math portion, but we did not further manipulate performance feedback about the math task. Future studies should determine if reactivity (or recovery post-stress) is further enhanced by math performance feedback in addition to standard prompts about incorrect math responses. We targeted manipulation about the speech component because of the inherent ambiguity (and thus uncontrollability) of speech performance, whereas math performance is objectively correct or incorrect. Lastly, while theories for cortisol and testosterone mechanisms were available in the literature, there were fewer guiding theories for DHEA. Empirically, there was indication that DHEA behaved somewhat as an androgen activated by incentive during the recovery period, yet DHEA differed from testosterone in its extended recovery and had some specificity to gain framing vs loss framing incentive instructions. Theories are needed for DHEA beyond describing its overlap with other hormones (van Hulle et al., 2015; Dismukes et al., 2014; Ruttle et al., 2015; Johnson et al., 2014).
The present findings have implications for future studies examining neurobiological mechanisms that could explain the processes of hormone-specific release to regulate behaviors associated with salient stressors. Our study fits with a growing literature that the dual-axis perspective of “coupling,” but more work is needed to mechanistically probe why the androgens are stress responsive. Future studies could perhaps address the dual role of the HPA and HPG axes (Mehta & Josephs, 2010) interacting to compensate for the differential patterns in responsivity, particularly with motivation to complete a stressful task through incentive conditions.
Conclusion
Although the TSST is well-characterized, this study adds incrementally to our understanding of how this social stressor impacts biopsychosocial processes. We showed that modifying social evaluative threat via negative verbal evaluation of speech performance within the confines of an already activated HPA axis still is capable of enhancing a stress response. Our interpretation about androgen reactivity broadly fits with a larger social neuroscience literature that has found that androgens are responsive to stressful contexts (Lee et al., 2015; Mehta, Jones, & Josephs, 2008; Sherman et al., 2015) but may be mechanistically tied with social status threats and opportunities (van Honk, Harmon-Jones, Morgan, & Schutter, 2010; van Honk et al., 2004), captured by the incentive manipulation, particularly the loss framing instructions. Time-sensitive modifications to stressors can inform scientists about the situational demands and motivations which influence neurobiological regulatory processes and add information about biopsychosocial processes.
Highlights.
Cortisol, testosterone and DHEA all showed TSST reactivity compared to basal levels
Verbal Evaluative Performance Feedback enhanced hormone reactivity to Social Stress
TSST modifications differentially affected cortisol, DHEA and testosterone reactivity
Gain- and loss-framing incentives impacted hormone reactivity, especially androgens
Within-person hormone coupling across TSST hinted at shared reactivity mechanisms
Acknowledgments
We thank the Stress Physiology Investigative Team (SPIT) for data collection, data entries, hormone assays, and writing assistance. This research was supported by K01 MH077687 (PI Shirtcliff) and a summer research program SCoRE award (PI Shirtcliff) from the Board of Regents of Louisiana.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews J, Wadiwalla M, Juster RP, Lord C, Lupien SJ, Pruessner JC. Effects of manipulating the amount of social-evaluative threat on the cortisol stress response in young healthy men. Behavioral Neuroscience. 2007;121(5):871. doi: 10.1037/0735-7044.121.5.871. http://dx.doi.org/10.1037/0735-7044.121.5.871. [DOI] [PubMed] [Google Scholar]
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neuroscience & Biobehavioral Reviews. 2006;30(3):319–345. doi: 10.1016/j.psyneuen.2014.05.007. 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Bedgood D, Boggiano MM, Turan B. Testosterone and social evaluative stress: the moderating role of basal cortisol. Psychoneuroendocrinology. 2014;47:107–115. doi: 10.1016/j.psyneuen.2014.05.007. doi. [DOI] [PubMed] [Google Scholar]
- Blascovich J. Challenge, threat, and health. Handbook of Motivation Science. 2008:481–493. [Google Scholar]
