Abstract
Previous work in our lab has shown that early-life infection affects female reproductive physiology and function (i.e., smaller ovaries, abnormal estrous cycles) and alters investigation and aggression towards male conspecifics in a reproductive context. Although many studies have investigated the effects of postnatal immune challenge on physiological and behavioral development, fewer studies have examined whether these changes have ultimate effects on reproduction. In the current study, we paired Siberian hamsters (Phodopus sungorus) and simulated a bacterial infection in early life by administering lipopolysaccharide (LPS) to male and female pups on pnd3 and pnd5. In adulthood, hamsters were paired with novel individuals of the same sex, and we scored an array of social behaviors (e.g., investigation, aggression). We then paired animals with individuals of the opposite sex for 5 consecutive nights, providing them with the opportunity to mate. We found that females exhibited impaired reproductive physiology and function in adulthood (i.e., smaller ovaries and abnormal estrous cycles), similar to our previous work. However, both LPS-treated males and females exhibited similar same-sex social behavior when compared with saline-treated controls, they successfully mated, and there were no significant changes in fecundity. These data suggest that the physiological changes in response to neonatal immune challenge may not have long-term effects on reproductive success in a controlled environment. Collectively, the results of this study are particularly important when investigating the relationships between physiology and behavior within an ultimate context. Animals exposed to early-life stress may in fact be capable of compensating for changes in physiology in order to survive and reproduce in some contexts.
Keywords: Development, Immune system, Lipopolysaccharide, Reproduction, Social behavior
1. Introduction
Early-life stressors (e.g., maternal care, social changes, sickness) can greatly influence physiology and behavior in adulthood (reviewed in Bilbo & Schwarz 2009). It is well-established that the neonatal period is an extremely sensitive time in the life of an individual [3], and infection during this time may increase susceptibility to a range of nervous system disorders, including autism and schizophrenia [4]. Further, infection during the neonatal period may affect the timing of puberty, as well as the development of the reproductive system and the immune system [1,5].
Treatment with lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria, is commonly employed to induce an immune response in animals. LPS administration mimics the actions of a live bacterial infection by binding to toll-like receptor (TLR)-4, which leads to the subsequent release of circulating cortisol and proinflammatory mediators in the body [6–8]. Previous work suggests that postnatal LPS treatment not only affects the development of the reproductive axis in males and females (e.g., early puberty, suppression of luteinizing hormone [LH] and testosterone [T]), but LPS treatment also alters reproductive behavior in adulthood [9]. Specifically, LPS-treated male Wistar rats show fewer mounts, and females exhibit more aggression and fewer hops towards male conspecifics [9]. Additionally, LPS-treated males produce lower levels of sperm present in female partners following an interaction, suggesting they may not be able to successfully reproduce. Similarly, recent work from our lab suggests that postnatal LPS affects reproductive physiology and opposite-sex social behavior in a sex-dependent manner as well (see Fig. 1). Specifically, Siberian hamster (Phodopus sungorus) females treated with LPS as neonates show no changes in consummatory reproductive behaviors (e.g., lordosis), however, they do show heightened levels of pre-copulatory investigation and aggression when paired with male conspecifics. Interestingly, however, there were no changes in adult male physiology or social behavior following postnatal LPS [10]. Additionally, LPS-treated females exhibited altered estrous cycles and smaller ovaries in adulthood, suggesting that they may not be capable of successfully reproducing [10]. Moreover, LPS-treated female Wistar rats exhibit reduced follicle stimulating hormone (FSH) levels in adolescence, early reproductive maturity, and decreased follicular reserves [11], further suggesting that LPS-treated females of various species may not be capable of successfully reproducing.
Figure 1.
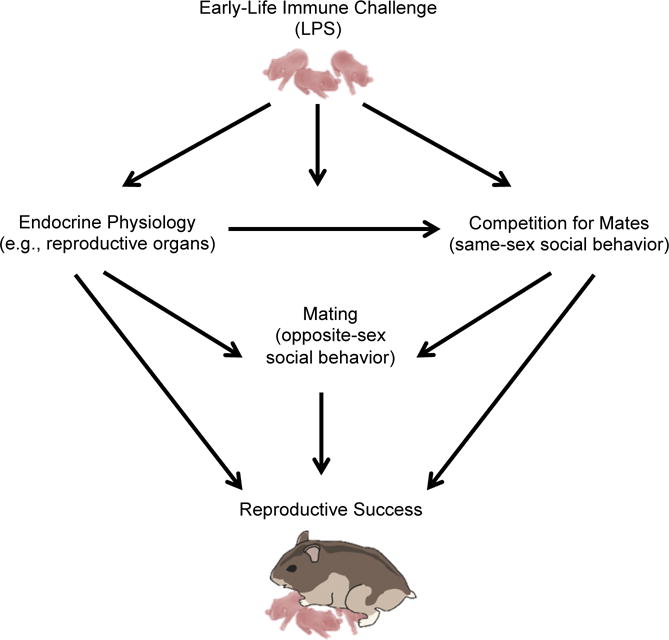
Graphical depiction of how early-life infection can influence physiology and behavior. Early-life immune activation can affect physiology and behavior in sex-dependent ways. We have previously shown that postnatal LPS affects reproductive physiology and pre-copulatory behavior (e.g., investigation and aggression) in female hamsters, but not in males [10]. In the present study, we investigated whether these changes in physiology consequently affect the ability to interact with same-sex individuals (e.g., investigation, aggression), behavior that is important for finding and attracting a mate, and whether early-life LPS treatment influences mating and reproductive success in males and females in a lab setting. There are complex interactions taking place among the endocrine systems and its effector organs, reproductive function, and behavior that is necessary for fitness and reproduction in various contexts.
Although many studies have investigated the effects of postnatal LPS on physiological and behavioral development (reviewed in Bilbo & Schwarz 2012a), fewer studies have investigated whether these developmental changes have ultimate (i.e., fitness) effects on reproduction. In one study, researchers found that female rats postnatally treated with LPS exhibited increased corticosterone concentrations in the juvenile, adolescent, and adult stages, suggesting a heightened hypothalamo-pituitary-adrenal (HPA) axis response [12]. Further, they found that when males were given the opportunity to mate with both LPS-treated and saline-treated females (in the same cage), novel males showed no preference for mating. Specifically, LPS- and saline-treated females did not differ in fecundity rate after being paired with a stud male for two weeks in adulthood, however, offspring born to LPS-treated females showed higher rates of mortality. The mating effects of LPS treatment on males, however, were not investigated in the study [12]. In a subsequent study, immediately following postnatal immune challenge, ovaries showed a significant up-regulation in genes important for immune cell signaling and inflammation, as well as reproductive development, suggesting that early-life immune activation may have severe implications for ovarian development and reproduction [13].
In order to investigate whether early-life immune activation affects the functioning of the hypothalamo-pituitary-gonadal (HPG) axis in adult males, we stimulated HPG activity using the RFamide peptide, kisspeptin. Kisspeptin is one of the primary regulators of gonadotropin releasing hormone (GnRH) neurons at the hypothalamus and ultimately serves as a crucial regulator of the entire HPG axis, including the onset of puberty and fertility [14]. Work in our lab has shown that male and female Siberian hamsters’ reproductive axes are reactive to exogenous kisspeptin at different stages of development. Specifically, males and females exhibit increased LH in response to exogenous kisspeptin, and males show increased testosterone in response to kisspeptin injection. Exogenous kisspeptin, however, does not affect normal seasonal changes in body mass or food intake [15,16].
