Abstract
A rapidly emerging set of catalytic reactions involves intermediates that contain a copper-substituted stereogenic carbon centre. Here, we demonstrate that intimate understanding of this distinction provides ways for addressing limitations in reaction scope and explaining why unexpected variations in enantioselectivity often occur. By using catalytic enantioselective Cu–boryl addition to alkenes as the model process, we have been able to elucidate several key mechanistic principles. We show that higher electrophile concentration can lead to elevated enantioselectivity; this is because diastereoselective Cu–H elimination may be avoided and/or achiral Cu–boryl intermediates can be converted to allyl–B(pin) rather than add to an alkene. We illustrate that lower alkene amounts and/or higher chiral ligand concentration can minimize the deleterious influence of achiral Cu–alkyl species, resulting in improved enantiomeric ratios. Moreover, and surprisingly, we find that enantioselectivities are higher with the less reactive allylphenyl carbonates as chemoselective copper–hydride elimination is faster with an achiral Cu-alkyl species.
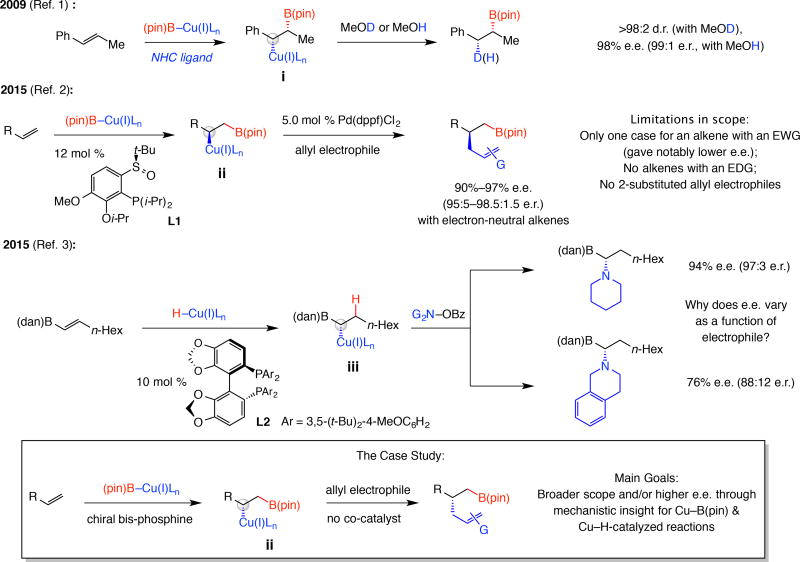
An early case of a transformation that proceeds via a compound that bears a Cu-substituted stereogenic centre entails addition of a Cu–B(pin) (pin, pinacolato) complex to an (E)-β-allkylstyrene (Fig. 1); the resulting Cu-alkyl species (i) then reacts with MeOH or MeOD in situ to give products in >98% e.e. (enantiomeric excess; >98:2 enantiomeric ratio or e.r.) and diastereomeric ratio (d.r.), respectively1. In such processes, the final e.e. depends on how stereoselectively an organometallic species is formed and by what mechanism the electrophile is trapped. Another example involves a chiral Cu complex along with an achiral bis-phosphine–Pd co-catalyst, and an allyl carbonate (via ii, Fig. 1)2. Generally, additions to aliphatic olefins are less efficient but aryl and heteroaryl olefins or alkenyl boronates and silanes are suitable, and Cu–C and/or C–B(pin) bonds can be further functionalized. Reactions that begin with enantioselective Cu–H addition (via iii)3 are a notable subset.
Figure 1. Key problems and the goals of this study.
Despite significant advances, notable questions remain unanswered. Cu–B(pin) addition to aryl alkenes is site- and syn-selective and the Cu-alkyl species can react in situ with an allyl electrophile. However, reactions require high ligand loading and a precious metal co-catalyst and scope is limited. Transformations involving Cu-H additions have also been developed, where enantioselectivities can vary widely, depending on electrophile identity despite organocopper formation being stereochemistry determining. With development of a catalytic site- and enantioselective boron-allyl additions as the model study, several key mechanistic issues will be examined that allow for scope of the reactions to be expanded considerably. NHC, N-heterocyclic carbene; Ln, ligands; e.r., enantiomeric ratio; pin, pinacolato; dppf, 1,1’-bis(diphenylphosphino)ferrocene; EWG, electron withdrawing group; EDG, electron donating group; dan, 1,8-diaminonaphthalene; Bz, benzoyl; G, functional group.
Despite numerous reports and notable advances, key shortcomings persist. In the disclosure corresponding to the two-catalyst protocol2 there is no mention of allyl–boron additions to electron-rich aryl alkenes or those involving more hindered (e.g., 2-substituted) electrophiles. Furthermore, enantioselective Cu–B(pin) or Cu–H additions with electron-deficient aryl olefins are either not mentioned4,5,6,7,8 or found to be less enantioselective9,10,11,12 (e.g., halo-, trifluoromethyl- or ester-substituted). It is unclear why enantioselectivity is lower with some substrates or at times depends on electrophile identity despite the fact that the Cu–B(pin)/Cu–H addition step is the stereochemistry-determining (e.g., 94% e.e. compared to 76% e.e., Fig. 1).
Electronic effects are central in these matters. Addition of a Cu–B(pin) or Cu–H complex to an electron-deficient alkene is faster1,13, but the resulting Cu-alkyl compound is probably less nucleophilic. Conversely, an electron-rich olefin likely generates a more reactive Cu-alkyl species slowly. It has been surmised9 that some kinetic enantioselectivity might be lost if an organocopper intermediate were to react slowly, whereas rapid trapping, for example with higher electrophile concentration, could improve e.e. The central question then is: exactly how does enantiomeric purity of a Cu-alkyl intermediate erode, and is the dearth of examples with strongly electron-rich and electron-deficient alkenes tied to these issues?
Results
We chose an enantioselective allyl–boron addition that would be promoted by a single Cu-based complex (i.e., no Pd-based co-catalyst) as the platform for this investigation. The lure of avoiding a precious metal notwithstanding, comparison of the one- versus two-catalyst approach would be more informative (e.g., does a co-catalyst help minimize enantioselectivity fluctuation?); the study would offer additional relevant data vis-à-vis Cu–H-catalyzed reactions14, which are also facilitated by one catalyst.
Efficient one-catalyst process
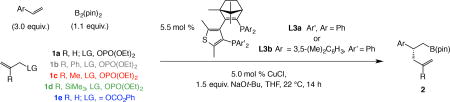
We used the transformation shown in entry 1, Table 1 to identify an appropriate chiral catalyst. NHC ligands and most phosphines were ineffective (e.g., <10% yield with L1 or L2, Fig. 1). The exceptions were L3a and L3b15, use of which led to the formation of 2a in 90% and 88% e.e., respectively. While, in this particular instance, e.e. was slightly lower compared to when the Cu/Pd regime2 was adopted (90% compared to 95% e.e.), this is a useful selectivity level, ligand loading was less (total of 5.5 as opposed to 17 mol %) and room temperature sufficed (rather than 0 °C). Still, our main goal was to expand the scope of the method through stronger appreciation of mechanistic details.
Table 1.