- Blascovich J, Tomaka J. The biopsychosocial model of arousal regulation. Advances in Experimental Social Psychology. 1996;28:1–51. [Google Scholar]
- Bobadilla L, Asberg K, Johnson M, Shirtcliff EA. Experiences in the military may impact dual-axis neuroendocrine processes in veterans. Developmental Psychobiology. 2015;57(6):719–730. doi: 10.1002/dev.21259. [DOI] [PubMed] [Google Scholar]
- Boksem MA, Mehta PH, Van den Bergh B, van Son V, Trautmann ST, Roelofs K, Sanfey AG. Testosterone inhibits trust but promotes reciprocity. Psychological Science. 2013;24(11):2306–2314. doi: 10.1177/0956797613495063. [DOI] [PubMed] [Google Scholar]
- Booth A, Granger DA, Mazur A, Kivlighan KT. Testosterone and social behavior. Social Forces. 2006;85(1):167–191. doi: 10.1353/sof.2006.0116. [DOI] [Google Scholar]
- Bos PA, Hermans EJ, Ramsey NF, van Honk J. The neural mechanisms by which testosterone acts on interpersonal trust. Neuroimage. 2012;61(3):730–737. doi: 10.1016/j.neuroimage.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluthé RM, Van Honk J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Frontiers in Neuroendocrinology. 2012;33(1):17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Bos PA, Terburg D, van Honk J. Testosterone decreases trust in socially naive humans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(22):9991–9995. doi: 10.1073/pnas.0911700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch JA, de Geus EJ, Carroll D, Goedhart AD, Anane LA, van Zanten JJV, Helmerhorst EJ, Edwards KM. A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosomatic Medicine. 2009;71(8):877. doi: 10.1097/PSY.0b013e3181baef05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ehlert U. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37(8):1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Carlström K, Brody S, Lunell NO, Lagrelius A, Möllerström G, Pousette Å, Rannevik G, Stege R, von Schoultz B. Dehydroepiandrosterone sulphate and dehydroepiandrosterone in serum: differences related to age and sex. Maturitas. 1988;10(4):297–306. doi: 10.1016/0378-5122(88)90065-5. [DOI] [PubMed] [Google Scholar]
- Carre JM, Campbell JA, Lozoya E, Goetz SM, Welker KM. Changes in testosterone mediate the effect of winning on subsequent aggressive behaviour. Psychoneuroendocrinology. 2013;38(10):2034–2041. doi: 10.1016/j.psyneuen.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Carre JM, Iselin AM, Welker KM, Hariri AR, Dodge KA. Testosterone reactivity to provocation mediates the effect of early intervention on aggressive behavior. Psychological Sciences. 2014;25(5):1140–1146. doi: 10.1177/0956797614525642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casto KV, Edwards DA. Testosterone, cortisol, and human competition. Hormones and Behavior. 2016;82:21–37. doi: 10.1016/j.yhbeh.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Jr, Vogelsong KM, Lu YC, Hudgens GA. Hormonal responses to psychological stress in men preparing for skydiving. The Journal of Clinical Endocrinology & Metabolism. 1997;82(8):2503–2509. doi: 10.1210/jc.82.8.2503. [DOI] [PubMed] [Google Scholar]
- Chichinadze K, Chichinadze N. Stress-induced increase of testosterone: contributions of social status and sympathetic reactivity. Physiology & Behavior. 2008;94(4):595–603. doi: 10.1016/j.physbeh.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47(3):864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience. 2005;30(5):319. [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Karst H, Joëls M. Corticosteroid hormones in the central stress response: quick-and-slow. Frontiers in Neuroendocrinology. 2008;29(2):268–272. doi: 10.1016/j.yfrne.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Denson TF, Mehta PH, Tan DH. Endogenous testosterone and cortisol jointly influence reactive aggression in women. Psychoneuroendocrinology. 2013;38(3):416–424. doi: 10.1016/j.psyneuen.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Mycek PJ, Zaldivar F. Negative social evaluation, but not mere social presence, elicits cortisol responses to a laboratory stressor task. Health Psychology. 2008;27(1):116. doi: 10.1037/0278-6133.27.1.116. [DOI] [PubMed] [Google Scholar]