While our previous findings and those of others have allowed an understanding of how an early-life immune challenge affects reproductive physiology and behavior, our work here provides insight into whether or not males and females from the same litters treated with postnatal LPS exhibit altered same-sex social behavior, an important aspect of finding potential mates. Further, we investigate if postnatal LPS treatment affects the ability for males and females to successfully mate when paired alone with a novel individual in the lab. As a result of the adverse effects of postnatal LPS on reproductive physiology that we found in our previous study, in this investigation, we hypothesized that females, but not males treated with postnatal LPS, would exhibit decreased mating success and reduced fecundity in adulthood.
2. Materials and methods
2.1 Animal housing and immune challenge
Adult male and female hamsters were paired (n=13 pairs) and housed in a 16:8 light:dark photoperiod, in polypropylene cages (28 × 17 × 12 cm). Ambient temperature was maintained at 20 ± 2°C, and relative humidity was maintained at 55 ± 5%. Hamsters were given ad libitum access to tap water and standard laboratory rodent chow (Lab Diet 5001, PMI Nutrition) throughout the experiment. Pups remained in their litters until weaning (postnatal day 24), when they were individually housed for the remainder of the study. On postnatal day (pnd) 3, approximately half of the litters were randomly assigned to either a treatment group, in which pups were given a single intraperitoneal (i.p.) injection (100μL) of 50 μg/kg of lipopolysaccharide (LPS, from Salmonella enterica serotype typhimurium, Sigma-Aldrich, St. Louis, MO, USA), suspended in 0.9% sterile saline (n=7 litters) or a control group, in which litters received i.p. injections of 0.9% sterile saline (n=6 litters). All pups received a second injection of LPS or saline on pnd5 according to a previously validated protocol, as there is heightened sensitivity of the GnRH pulse generator at these time points [5,10]. All pups in an individual litter received the same treatment (LPS or saline). Once injected, pups were monitored throughout the study, and all animals were weighed weekly for the remainder of experimentation. At the conclusion of the study, all animals were euthanized and organs were weighed. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Bloomington Institutional Animal Care and Use Committee (BIACUC) at Indiana University.
2.2 Reproductive physiology
2.2.1 Estimated Testis Volume
Beginning at pnd25 and once per week thereafter until reproductive maturity males (n=16 saline; n=10 LPS) were lightly anesthetized with isoflurane, and the length and width of the left testis was measured externally (+/− 0.1 mm) with calipers, as a proxy for reproductive maturity [10,17]. Estimated testis volume (ETV) was calculated as the length × width2, which is directly correlated with testis mass and spermatogenesis [17,18]. An ETV of 400mm3 indicates a mass of approximately 200mg, which is correlated with the critical mass for production of viable spermatids [17,18].
2.2.2 Vaginal patency and estrous cycling
Beginning at pnd25, and every five days thereafter, all female offspring (n=17 saline; n=16 LPS) were monitored for initial vaginal opening [19]. Because some females were euthanized at pnd45 (see 2.3 Hypothalamo-pituitary challenge), and five other females did not have vaginal openings at the time of estrous cycle monitoring in adulthood, estrous cycles were monitored in twenty-nine females (n=14 saline, n=15 LPS) via vaginal cytology [20,21]. Estrous cycling provides a measure of reproductive functioning, specifically ovarian functioning, in Siberian hamsters [22].
Vaginal cell samples were obtained via vaginal lavage. Following lavage, samples were transferred to microscope slides, fixed with methanol, and stained with Giemsa. Samples were then evaluated for estrous stage (diestrus, proestrus, estrus, and metestrus) under 100× magnification [20,21,23]. We determined estrous stage using the following characteristics: diestrus (presence of many polymorphonuclear leucocytes, some non-nucleated keratinized cells, and some parabasal cells), proestrus (clumps of lightly staining nucleated epithelial cells), estrus (many non-nucleated keratinized cells), and metestrus (flakes of keratinized cells and some leucocytes) [20,21,23]. Once we determined the stage of the estrous cycle, females exhibiting cycles in which no pattern was seen (no presence of particular cell types or the presence of all one cell type), or those in which the cycle appeared to be incomplete (those not showing more than one stage of the cycle over a five-day period) were considered to be cycling “abnormally.” Siberian hamsters have a cycle lasting approximately four days [24], therefore, animals who had not shown evidence of cycling within the five day period were confidently grouped into the “abnormally cycling” group.
2.2.3 Uterine horn scarring
At the end of the study, uterine horns from females placed in staged mating pairs (n=11 saline females, n=12 LPS females; n=16 females paired with saline males, n=11 females paired with LPS males) were stained to visualize embryo implantation sites by immersing uterine horns in 10% ammonium sulfide solution for 10 minutes followed by rinsing with distilled water [25,26]. Implantation sites appeared as dark spots along the uterine horns, and females that did not become pregnant showed no stained scarring. We measured the ratio of pups born to uterine horn implantation sites [27], in order to determine the difference between scars and number of offspring carried to full term (scarring-to-offspring ratio).
2.3 Hypothalamo-pituitary challenge
On pnd45 and pnd75, in a subset of animals in both postnatal treatment groups (LPS and saline), a baseline blood sample was collected prior to the animals receiving a single i.p. injection of 100μL of 10 μM kisspeptin-10 (Phoenix Pharmaceuticals, Inc.). Animals were immediately placed back into their home cage, and a second terminal blood sample was taken 30 minutes after kisspeptin injection for hormone analysis. To collect blood samples, animals were lightly anesthetized with isoflurane vapors, and blood samples were drawn from the retro-orbital sinus. Blood samples were allowed to clot at room temperature for 1h, the clots were removed, and samples were centrifuged at 4°C for 30min at 2500 rpm. Plasma was stored at −20°C until assayed for testosterone (males: saline × kisspeptin [n=4], LPS × kisspeptin [n=6]) and secondarily assayed for cortisol (males: saline [n=4], LPS [n=6]; females: saline [n=9], LPS: [n=8]. All blood samples were collected within 3min of initial handling.
2.4 Hormone analysis
2.4.1 Testosterone enzyme immunoassay (EIA)
To determine how postnatal LPS affected adult reproductive function in males, testosterone levels were measured before and after kisspeptin injections via a commercial EIA kit (Correlate-EIA Kit #900–065; Assay Designs, Ann Arbor, MI, USA) that has been previously validated for use in Siberian hamsters [28,29]. Samples were diluted 1:20 and run in duplicate for each sample. The sensitivity of the assay is 3.82 pg/mL. The intra-assay coefficient of variation was 3.2%.
2.4.2 Cortisol enzyme immunoassay (EIA)
As a secondary measure, we assessed circulating cortisol levels before and after injection stress to determine if postnatal LPS treatment affected HPA axis activation in males and females. Although not a typical method for activating the HPA axis, we assessed cortisol after an injection stressor, since it has been observed that the injection alone is associated with an increase in cortisol levels in control animals (personal observations). Cortisol is the predominant glucocorticoid in Siberian hamsters, with concentrations ~100× that of corticosterone [30]. Serum cortisol concentrations were determined in multiple enzyme immunoassays (EIAs) from a commercially prepared kit (Cortisol EIA Kit; Enzo Life Sciences, Inc., Farmingdale, NY, USA) that was previously validated for use in Siberian hamsters [31,32]. The assay is highly specific for cortisol, with corticosterone cross-reactivity 27.7% and <4.0% for other steroid hormones. The sensitivity of the assay is 56.72 pg/mL. Samples were diluted 1:80 with assay buffer and run in duplicate. Male and female samples were run on the same plates. The intra-assay coefficient of variation was 6.7%, and the inter-assay coefficient of variation was 5.2%.
