Abstract
Background and Aim
Although methadone, an opioid agonist, has been an effective medication used to treat opioid use disorder for over 40 years, recent studies have found that methadone was identified in more than a quarter of prescription opioid-related deaths among people who use illicit drugs in Vancouver, Canada. Thus, we sought to longitudinally examine the availability of diverted methadone among people who inject drugs (PWID).
Design and Methods
Data were collected from three prospective cohorts of PWID in Vancouver, Canada between December 2005 and May 2015. Multivariable generalized estimating equation logistic regression was used to identify temporal trends in the immediate availability of diverted methadone (defined as the ability to acquire illicit methadone in <10 minutes).
Results
A total of 2092 participants, including 727 (34.8%) women, were included in the present study. In the multivariable analyses after adjusting for a range of potential confounders, later calendar year (adjusted odds ratio [AOR] = 1.21 per year; 95% confidence interval [CI]: 1.19–1.23) was independently and positively associated with reporting immediate availability of diverted methadone.
Conclusions
We observed a significant increase in the reported availability of diverted methadone among PWID over a ten-year follow-up period. Further research is needed to identify strategies to limit methadone diversion and assess the impact of alternative medications that are equally effective but safer, such as buprenorphine/naloxone.
Keywords: methadone, diversion, opioid, overdose, illicit drug use
1. INTRODUCTION
In the past decade, opioid use disorder (OUD) has become a major public health challenge in Canada and the United States (Gomes et al., 2011; United Nations Office on Drugs and Crime, 2014). Increases in the availability of heroin and other illicit opioids, significant increase in opioid prescription, and diversion of prescription opioids have contributed to the morbidity and mortality associated with OUD (Carter, 2012; Compton, Jones, & Baldwin, 2016; King, Fraser, Boikos, Richardson, & Harper, 2014; Nosyk et al., 2012). Specific consequences of OUD include increased risk of blood-borne disease including viral hepatitis and HIV, as well as opioid related overdose and death (Peters et al., 2016; Suryaprasad et al., 2014; Zibbell, Hart-Malloy, Barry, Fan, & Flanigan, 2014) (Dart et al., 2015; Fischer, Jones, & Rehm, 2013; Gomes et al., 2014).
Methadone maintenance therapy (MMT) is a longstanding pharmacotherapy prescribed for OUD (Nosyk, Marsh, Sun, Schechter, & Anis, 2010). In British Columbia, Canada, MMT clinical practice guidelines were introduced in 1995 and have been expanded to include strategies to monitor and titrate daily dosing, treatment initiation, maintenance dosing, and take-home doses (College of Physicians and Surgeons of British Columbia, 2005; Payte, 1995). With an exemption under section 56 of the Controlled Drugs and Substances Act, primary care physicians can prescribe methadone that is dispensed through community pharmacies. Between 1996 and 2016, the number of patients receiving methadone has increased nearly six-fold in British Columbia (Nosyk et al., 2010; Office of the Provincial Health Officer, 2017). MMT has been shown to effectively decrease illicit opioid use, reduce rates of hepatitis C and HIV infection among people who inject drugs (PWID) and improve antiretroviral adherence and virologic outcomes among people living with HIV (Gowing, Farrell, Bornemann, Sullivan, & Ali, 2011; Low et al., 2016; MacArthur et al., 2012; Perlman et al., 2015).
However, the risk of toxicity and adverse events associated with methadone is greater than other prescription opioids due to the narrow therapeutic index, long and highly variable half-life, and potential for drug-drug interactions (British Columbia Centre on Substance Use, 2017; Webster et al., 2011). In the United States, the rate of methadone related emergency room visits has been found to be approximately six times greater than prescription oxycodone and 23 times greater than prescription hydrocodone after adjusting for the total number of prescriptions dispensed (Substance Abuse and Mental Health Services Administration 2013; Webster et al., 2011). While methadone prescription has been linked to diversion and methadone-related overdose in several countries, some settings have successfully expanded methadone programs without an increase in mortality risk (Fugelstad, Lars, & Thiblin, 2010; Iwersen-Bergmann et al., 2014; Morgan, Griffiths, & Hickman, 2006; Strang, Hall, Hickman, & Bird, 2010). In the United States, methadone was identified in more than a third of opioid-related deaths in 2009 despite representing 5–19% of all opioid prescriptions per year (Centers for Disease & Prevention, 2012). Although recent data have shown a decrease in methadone-related overdose deaths in the United States, they still accounted for 3400 overdose deaths in 2014 (Jones, Baldwin, Manocchio, White, & Mack, 2016). A recent study from British Columbia, Canada, reported similar results and found that methadone was involved in 25% of opioid-related deaths (Gladstone, Smolina, & Morgan, 2016). Thus, there seems to be regional differences in whether or not methadone prescription is associated with increases in overdose mortality.
Many factors have been consistently associated with methadone-related overdose. These factors include non-prescribed, diverted and illicit use of methadone, as well as polysubstance use involving alcohol and benzodiazepines (Duffy P, 2014; Jones et al., 2016; Strang et al., 2010; Tjagvad et al., 2016). However, few studies have examined the diverted methadone market. A recent study from the United States indicated methadone prescription for pain, as opposed to treatment for OUD, was the primary source of diversion, although other evidence indicates that this may be moderated by regional differences in prescription programs and the accessibility of methadone (Johnson & Richert, 2015b; Jones et al., 2016). While the primary source of methadone diversion in Vancouver, Canada is uncertain, previous studies reported that there were 344 active prescribers of methadone for OUD in 2012, compared to 685 who were authorized to prescribe methadone for analgesia, with a significant proportion being pain or palliative care specialists (Hawley, 2012; Office of the Provincial Health Officer, 2017). Given the association between methadone diversion and methadone-related overdose, we sought to longitudinally examine the availability of diverted methadone among three community-recruited prospective cohortsof PWI D in Vancouver, British Columbia.