- Dismukes AR, Johnson MM, Vitacco MJ, Iturri F, Shirtcliff EA. Coupling of the HPA and HPG axes in the context of early life adversity in incarcerated male adolescents. Developmental Psychobiology. 2014 doi: 10.1002/dev.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismukes AR, Shirtcliff EA, Hanson JL, Pollak SD. Context influences the interplay of endocrine axes across the day. Developmental Psychobiology. 2015;57(6):731–741. doi: 10.1002/dev.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom JR, Cicchetti D, Rogosch FA, Dackis MN. Child maltreatment and gender interactions as predictors of differential neuroendocrine profiles. Psychoneuroendocrinology. 2013;38(8):1442–1454. doi: 10.1016/j.psyneuen.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doron R, Fridman L, Gispan-Herman I, Maayan R, Weizman A, Yadid G. DHEA, a neurosteroid, decreases cocaine self-administration and reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2006;31(10):2231–2236. doi: 10.1038/sj.npp.1301013. [DOI] [PubMed] [Google Scholar]
- Drury SS, Shirtcliff EA, Shachet A, Phan JM, Mabile E, Brett ZH, Wren M, Esteves K, Theall KP. Growing up or growing old? Cellular aging linked with testosterone reactivity to stress in youth. The American Journal of the Medical Sciences. 2014;348(2):92–100. doi: 10.1097/MAJ.0000000000000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology. 2009;34:1242–1246. doi: 10.1016/j.psyneuen.2009.03.006. S0306-4530(09)00089-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch T, Stefano GB. Endogenous reward mechanisms and their importance in stress reduction, exercise and the brain. Archives of Medical Science. 2010;6(3):447–55. doi: 10.5114/aoms.2010.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Flügge G. Chronic social stress: effects on limbic brain structures. Physiology & Behavior. 2003;79(3):417–427. doi: 10.1016/S0031-9384(03)00161-6. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA, Gao Y, Granger DA. Increased testosterone-to-cortisol ratio in psychopathy. Journal of Abnormal Psychology. 2011;120(2):389. doi: 10.1037/a0021407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joëls M. Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Molecular and Cellular Endocrinology. 2012;350(2):299–309. doi: 10.1016/j.mce.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Kemeny ME, Aziz N, Fahey JL. Acute threat to the social self: shame, social self-esteem, and cortisol activity. Psychosomatic Medicine. 2004;66(6):915–924. doi: 10.1097/01.psy.0000143639.61693.ef. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Han G, Miller JG, Cole PM, Zahn-Waxler C, Hastings PD. Adolescents’ internalizing and externalizing problems predict their affect-specific HPA and HPG axes reactivity. Developmental Psychobiology. 2015 doi: 10.1002/dev.21268. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary- adrenal axis. Frontiers in Neuroendocrinology. 2014;35(2):197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Wrzus C, Luong G, Grotzinger A, Bajbouj M, Rauers A, Wagner G, Riediger M. Diurnal coupling between testosterone and cortisol from adolescence to older adulthood. Psychoneuroendocrinology. 2016;73:79–90. doi: 10.1016/j.psyneuen.2016.07.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Ruttle PL, Serbin LA, Mills RS, Stack DM, Schwartzman AE. Adrenocortical responses to strangers in preschoolers: Relations with parenting, temperament, and psychopathology. Developmental Psychobiology. 2011;53(7):694–710. doi: 10.1002/dev.20545. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Ramsey NF, van Honk J. Exogenous testosterone enhances responsiveness to social threat in the neural circuitry of social aggression in humans. Biological Psychiatry. 2008;63(3):263–270. doi: 10.1016/j.biopsych.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT. Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test’. Psychoneuroendocrinology. 2009;34(7):1075–1086. doi: 10.1016/j.psyneuen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Huovinen J, Tulppo M, Nissilä J, Linnamo V, Häkkinen K, Kyrolainen H. Relationship between heart rate variability and the serum testosterone-to-cortisol ratio during military service. European Journal of Sport Science. 2009;9(5):277–284. [Google Scholar]