2.5 Same-sex social interaction trials
On pnd65, all animals were paired with a novel individual of the same-sex for social behavioral assessments, in which the pairs were scored for social behaviors within a 5min testing period [21,32–34]. Behavioral assessments were completed in the home cage of the experimental animal, and each animal was placed back into his or her home cage following behavioral assessment. All experimental trials were video recorded (Sony Handycam HD R-SR7) under low illumination (25W), red light conditions. To identify the intruders, small patches of fur were shaved on the dorsal surface at least 24h prior to behavioral assessment. Aggressive behaviors (i.e., chases, attacks, latency to first attack), investigative behaviors (i.e., nose-to-nose sniffing, nose-to-anogenital sniffing), and grooming were scored by a trained observer blind to the treatment group, using ODlog™ software (Macropod).
2.6 Mating success
On pnd71, all animals were paired with a novel animal of the opposite sex for five consecutive nights (pnd71–75), providing them for the opportunity to breed. On the morning of the sixth day, males were removed from the cage, and all males were euthanized. All females remained in their home cage and were monitored daily for signs of pregnancy. Litter sizes and weights were tracked until pnd5. Litter mass and infanticide were assessed across all groups.
2.7 Tissue collection
Experimental adults were euthanized (adult males at pnd45, pnd75, or pnd76; adult females on pnd 45, pnd75, or pnd5 of F2 generation) via a lethal i.p. injection of a ketamine and xylazine cocktail in 0.9% saline, and all adult (≥pnd75) livers, spleens, and reproductive organs were collected and weighed. All litters were euthanized by anesthetization with an i.p. injection of a ketamine and xylazine cocktail in 0.9% saline followed by rapid decapitation five days after day of birth.
3. Statistical analyses
All statistical analyses were performed in R v. 3.3.3 (R Core Team 2016), and we attributed statistical significance at p < 0.05 after adjusting to control for false discovery rate (FDR) when making multiple comparisons [35]. Organ mass and behavioral data were analyzed using a generalized linear mixed effects model (GLMM), including the fixed effects of the model (e.g., treatment) as well as the random effect of litter, enabling us to take into account the fact that individual pups from the same litter may not have truly been independent samples. Differences in repeated measures (i.e., body mass, food intake) were assessed via repeated-measures GLMMs. Because all males in this study reached reproductive maturity by pnd25, we compared the values of estimated testis volume at pnd25 between LPS- and saline-treated males using a GLMM as well. If a model reported a significant interaction effect, two-tailed t-tests were run to determine pair-wise relationships.
Data were checked for normality and homogeneity of variance and those data that were non-normally distributed were log or square root transformed to attain normality and equal variances. Data that could not be transformed to attain normality were analyzed using non-parametric tests. Specifically, to test whether there was a difference in time to return to nursing after injection 1 and injection 2, and to test whether mass differed between LPS- and saline-treated litters, we ran Mann-Whitney U tests to compare groups.
We found including litter as a random effect in a GLMM for assessing the effects of LPS treatment on estrous cycling and mating success were not appropriate, and when tested, litter did not significantly affect the outcome of the models. Therefore, the effect of LPS treatment on estrous cycling and mating success were assessed with Fisher’s Exact Tests.
Serum samples collected at pnd45 and pnd75 for hormone analysis were collapsed in analysis, since it was determined that samples within each sex at these two time points were not significantly different from one another in the initial analysis (p > 0.05). It has previously been shown that testosterone concentrations in males at pnd45 and pnd75 are not significantly different from each other, as males reach puberty before pnd45 [16]. Ovaries from one LPS-treated female were excluded from analysis because the ovaries were incorrectly collected during necropsy. Another female from this litter, however, was represented in the final analysis.
4. Results
4.1 Early-life immune activation did not affect litter physiology
The total number of pups in each litter did not differ across treatment groups (W=17.5, Z=−0.535, P=0.647). Further, the time to retrieve pups and return to nursing after the first injection (W=12.5, Z=−1.216, P=0.504) and after the second injection (W=10, Z=1.714, P=0.504) was not affected by treatment. The average offspring mass from pnd2 through pnd24 in LPS- and saline-treated litters was not different across treatments as well (t92=0.750, P=0.607).
4.2 Early-life immune activation affected food intake, but not body mass in females only
In females, there were no significant effects of treatment (t17=1.216, P=0.321) or the time × treatment interaction (t206=−1.720, P=0.209) on body mass, however, there was a significant effect of time (postnatal week) on body mass in both LPS- and saline-treated females (t206=21.152, P<0.001) (Fig. 2). Additionally, there was no significant effect of treatment alone on food intake in females (t19=1.644, P=0.234); however, there were significant effects of time (t173=16.518, P<0.001) and the time × treatment interaction (t173=−2.425, P=0.048) on food intake in females. Two-tailed t-tests determined that individually during weeks 2–6, there were no significant effects of treatment on food intake directly (p > 0.05 in all cases).
Figure 2.
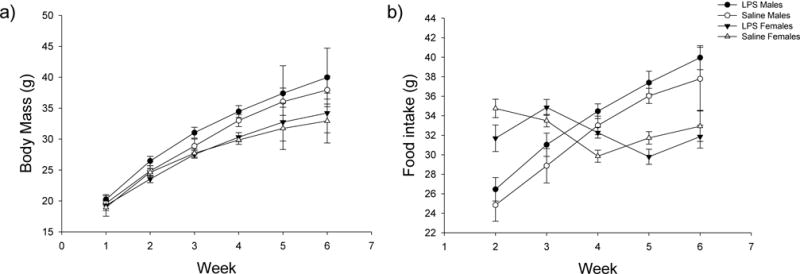
Mean ± SEM of (a) body mass and (b) food intake over time in male and female hamsters. There were no significant effects of treatment or the time × treatment interaction on body mass in males, however, there was a significant effect of time (postnatal week) on body mass in males in both treatment groups. There were no significant effects of treatment, time, or the time × treatment interaction on food intake in males. In females, there were no significant effects of treatment or the time × treatment interaction on body mass, however, there was a significant effect of time (postnatal week) on body mass in both LPS- and saline-treated females. There was no significant effect of treatment alone on food intake in females; however, there were significant effects of time and the time × treatment interaction on food intake in females. White circles represent saline-treated males; white triangles represent saline-treated females; black circles represent LPS-treated males; and black triangles represent LPS-treated females.
In males, there were no significant effects of treatment (t28=−1.206, P=0.321) or the time × treatment interaction (t182=−0.310, P=0.826) on body mass, however, there was a significant effect of time (postnatal week) on body mass in both treatment groups (t183=19.722, P<0.001) (Fig. 2). Further, there were no significant effects of treatment (t139=1.031, P=0.365), time (t149=0.059, P=0.953), or the time × treatment interaction (t149=−1.204, P=0.321) on food intake in males.