Scope of the catalytic process and the effect of alkene:electrophile ratio.
| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Ar; Electrophile | Alkene: Electrophile | Product | Conv. (%)*; Yield (%)† | e.e.§ |
| 1 | Ph; 1a | 3:1 | 2a | >98; 67 | 90 |
| 2 | o-MeOC6H4; 1a | 3:1 | 2b | >98; 55 | 90 |
| 3 | o-FC6H4; 1a | 3:1 | 2c | 98; 54 | 78 |
| 4 | o-F3CC6H4; 1a | 3:1 | 2d | >98; 46 | 66 |
| 5 | 2-naphthyl; 1a | 3:1 | 2e | >98; 65 | 78 |
| 6 | m-(pin)BC6H4; 1a | 3:1 | 2f | >98; 44 | 66 |
| 7 | m-tert-BuO2CC6H4; 1a | 3:1 | 2g | >98; 69 | 64 |
| 8 | p-MeOC6H4; 1a | 3:1 | 2h | >98; 28 | 94 |
| 9 | p-FC6H4; 1a | 3:1 | 2i | >98; 66 | 84 |
| 10 | p-(pin)BC6H4; 1a | 3:1 | 2j | >98; 48 | 64 |
| 11 | p-tert-BuO2CC6H4; 1a | 6:1 | 2k | 22; 14 | 2 |
| 12 | p-F3CC6H4; 1a | 3:1 | 2l | >98; 70 | 16 |
| 13 | 3-Boc-indolyl; 1a | 3:1 | 2m | >98; 58 | 96 |
|
| |||||
| 14 | 2-naphthyl; 1a | 1:3 | 2e | 80; 50 | 92 |
| 15 | m-(pin)BC6H4; 1a | 1:3 | 2f | 71; 52 | 93 |
| 16 | m-tert-BuO2CC6H4; 1a | 1:3 | 2g | 83; 62 | 90 |
| 17 | p-(pin)BC6H4; 1a | 1:3 | 2j | 66; 56 | 84 |
| 18 | p-tert-BuO2CC6H4; 1a | 1:3 | 2k | >98; 72 | 4 |
| 19 | p-F3CC6H4; 1a | 1:3 | 2l | >98; 79 | 34 |
|
| |||||
| 20 | Ph;
|
3:1 | 2n | >98; 60 | 80 |
| 21 | Ph;
|
1:3 | 2n | >98; 51 | 92 |
| 22 | Ph;

|
3:1 | 2o | 45; 29 | 80 |
| 23 | Ph;

|
1:6 | 2o | >98; 84 | 90 |
| 24 | Ph;

|
3:1 | 2p | >98; 63 | 90 |
| 25 | Ph;

|
1:3 | 2p | 88; 73 | 92 |
|
| |||||
| 26 |
o-FC6H4;

|
1:3 | 2c | 79; 64 | 92 |
| 27 |
o-F3CC6H4;

|
1:3 | 2d | 68; 50 | 92 |
| 28 |
p-FC6H4;

|
3:1 | 2i | >98; 65 | 96 |
| 29 |
p-F3CC6H4;

|
3:1 | 2l | >98; 68 | 92 |
Reactions were carried out under N2 atmosphere with L3a as the chiral ligand, except for L3b in the case of 2n, 2o and 2p).
Conv. determined by analysis of the 1H NMR spectra of the unpurified mixtures (±2%).
Yields are of isolated and purified product (±5%); differences between conv. and yield is due to allyl–B(pin) formation (excess alkene) or of proto-boryl addition products (excess allyl electrophile).
Enantiomeric excess (e.e.) determined by HPLC analysis (±1%). Experiments were performed at least in triplicate. See the Supplementary Information (Section 5) for experimental and analytical details. pin, pinacolato; Boc, tert-butoxycarbonyl.
Electronic properties of alkenes and e.e
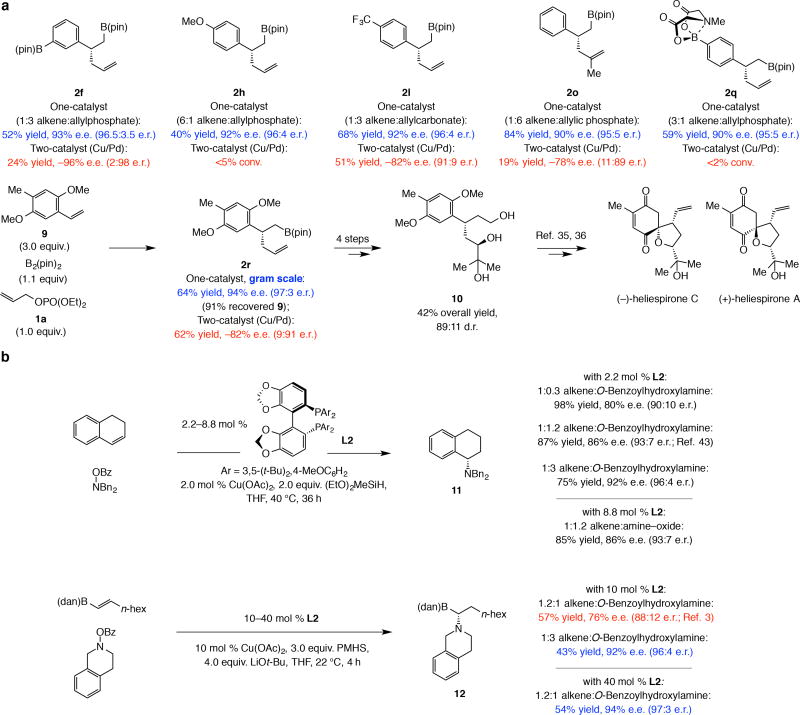
Organoboron products were in most instances obtained in ≥55% yield and ≥90% e.e. (Table 1; for additional examples, see the Supplementary Information, Section 5). As expected, there were several shortcomings: (1) Reactions with electron-deficient olefins were much less enantioselective. ortho-Trifluoromethyl 2d (entry 4) and meta-carboxylic ester styrene 2g (entry 7) were formed in 66% and 64% e.e., respectively, and para-ester- and trifluoromethyl-substituted 2k and 2l (entries 11–12) were generated in 2% and 16% e.e., respectively. The same was true with the Cu/Pd approach. For the sole reported example with a clearly electron-deficient alkene, 2l was formed in 82% e.e.2 (compared to 95% e.e. for 2a; more on this below); no rationale was provided for this significant selectivity gap. (2) para-Methoxy-substituted 2h (entry 8; not reported previously2) was obtained in 94% e.e. and 28% yield; the lower efficiency is probably because reaction of a Cu–B(pin) complex to a more electron-rich substrate is slower and its addition to an allylic phosphate [to give allyl–B(pin)]16,17 becomes the major side reaction (see Fig. 5a for optimal conditions). It merits note that the data regarding 2d, 2g, and 2k were not included in the disclosure on the Cu/Pd approach2 (the same for 2f–j and 2m–o, Table 1).
Figure 5. Better mechanistic understanding leads to broader scope.