- Johnson MM, Dismukes AR, Vitacco MJ, Breiman C, Fleury D, Shirtcliff EA. Psychopathy’s influence on the coupling between hypothalamic-pituitary-adrenal and-gonadal axes among incarcerated adolescents. Developmental Psychobiology. 2014;56(3):448–458. doi: 10.1002/dev.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, Raymond C, Desrochers AB, Bourdon O, Durand N, Wan N, Pruessner J, Lupien SJ. Sex hormones adjust “sex-specific” reactive and diurnal cortisol profiles. Psychoneuroendocrinology. 2016;63:282–290. doi: 10.1016/j.psyneuen.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA) Molecular and Cellular Biochemistry. 1994;131(2):99–104. doi: 10.1007/BF00925945. [DOI] [PubMed] [Google Scholar]
- Kelly O, Matheson K, Martinez A, Merali Z, Anisman H. Psychosocial stress evoked by a virtual audience: relation to neuroendocrine activity. CyberPsychology & Behavior. 2007;10(5):655–662. doi: 10.1089/cpb.2007.9973. [DOI] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33(4):517–529. doi: 10.1016/j.psyneuen.2008.01.010. S0306-4530(08)00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinner VL, Wolf OT, Merz CJ. Cortisol alters reward processing in the human brain. Hormones and Behavior. 2016;84:75–83. doi: 10.1016/j.yhbeh.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. http://dx.doi.org/10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Knight EL, Mehta PH. Hierarchy stability moderates the effect of status on stress and performance in humans. Proceedings of the National Academy of Sciences. 2017;114(1):78–83. doi: 10.1073/pnas.1609811114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C, Harmon-Jones E, Winkielman P. Ten years of research with the Trier Social Stress Test—revisited. Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior. 2007:56–83. [Google Scholar]
- Lee JJ, Gino F, Jin ES, Rice LK, Josephs RA. Hormones and ethics: Understanding the biological basis of unethical conduct. Journal of Experimental Psychology General. 2015;144(5):891–897. doi: 10.1037/xge0000099. [DOI] [PubMed] [Google Scholar]
- Lennartsson AK, Kushnir MM, Bergquist J, Jonsdottir IH. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biological Psychology. 2012;90(2):143–149. doi: 10.1016/j.biopsycho.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Maayan R, Lotan S, Doron R, Shabat-Simon M, Gispan-Herman I, Weizman A, Yadid G. Dehydroepiandrosterone (DHEA) attenuates cocaine-seeking behavior in the self-administration model in rats. European Neuropsychopharmacology. 2006;16(5):329–339. doi: 10.1016/j.euroneuro.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Frontiers in Neuroendocrinology. 2009;30(1):65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Ruttle PL, Shirtcliff EA, Hastings PD, Klimes-Dougan B, Zahn-Waxler C. Within-person coupling of changes in cortisol, testosterone, and DHEA across the day in adolescents. Developmental psychobiology. 2015;57(6):654–669. doi: 10.1002/dev.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Shirtcliff EA, Hastings PD, Klimes-Dougan B, Zahn-Waxler C, Dorn LD, Susman EJ. Within-adolescent coupled changes in cortisol with DHEA and testosterone in response to three stressors during adolescence. Psychoneuroendocrinology. 2014;41:33–45. doi: 10.1016/j.psyneuen.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behavioral and Brain Sciences. 1998;21(03):353–363. [PubMed] [Google Scholar]
- Mehta PH, Jones AC, Josephs RA. The social endocrinology of dominance: basal testosterone predicts cortisol changes and behavior following victory and defeat. Journal of Personality and Social Psychology. 2008;94(6):1078–1093. doi: 10.1037/0022-3514.94.6.1078. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Josephs RA. Testosterone change after losing predicts the decision to compete again. Hormones and Behavior. 2006;50(5):684–692. doi: 10.1016/j.yhbeh.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Josephs RA. Testosterone and cortisol jointly regulate dominance: Evidence for a dual-hormone hypothesis. Hormones and Behavior. 2010;58(5):898–906. doi: 10.1016/j.yhbeh.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Welker KM, Zilioli S, Carré JM. Testosterone and cortisol jointly modulate risk-taking. Psychoneuroendocrinology. 2015;56:88–99. doi: 10.1016/j.psyneuen.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Serbin LA, Stack DM, Schwartzman AE, Shirtcliff EA. Adrenocortical attunement in mother-child dyads: Importance of situational and behavioral characteristics. Biological Psychology. 2011;88(1):104–111. doi: 10.1016/j.biopsycho.