4.3 Early-life immune activation affected female but not male reproductive physiology
Though postnatal LPS did not significantly affect the timing of female vaginal opening (t17=−1.834, P=0.112), postnatal LPS disrupted female estrous cycles. Specifically, only 20.0% of LPS-treated females exhibited normal 4–5 day estrous cycles, whereas 78.6% of the saline-treated females displayed normal estrous cycles. This difference in estrous cycling between saline- and LPS-treated females was significant (P =0.020) (Fig. 3). Further, LPS-treated females had significantly smaller ovaries when compared with saline-treated females (t11=2.362, P=0.022). There was no significant difference, however, between uterine horns in either treatment group (t11=0.402, P=0.695). There was no significant difference between the masses of livers (t11=−1.105, P=0.293) or spleens (t11=−0.462, P=0.653) in LPS-treated females when compared with saline-treated females as well.
Figure 3.
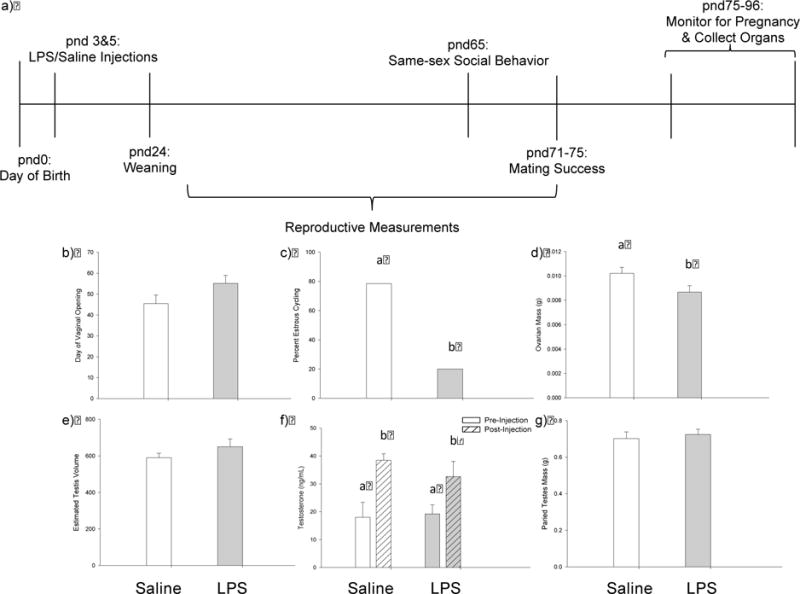
Experimental timeline and effects of treatment on reproductive measures in males and females. (a) Experimental timeline demonstrating when treatments were performed and when physiological and behavioral measures were collected. Postnatal day (pnd) 0 represents the time point at which pups were born, and pnd24 represents the time point at which each animal was individually housed for the remainder of the study. (b-g) Effects of LPS treatment on reproductive physiology in female and male hamsters. (b) LPS-treated females reached reproductive maturity later than saline-treated females, however, there was no significant difference in day of vaginal opening; (c) there was a lower percentage of normal estrous cycles in LPS-treated females (gray bars) when compared with saline-treated females (white bars); (d) LPS-treated females had significantly smaller ovaries when compared with saline-treated females; (e) LPS-treatment did not affect estimated testis volume (ETV) at pnd25; (f) there were no effects of treatment or the treatment × time interaction on testosterone concentrations following kisspeptin challenge; however, both treatment groups exhibited a significant increase in testosterone concentrations after kisspeptin injection; and (g) male paired testes mass was not affected by LPS treatment. Bar heights represent mean ± SEM Groups with different letters indicate statistically significant differences between group means (p < 0.05); groups sharing the same letter are statistically equivalent.
In contrast, postnatal LPS did not affect any of the measures of reproductive physiology taken in males. Estimated testis volume (ETV) at pnd25 did not differ between groups (t8=−1.368, P=0.209), and paired testes mass in adulthood did not differ between LPS-treated males and saline-treated males (t10=−0.431, P=0.675). Postnatal LPS did not affect testosterone concentrations before or after kisspeptin challenge. Specifically, there were no effects of treatment (t6=0.899, P=0.403) or the treatment × time interaction (t10=−0.765, P=0.462) on testosterone concentrations; however, there was a significant effect of time (pre and post injection) on testosterone in both LPS- and saline-treated males (t10=−2.299, P=0.044). Both treatment groups exhibited an increase in testosterone concentrations after kisspeptin injection (Fig. 3). Further, there was no significant difference between the masses of livers (t10=−1.306, P=0.221) or spleens (t10=−0.876, P=0.401) in LPS-treated males when compared with saline-treated males.
4.4 Early-life immune challenge did not affect cortisol
LPS-treatment did not affect the baseline concentration of cortisol, nor did it affect the concentration of cortisol following injection stress in males or in females. In females, there were no significant effects of treatment (t7=−0.426, P=0.683) or the time × treatment interaction (t18=1.691, P=0.162), however, there was a significant effect of time (pre or post injection) on cortisol concentration in both saline- and LPS-treated females (t18=−2.669, P=0.048) (Fig. 4). In males, there were no significant effects of time (t15=−0.692, P=0.667), treatment (t7=−0.631, P=0.667), or the time × treatment interaction (t15=−0.439, P=0.667) on cortisol concentrations (Fig. 4). Cortisol concentrations in both sexes were similar to those seen in previous studies [31].
Figure 4.
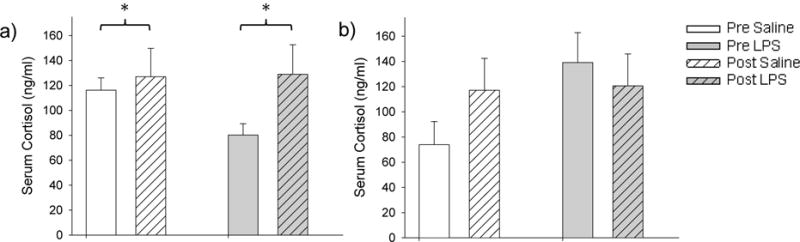
Mean ± SEM of serum cortisol pre and post injection stress in female (a) and male (b) hamsters. LPS-treatment did not affect the baseline concentration of cortisol, nor did it affect the concentration of cortisol following injection stress in males or in females. In females, however, there was a significant effect of time (pre or post injection) on cortisol concentration in both saline- and LPS-treated females. No other values were significantly different (p > 0.05).
4.5 Early-life immune activation did not affect mating success or fecundity
Mating success in experimental females was not affected by treatment. Specifically, 50.00% of LPS-treated females and 45.45% of saline-treated females became pregnant. This difference in rates of pregnancy between saline- and LPS-treatment was not significant (P=0.737) (Fig. 5). Of the experimental females that successfully reproduced, the number of offspring did not differ across groups (t8=−0.656, P=0.530). There was also no difference in rate of infanticide across females treated with LPS or saline (t8=−0.553, P=0.595), and there was no difference in uterine horn scarring ratio across treatments groups (t8=−0.556, P=0.594). Similarly, we found no difference in the average mass of offspring at pnd5 in the second generation (t8=−0.181, P=0.861).
Figure 5.