2. METHODS
2.1 Study Procedure
The data for this investigation were obtained from three ongoing open prospective cohort studies of people who use drugs: the Vancouver Injection Drug Users Study (VIDUS), the AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS), and the At-Risk Youth Study (ARYS). These studies have been described in detail previously (Tyndall et al., 2003; Wood, Stoltz, Montaner, & Kerr, 2006). To provide a brief overview, VIDUS enrolls HIV-negative people who inject drugs, ACCESS enrolls HIV-positive people who use illicit drugs other than or in addition to cannabis (Strathdee et al., 1998; Wood et al., 2009) and ARYS enrolls street-involved youth who use illicit drugs, other than or in addition to cannabis, and are 14–26 years old (Wood et al., 2006). Participants in all three studies have to reside in the Greater Vancouver region and provide informed consent at study enrollment.
Data related to sociodemographic information, substance use patterns, HIV risk behavior and engagement with health and social services including addiction treatment were collected through an interview-administered questionnaire at baseline and semi-annually over follow-up. All three studies applied harmonized recruitment and data collection methods to facilitate pooled analyses. Participants are remunerated $30 CAD for each study visit. These studies have been approved by the University of British Columbia/Providence Health Care Research Ethics Board.
2.2 Participants and Outcome Measure
This study included all VIDUS, ACCESS and ARYS participants aged ≥18 years who had reported ever injecting drugs or initiated injecting during follow-up, completed at least one study visit between December 2005 and May 2015, had ever used heroin or any prescription opioid by injection or non-injection, and provided a valid answer to questions regarding the availability of diverted methadone. The sample was restricted to people who inject drugs or people with a history of injection drug use since they are at a high risk of experiencing harms associated with diverted methadone such as overdosing (Kerr et al., 2007). The primary outcome of interest was diverted methadone availability from street-based sources, which was derived from a question, “How difficult would it be for you to get street methadone right now in the area where you typically obtain your drugs?” The five response options included (1) within 10 minutes; (2) within 90 minutes; (3) within a day; (4) in more than a day; (5) could not access this drug. These categories were collapsed to create a three-level categorical variable for this study: (1) immediate availability (within 10 minutes); (2) delayed availability (i.e., > 10 minutes); and (3) not available. Since very few participants selected responses (2)–(4) (i.e., within 90 minutes; within a day; in more than a day), these categories were collapsed to create a three-level categorical variable for this study: 1) immediate availability (within 10 minutes); (2) delayed availability (i.e., > 10 minutes); and (3) not available. Retaining response options with small sample sizes can lead to unstable effect estimates, and combining these response options mitigated this issue (MacCallum, 1999). We examined correlates of immediate and delayed availability of diverted methadone, respectively, against no availability.
2.3 Explanatory Variables
In order to examine temporal trends of the availability of diverted methadone, we included calendar year of interview (per year later) as the primary explanatory variable. The selection of additional explanatory variables that may impact the association between the primary explanatory variable and the availability of diverted methadone was based on existing literature (Hadland et al., 2010; Lake et al., 2015; Nosyk et al., 2012). These variables included socio-demographic factors such as age (per 10 years older), sex (male vs. female), ethnicity/ancestry (white vs. others), residing in the Downtown Eastside (DTES) neighborhood of Vancouver which contains a large open drug scene (yes vs. no), cohort designation (ARYS vs. ACCESS vs. VIDUS [the reference category]) and homelessness (yes vs. no). Drug use patterns included: crack smoking (≥ daily vs. < daily); injection heroin use (≥ daily vs. < daily); injection cocaine use (≥ daily vs. < daily); injection crystal methamphetamine use (≥ daily vs. < daily); injection of prescription opioids (≥ daily vs. < daily); and non-fatal overdose (yes vs. no). Variables related to health and social service utilization included: enrollment in MMT (yes vs. no), and having tried but been unable to access addiction treatment services (yes vs. no or never tried). Other social and structural exposures included: incarceration (yes vs. no); involvement in drug dealing (yes vs. no); and involvement in sex work (yes vs. no). Variable definitions were consistent with previous studies and all behavioral variables referred to the previous 6 months (Wood et al., 2001).
2.4 Statistical Analysis
As a first step, we analyzed the baseline sample characteristics stratified by availability of diverted methadone (immediate vs. delayed vs. no availability), using Cochran-Armitage trend test for categorical variables and Kruskal-Wallis test for continuous variables. We also graphically illustrated temporal trends in the availability during the study period. Since repeated measures were available for each participant, we applied generalized estimating equation (GEE) logistic regression for the analysis of correlated data. By using an exchangeable correlation structure that adjusts for multiple observations for each participant, this method identified factors associated with the outcome across the entire study period. The initial multivariable model included all explanatory variables associated with diverted methadone availability at the level of p < 0.10 in bivariable analyses. Through a manual stepwise approach, reduced models were built by removing one secondary explanatory variable at a time that produced the smallest relative change in the calendar year coefficient. This process was repeated until the minimum change in the calendar year coefficient surpassed five percent. The objective of this technique is to retain covariates with a larger relative impact on the association between the primary explanatory variable and the outcome (Maldonado & Greenland, 1993).
We also performed a sub-analysis to examine changes in the self-reported price of diverted methadone during the study period. The median price of diverted methadone was presented for each calendar year. All statistical analyses were performed using SAS version 9.4 (SAS Institute, USA). All tests of significance were two sided.
3. RESULTS
During the study period (December 2005 and May 2015), a total of 2092 participants enrolled in the VIDUS, ACCESS or ARYS were eligible for the present study. The median age of participants was 37.3 (interquartile range [IQR] = 26.2–45.9) years at baseline. Seven hundred and twenty-seven (34.8%) were females and 1324 (63.3%) were white. Participants completed a median of six study visits (IQR = 2–12) and the median observation time per participant was 49.0 months (IQR = 12.0–92.0). At baseline, 601 (28.7%) participants reported having immediate access to diverted methadone, 387 (18.5%) individuals reported delayed availability, and 1104 (52.8%) participants were not able to access diverted methadone.