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, Essex MJ. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Developmental Psychobiology. 2015;57(6):688–704. doi: 10.1002/dev.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador A. Coping with competitive situations in humans. Neuroscience & Biobehavioral Reviews. 2005;29(1):195–205. doi: 10.1016/j.neubiorev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions 1. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/er.21.1.55. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Wolf OT. Are salivary gonadal steroid concentrations influenced by acute psychosocial stress? A study using the Trier Social Stress Test (TSST) International Journal of Psychophysiology. 2011;80(1):36–43. doi: 10.1016/j.ijpsycho.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008 doi: 10.1016/j.psyneuen.2008.03.001. S0306-4530(08)00064-4. [DOI] [PubMed] [Google Scholar]
- Seery MD. Challenge or threat? Cardiovascular indexes of resilience and vulnerability to potential stress in humans. Neuroscience & Biobehavioral Reviews. 2011;35(7):1603–1610. doi: 10.1016/j.neubiorev.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Seery MD, Weisbuch M, Blascovich J. Something to gain, something to lose: The cardiovascular consequences of outcome framing. International Journal of Psychophysiology. 2009;73(3):308–312. doi: 10.1016/j.ijpsycho.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Sherman GD, Lerner JS, Josephs RA, Renshon J, Gross JJ. The interaction of testosterone and cortisol is associated with attained status in male executives. Journal of Personality Social Psychology. 2015 doi: 10.1037/pspp0000063. [DOI] [PubMed] [Google Scholar]
- Shirotsuki K, Izawa S, Sugaya N, Yamada KC, Ogawa N, Ouchi Y, Nagano Y, Nomura S. Salivary cortisol and DHEA reactivity to psychosocial stress in socially anxious males. International Journal of Psychophysiology. 2009;72(2):198–203. doi: 10.1016/j.ijpsycho.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dismukes AR, Marceau K, Ruttle PL, Simmons JG, Han G. A dual-axis approach to understanding neuroendocrine development. Developmental Psychobiology. 2015;57(6):643–653. doi: 10.1002/dev.21337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E, Zahn-Waxler C, Klimes-Dougan B, Slattery M. Salivary dehydroepiandrosterone responsiveness to social challenge in adolescents with internalizing problems. Journal of Child Psychology and Psychiatry. 2007;48(6):580–591. doi: 10.1111/j.1469-7610.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- Stárka L, Dušková M, Hill M. Dehydroepiandrosterone: a neuroactive steroid. The Journal of Steroid Biochemistry and Molecular Biology. 2015;145:254–260. doi: 10.1016/j.jsbmb.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Stephens MAC, Mahon PB, McCaul ME, Wand GS. Hypothalamic- pituitary-adrenal axis response to acute psychosocial stress: Effects of biological sex and circulating sex hormones. Psychoneuroendocrinology. 2016;66:47–55. doi: 10.1016/j.psyneuen.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21(1):47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šulcová J, Hill M, Hampl R, Starka L. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. Journal of Endocrinology. 1997;154(1):57–62. doi: 10.1677/joe.0.1540057. [DOI] [PubMed] [Google Scholar]
- Sullivan LM, Dukes KA, Losina E. Tutorial in biostatistics. An introduction to hierarchical linear modelling. Statistics in Medicine. 1999;18(7):855–888. doi: 10.1002/(SICI)1097-0258(19990415)18:7<855::AID-SIM117>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Peckins MK, Bowes JL, Dorn LD. Longitudinal synergies between cortisol reactivity and diurnal testosterone and antisocial behavior in young adolescents. Development and Psychopathology. 2017:1–17. doi: 10.1017/S0954579416001334. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Seeman TE, Eisenberger NI, Kozanian TA, Moore AN, Moons WG. Effects of a supportive or an unsupportive audience on biological and psychological responses to stress. Journal of Personality and Social Psychology. 2010;98(1):47. doi: 10.1037/a0016563. [DOI] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J, Kibler J, Ernst JM. Cognitive and physiological antecedents of threat and challenge appraisal. Journal of Personality and Social Psychology. 1997;73(1):63. doi: 10.1037//0022-3514.73.1.63. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory Data Analysis. Don Mills, Ontario: Addison-Wesley; 1997. [Google Scholar]