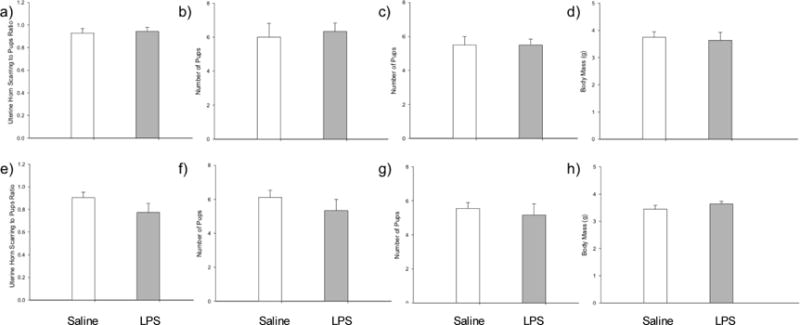
Mean ± SEM of measures of fecundity in adult male and female hamsters. (a) Uterine horn scarring-to-offspring ratio in female hamsters; (b) number of pups born to experimental females; (c) number of offspring lost to infanticide by experimental females; (d) average mass of offspring at pnd5 born to experimental females; (e) uterine horn scarring-to-offspring ratio in female hamsters mated with experimental males; (f) number of pups born to females paired with experimental males; (g) number of offspring lost to infanticide by females paired with experimental males; and (h) average mass of offspring at pnd5 born to females paired with experimental males. No values were significantly different across treatment groups (p > 0.05).
Similar to experimental females, experimental males paired with a novel female showed no difference in mating success. Specifically, 54.55% of females paired with LPS-treated males and 56.25% of females paired with saline-treated males became pregnant. This difference in rates of pregnancy between saline- and LPS-treatment was not significant (P=0.619) (Fig. 5). Of the females that did successfully reproduce with an experimental male, there was no significant difference in size of the litters (t7=1.027, P=0.339), nor was there a significant difference in rate of infanticide (t7=0.580, P=0.580). There was also no difference in uterine horn scarring ratio across treatments (t7=1.513, P=0.174), and similarly, we found no difference in the average mass of offspring at pnd5 in the second generation across treatments (t7=−0.569, P=0.587).
4.6 Early-life immune activation did not affect investigation
The frequency (t8=1.477, P=0.178) and duration (t8=1.155, P=0.281) of nose-to-nose investigation was not affected by LPS treatment in females, and the frequency (t8=1.396, P=0.200) and duration (t8=0.287, P=0.782) of nose-to-anogenital investigation was not affected by LPS treatment in females (Table 1).
Table 1.
Means ± SEM of investigation, aggression, and grooming behaviors in female hamsters across treatment groups. No values were significantly different across treatment groups (p > 0.05).
| Female Behavior | Saline | LPS |
|---|---|---|
| Frequency of Anogenital Sniffing | 9.455 ± 1.846 | 6.417 ± 1.069 |
| Duration of Anogenital Sniffing | 17.009 ± 3.882 | 15.433 ± 3.88 |
| Frequency of Head/Neck Sniffing | 13.182 ± 1.976 | 7.250 ± 0.986 |
| Duration of Head/Neck Sniffing | 21.345 ± 4.181 | 13.050 ± 4.198 |
| Total Frequency of Investigation | 22.636 ± 3.599 | 13.667 ± 1.734 |
| Total Duration of Investigation | 38.355 ± 6.92 | 28.483 ± 6.886 |
| Frequency of Chases | 0.091 ± 0.091 | 0.333 ± 0.333 |
| Duration of Chases | 0.036 ± 0.036 | 0.475 ± 0.475 |
| Frequency of Attacks | 6.091 ± 1.217 | 5.333 ± 1.333 |
| Duration of Attacks | 4.482 ± 0.890 | 5.317 ± 1.999 |
| Latency to Attack | 84.091 ± 21.959 | 97.727 ± 21.652 |
| Frequency of Grooming | 2.00 ± 0.714 | 1.667 ± 0.284 |
| Duration of Grooming | 2.182 ± 0.826 | 2.05 ± 0.546 |
Similarly, in males, the frequency (t8=0.836, P=0.428) and duration (t8=0.304, P=0.769) of nose-to-nose investigation was not affected by LPS, and the frequency (t8=0.610, P=0.559) and duration (t8=0.009, P=0.993) of nose-to-anogenital investigation was not affected by LPS treatment in males (Table 2).
Table 2.
Means ± SEM of investigation, aggression, and grooming behaviors in male hamsters across treatment groups. No values were significantly different across treatment groups (p > 0.05).
| Male Behavior | Saline | LPS |
|---|---|---|
| Frequency of Anogenital Sniffing | 14.313 ± 2.271 | 11.545 ± 1.875 |
| Duration of Anogenital Sniffing | 34.763 ± 4.869 | 35.236 ± 6.698 |
| Frequency of Head/Neck Sniffing | 16.938 ± 2.308 | 15.091 ± 2.588 |
| Duration of Head/Neck Sniffing | 36.750 ± 6.455 | 40.873 ± 7.159 |
| Total Frequency of Investigation | 31.250 ± 4.193 | 26.636 ± 4.235 |
| Total Duration of Investigation | 71.513 ± 10.615 | 76.109 ± 12.442 |
| Frequency of Chases | 1.375 ± 0.491 | 0.909 ± 0.368 |
| Duration of Chases | 1.963 ± 0.763 | 1.173 ± 0.578 |
| Frequency of Attacks | 8.625 ± 1.008 | 5.818 ± 1.476 |
| Duration of Attacks | 12.281 ± 2.880 | 8.900 ± 2.685 |
| Latency to Attack | 74.375 ± 16.361 | 73.6 ± 19.725 |
| Frequency of Grooming | 1.688 ± 0.373 | 1.455 ± 0.282 |
| Duration of Grooming | 1.625 ± 0.382 | 1.600 ± 0.352 |
4.7 Early-life immune activation did not affect aggression
There was no significant difference in the frequency (t8=0.710, P=0.498) or duration (t8=−0.725, P=0.489) of attacks, and there was no difference in the frequency (t8=−0.692, P=0.509) or duration (t8=0.993, P=0.350) of chases in LPS-treated females. Additionally, there was no difference in the latency to first attack in LPS-treated females (t8=−0.513, P=0.622) (Table 1).
Similarly, in males, there was no significant difference in the frequency (t8=1.140, P=0.287) or duration (t8=0.818, P=0.437) of attacks in LPS-treated animals. There was also no difference in the frequency (t8=0.691, P=0.509) or duration (t8=0.737, P=0.482) of chases, and there was no difference in the latency to first attack in LPS-treated males (t8=0.030, P=0.977) (Table 2).
4.8 Early-life immune activation did not affect grooming behavior
There was no difference in the frequency (t8=1.446, P=0.186) or duration (t8=0.883, P=0.403) of grooming in LPS-treated females (Table 1). Similarly, there was no difference in the frequency (t8=0.458, P=0.659) or duration (t8=0.046, P=0.965) of grooming in LPS-treated males (Table 2).
5. Discussion
Environmental stressors, including those that activate the immune system, often influence physiology and behavior; however, the long-term consequences of those stressors are not fully understood. In the current study, we built upon our previous findings, showing that an early-life immune challenge influences female physiology and opposite-sex behavior [10]. In doing so, we tested the effects of early-life immune activation on adult same-sex social behavior and fecundity (e.g., mating success and infanticide). We paired hamsters and mimicked a bacterial infection in early life, and in adulthood, hamsters were paired with a novel animal of the same sex to assay social behaviors and were given the opportunity to mate with a novel individual. We found that although LPS-treated females exhibited impaired reproductive physiology and function in adulthood, similar to the results we found in our previous study, LPS-treated females successfully reproduced, and their offspring showed no significant differences (e.g., no difference in body mass or infanticide). This may suggest that the physiological changes in response to neonatal immune challenge may not have long-term effects on reproductive functioning in some contexts. Further, when given the opportunity to mate, affected females are able to compensate for their impaired reproductive physiology. These results are particularly important when investigating the effects of early-life stress on physiology alone, as we have found that reproductive success (i.e., fecundity) is not directly associated with measures of reproductive physiology in some cases.