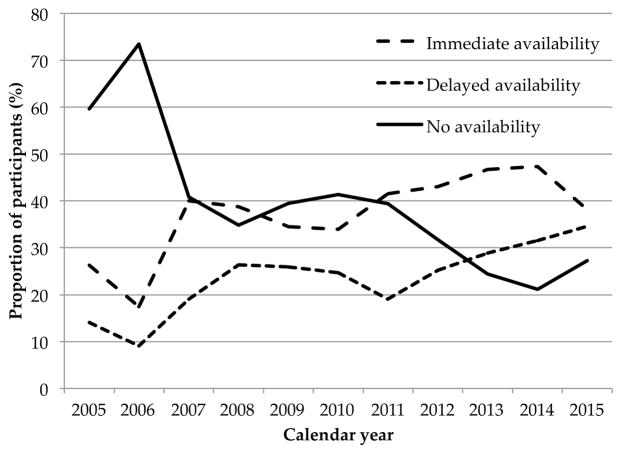
Baseline characteristics of the study sample stratified by diverted methadone availability are shown in Table 1. Factors that differed across diverted methadone availability strata included age, sex, homelessness, DTES residence, involvement in drug dealing, prescription opioid injection, participation in MMT and being unable to access addiction treatment (all p < 0.05). Unadjusted temporal trends in the availability of diverted methadone are illustrated in Figure 1.
Table 1.
Baseline characteristics stratified by diverted methadone availability among opioid users in Vancouver, Canada, 2005–2015 (n = 2092).
| Diverted methadone Availability
|
p-value | |||
|---|---|---|---|---|
| Not Available (n=1104) n (%) |
Delayed Availability (n=387) n (%) |
Immediate Availability (n=601) n (%) |
||
| Age (per additional year) | ||||
| Median | 37.9 | 34.1 | 38.8 | <0.001 |
| IQR | (26.8 – 45.9) | (23.7 – 44.3) | (28.3 – 46.9) | |
| White | ||||
| Yes | 671 (50.7) | 271 (20.5) | 382 (28.9) | 0.126 |
| No | 433 (56.4) | 116 (15.1) | 219 (28.5) | |
| Female | ||||
| Yes | 412 (56.6) | 119 (16.4) | 196 (27.0) | 0.030 |
| No | 692 (50.7) | 268 (19.6) | 405 (29.7) | |
| HomelessA | ||||
| Yes | 472 (49.9) | 186 (19.6) | 289 (30.5) | 0.022 |
| No | 630 (55.3) | 199 (17.5) | 310 (27.2) | |
| DTES residenceA,B | ||||
| Yes | 662 (49.8) | 212 (16.0) | 455 (34.2) | <0.001 |
| No | 442 (57.9) | 175 (22.9) | 146 (19.2) | |
| Dealing drugsA | ||||
| Yes | 377 (44.8) | 155 (18.4) | 309 (36.8) | <0.001 |
| No | 727 (58.1) | 232 (18.5) | 292 (23.4) | |
| Sex work involvementA | ||||
| Yes | 186 (53.7) | 65 (18.8) | 95 (27.5) | 0.570 |
| No | 911 (52.5) | 320 (18.4) | 505 (29.1) | |
| Prescription opioid injectionA | ||||
| ≥ daily | 65 (44.5) | 23 (15.8) | 58 (39.7) | 0.006 |
| < daily | 1036 (53.4) | 364 (18.8) | 541 (27.8) | |
| Injection heroin useA | ||||
| ≥ daily | 346 (52.5) | 111 (16.9) | 201 (30.6) | 0.491 |
| < daily | 756 (52.8) | 276 (19.3) | 400 (27.9) | |
| Injection cocaine useA | ||||
| ≥ daily | 90 (53.6) | 19 (11.3) | 59 (35.1) | 0.398 |
| < daily | 1010 (52.6) | 368 (19.2) | 542 (28.2) | |
| Injection methamphetamine useA | ||||
| ≥ daily | 58 (40.6) | 37 (25.8) | 48 (33.6) | 0.152 |
| < daily | 1042 (53.6) | 350 (18.0) | 552 (28.4) | |
| Non-injection crack useA | ||||
| ≥ daily | 402 (52.3) | 121 (15.8) | 245 (31.9) | 0.152 |
| < daily | 701 (53.0) | 265 (20.1) | 356 (26.9) | |
| Non-fatal overdoseA | ||||
| Yes | 121 (48.7) | 48 (19.4) | 79 (31.9) | 0.174 |
| No | 980 (53.3) | 339 (18.4) | 521 (28.3) | |
| Participated in MMTA | ||||
| Yes | 402 (48.2) | 162 (19.5) | 269 (32.3) | <0.001 |
| No | 697 (55.9) | 223 (17.9) | 328 (26.2) | |
| Unable to access addiction treatmentA | ||||
| Yes | 83 (40.7) | 49 (24.0) | 72 (35.3) | 0.001 |
| No | 1015 (54.1) | 336 (17.9) | 525 (28.0) | |
| IncarcerationA | ||||
| Yes | 214 (50.8) | 82 (19.5) | 125 (29.7) | 0.481 |
| No | 882 (53.1) | 305 (18.3) | 475 (28.6) | |
| Cohort designation | ||||
| ACCESS | 301 (50.3) | 114 (19.0) | 184 (30.7) | 0.307 |
| ARYS | 239 (49.4) | 126 (26.0) | 119 (24.6) | |
| VIDUS | 564 (55.9) | 147 (14.6) | 298 (29.5) | |
denotes activities in the six months prior to follow-up interview
DTES – Downtown Eastside; MMT – Methadone Maintenance Therapy; ACCESS – AIDS Care Cohort to Evaluate Exposure to Survival Services; VIDUS – Vancouver Injection Drug Users Study; ARYS – At-Risk Youth Study.
Figure 1.
Trends in diverted methadone availability over the study period.