The mechanistic knowledge obtained through this study allows for a broader scope. a, Without a Pd-based co-catalyst and by adjusting the alkene:electrophile ratio based on the structural attributes of the substrates, the scope of a catalytic process may be significantly broadened. This is illustrated by the representative examples shown here. Experiments were performed at least in triplicate. b, The impact of the findings resulting from the present study extend to transformations involving enantioselective Cu–H addition as the initial step. Thus, higher enantioselectivity, at times significant (e.g., 12 obtained in 92% vs. 76% e.e.) can be achieved by modifying the reaction conditions. Moreover, it is now easier to understand why higher catalyst loading makes little impact in reactions involving an electron rich alkene by have a more substantial effect when an electron-deficient olefin is involved. See the Supplementary Information, Sections 7 and 12, for the complete synthesis route and all experimental and analytical details. pin, pinacolato; Mes, 2,4,6-trimethylphenyl; dppf, 1,1’-bis(diphenylphosphino)ferrocene.
Conditions the same as those in Table 1. Conv. determined by analysis of the 1H NMR spectra of the unpurified mixtures (±2%). Yields are of isolated and purified product (±5%); differences between conv. and yield is due to allyl–B(pin) formation (excess alkene) or of proto-boryl addition products (excess allyl electrophile). Enantioselectivity determined by HPLC analysis (±1%). Experiments were performed at least in triplicate. See the Supplementary Information (Section 5) for details.
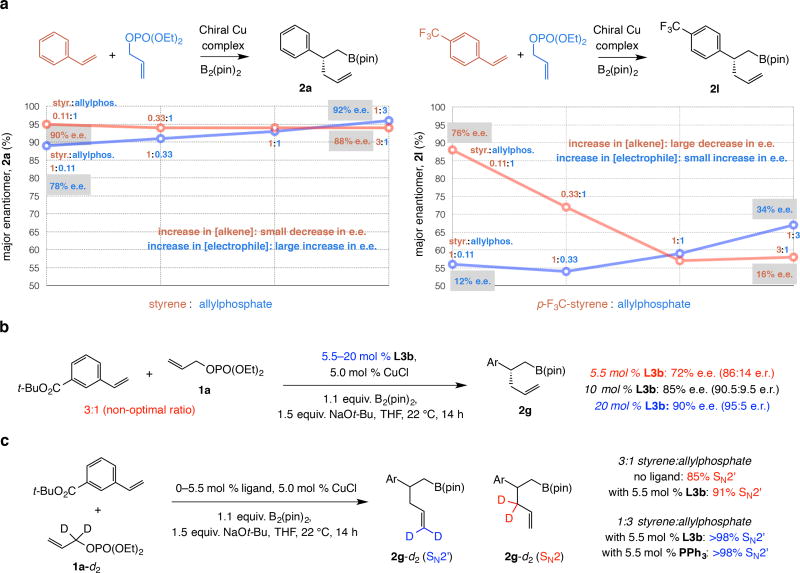
Alkene:electrophile ratio and e.e
Enantioselectivity variations might arise from a difference in kinetic selectivity in the Cu–B(pin) addition step, or it might be that a slower forming but more nucleophilic Cu-alkyl intermediate (see 2h, entry 8, Table 1) reacts faster so that the initial (and high) enantioselectivity is better preserved. The less nucleophilic Cu-alkyl species derived from electron-deficient alkenes, would react less readily and there could be more e.e. loss before C–C bond formation. In this latter scenario, higher allylphosphate concentration should lead to faster alkylation and the loss in kinetic selectivity would be diminished9. Indeed, whereas 2g was formed in 64% e.e. with a 3:1 aryl olefin:1a mixture (entry 7), when the ratio was reversed selectivity improved to 90% e.e. (entry 16). 2-Naphthyl-substituted 2e (92% e.e., entry 14 compared to 78% e.e., entry 5), meta-B(pin)-substituted 2f (93% e.e., entry 15 compared to 66% e.e., entry 6), and para-B(pin)-substituted 2j (84% e.e., entry 17 compared to 64% e.e., entry 10) were also generated with notably higher enantioselectivity. Therefore, the differences in kinetic selectivity in the initial Cu–B(pin) addition step are not the reason for the e.e. variations.
Nonetheless, we soon discovered that matters are more complex. On several occasions, increasing allylphosphate concentration did not improve e.e. For example, while the yield for 2k was much higher (72%, entry 18 compared to 14%, entry 11) there was little impact on its enantioselectivity or that of para-trifluoromethyl-substituted 2l (entries 12 and 19; see the Epimerization section for rationale). With para-methoxy-substituted 2h (entry 8) the Cu-alkyl species is exceedingly nucleophilic and reversing the alkene:electrophile ratio does not improve e.e.
Electrophile size and e.e
With a 2-substituted allylphosphate, Cu-alkyl trapping should be slower and enantioselectivity is expected to suffer. Indeed, transformations leading to 2n (entries 20–21) and 2o (entries 22–23), which contain 2-substituted alkenes, were more enantioselective when more electrophile was present (92% e.e. compared to 80% e.e. and 90% e.e. compared to 80% e.e.). With alkenylsilane 2p (entries 24–25) the same alteration was less consequential (see the Supplementary Information, Section 5, for analysis).
Advantage of the one-catalyst method
Under the Cu/Pd conditions, where higher electrophile concentration means increasing the amount of the allyl carbonate as well as the co-catalyst, e.e. could not be improved by adjusting electrophile and co-catalyst concentration (see the Supplementary Information, Section 13, for details). This might be because the presence of achiral bis-phosphine Pd species causes an achiral Cu–B(pin) complex to be generated by ligand exchange18, and the resulting non-enantioselective pathways offset any advantage that might result from a change in conditions.
Higher e.e. with a less reactive electrophile
Faster Cu-alkyl trapping is not the only way to obtain high enantioselectivity. Regardless of the alkene:electrophile ratio, use of allylphenyl carbonate (1e), shown to be less reactive than allylphosphate (see the Supplementary Information, Section 11)19,20, generally led to higher e.e. (compared to 1a; entries 26–29, Table 1). To counter the lower reactivity of 1e, larger amounts of this electrophile were used with the more electron-withdrawing aryl olefins 2d and 2l (less nucleophilic copper–alkyl intermediates). Thus, 2c, 2d, 2i, and 2l were obtained in 92–96% e.e. (entries 26–29, compared to 16%–84% e.e., entries 3, 4, 9 and 12 with 1a). With electron- neutral or electron-rich olefins use of allylphosphate was often preferred owing to better yields as opposed to higher e.e. (see the Supplementary Information, Sections 5 and 11, for details).
Mechanism
We then carried out labeling, spectroscopic and computational experiments to elucidate the basis of the reactivity and enantioselectivity trends. These studies revealed that while epimerization at the Cu-substituted stereogenic center have limited relevance, alkene concentration and Cu–H elimination have considerably broader influence.