5.1 Changes in reproductive physiology may not predict mating success
Though females in our study showed impaired reproductive physiology and function, we found that those same females showed no changes in their ability to mate or in the measures of fecundity studied here. These data suggest that changes in physiology may not necessarily correspond to changes in fitness. Previous studies have shown similar connections. For example, both male meadow voles and prairie voles show a decrease in testosterone and an increase in corticosterone and interleukin-1β (IL-1β) in response to LPS, however, when novel females are paired with LPS-treated prairie voles, but not meadow voles, females spend less time with LPS-treated males when compared with saline-treated males. Further, both LPS-injected male prairie voles and meadow voles spend less time engaging in social behavior with females [36]. These data suggest that although LPS treatment influenced measures of physiology in both species, those physiological measures do not necessarily coincide with behavioral responses in both species, including those important in mating and reproduction. In another study, infection with Trichinella spiralis affected female odor preference of male meadow voles, however, infection did not affect mate preference, suggesting that although there were clear changes in physiology as shown in the changes in odor preference, those physiological changes did not correspond with changes in mating abilities [37].
In a different study, male and female prairie voles exhibited an increase in corticosterone concentrations in response to exogenous LPS. Following treatment, female prairie voles were paired with a novel male for 6 hours. During a subsequent partner-preference test, they found that LPS-treated females spent significantly more time with familiar males, and saline-treated females spent significantly more time with unfamiliar males. Males, however, exhibited no partner preference [38]. These data suggest that behavioral changes may be sex-specific, and although they found changes in physiology in both sexes, behavioral changes may not always coincide with alterations in physiology.
In our current study, however, we did not provide a choice between LPS- and saline-treated individuals, nor did we challenge the individuals with other environmental factors that may be disadvantageous. For example, in the wild, conditions are unpredictable (e.g., scarce food sources, temperature fluctuations) and can greatly influence the physiological response to an immune challenge. Further studies should therefore be done to investigate whether early-life infection would affect mating success in a natural environment, where conditions may be unfavorable.
5.2 Sex modulates the response to stressors
Sexually dimorphic responses to both physiological and behavioral stressors have been seen in various species [39]. In particular, the HPA response to stressors is often sex-dependent and is connected to the overall immune response following such a challenge [40]. On average, female rats have a greater endocrine response to both physiological and psychological stressors, and females often show a greater average concentration of glucocorticoids than males [41]. During an immune challenge, not only are the HPA axis and the immune system mediating an appropriate response, but the HPG axis also plays a vital role influencing the physiological reaction [6–8].
Interestingly, it is believed that gonadal steroid hormones may play an important role in regulating these effects [42–44], and testosterone may actually decrease the amount of circulating ACTH and glucocorticoids in response to a stressor, whereas estradiol may be associated with an increase in these molecules [42,45]. The paraventricular nucleus (PVN) of the hypothalamus exhibits estrogen and androgen receptors, suggesting that sex steroids may be acting at the level of the hypothalamus to regulate the physiological response to stress, however, the precise manner in which the HPA and the HPG axis interact is not completely understood [46]. These studies and others suggest that the HPA and HPG axis are important in the regulation of appropriate physiological and behavioral responses in the face of infection and inflammation, and the interaction of these two axes may be playing a significant role in the sex-dependent responses to early-life immune activation that we saw in the current study.
5.3 Social context influences physiology and behavior
It has long been suggested that an individual’s social environment can greatly influence an animal’s physiological and behavioral response [reviewed in 47,48]. For example, zebra finches that are group housed show no change in social behavior when treated with exogenous LPS, however, socially-isolated finches show reduced activity. Though finches in both social environments exhibit an increase in proinflammatory cytokine, IL-6, suggesting that there may be a tradeoff between showing sickness behaviors in response to infection and exhibiting social behavior in these different contexts (Lopes, Adelman, Wingfield, & Bentley, 2012). In a different study, male Aztec mice (Peromyscus aztecus) housed with females had significantly larger paired testes, higher spermatozoon counts, and higher serum testosterone levels when compared with males housed individually [50], suggesting that social context may play a particularly important role in reproductive physiology.
Further, animals may avoid exhibiting sickness behaviors in order to put themselves in a better position for territory defense, mating, or offspring survival. For example, house finches choose to avoid an infected partner more often, however, those individuals that avoid sick conspecifics more often show lower natural antibody levels than non-avoiders [51]. These data suggest that finches alter their behavior based on not only social environment but also their ability to fight off infection in that social environment. In our previous work, we have shown that early-life immune activation affects female investigation and aggression towards a male conspecific and female reproductive physiology. In the present study, however, we found that though females showed similar alterations in their reproductive physiology, they were still able to successfully mate and reproduce. These data suggest that there may not be dichotomous trade-offs between immunity and reproduction; but rather, the context in which an animal is placed in may impact their behavior to a greater degree.
5.4 Timing of stressors can impact physiological and behavioral responses
In many animal models, sex differences in behavior emerge later in life, often after a secondary stressor [52], and therefore further investigation into how subsequent stressors might affect physiological and behavioral outcomes is important. Though we did not find LPS to be associated with a significant difference in the concentration of cortisol following injection stress, it is possible that continual handling stress instead of this one-time stressor could yield different results. Further, it is possible that changes in HPA responsiveness due to early-life immune activation may occur after a secondary stressor. For example, in one study, postnatal LPS treatment had no immediate effect on anxiety-like behavior or spatial memory in adult mice, but following a second LPS challenge in adulthood, animals showed impaired spatial memory and neurogenesis [53]. Additionally, neonatal rats infected with E. coli show no immediate memory changes in adulthood, however, when infected neonates experience an LPS challenge in adulthood, they also exhibit impaired recent memory, decreased hippocampal astrocytes, and decreased brain IL-1 [54]. These studies suggest that some physiological and behavioral effects of postnatal infection may not take place until a secondary stressor is introduced. Further work suggests that these changes may be partially reversible. In a subsequent study, rats treated with neonatal E.coli or saline received caspase-1 inhibitor as adults, which prevents the synthesis of the proinflammatory cytokine, IL-1. Treatment with this caspase-1 inhibitor prevented LPS-induced memory impairment, providing evidence that the changes seen in behavior may be, in part, mediated by the inflammatory response that occurs during a neonatal infection (Bilbo, et al., 2005).
Furthermore, there has been considerable research investigating the effects of maternal immune challenge during pregnancy on offspring physiology and behavior. Specifically, work in our lab suggests that prenatal LPS is associated with a greater cortisol response following an intruder encounter and higher frequency of bites during that agonistic encounter in male offspring when compared with male offspring from control-treated dams (French et al., 2013). In another study, prenatal LPS was associated with altered male sexual behavior and physiology. Specifically, the number of males that successfully ejaculated and testis mass were both reduced in males prenatally exposed to LPS (Bernardi et al., 2010). Additionally, in separate study, during the fetal period, there were alterations in males whose mothers were exposed to LPS during pregnancy. Specifically, there were significant abnormalities in Leydig cells; anogenital distances were reduced; and in adulthood, testis, prostate, and seminal vesicle mass were reduced in males whose mothers were treated with LPS during pregnancy. These studies and others suggest that prenatal exposure to LPS can have long-lasting effects on the reproductive system. Our current research, however, examines the effects of postnatal LPS on reproductive development a nd behavior, and future research should investigate the effects of immune activation on development at varying times in the developmental process (e.g., prenatally), as well as in conjunction with secondary stressors.