- Turan B, Tackett JL, Lechtreck MT, Browning WR. Coordination of the cortisol and testosterone responses: a dual axis approach to understanding the response to social status threats. Psychoneuroendocrinology. 2015;62:59–68. doi: 10.1016/j.psyneuen.2015.07.166. [DOI] [PubMed] [Google Scholar]
- Van Honk J, Harmon-Jones E, Morgan BE, Schutter DJ. Socially explosive minds: the triple imbalance hypothesis of reactive aggression. Journal of Personality. 2010;78(1):67–94. doi: 10.1111/j.1467-6494.2009.00609.x. [DOI] [PubMed] [Google Scholar]
- Van Honk J, Schutter DJ, Hermans EJ, Putman P, Tuiten A, Koppeschaar H. Testosterone shifts the balance between sensitivity for punishment and reward in healthy young women. Psychoneuroendocrinology. 2004;29(7):937–943. doi: 10.1016/j.psyneuen.2003.08.007. S0306453003001628 [pii] [DOI] [PubMed] [Google Scholar]
- Van Honk J, Tuiten A, van den Hout M, Koppeschaar H, Thijssen J, de Haan E, Verbaten R. Conscious and preconscious selective attention to social threat: different neuroendocrine response patterns. Psychoneuroendocrinology. 2000;25(6):577–591. doi: 10.1016/s0306-4530(00)00011-1. [DOI] [PubMed] [Google Scholar]
- Van Hulle CA, Moore MN, Shirtcliff EA, Lemery-Chalfant K, Goldsmith HH. Genetic and environmental contributions to covariation between dhea and testosterone in adolescent twins. Behavior Genetics. 2015;45(3):324–340. doi: 10.1007/s10519-015-9709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and-adrenal axes. Journal of Neuroendocrinology. 2002;14(6):506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Von Dawans B, Kirschbaum C, Heinrichs M. The Trier Social Stress Test for Groups (TSST-G): A new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology. 2011;36(4):514–522. doi: 10.1016/j.psyneuen.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Wadiwalla M, Andrews J, Lai B, Buss C, Lupien SJ, Pruessner JC. Effects of manipulating the amount of social-evaluative threat on the cortisol stress response in young healthy women. Stress. 2010;13(3):214–220. doi: 10.3109/10253890903277561. [DOI] [PubMed] [Google Scholar]
- Welker KM, Lozoya E, Campbell JA, Neumann CS, Carré JM. Testosterone, cortisol, and psychopathic traits in men and women. Physiology & Behavior. 2014;129:230–236. doi: 10.1016/j.physbeh.2014.02.057. [DOI] [PubMed] [Google Scholar]
- Westenberg PM, Bokhorst CL, Miers AC, Sumter SR, Kallen VL, van Pelt J, Blote AW. A prepared speech in front of a pre-recorded audience: subjective, physiological, and neuroendocrine responses to the Leiden Public Speaking Task. Biological Psychology. 2009;82(2):116–124. doi: 10.1016/j.biopsycho.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Wiemers US, Schoofs D, Wolf OT. A friendly version of the Trier Social Stress Test does not activate the HPA axis in healthy men and women. Stress. 2013;16(2):254–260. doi: 10.3109/10253890.2012.714427. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. The challenge hypothesis: where it began and relevance to humans. Hormones and Behavior. 2017;92:9–12. doi: 10.1016/j.yhbeh.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Jr, Ball GF. The” challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. The American Naturalist. 1990;136(6):829–846. [Google Scholar]
- Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: when and how. Journal of Neuroendocrinology. 2003;15(8):711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Wobber V, Hare B, Maboto J, Lipson S, Wrangham R, Ellison PT. Differential changes in steroid hormones before competition in bonobos and chimpanzees. Proceedings of the National Academy of Sciences. 2010;107(28):12457–12462. doi: 10.1073/pnas.1007411107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annual Review Clinical Psychology. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- Zilioli S, Watson NV. Winning isn’t everything: mood and testosterone regulate the cortisol response in competition. PLoS One. 2013;8(1):e52582. doi: 10.1371/journal.pone.0052582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilioli S, Watson NV. Testosterone across successive competitions: evidence for a ‘winner effect’ in humans? Psychoneuroendocrinology. 2014;47:1–9. doi: 10.1016/j.psyneuen.2014.05. [DOI] [PubMed] [Google Scholar]