Table 2 includes two models showing the bivariable and multivariable GEE analyses of the temporal trends of the availability of diverted methadone. Model 1 analyzed trends of the immediate availability (vs. not available) and the adjusted analysis revealed that calendar year remained independently and positively associated with immediate availability (adjusted odds ratio [AOR] = 1.21; 95% confidence interval [CI]: 1.19–1.23). Model 2 analyzed trends of the delayed availability (vs. not available) and in the adjusted model, the calendar year (AOR = 1.21; 95% CI: 1.19–1.23) was also independently and positively associated with delayed availability. The median self-reported price of diverted methadone remained at$10 CADper 100mgin every calendar year of follow-up.
Table 2.
Bivariable and multivariable GEE analysis of factors associated with methadone availability
| Characteristic | Model 1. Immediate availability vs. not available | Model 2. Delayed availability vs. not available | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
|
| ||||||||
| Odds Ratio (95% CI) | p - value | Odds Ratio (95% CI) | p - value | Odds Ratio (95% CI) | p - value | Odds Ratio (95% CI) | p - value | |
| Calendar year | ||||||||
| (per year later) | 1.18 (1.16 – 1.20) | <0.001 | 1.21 (1.19 – 1.23) | <0.001 | 1.28 (1.18 – 1.22) | <0.001 | 1.21 (1.19 – 1.23) | <0.001 |
| Age | ||||||||
| (per 10 years older) | 1.45 (1.37 – 1.53) | <0.001 | 1.16 (1.08 – 1.24) | <0.001 | 1.16 (1.10 – 1.22) | <0.001 | ||
| White | ||||||||
| (yes vs. no) | 1.14 (1.01 – 1.28) | 0.029 | 1.45 (1.28 – 1.64) | <0.001 | ||||
| Sex | ||||||||
| (male vs. female) | 1.26 (1.12 – 1.42) | <0.001 | 1.28 (1.13 – 1.45) | <0.001 | ||||
| HomelessA | ||||||||
| (yes vs. no) | 0.83 (0.76 – 0.91) | <0.001 | 0.80 (0.72 – 0.88) | <0.001 | ||||
| DTES residenceA,B | ||||||||
| (yes vs. no) | 1.51 (1.37 – 1.66) | <0.001 | 1.65 (1.50 – 1.82) | <0.001 | 0.90 (0.82 – 1.00) | 0.041 | ||
| Dealing drugsA | ||||||||
| (yes vs. no) | 1.25 (1.14 – 1.37) | <0.001 | 1.62 (1.47 – 1.78) | <0.001 | 1.28 (1.15 – 1.42) | <0.001 | 1.57 (1.41 – 1.75) | <0.001 |
| Sex work involvement | ||||||||
| (yes vs. no)A | 0.92 (0.79 – 1.06) | 0.255 | 0.99 (0.85 – 1.16) | 0.905 | ||||
| Prescription opioid injectionA | ||||||||
| (≥ daily vs. <daily) | 1.27 (1.07 – 1.51) | 0.005 | 1.18 (0.96 – 1.47) | 0.125 | ||||
| Injection heroin useA | ||||||||
| (≥ daily vs. <daily) | 1.01 (0.91 – 1.12) | 0.827 | 0.93 (0.83 – 1.04) | 0.214 | ||||
| Injection cocaine useA | ||||||||
| (≥ daily vs. <daily) | 1.07 (0.92 – 1.25) | 0.362 | 0.82 (0.69 – 0.98) | 0.032 | ||||
| Injection methamphetamine useA | ||||||||
| (≥ daily vs. <daily) | 1.17 (0.98 – 1.41) | 0.078 | 1.21 (0.99 – 1.47) | 0.063 | ||||
| Non-injection crack useA | ||||||||
| (≥ daily vs. <daily) | 0.92 (0.84 – 1.01) | 0.075 | 0.78 (0.70 – 0.87) | <0.001 | ||||
| Non-fatal overdoseA | ||||||||
| (yes vs. no) | 1.01 (0.88 – 1.17) | 0.891 | 1.13 (0.96 – 1.33) | 0.147 | ||||
| Participated in MMTA | ||||||||
| (yes vs. no) | 1.51 (1.37 – 1.66) | <0.001 | 1.57 (1.42 – 1.75) | <0.001 | ||||
| Unable to access addiction treatmentA | ||||||||
| (yes vs. no) | 1.03 (0.89 – 1.19) | 0.695 | 1.19 (1.00 – 1.41) | 0.049 | ||||
| IncarcerationA | ||||||||
| (yes vs. no) | 0.82 (0.73 – 0.93) | 0.001 | 0.89 (0.77 – 1.02) | 0.101 | ||||
| Cohort designation | ||||||||
| (ACCESS vs. VIDUS) | 0.98 (0.86 – 1.10) | 0.710 | 0.85 (0.75 – 0.96) | 0.010 | 0.98 (0.86 – 1.11) | 0.721 | ||
| (ARYS vs. VIDUS) | 0.50 (0.42 – 0.60) | <0.001 | 1.16 (1.08 – 1.24) | <0.001 | 0.99 (0.84 – 1.17) | 0.931 | ||
Refers to the 6-month period before the interview.
DTES – Downtown Eastside; MMT – Methadone Maintenance Therapy; ACCESS – AIDS Care Cohort to Evaluate Exposure to Survival Services; VIDUS – Vancouver Injection Drug Users Study; ARYS – At-Risk Youth Study.
4. DISCUSSION
In the present study, we observed a significant increase in the reported availability of diverted methadone among a community-recruited sample of PWID in Vancouver between 2005 and 2015 even after adjustment for a range of potential demographic, behavioural and social-structural confounders. The price of diverted methadone remained consistent at $10 CAD per 100mg over the ten-year follow-up period, with a median price of $10 CAD per 100mg reported at every year of the study period. This price was substantially less than that identified from studies in the United States, which reported a price of approximately $20 US per 80mg dose (Gwin Mitchell et al., 2009).