Conclusions
The neonatal period is an extremely sensitive time in the life of an individual, and infection during this critical time can greatly influence the development of the reproductive system and the immune system. Our work suggests that, although female physiology is more strongly affected by an early-life immune challenge, both LPS-treated males and females exhibit no significant changes in fecundity (e.g., no difference in litter size, mass, or infanticide). These data indicate that even when a stressor affects physiology, there may not be long-term effects on reproduction or survival, at least under the conditions of the present study. Taken together, the results of this study are particularly important when investigating the intricate relationships between physiology and behavior. Animals exposed to early-life stress may in fact be capable of compensating for changes in physiology in order to survive and reproduce.
Highlights.
LPS treatment impaired reproductive physiology and function in females.
LPS treatment did not affect male reproductive physiology or function.
Male and female same-sex social behavior was not affected by early-life sickness.
LPS-treated males and females exhibited no changes in fecundity.
Acknowledgments
The authors thank Dr. Kim Rosvall for her guidance in conceptualizing the data collected from this study and L. Beck, D. Boyes, N.M. Rendon, and E.A. St. John for assistance in behavioral filming, necropsies, and general animal procedures.
Funding
This work was supported by a Sigma Xi Grant in Aid of Research [KES]; a Kinsey Institute Graduate Student Research Award [KES]; the National Institute of Child Health and Human Development [T32HD49336 to KES]; Indiana University; and in part by a National Science Foundation Research Experience for Undergraduates (REU) in Animal Behavior [1460949 to PBR].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
KES and GED conceived the ideas and designed the methodology; KES and PBR collected the data; KES analyzed the data and led the writing of the manuscript; and all authors contributed critically to the drafts and gave final approval for publication.
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system., Front. Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocr. 2012;33:267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/S0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 4.Harvey L, Boksa P. Prenatal and postnatal animal models of immune activation: Relevance to a range of neurodevelopmental disorders. Dev Neurobiol. 2012;72:1335–1348. doi: 10.1002/dneu.22043. [DOI] [PubMed] [Google Scholar]
- 5.Knox AM, Li XF, Kinsey-Jones JS, Wilkinson ES, Wu XQ, Cheng YS, Milligan SR, Lightman SL, O’Byrne KT. Neonatal lipopolysaccharide exposure delays puberty and alters hypothalamic Kiss1 and Kiss1r mRNA expression in the female rat. J Neuroendocr. 2009;21:683–689. doi: 10.1111/j.1365-2826.2009.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33:267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French SS, Chester EM, Demas GE. Maternal immune activation affects litter success, size and neuroendocrine responses related to behavior in adult offspring. Physiol Behav. 2013;119:175–184. doi: 10.1016/j.physbeh.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker AK, Hiles SA, Sominsky L, McLaughlin EA, Hodgson DM. Neonatal lipopolysaccharide exposure impairs sexual development and reproductive success in the Wistar rat. Brain Behav Immun. 2011;25:674–684. doi: 10.1016/j.bbi.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Sylvia KE, Demas GE. Overcoming neonatal sickness: Sex-specific effects of sickness on physiology and social behavior. Physiol Behav. 2017;179:324–332. doi: 10.1016/j.physbeh.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sominsky L, Meehan CL, Walker AK, Bobrovskaya L, McLaughlin EA, Hodgson DM. Neonatal immune challenge alters reproductive development in the female rat. Horm Behav. 2012;62:345–55. doi: 10.1016/j.yhbeh.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Sominsky L, Walker AK, Ong LK, Tynan RJ, Walker FR, Hodgson DM. Increased microglial activation in the rat brain following neonatal exposure to a bacterial mimetic. Behav Brain Res. 2012;226:351–356. doi: 10.1016/j.bbr.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Sominsky L, Sobinoff AP, Jobling MS, Pye V, McLaughlin EA, Hodg Immune regulation of ovarian development : programming by neonatal immune challenge. Front Neurosci. 2013;7:1–20. doi: 10.3389/fnins.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellano JM, Bentsen AH, Mikkelsen JD, Tena-Sempere M. Kisspeptins: bridging energy homeostasis and reproduction. Brain Res. 2010;1364:129–38. doi: 10.1016/j.brainres.2010.08.057. [DOI] [PubMed] [Google Scholar]
- 15.Bailey AM, Legan SJ, Demas GE. Exogenous Kisspeptin Enhances Seasonal Reproductive Function in Male Siberian Hamsters. Funct Ecol. 2017:1–11. doi: 10.1111/1365-2435.12846. [DOI] [Google Scholar]
- 16.Greives TJ, Long KL, Burns CMB, Demas GE. Response to exogenous kisspeptin varies according to sex and reproductive condition in Siberian hamsters (Phodopus sungorus) Gen Comp Endocrinol. 2011;170:172–9. doi: 10.1016/j.ygcen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorman MR. Seasonal adaptations of Siberian hamsters. I. Accelerated gonadal and somatic development in increasing versus static long day lengths. Biol Reprod. 1995;53:110–5. doi: 10.1095/biolreprod53.1.110. http://www.ncbi.nlm.nih.gov/pubmed/7669841. [DOI] [PubMed] [Google Scholar]
- 18.Schlatt S, De Geyter M, Kliesch S, Nieschlag E, Bergmann M. Spontaneous recrudescence of spermatogenesis in the photoinhibited male Djungarian hamster, Phodopus sungorus. Biol Reprod. 1995;53:1169–77. doi: 10.1095/biolreprod53.5.1169. http://www.ncbi.nlm.nih.gov/pubmed/8527522. [DOI] [PubMed] [Google Scholar]
- 19.Adam CL, Moar KM, Logie TJ, Ross AW, Barrett P, Morgan PJ, Mercer JG. Photoperiod Regulates Growth, Puberty and\nHypothalamic Neuropeptide and Receptor Gene\nExpression. Female Siberian Hamsters,\nEndocrinology. 2000;141:4349–4356. doi: 10.1210/endo.141.12.7807. [DOI] [PubMed] [Google Scholar]
- 20.Carlton ED, Cooper CL, Demas GE. Metabolic stressors and signals differentially affect energy allocation between reproduction and immune function. Gen Comp Endocrinol. 2014;208:21–29. doi: 10.1016/j.ygcen.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scotti MAL, Place NJ, Demas GE. Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus) Horm Behav. 2007;52:183–90. doi: 10.1016/j.yhbeh.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Shahed A, Young KA. Differential Ovarian Expression of KiSS-1 and GPR-54 During the Estrous Cycle and Photoperiod Induced Recrudescence in Siberian Hamsters (Phodopus sungorus) Mol Reprod Dev. 2009;76:444–452. doi: 10.1124/dmd.107.016501.CYP3A4-Mediated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moffatt-Blue CS, Sury JJ, Young KA. Short photoperiod-induced ovarian regression is mediated by apoptosis in Siberian hamsters (Phodopus sungorus) Reproduction. 2006;131:771–82. doi: 10.1530/rep.1.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMillan HJ, Wynne-Edwards KE. Divergent reproductive endocrinology of the estrous cycle and pregnancy in dwarf hamsters (phodopus) Comp Biochem Physiol A Mol Integr Physiol. 1999;124:53–67. doi: 10.1016/s1095-6433(99)00090-2. http://www.ncbi.nlm.nih.gov/pubmed/10605068. [DOI] [PubMed] [Google Scholar]