Epimerization
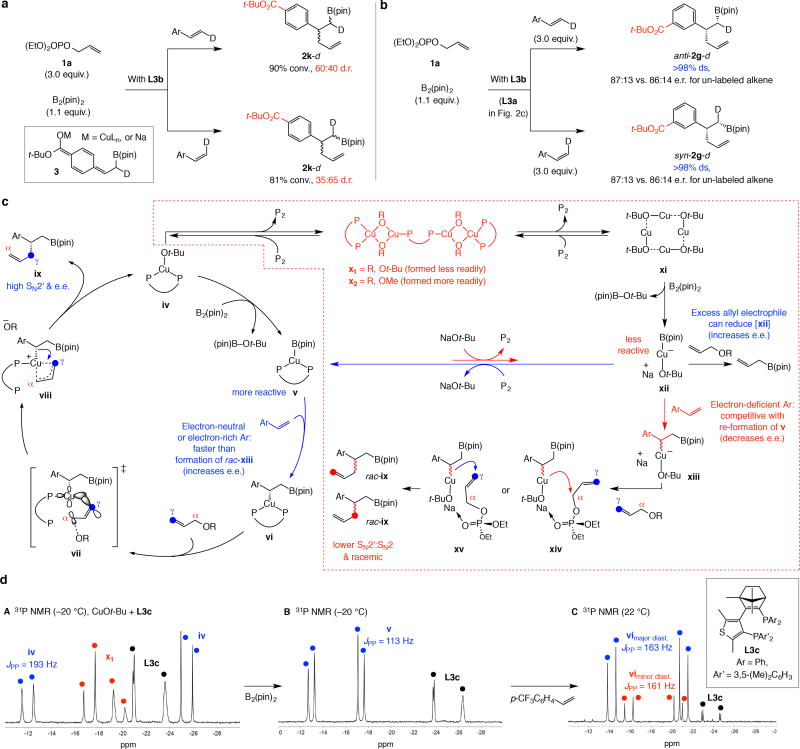
Reactions with (E)- and (Z)-β-deuterio-para-tert-butyl ester substituted styrene afforded 2k-d in low d.r. (Fig. 2a), indicating that epimerization is fast21. The same was observed with the Cu/Pd system2 (see the Supplementary Information, Section 13, for details). [For supporting “radical clock” experiments, see the Supplementary Information (Section 10)]. Changes in catalyst concentration did not impact e.e., showing that epimerization does not proceed via a bimetallic transition state22. The electron-deficient aryl unit probably stabilizes electron density at the benzylic site, facilitating heterolytic cleavage/re-formation of the Cu–C bond through epimerization via metal-enolate 3 (Fig. 2a). A para-ester-substituted aryl olefin was the only case where increasing electrophile concentration (Table 1, entries 11 and 18) or the use of allylphenyl carbonate did not enhance e.e. Loss of enantioselectivity is too facile in this particular case.
Figure 2. Labeling studies, the catalytic cycle and temporary loss of chiral ligand.
Labeling experiments shed critical light and it appears that at one point in the catalytic cycle the chiral ligand might dissociate from the Cu centre to cause lowering of e.e. a, Labeling experiments reveal that e.e. variations have different origins, depending on the nature of the aryl olefin involved. b, The most efficient catalytic cycle entails transition structure vii to afford ix with high enantioselectivity. However, a competitive pathway consistent with the experimental data involves dislodging the bis-phosphine ligand (iv →×→ xi) and formation of an achiral complex (xii) resulting in lower SN2’ selectivity and formation of rac-ix (via xiv or xv). c, Spectroscopic analysis (A–C, with L3c) reveals that there is considerable amount of free chiral ligand except at the Cu-alkyl stage of the catalytic cycle and at certain points competitive addition by an achiral Cu–B(pin) species can lead to lower SN2’ and enantioselectivity. Reducing the concentration of the alkene thus leads to higher e.e. See the Supplementary Information, Section 14, for details. pin, pinacolato; P, diaryl-phosphine ligand; P2, bis-phosphine ligand; R, PO(OEt)2 or CO2Ph; bis-phos., unbound bis-phosphine.
Temporary ligand loss at the Cu–alkoxide stage
Reactions with other mono-deuterated alkenes, such as those shown in Fig. 2b, were completely diastereospecific (>98% ds; see the Supplementary Information, Section 9, for details); again, similar results were obtained under the Cu/Pd conditions2. Thus, in most instances, diminution in e.r. does not arise from Cu–alkyl trapping with inversion of stereochemistry23 or Cu–C bond rupture24, as, otherwise, d.r. would be lower when labeled alkenes were used.
What might be behind loss of enantioselectivity? Why does increasing electrophile concentration does not lead to higher e.e. with the more strongly electron-deficient olefins [e.g., para-ester-substituted 2k (entries 11 and 18, Table 1) in contrast to para-trifluoromethylphenyl-substituted 2l (entries 12, 19 and 29)]? Could it be that in some cases, e.e. improves (e.g., 2g, entries 7 and 16, Table 1) by reversal of alkene:electrophile ratio because the alkene concentration is reduced and not simply as a result of higher electrophile concentration?
The most likely enantioselective route is shown in Fig. 2c: Conversion of chiral Cu-alkoxide iv to Cu–alkyl vi would deliver ix via transition structure vii, which in turn affords Cu-alkyl viii (more on this later); reductive elimination would then generate the final product ix. Spectroscopic studies show that the chiral ligand dissociates from the metal centre at the Cu–Ot-Bu stage. Subjection of Cu–Ot-Bu to 1.1 equivalents of L3c (spectrum A, Fig. 2d) generated an equal mixture of iv, the derived aggregates (e.g., x1), 30 mol % of unbound ligand and thus in all likelihood the well characterized copper-alkoxide, xi25 (Fig. 5a). Addition of B2(pin)2 yielded L3c–Cu complex v, which is stable enough for analysis at −20 °C (spectrum B). There was also ~30% unbound bis-phosphine ligand, the same amount present at the Cu–Ot-Bu stage; this indicates that there is no chiral ligand re-association upon Cu–B bond formation and that at this point achiral Cu–(pin) complex is available (see the Supplementary Information, Section 14, for spectroscopic analysis). Addition of para-CF3-styrene at −20 °C afforded vi in 99:1 d.r. (~75% conv.); when the mixture was allowed to warm to 22 °C (C, Fig. 2d) there was complete conversion and stereoselectivity was reduced to 72:28 d.r. with ~10% of un-bound L3c remaining, which is equal to the excess 0.1 equivalent used initially.
A Cu–alkoxide oxygen atom can form a bridge between transition metals and facilitate aggregation26,27,28, ligand dissociation, and generation of achiral Cu–B(pin) species. Accordingly, when bis-phosphine–Cu–Ot-Bu iv is re-generated ligand loss becomes problematic. Spectroscopic studies confirm that with excess chiral ligand the equilibrium shifts towards the bis-phosphine–Cu complex (see the Supplementary Information, Section 14, for details). An achiral Cu–B(pin) complex (xii) lacks the Lewis basic phosphine ligand, is less nucleophilic, and cannot readily react with an electron-rich alkene (xii → xiii). DFT calculations confirm that addition of an achiral Cu–B(pin) to an electron-poor olefin is more favourable and can be problematic (Fig. 4a; for full-system calculations, see the Supplementary Information, Section 18). Faster reaction between an achiral Cu–B(pin) complex and an electron-poor alkene suggests that olefin concentration must be kept low for higher e.e.; this allows ligand association to convert xii to v before Cu–B(pin) reacts with an alkene (Curtin–Hammett kinetics). This is unlike when an electron-neutral or electron-rich olefin is used where altering alkene concentration is largely inconsequential and only increasing the electrophile amount positively impacts e.e. The involvement of achiral Cu–B(pin) species (xii) explains why higher electrophile concentration elevates e.e. Increasing the amount of allylic phosphate facilitates conversion of chiral as well as achiral Cu–B(pin) complexes (v and xii) to allyl–B(pin) byproduct (Fig. 2c)16,17. However, the net effect would be more diminution in the concentration of achiral xii, which probably alkylates faster than bis-phosphine-containing v because, as supported by DFT calculations, in the chiral complex one arm of the bidentate ligand must first dissociate before allylic substitution can occur (see vi → vii → viii, Fig. 2c). Less Cu–alkyl intermediate xiii is thus formed and enantioselectivity improves. The following observations offer further clarification.