A number of existing studies have contextualized the illicit market for methadone and buprenorphine (Cicero & Inciardi, 2005; Spunt, 1986). This market is different from the trafficking of other illicit opiates such as heroin, in that it consists of several individuals, primarily, patients undergoing opioid agonist therapy, who are selling small quantities of their prescription medication (Agar, 1977; Fountain, Strang, Gossop, Farrell, & Griffiths, 2000). A number of individual, social and structural risk factors for diversion have also been identified. Three consistently recognized risk factors for a patient diverting their medication include current illicit drug use, previous experience of purchasing illicit methadone or buprenorphine and socializing with active drug users (Johnson & Richert, 2015b). Supervised dosing and strict collection routines were associated with a decreased risk of diversion in some studies, yet other studies did not report this association to be significant (Dale-Perera, 2012; Duffy & Baldwin, 2012; Spunt, 1986; Winstock, Lea, & Sheridan, 2008). Specific diversion methods have been understudied, but obtaining more than one prescription (multiple prescription) and exaggerating the severity of drug addiction to receive a higher dose (overprescription) have been reported as strategies by methadone patients (Fountain, 1998). The two primary motives for diversion include a financial need, which may or may not involve supporting existing drug use, and altruistic motives to support others who are experiencing withdrawal. Ethnographic researchers have identified that people who use drugs often develop a norm system where it is considered unethical to not share drugs with friends or acquaintances who are suffering from withdrawal. This has been termed ‘a moral economy of sharing’ that is based on empathizing with others in withdrawal and can also be influenced by the expectation that the recipient will pay for the medication (Bourgois, 1998; Bourgois, & Schonberg, 2009; Havnes, Clausen, & Middelthon, 2013). The altruistic motive may be a more significant factor since a previous study reported ‘helping others’ was cited as a motive for diversion by 90% of participants while only 40% reported ‘financial need’ as the purpose (Johnson & Richert, 2015c)
Increased diversion of methadone from opioid agonist therapy has been associated with methadone-related fatalities in several countries (Jones et al., 2016; Morgan et al., 2006; Seymour et al., 2003; Strang et al., 2010). Our results suggest that methadone diversion may have increased between 2005 and 2015, coinciding with a steady expansion of MMT programs in this setting during this period (College of Physicians and Surgeons of British Columbia, 2014). During the study period, the total number of patients receiving methadone in British Columbia increased from approximately 8,000 in 2005, to 16,900 in 2015 (Office of the Provincial Health Officer, 2017) (Office of the Provincial Health Officer, 2013). However, between 2006/2007 and 2007/2008, the number of active methadone prescribers decreased from over 400 to less than 300. This may be reflected in our trend data (Figure 1), which indicate a plateau in the availability of illicit methadone from 2007 to 2011. Between 2011 and 2015, the number of active opioid agonist therapy prescribers increased from 328 to 401 (Office of the Provincial Health Officer, 2013; Office of the Provincial Health Officer, 2017).
In addition to concerns regarding methadone diversion, the risk of toxicity and adverse events associated with methadone provoked policy change for the treatment of OUD. In 2017, health authorities in British Columbia also released new OUD treatment guidelines, which recommended replacing methadone with buprenorphine-naloxone as the preferred first-line opioid agonist therapy due to the improved public safety profile with similar treatment efficacy (British Columbia Centre on Substance Use, 2017; Dunlap & Cifu, 2016). This guideline also proposed a range of more stringent guidelines including urine drug screening and unannounced medication checks for those with take home dosing as a strategy to reduce diversion. While our findings provide support for such change, the control and support measures should incorporate existing evidence from illicit methadone markets. Increased dosing supervision significantly reduced methadone-related mortality among methadone programs in Scotland and England, yet stringent supervision can also decrease the autonomy of patients, be perceived as an indignity and create an obstacle to gainful employment (Johnson & Richert, 2015b; Morgan et al., 2006; Strang et al., 2010). Patients who are regular diverters also seem to be relatively unaffected by the threat of sanctions, which suggests that broad increases in controls may have little effect on these diverters and impose unnecessary restrictions on rule following patients (Johnson & Richert, 2015b). A more promising strategy for minimizing diversion may be improved management of comorbid psychological and physical health issues among opioid agonist therapy patients. Ongoing illicit drug use and heavy alcohol consumption are associated with diversion, and may represent forms of self-medication for unmanaged psychological and physical symptoms. Sustained follow-up with patients to evaluate dosing protocols and side effects is also recommended since these issues are potential triggers for diversion (Johnson & Richert, 2015b). Increasing the accessibility of buprenorphine-naloxone in opioid agonist therapies is also likely to improve health outcomes among patients with OUD.
Buprenorphine-naloxone is generally viewed to be equally effective for reducing illicit opioid use and sustaining treatment enrolment as methadone (Kakko et al., 2007; Mattick, Breen, Kimber, & Davoli, 2014; Nielsen et al., 2016). The advantages of buprenorphine-naloxone include a decreased risk of overdose due to its partial agonist effect, reduced public-health risk with diversion and injection based on the naloxone component, milder side effect profile and fewer drug-drug interactions (Bell, Butler, Lawrance, Batey, & Salmelainen, 2009; Chou, Weimer, & Dana, 2014; D’Amore et al., 2011). In addition, buprenorphine-naloxone may be more cost effective than methadone since the shorter induction period and more flexible take-home dosing schedules reduce pharmacy dispensing costs and require fewer clinical visits to achieve a stable dose (Auriacombe, Franques, & Tignol, 2001; CADTH Rapid Response Reports, 2013). Although there is a decreased risk of diversion with buprenorphine-naloxone due to a lower abuse potential and lower street value, diversion and misuse of buprenorphine-naloxone is still a concern for opioid agonist therapy programs (Johnson & Richert, 2015a). A recent study of patients receiving buprenorphine for opioid detoxification found that sharing medication was perceived as a normative behaviour and 50% of the patients studied reported sharing their medication in the past (Kenney, Anderson, Bailey, & Stein, 2017). There is evidence that methadone may be preferable to buprenorphine-naloxone for individuals at a high risk of dropout or for those who continue to experience withdrawal symptoms despite receiving an optimal buprenorphine-naloxone dose (Srivastava, Kahan, & Nader, 2017). Buprenorphine-naloxone induction can also produce severe precipitated withdrawal if the induction is done incorrectly, which does not occur with methadone treatment (Rosado, Walsh, Bigelow, & Strain, 2007). However, the partial agonist effect of buprenorphine-naloxone makes it more manageable for patients to switch from buprenorphine-naloxone to methadone if needed, which supports buprenorphine-naloxone as the preferred first-line option if contraindications are not present (Breen et al., 2003; Walsh et al., 1995). Based on the many advantages of buprenorphine-naloxone over methadone, these guidelines may help to alleviate morbidity and mortality among PWID living with OUD in our setting.