- 25.Kopf R, Lorenz D, Salewski E. Der Einfluβ von Thalidomid auf die Fertilität von Ratten im Generationsversuch über zwei Generationen. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964;247:360–361. doi: 10.1007/BF02308457. [DOI] [PubMed] [Google Scholar]
- 26.Bailey AM, Rendon NM, O’Malley KJ, Demas GE. Food as a supplementary cue triggers seasonal changes in aggression, but not reproduction, in Siberian hamsters. Physiol Behav. 2016;167:298–308. doi: 10.1016/j.physbeh.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roosa KA, Zysling DA, Place NJ. An assessment of anti-Mullerian hormone in predicting mating outcomes in female hamsters that have undergone natural and chemically-accelerated reproductive aging. Gen Comp Endocrinol. 2015;214:56–61. doi: 10.1016/j.ygcen.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Demas GE, Johnson C, Polacek KM. Social interactions differentially affect reproductive and immune responses of Siberian hamsters. Physiol Behav. 2004;83:73–79. doi: 10.1016/j.physbeh.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 29.Greives TJ, Humber SA, Goldstein AN, Scotti MAL, Demas GE, Kriegsfeld LJ. Photoperiod and testosterone interact to drive seasonal changes in kisspeptin expression in siberian hamsters (Phodopus sungorus) J Neuroendocrinol. 2008;20:1339–1347. doi: 10.1111/j.1365-2826.2008.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reburn CJ, Wynne-Edwards KE. Cortisol and prolactin concentrations during repeated blood sample collection from freely moving, mouse-sized mammals (Phodopus spp.) Comp Med. 2000;50:184–198. [PubMed] [Google Scholar]
- 31.Carlton ED, Demas GE. Body mass affects seasonal variation in sickness intensity in a seasonally-breeding rodent. J Exp Biol. 2015:1667–1676. doi: 10.1242/jeb.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rendon NM, Rudolph LM, Sengelaub DR, Demas GE. The agonistic adrenal : melatonin elicits female aggression via regulation of adrenal androgens. Proc R Soc B Biol Sci. 2015;282:20152. doi: 10.1098/rspb.2015.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short-day increases in aggression are inversely related to circulating testosterone concentrations in male Siberian hamsters (Phodopus sungorus) Horm Behav. 2000;38:102–110. doi: 10.1006/hbeh.2000.1604. [DOI] [PubMed] [Google Scholar]
- 34.Sylvia KE, Jewell CP, Rendon NM, St John EA, Demas GE. Sex-specific modulation of the gut microbiome and behavior in Siberian hamsters. Brain Behav Immun. 2016;60:51–62. doi: 10.1016/j.bbi.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 35.Verhoeven KJF, Simonsen KL, McIntyre LM. Implementing false discovery rate control: Increasing your power. Oikos. 2005;108:643–647. doi: 10.1111/j.0030-1299.2005.13727.x. [DOI] [Google Scholar]
- 36.Klein SL, Nelson RJ. Activation of the immune-endocrine system with lipopolysaccharide reduces affiliative behaviors in voles. Behav Neurosci. 1999;113:1042–1048. doi: 10.1037/0735-7044.113.5.1042. [DOI] [PubMed] [Google Scholar]
- 37.Klein SL, Gamble HR, Nelson RJ. Trichinella spiralis Infection in Voles Alters Female Odor Preference but Not Partner Preference Stable. Behav Ecol Sociobiol. 1999;45:323–329. URL : http://www.jstor.org/stable/4601610 REFERENCES Linked references are available on JSTOR for this article : Trichinella spiralis incon in voles al. [Google Scholar]
- 38.Bilbo SD, Klein SL, Devries AC, Nelson RJ. Lipopolysaccharide facilitates partner preference behaviors in female prairie voles. Physiol Behav. 1999;68:151–156. doi: 10.1016/S0031-9384(99)00154-7. [DOI] [PubMed] [Google Scholar]
- 39.Verma R, Balhara YPS, Gupta CS. Gender differences in stress response: Role of developmental and biological determinants. Ind Psychiatry J. 2011;20:4–10. doi: 10.4103/0972-6748.98407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo – pituitary – adrenal axis. Front Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- 42.Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 43.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal Steroid Hormone Receptors and Sex Differences in the Hypothalamo-Pituitary-Adrenal Axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 44.Viau V. YOUNG INVESTIGATOR PERSPECTIVES Functional Cross-Talk Between the Hypothalamic-Pituitary-Gonadal and -Adrenal Axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 45.Fuxjager MJ, Foufopoulos J, Diaz-Uriarte R, Marler CA. Functionally opposing effects of testosterone on two different types of parasite: implications for the immunocompetence handicap hypothesis. Funct Ecol. 2011;25:132–138. doi: 10.1111/j.1365-2435.2010.01784.x. [DOI] [Google Scholar]
- 46.Panagiotakopoulos L, Neigh GN. Frontiers in Neuroendocrinology Development of the HPA axis : Where and when do sex differences manifest? Front Neuroendocrinol. 2014;35:285–302. doi: 10.1016/j.yfrne.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Sylvia KE, Demas GE. A Return to Wisdom: Using Sickness Behaviors to Integrate Ecological and Translational Research. Integr Comp Biol. 2017:1–10. doi: 10.1093/glycob/cwx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopes PC. When is it socially acceptable to feel sick? Proc R Soc B 2014. 2014;281:20140218. doi: 10.1098/rspb.2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopes PC, Adelman J, Wingfield JC, Bentley GE. Social context modulates sickness behavior. Behav Ecol Sociobiol. 2012;66:1421–1428. doi: 10.1007/s00265-012-1397-1. [DOI] [Google Scholar]
- 50.Demas GE, Nelson RJ. Social, but not photoperiodic, influences on reproductive function in male Peromyscus aztecus. Biol Reprod. 1998;58:385–389. doi: 10.1095/biolreprod58.2.385. [DOI] [PubMed] [Google Scholar]
- 51.Zylberberg M, Klasing KC, Hahn TP. House finches (Carpodacus mexicanus) balance investment in behavioural and immunological defences against pathogens. 2012 doi: 10.1098/rsbl.2012.0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tenk CM, Kavaliers M, Ossenkopp KP. Sexually dimorphic effects of neonatal immune system activation with lipopolysaccharide on the behavioural response to a homotypic adult immune challenge. Int J Dev Neurosci. 2008;26:331–8. doi: 10.1016/j.ijdevneu.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Dinel AL, Joffre C, Trifilieff P, Aubert A, Foury A, Le Ruyet P, Layé S. Inflammation early in life is a vulnerability factor for emotional behavior at adolescence and for lipopolysaccharide-induced spatial memory and neurogenesis alteration at adulthood. J Neuroinflammation. 2014;11:155. doi: 10.1186/s12974-014-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- 55.Bilbo D, Staci, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal Infection-Induced Memory Impairment after Lipopolysaccharide in Adulthood Is Prevented via Caspase-1 Inhibition. J Neurosci. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