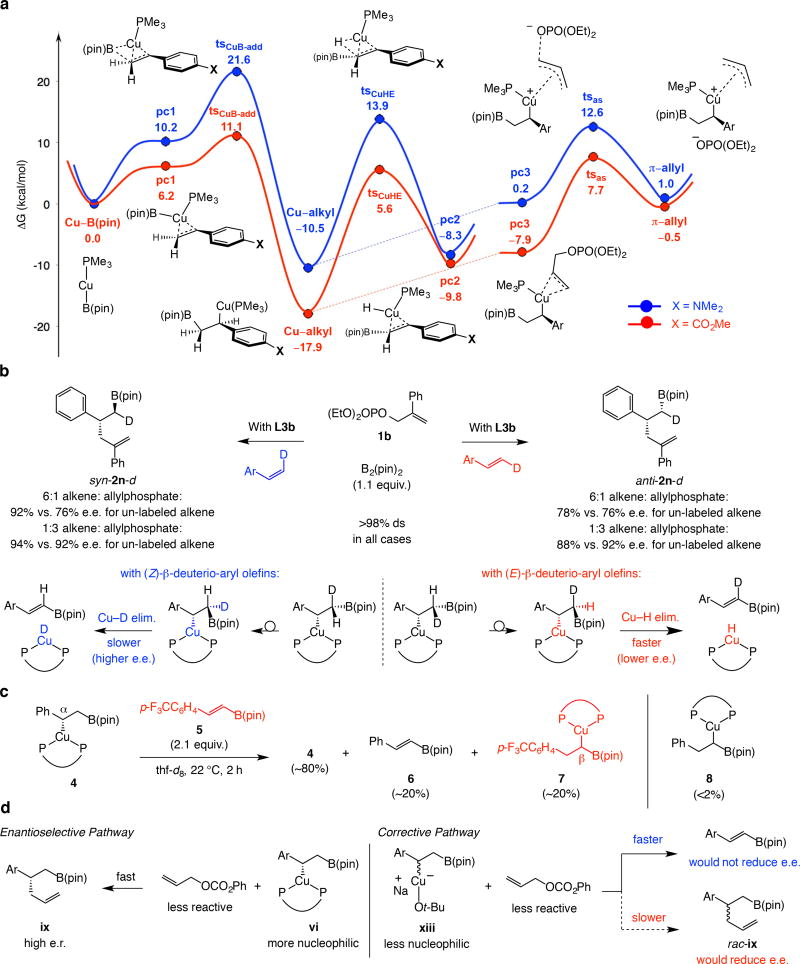
Figure 4. Different roles of Cu–H elimination.
Cu–H elimination can have a negative or a positive impact on e.e. a, DFT calculations (PMe3 as model ligand as monodentate complex alkylates) suggest that Cu–H elimination can be competitive with allylic substitution (Cu–alkyl → tsCHE → pc2 vs. Cu-alkyl → pc3 → tsas → π-alkyl). Cu–H elimination can thus impact e.e. when C–C bond formation is slower. These studies also show that Cu–B(pin) addition to an electron-deficient alkene is favored, leading to decrease in e.e. due to temporary chiral ligand dissociation (see Fig. 5). DFT calculations were performed at MN12SX/Def2TZVPP//wB97XD/Def2SVP level in THF (SMD; Solvation Model based on Density). b, Indeed, with a slower reacting 2-substituted allylic phosphate (with lower electrophile concentration) reaction with (Z)-β-deuterioalkene is more enantioselective, indicating that inhibiting Cu–H elimination can lead to higher e.e. In one D-labeled aryl olefin Cu–D elimination/re-addition is slower for (Z)-β-deuterioalkenes and less prone to loss of enantioselectivity. c, A cross-over experiment (with L3c) support the involvement of Cu–H elimination, also demonstrating that re-addition does not cause diminution in e.e. (i.e., β-Cu–C bond is formed). d, Since an achiral Cu–alkyl intermediate is less nucleophilic (xiii vs. vi) but might undergo Cu–H elimination readily, use of a less reactive electrophile can lead to higher e.r. See the Supplementary Information for details (for Fig. 6a, see Section 18; for Fig. 7b-c, see Sections 9 and 14). pin, pinacolato; pc, π complex; ts, transition state; B-add, boryl addition; CuHE, Cu–H elimination; as, allylic substitution.
Systematic study of concentration effect (i.e., changing only one substrate concentration at a time; Fig. 3a) indicated that while increasing electrophile amount can lead to higher e.e. with an electron-neutral styrene, variations in olefin concentration have stronger influence on reactions with a more electron-deficient olefin (90% to 88% e.e. for 2a compared to 76% to 16% e.e. for 2l; pathways shown in red). With excess alkene, addition of achiral Cu–B(pin) to the more reactive electron-deficient π-bond is faster than its association with the chiral ligand (xii → v) and there is further decrease in e.e.; above a certain point in olefin concentration (i.e., 1:1–3:1 alkene:allylphosphate, sequence shown in red) the adventitious pathway becomes sufficiently competitive such there is no longer a major change in enantioselectivity (non-Curtin-Hammett regime). The following data support the proposed scenario: 1) With 20 mol % L3b (Fig. 3b) 2g was obtained in 90% e.e. (compared to 72% e.e. at 5.5 mol % ligand and 3:1 alkene:allylphosphate). 2) With the smaller NaOMe (vs. NaOt-Bu), expected to bridge Cu centers and cause ligand dissociation more efficiently (x2 vs. x1, Fig. 2c), e.e. was lower (2g in 44% e.e. with NaOMe compared to 72% e.e. with NaOt-Bu and 5.5 mol % L3b).
Figure 3. Effect of olefin concentration on e.e. and SN2’ selectivity.
Lower alkene concentration and higher electrophile concentration can improve e.e. a, Changing the concentration of aryl olefin and/or allylic phosphate can impact e.e. With electron-neutral olefins the concentration of the allylic electrophile is mildly influential. However, with electron-deficient alkenes it is the olefin concentration that plays a major role. b, Higher chiral bis-phosphine loading leads to significant increase in e.e. See the Supplementary Information, Section 5, for experimental and analytical details. pin, pinacolato. c, Studies with D-labeled allylphosphate show that a phosphine ligand is needed for high SN2’selectivity and that selectivity is reduced when there is excess aryl olefin, implying competitive reaction by an achiral Cu–B(pin) species.