The major strength of this study is the prospective cohort design, which permitted the analysis of multiple independent correlates of diverted methadone availability over a ten-year follow-up period. This study also has several limitations. Since the participants were not randomly recruited, these results may not be generalizable to PWID in this and other settings. While self-report methods generally provide valid and reliable measurements among PWID, socially desirable responding and recall bias remain concerns (Darke, 1998). Lastly, there is a possibility that residual confounding may have impacted the associations with diverted methadone availability since this is an observational design.
In summary, we found that the availability of diverted methadone among PWID significantly increased between 2005 and 2015. Given the increasing concerns of diverted methadone and methadone-related overdose, it is encouraging that buprenorphine-naloxone has been endorsed as a preferred first-line treatment for OUD. Since methadone is still recommended for patients who are ineligible for buprenorphine-naloxone, interventions to reduce methadone diversion must consider how treatment access for those seeking treatment for OUD will be affected. Local guidelines have recently recommended strategies to reduce diversion, and future evaluation should examine the impacts of these changes (British Columbia Centre on Substance Use, 2017).
HIGHLIGHTS.
Methadone has been implicated in opioid-related deaths among illicit drug users.
We examined the availability of diverted methadone among people who inject drugs.
The availability of diverted methadone significantly increased between 2005 and 2015.
Strategies to limit methadone diversion and evaluate alternatives are needed.
Acknowledgments
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff. The study was supported by the US National Institutes of Health (NIH) (U01DA038886, R01DA021525). This research was undertaken, in part, thanks to funding from the Canada Research Chairs program through a Tier 1 Canada Research Chair in Inner City Medicine which supports Dr. Evan Wood, as well as a Canadian Institutes of Health Research (CIHR) Foundation grant supporting Dr. Thomas Kerr (FDN-148476). Dr. Kanna Hayashi is supported by a CIHR New Investigator Award (MSH-141971). Dr. Kora DeBeck is supported by a Michael Smith Foundation for Health Research (MSFHR)/St. Paul’s Hospital Foundation– Providence Health Care Career Scholar Award and a CIHR New Investigator Award. Dr. M-J Milloy is supported by a CIHR New Investigator Award, a MSFHR Scholar Award, and the NIH (R01-DA0251525). His institution has received an unstructured gift from NG Biomed, Ltd., to support his research. Dr. Keith Ahamad is supported by a CIHR Embedded Clinician Researcher Award (TIC-368775).
Footnotes
Declaration of interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agar MH. Going through the changes: methadone in New York City. Human Organ. 1977;36(3):291–295. [Google Scholar]
- Auriacombe M, Franques P, Tignol J. Deaths attributable to methadone vs buprenorphine in France. JAMA. 2001;285(1):45. doi: 10.1001/jama.285.1.45. [DOI] [PubMed] [Google Scholar]
- Bell JR, Butler B, Lawrance A, Batey R, Salmelainen P. Comparing overdose mortality associated with methadone and buprenorphine treatment. Drug Alcohol Depend. 2009;104(1–2):73–77. doi: 10.1016/j.drugalcdep.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Bourgois P. The moral economies of homeless heroin addicts: confronting ethnography, HIV risk, and everyday violence in San Francisco shooting encampments. Subst Use Misuse. 1998;33(11):2323–2351. doi: 10.3109/10826089809056260. [DOI] [PubMed] [Google Scholar]
- Bourgois P, Schonberg J. Righteous dopefiend. Berkeley: University of California Press; 2009. [Google Scholar]
- Breen CL, Harris SJ, Lintzeris N, Mattick RP, Hawken L, Bell J, … Mendoza E. Cessation of methadone maintenance treatment using buprenorphine: transfer from methadone to buprenorphine and subsequent buprenorphine reductions. Drug Alcohol Depend. 2003;71(1):49–55. doi: 10.1016/s0376-8716(03)00071-1. [DOI] [PubMed] [Google Scholar]
- British Columbia Centre on Substance Use. A Guideline for the Clinical Management of Opioid Use Disorder. 2017 Retrieved from http://www.bccsu.ca/care-guidance-publications/
- CADTH Rapid Response Reports. Suboxone versus Methadone for the Treatment of Opioid Dependence: A Review of the Clinical and Cost-effectiveness. Ottawa (ON): 2013. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24716256. [PubMed] [Google Scholar]
- Carter CI, Graham B. Opioid overdose prevention & response in Canada. Vancouver, BC, Canada: Canadian Drug Policy Coalition; 2012. [Google Scholar]
- Centers for Disease C & Prevention. Vital signs: risk for overdose from methadone used for pain relief - United States, 1999–2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493–497. [PubMed] [Google Scholar]
- Chou R, Weimer MB, Dana T. Methadone overdose and cardiac arrhythmia potential: findings from a review of the evidence for an American Pain Society and College on Problems of Drug Dependence clinical practice guideline. J Pain. 2014;15(4):338–365. doi: 10.1016/j.jpain.2014.01.495. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA. Diversion and abuse of methadone prescribed for pain management. JAMA. 2005;293(3):297–298. doi: 10.1001/jama.293.3.297. [DOI] [PubMed] [Google Scholar]
- College of Physicians and Surgeons of British Columbia. Methadone maintenance handbook. 2005 Retrieved from http://www.bcguidelines.ca/gpac/guideline_methadone.html.