Fluctuations in SN2’ selectivity elucidate matters further. A bis-phosphine–Cu complex would deliver high SN2’ selectivity due to maximum overlap between the largest HOMO and LUMO coefficients in transition structure vii (Fig. 2c, dxy orbital and at Cγ, respectively)29. The Cu–alkyl bond in square planar viii is thus formed syn to Cγ, favoring the SN2’ mode of addition (ix). With an achiral alkyl-Cu complex (xiv–xv, Fig. 2c) SN2’ selectivity is lower because sodium ion chelation with the alkoxide oxygen30 and phosphate moiety directs the reaction. These scenarios are supported by DFT calculations (see the Supplementary Information, Section 18). In the reaction with meta-tert-butylester-substituted styrene and 1a-d2 (non-optimal conditions, 3:1 alkene:electrophile) without a ligand and with L3b present, SN2’:SN2 ratio was 85:15 and 91:9, respectively (Fig. 3c). In contrast, with optimal alkene:electrophile ratio (1:3) and a chiral bis-phosphine present, SN2’:SN2 ratio was >98:2. As long as alkene concentration was kept low, regardless of the ligand identity (L3b or PPh3), the SN2 product could not be detected.
The above findings show that for higher e.e., particularly with electron-deficient alkenes, it is more crucial to counter the deleterious effect of achiral Cu–B(pin) complex formation by lowering alkene concentration an/or increasing ligand loading. There was less increase in e.e. at higher electrophile concentration for products such as 2l because alkene concentration was still too high (unlike electron-neutral alkenes; see Table 1).
Cu–H elimination
Cu–H elimination is well-documented13,31,32, 33 and has been used in reaction development34. Through computational studies (Fig. 4a) we find that, especially with an electron-deficient alkene substrate (red vs. blue pathway), Cu–H elimination is able to compete with allylic substitution (Cu–alkyl → tsCuHE → pc2 vs. Cu–alkyl → pc3 → tsas → π-allyl). Below, we illustrate that such processes can adversely or favorably impact enantioselectivity, depending on the alkene and/or the electrophile involved.
Cu–H elimination can reduce e.e
The data in Fig. 4b show that adventitious Cu–H elimination is one reason why enantioselectivity is lower if Cu–alkyl trapping is slow (i.e., when excess alkene is used). With the unlabled alkene or E-β-deuteriostyrene there was only a small change in e.e., especially considering experimental error, irrespective of the relative amounts of styrene or its 2-phenyl-substituted variant, 1b. With excess Z-β-deuteriostyrene, on the other hand, syn-2n-d was generated in notably higher e.e. compared to when un-labeled styrene was used (92% vs. 76%). Thus, while reaction for the Z isomer would either involve a slower Cu–D elimination (primary isotope effect) or Cu–H elimination via a sterically hindered intermediate (eclipsing Ar and B(pin)), β-hydride elimination can proceed readily with the E isomer (Fig. 4b).
Additional insight was gained by spectroscopic studies (Fig. 4c). Treatment of a sample of Cu-alkyl complex 4 (82:18 d.r.) with para-trifluoromethyl alkenyl–B(pin) (5) afforded ~20% alkenyl–B(pin) 6 and isomeric species 7 (22 °C, 2 h; see the Supplementary Information, Section 14). Hence, Cu–H elimination converts 4 to 6 and the metal–hydride complex generated adds preferentially to the more electrophilic alkene (5 vs. styrene; <2% 8).
Because of the polarity reversal in the olefin in alkenyl–B(pin) 5 [vs. an aryl olefin or phenyl alkenyl–B(pin)] Cu–H re-addition yields a homobenzylic Cu–C bond13. This indicates that, under unoptimal conditions (excess alkene vs. allyl electrophile), lowering of e.e. does not originate from Cu–H re-addition with the same regioselectivity but on the opposite enantiotopic face of the alkenyl–B(pin) (see the Supplementary Information, Section 18, for DFT studies). Instead, diminished enantioselectivity might be attributed to the major Cu-alkyl diastereomer undergoing faster Cu–H elimination. At higher electrophile concentration, Cu-alkyl trapping can compete better with diastereoselective Cu–H elimination and e.e. improves. It would be difficult to anticipate to what extent and how much faster one isomer might undergo Cu–H elimination. What is key is the feasibility of Cu–H elimination, which, as will be shown below, has a more general influence on product enantiopurity.
High e.e. due to Cu–H elimination
Although counter-intuitive, use of the less reactive allylphenyl carbonate Cu–H elimination gives rise to higher e.e. One clue to the reason for this effect was the larger amounts of alkenyl–B(pin) formed in reactions with allylphenyl carbonate (~40% compared to <5% with allylphosphate without a chiral bis-phosphine, and ~10% vs. ~2% with allylphosphate under catalytic enantioselective conditions). Unlike when an allylic phosphate is involved (see above), there is minimal trapping of the achiral Cu–B(pin) species (xiii, Fig. 4d, namely, insignificant formation of allyl–B(pin) byproduct).
The racemic pathway may be circumvented by chemoselective Cu–H elimination of an achiral Cu-alkyl intermediate (vs. the derived bis-phosphine complex). This is for the two reasons. 1) A bis-phosphine–Cu-alkyl species is less prone to undergo Cu–H elimination than its unbound, achiral variant32. 2) With the less reactive carbonate and under the more dilute catalytic conditions, intramolecular Cu–H elimination in xiii (Fig. 4d) is bound to be faster compared to intermolecular events, such as allylic substitution (to give rac-ix), or re-association with the chiral ligand (xiii → rac-vi). Thus, especially for 2d and 2l, e.e. is high only when carbonate 1e is used (Table 1, entries 26–29). The adverse effect of Cu–H elimination when Cu-alkyl trapping is slow is applicable here (see Fig. 4c), but the ability of the same process to block an achiral Cu-alkyl complex to generate racemic products appears to be the dominant factor.
Broader scope
A rational platform is now available for achieving broader applicability. The cases in Fig. 5a are illustrative; except for 2l, none were previously reported under the two-catalyst conditions2. With relatively electron-rich substrates (e.g., 2h or 2r), where Cu–alkyl formation is more sluggish, higher alkene concentration led to high yield and e.e. The positive effect of a less reactive electrophile in reactions with a strongly electron-deficient alkene is underscored by the improved yield and e.e. for 2l. When electron-neutral styrene is used (e.g., 2o), larger amounts of allylic phosphate reduce the possibility of diastereoselective Cu–H elimination and lower the concentration of achiral Cu–B(pin), resulting in higher e.e. Gram-scale synthesis of 2r proceeded in higher enantioselectivity (94% e.e. compared to 82% e.e. with the two-catalyst method). Diol 10, applicable to synthesis of heliespirones A and C35,36, was prepared from 2r in four steps and 42% overall yield [89:11 d.r.; see the Supplementary Information, Section 7, for details]. Unreacted 9 could be recovered easily (91% yield). Compounds 2r or 10 cannot be accessed by enantioselective hydroboration37,38,39,40,41 or conjugate addition of an aryl or a prenyl group to an enoate42.
Relevance to Cu–H-catalyzed processes
Reactions with electronic-neutral dihydronaphthalene and electron-deficient alkenyl–B(dan) were investigated to see if the abovementioned principles apply to Cu–H additions as well (Fig. 5b). With dihydronaphthalene6, increasing the hydroxyamine concentration led to improvement in enantioselectivity (11 from 80% to 92% e.e.); similarly, in the reaction with alkenyl–B(dan)3 there was significant increase in e.e. when larger electrophile amounts were present (from 76% to 92% e.e.). However, based on the above studies, with the more electron-deficient alkenyl–B(dan), e.e. variations are more likely caused by lesser contribution by adventitious addition of an achiral Cu–H complex. This is supported by the distinct way by which increased ligand loading impacts these reactions: with dihydronaphthalene there was no change in e.e. when 2.0 or 8.0 mol % L2 was used, but 12 was generated in 94% e.e. compared to 76% e.e. with four-fold lower catalyst loading. Only in the latter instance competitive addition by an achiral Cu–B(pin) complex is problematic and a shift in equilibrium away from the achiral Cu–H complex becomes consequential.
Conclusions
These investigations shed light on several factors that directly impact the efficiency and enantioselectivity of a rapidly developing class of transformations, offering cogent strategies for maximizing efficiency and/or enantioselectivity. The study reveals that enantioselectivity increases with higher electrophile concentration due to minimization of diastereoselective Cu–H elimination within the major chiral Cu-alkyl intermediate and largely for reactions with electron-neutral or -rich alkenes (Table 1). There are other notable (and less expected) findings. One is that because a bis-phosphine–Cu–alkoxide species is especially vulnerable to ligand loss, the resulting achiral Cu–B(pin) or Cu–H complex can lead to lower e.e. (Fig. 2); a consequence is that lower alkene concentration can enhance enantioselectivity when electron-deficient olefin alkenes are involved (Fig. 3). Another discovery is that Cu–H elimination may elevate e.e. by channeling racemic pathways towards formation of other byproducts (Fig. 4). Thus, product enantio-purity may be improved when a less reactive electrophile is employed – a surprising twist considering that in certain cases e.e. is boosted by faster Cu-alkyl trapping (Table 1). As highlighted by the representative applications in Cu–H-catalyzed processes (Fig. 5b), the newly acquired understanding and its strategic implications are likely to be instrumental in the success of future endeavors in this area.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institutes of Health (GM-47480) and the National Science Foundation (CHE-1362763). J. d. P. is an Alfonso Martin Escudero Foundation postdoctoral fellow. We are grateful to Professor M. Miura, Dr. K. Hirano and Mr. D. Nishikawa (University of Osaka) for their generous assistance in measuring enantioselectivity for the formation of compound 16. We thank F. Romiti and Y. Shi for helpful discussions.
Footnotes
Data availability
X-ray crystallographic data for compound 38 (see the Supporting Information) is freely available from the Cambridge Crystallographic Data Centre (CCDC 1547732).
Author Contributions
J. L. and S. R. identified the optimal catalyst and conditions, developed the catalytic enantioselective transformations and performed the labeling and related experiments. S. T. and J. d. P. designed and performed the computational and spectroscopic studies, respectively, and developed the related mechanistic hypotheses. A. H. H. directed the investigations and composed the manuscript with revisions provided by the other authors.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Lee Y, Hoveyda AH. Efficient boron–copper additions to aryl-substituted alkenes promoted by NHC-based catalysts. Enantioselective Cu-catalyzed hydroboration reactions. J. Am. Chem. Soc. 2009;131:3160–3161. doi: 10.1021/ja809382c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jia T, et al. A Cu/Pd cooperative catalysis for enantioselective allylboration of alkenes. J. Am. Chem. Soc. 2015;137:13760–13763. doi: 10.1021/jacs.5b09146. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa D, Hirano K, Miura M. Asymmetric synthesis of α-aminoboronic acid derivatives by copper-catalyzed enantioselective hydroamination. J. Am. Chem. Soc. 2015;137:15620–15623. doi: 10.1021/jacs.5b09773. [DOI] [PubMed] [Google Scholar]
- 4.Noh D, Chea H, Ju J, Yun J. Highly regio- and enantioselective copper-catalyzed hydroboration of styrenes. Angew. Chem. Int. Ed. 2009;48:6062–6064. doi: 10.1002/anie.200902015. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda N, Hirano K, Satoh T, Miura M. Regioselective and stereospecific copper-catalyzed aminoboration of styrenes with bis(pinacolato)diboron and O-benzoyl-N,N-dialkylhydroxylamines. J. Am. Chem. Soc. 2013;135:4934–4937. doi: 10.1021/ja4007645. [DOI] [PubMed] [Google Scholar]
- 6.Zhu S, Niljianskul N, Buchwald SL. Enantio- and regioselective CuH-catalyzed hydroamination of alkenes. J. Am. Chem. Soc. 2013;135:15746–15749. doi: 10.1021/ja4092819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi SL, Buchwald SL. Copper-catalysed selective hydroamination reactions of alkynes. Nat. Chem. 2015;7:38–44. doi: 10.1038/nchem.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logan KM, Brown MK. Catalytic enantioselective arylboration of alkenylarenes. Angew. Chem. Int. Ed. 2017;56:851–855. doi: 10.1002/anie.201609844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gribble MW, Pirnot MT, Bandar JS, Liu RY, Buchwald SL. Asymmetric copper hydride-catalyzed Markovnikov hydrosilylation of vinylarenes and vinyl heterocycles. J. Am. Chem. Soc. 2017;139:2192–2195. doi: 10.1021/jacs.6b13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandar JS, Pirnot MT, Buchwald SL. Mechanistic studies lead to dramatically improved reaction conditions for the Cu-catalyzed asymmetric hydroamination of olefins. J. Am. Chem. Soc. 2015;137:14812–14818. doi: 10.1021/jacs.5b10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friis SD, Pirnot MT, Buchwald SL. Asymmetric hydroarylation of vinylarenes using a synergistic combination of CuH and Pd catalysis. J. Am. Chem. Soc. 2016;138:8372–8375. doi: 10.1021/jacs.6b04566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bandar JS, Ascic E, Buchwald SL. Enantioselective CuH-catalyzed reductive coupling of aryl alkenes and activated carboxylic acids. J. Am. Chem. Soc. 2016;138:5821–5824. doi: 10.1021/jacs.6b03086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laitar DS, Tsui EY, Sadighi JP. Copper(I) β-boroalkyls from alkene insertion: Isolation and rearrangement. Organometallics. 2006;25:2405–2408. [Google Scholar]