- College of Physicians and Surgeons of British Columbia. An important update from the methadone maintenance program. 2014 Retrieved from https://http://www.cpsbc.ca/files/pdf/Methadone-Methadose-Update-2014-01.pdf.
- Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med. 2016;374(2):154–163. doi: 10.1056/NEJMra1508490. [DOI] [PubMed] [Google Scholar]
- D’Amore MM, Cheng DM, Kressin NR, Jones J, Samet JH, Winter M, … Saitz R. Oral health of substance-dependent individuals: impact of specific substances. J Subst Abuse Treat. 2011;41(2):179–185. doi: 10.1016/j.jsat.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Perera A, Goulão J, Stöver H. Quality of care provided to patients receiving opioid maintenance treatment in Europe: Results from the EQUATOR analysis. Heroin Addiction and Related Clinical Problems. 2012;14:23–38. [Google Scholar]
- Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend. 1998;51(3):253–263. doi: 10.1016/s0376-8716(98)00028-3. discussion 267-258. [DOI] [PubMed] [Google Scholar]
- Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- Duffy P, Baldwin H. The nature of methadone diversion in England: a Merseyside case study. Harm Reduct J. 2012;9:3. doi: 10.1186/1477-7517-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy PMA. Use and diversion of illicit methadone — under what circumstances does it occur, and potential risks associated with continued use of other substances. J Subst Abuse. 2014;19(1–2):48–55. [Google Scholar]
- Dunlap B, Cifu AS. Clinical Management of Opioid Use Disorder. JAMA. 2016;316(3):338–339. doi: 10.1001/jama.2016.9795. [DOI] [PubMed] [Google Scholar]
- Fischer B, Jones W, Rehm J. High correlations between levels of consumption and mortality related to strong prescription opioid analgesics in British Columbia and Ontario, 2005–2009. Pharmacoepidemiol Drug Saf. 2013;22(4):438–442. doi: 10.1002/pds.3404. [DOI] [PubMed] [Google Scholar]
- Fountain JGP, Farrell M, Gossop M, Strang J. Diversion tactics: how a sample of drug misusers in treatment obtained surplus drugs to sell on the illicit market. Int J Drug Policy. 1998;9(3):159–167. [Google Scholar]
- Fountain J, Strang J, Gossop M, Farrell M, Griffiths P. Diversion of prescribed drugs by drug users in treatment: analysis of the UK market and new data from London. Addiction. 2000;95(3):393–406. doi: 10.1046/j.1360-0443.2000.95339310.x. [DOI] [PubMed] [Google Scholar]
- Fugelstad A, Lars AJ, Thiblin I. More and more methadone deaths. “Leakage” from ongoing more liberal treatment programs might be a cause. Lakartidningen. 2010;107(18):1225–1228. [PubMed] [Google Scholar]
- Gladstone EJ, Smolina K, Morgan SG. Trends and sex differences in prescription opioid deaths in British Columbia, Canada. Inj Prev. 2016;22(4):288–290. doi: 10.1136/injuryprev-2015-041604. [DOI] [PubMed] [Google Scholar]
- Gomes T, Juurlink DN, Dhalla IA, Mailis-Gagnon A, Paterson JM, Mamdani MM. Trends in opioid use and dosing among socioeconomically disadvantaged patients. Open Med. 2011;5(1):e13–22. [PMC free article] [PubMed] [Google Scholar]
- Gomes T, Mamdani MM, Dhalla IA, Cornish S, Paterson JM, Juurlink DN. The burden of premature opioid-related mortality. Addiction. 2014;109(9):1482–1488. doi: 10.1111/add.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2011;(8):CD004145. doi: 10.1002/14651858.CD004145.pub4. [DOI] [PubMed] [Google Scholar]
- Gwin Mitchell S, Kelly SM, Brown BS, Schacht Reisinger H, Peterson JA, Ruhf A, … Schwartz RP. Uses of diverted methadone and buprenorphine by opioid-addicted individuals in Baltimore, Maryland. Am J Addict. 2009;18(5):346–355. doi: 10.3109/10550490903077820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland SE, Kerr T, Marshall BD, Small W, Lai C, Montaner JS, Wood E. Non-injection drug use patterns and history of injection among street youth. Eur Addict Res. 2010;16(2):91–98. doi: 10.1159/000279767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havnes IA, Clausen T, Middelthon AL. ‘Diversion’ of methadone or buprenorphine: ‘harm’ versus ‘helping’. Harm Reduct J. 2013;10:24. doi: 10.1186/1477-7517-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley P. Methadone for pain in palliative care. British Columbia Medical Journal. 2012;54(6):298–301. [Google Scholar]
- Iwersen-Bergmann S, Jungen H, Andresen-Streichert H, Muller A, Elakkary S, Puschel K, Heinemann A. Intravenous methadone application as a serious risk factor for an overdose death: methadone-related fatalities in Hamburg from 2007 to 2012. Int J Legal Med. 2014;128(5):751–764. doi: 10.1007/s00414-014-1017-x. [DOI] [PubMed] [Google Scholar]
- Johnson B, Richert T. Diversion of methadone and buprenorphine by patients in opioid substitution treatment in Sweden: prevalence estimates and risk factors. Int J Drug Policy. 2015a;26(2):183–190. doi: 10.1016/j.drugpo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Johnson B, Richert T. Diversion of methadone and buprenorphine from opioid substitution treatment: patients who regularly sell or share their medication. J Addict Dis. 2015b;34(1):1–17. doi: 10.1080/10550887.2014.975617. [DOI] [PubMed] [Google Scholar]
- Johnson B, Richert T. Diversion of methadone and buprenorphine from opioid substitution treatment: the importance of patients’ attitudes and norms. J Subst Abuse Treat. 2015c;54:50–55. doi: 10.1016/j.jsat.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Jones CM, Baldwin GT, Manocchio T, White JO, Mack KA. Trends in Methadone Distribution for Pain Treatment, Methadone Diversion, and Overdose Deaths - United States, 2002–2014. MMWR Morb Mortal Wkly Rep. 2016;65(26):667–671. doi: 10.15585/mmwr.mm6526a2. [DOI] [PubMed] [Google Scholar]