- 14.Wang Y-M, Buchwald SL. Enantioselective CuH-catalyzed hydroallylation of vinylarenes. J. Am. Chem. Soc. 2016;138:5024–5027. doi: 10.1021/jacs.6b02527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadyrov R, Iladinov IZ, Almena J, Monsees A, Riermeier TH. Chiral diphosphine ligands based on camphor: synthesis and applications in asymmetric hydrogenations. Tetrahedron Lett. 2005;46:7397–7400. [Google Scholar]
- 16.Guzman-Martinez A, Hoveyda AH. Enantioselective synthesis of allylboronates bearing a tertiary or quaternary B-substituted stereogenic carbon by NHC–Cu-catalyzed substitution reactions. J. Am. Chem. Soc. 2010;132:10634–10637. doi: 10.1021/ja104254d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito H, Ito S, Sasaki Y, Matsuura K, Sawamura M. Copper-catalyzed enantioselective substitution of allylic carbonates with diboron: An efficient route to optically active α-chiral allylboronates. J. Am. Chem. Soc. 2007;129:14856–14857. doi: 10.1021/ja076634o. [DOI] [PubMed] [Google Scholar]
- 18.delPozo J, Casares JA, Espinet P. The decisive role of ligand metathesis in Au/Pd bimetallic catalysis. Chem. Sci. 2013;49:7246–7248. doi: 10.1039/c3cc43133a. [DOI] [PubMed] [Google Scholar]
- 19.Zhong C, Kunii S, Kosaka Y, Sawamura M, Ito H. Enantioselective synthesis of trans-aryl- and -heteroaryl-substituted cyclopropylboronates by copper(I)-catalyzed reactions of allylic phosphates with a boron derivative (Supporting Information, Section 3,) J. Am. Chem. Soc. 2010;132:11440–11442. doi: 10.1021/ja103783p. [DOI] [PubMed] [Google Scholar]
- 20.Bayer A, Kazmaier U. [(p-Cymene)RuCl2]2: An efficient catalyst for highly regioselective allylic alkylations of chelated amino acid ester enolates. Chem. Eur. J. 2014;20:10484–10491. doi: 10.1002/chem.201402825. [DOI] [PubMed] [Google Scholar]
- 21.Maity P, Shacklady-McAtee DM, Yap GPA, Sirianni ER, Watson MP. Nickel-catalyzed cross coupling of benzylic ammonium salts and boronic acids: Stereospecific formation of diarylethanes via C–N bond activation. J. Am. Chem. Soc. 2013;135:280–285. doi: 10.1021/ja3089422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suess AM, Uehling MR, Kaminsky W, Lalic G. Mechanism of copper-catalyzed hydroalkylation of alkynes: An unexpected role of dinuclear copper complexes. J. Am. Chem. Soc. 2015;137:7747–7753. doi: 10.1021/jacs.5b03086. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Perry IB, Buchwald SL. Copper-catalyzed enantioselective addition of styrene-derived nucleophiles to imines enabled by ligand-controlled chemoselective hydrocupration. J. Am. Chem. Soc. 2016;138:9787–9790. doi: 10.1021/jacs.6b06299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitesides GM, Panek EJ, Stedronsky ER. Radical intermediates in the thermal decomposition of neophyl(tri-n-butylphosphine)copper(I) and neophyl(tri-n-butylphosphinesilver(I) J. Am. Chem. Soc. 1972;94:232–239. [Google Scholar]
- 25.Greiser T, Weiss E. Kristallstruktur des kupfer(I)-tert-butoxids, [(CH3)3COCu]4. Chem. Ber. 1976;109:3142–3146. [Google Scholar]
- 26.Lemmen TH, Goeden GV, Huffman JC, Geerts RL, Caulton KG. Alcohol elimination chemistry of tetrakis(tert-butoxocopper) Inorg. Chem. 1990;29:3680–3685. [Google Scholar]
- 27.Dubinina GG, Furutachi H, Vicic DA. Active trifluoromethylating agents from well-defined copper(I)–CF3 complexes. J. Am. Chem. Soc. 2008;130:8600–8601. doi: 10.1021/ja802946s. [DOI] [PubMed] [Google Scholar]
- 28.Bradley DC, Mehrotra RC, Rothwell IP, Singh A. Alkoxo and Aryloxo Metal Derivatives. Elsevier; 2001. pp. 329–332. [Google Scholar]
- 29.Yoshikai N, Nakamura E. Mechanisms of nucleophilic organocopper(I) reactions. Chem. Rev. 2012;112:2339–2372. doi: 10.1021/cr200241f. [DOI] [PubMed] [Google Scholar]
- 30.Konovalov AI, Benet-Buchholz J, Martin E, Grushin VV. The critical effect of the counterion in the direct cupration of fluoroform with [Cu(OR)2]−. Angew. Chem. Int. Ed. 2013;52:11637–11641. doi: 10.1002/anie.201306272. [DOI] [PubMed] [Google Scholar]
- 31.Whitesides GM, Stedronsky ER, Casey CP, San Filippo J., Jr Mechanism of decomposition of nbutyl) tri-n-butylphosphine)copper(I) J. Am. Chem. Soc. 1970;92:1426–1427. [Google Scholar]
- 32.Miyashita A, Yamamoto T, Yamamoto A. Thermal stability of alkylcopper(I) complexes coordinated with tertiary phosphines. Bull. Chem. Soc. Jpn. 1977;50:1109–1117. [Google Scholar]
- 33.Van Koten G, Noltes JG. In: Comprehensive Organometallic Chemistry. The Synthesis, Reactions and Structures of Organometallics Compounds. Wilkinbson G, Stone FGA, Abel EW, editors. Vol. 2. Pergamon; 1982. p. 746. [Google Scholar]
- 34.Mazzacano TJ, Mankad NP. Dehydrogenative borylation and silylation of styrenes catalyzed by copper carbenes. ACS Catal. 2017;7:146–149. [Google Scholar]
- 35.Huang C, Liu B. Asymmetric total synthesis of ent-heliespirones A & C. Chem. Commun. 2010;46:5280–5282. doi: 10.1039/c0cc00481b. [DOI] [PubMed] [Google Scholar]
- 36.Bai W-J, Green JC, Pettus TRR. Total syntheses of ent-Heliespirones A and C. J. Org. Chem. 2012;77:379–387. doi: 10.1021/jo201971g. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez AZ, et al. 9-Borabicyclo[3.3.2]decanes and the asymmetric hydroboration of 1,1-disubstituted alkenes. J. Am. Chem. Soc. 2008;130:9218–9219. doi: 10.1021/ja803119p. [DOI] [PubMed] [Google Scholar]
- 38.Thomas SP, Aggarwal VK. Asymmetric hydroboration of 1,1-disubstituted alkenes. Angew. Chem. Int. Ed. 2009;48:1896–1898. doi: 10.1002/anie.200805604. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Zuo Z, Wan X, Huang Z. Cobalt-catalyzed enantioselective hydroboration of 1,1-disubstituted aryl alkenes. J. Am. Chem. Soc. 2014;136:15501–15504. doi: 10.1021/ja5093908. [DOI] [PubMed] [Google Scholar]
- 40.Mazet C, Gérard D. Highly regio- and enantioselective catalytic asymmetric hydroboration of α-substituted styrenyl derivatives. Chem. Commun. 2011;47:298–300. doi: 10.1039/c0cc01547d. [DOI] [PubMed] [Google Scholar]
- 41.Corberán R, Mszar NW, Hoveyda AH. NHC-Cu-catalyzed enantioselective hydroboration of acyclic and exocyclic 1,1-disubstituted aryl alkenes. Angew. Chem. Int. Ed. 2011;50:7079–7082. doi: 10.1002/anie.201102398. [DOI] [PubMed] [Google Scholar]
- 42.Alexakis A, Krause N, Woodward S. In: Copper-Catalyzed Asymmetric Synthesis. Alexakis A, Krause N, Woodward S, editors. VCH–Wiley; 2014. pp. 33–68. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.