- Kakko J, Gronbladh L, Svanborg KD, von Wachenfeldt J, Ruck C, Rawlings B, … Heilig M. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry. 2007;164(5):797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]
- Kenney SR, Anderson BJ, Bailey GL, Stein MD. The relationship between diversion-related attitudes and sharing and selling buprenorphine. J Subst Abuse Treat. 2017;78:43–47. doi: 10.1016/j.jsat.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr T, Fairbairn N, Tyndall M, Marsh D, Li K, Montaner J, Wood E. Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend. 2007;87(1):39–45. doi: 10.1016/j.drugalcdep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- King NB, Fraser V, Boikos C, Richardson R, Harper S. Determinants of increased opioid-related mortality in the United States and Canada, 1990–2013: a systematic review. Am J Public Health. 2014;104(8):e32–42. doi: 10.2105/AJPH.2014.301966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake S, Hayashi K, Buxton J, Milloy MJ, Dong H, Wood E, … Kerr T. The effect of prescription opioid injection on the risk of non-fatal overdose among people who inject drugs. Drug Alcohol Depend. 2015;156:297–303. doi: 10.1016/j.drugalcdep.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low AJ, Mburu G, Welton NJ, May MT, Davies CF, French C, … Vickerman P. Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2016;63(8):1094–1104. doi: 10.1093/cid/ciw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, … Hickman M. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Widaman KF, Zhang S, Hong S. Sample size in factor analysis. Psychol Methods. 1999;4(1):84–99. [Google Scholar]
- Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PubMed] [Google Scholar]
- Morgan O, Griffiths C, Hickman M. Association between availability of heroin and methadone and fatal poisoning in England and Wales 1993–2004. Int J Epidemiol. 2006;35(6):1579–1585. doi: 10.1093/ije/dyl207. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database Syst Rev. 2016;(5):CD011117. doi: 10.1002/14651858.CD011117.pub2. [DOI] [PubMed] [Google Scholar]
- Nosyk B, Marsh DC, Sun H, Schechter MT, Anis AH. Trends in methadone maintenance treatment participation, retention, and compliance to dosing guidelines in British Columbia, Canada: 1996–2006. J Subst Abuse Treat. 2010;39(1):22–31. doi: 10.1016/j.jsat.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Nosyk B, Marshall BD, Fischer B, Montaner JS, Wood E, Kerr T. Increases in the availability of prescribed opioids in a Canadian setting. Drug Alcohol Depend. 2012;126(1–2):7–12. doi: 10.1016/j.drugalcdep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Provincial Health Officer. BC Methadone Maintenance System Performance Measures 2011/2012. 2013 Retrieved from http://www2.gov.bc.ca/assets/gov/health/about-bc-s-health-care-system/office-of-the-provincial-health-officer/reports-publications/special-reports/methadone-2011-12.pdf.
- Office of the Provincial Health Officer. BC Opioid Substitution Treatment System: Performance Measures 2014/2015 and 2015/2016. 2017 Retrieved from http://www2.gov.bc.ca/assets/gov/health/about-bc-s-health-care-system/office-of-the-provincial-health-officer/reports-publications/special-reports/bc-ost-system-measures-14-15-and-15-16.pdf.
- Payte JT. Opioid dependence: A practical guide to pharmacotherapy. Vancouver, British Columbia: 1995. [Google Scholar]
- Perlman DC, Jordan AE, Uuskula A, Huong DT, Masson CL, Schackman BR, Des Jarlais DC. An international perspective on using opioid substitution treatment to improve hepatitis C prevention and care for people who inject drugs: Structural barriers and public health potential. Int J Drug Policy. 2015;26(11):1056–1063. doi: 10.1016/j.drugpo.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters PJ, Pontones P, Hoover KW, Patel MR, Galang RR, Shields J … Indiana HIVOIT. HIV Infection Linked to Injection Use of Oxymorphone in Indiana, 2014–2015. N Engl J Med. 2016;375(3):229–239. doi: 10.1056/NEJMoa1515195. [DOI] [PubMed] [Google Scholar]
- Rosado J, Walsh SL, Bigelow GE, Strain EC. Sublingual buprenorphine/naloxone precipitated withdrawal in subjects maintained on 100mg of daily methadone. Drug Alcohol Depend. 2007;90(2–3):261–269. doi: 10.1016/j.drugalcdep.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour A, Black M, Jay J, Cooper G, Weir C, Oliver J. The role of methadone in drug-related deaths in the west of Scotland. Addiction. 2003;98(7):995–1002. doi: 10.1046/j.1360-0443.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- Small W, Maher L, Lawlor J, Wood E, Shannon K, Kerr T. Injection drug users’ involvement in drug dealing in the downtown eastside of Vancouver: social organization and systemic violence. Int J Drug Policy. 2013;24(5):479–487. doi: 10.1016/j.drugpo.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt BHD, Lipton DS, Goldsmith DS. Methadone diversion: a new look. J Drug Issues. 1986;16(4):569–583. [Google Scholar]
- Srivastava A, Kahan M, Nader M. Primary care management of opioid use disorders: Abstinence, methadone, or buprenorphine-naloxone? Can Fam Physician. 2017;63(3):200–205. [PMC free article] [PubMed] [Google Scholar]
- Strang J, Hall W, Hickman M, Bird SM. Impact of supervision of methadone consumption on deaths related to methadone overdose (1993–2008): analyses using OD4 index in England and Scotland. BMJ. 2010;341:c4851. doi: 10.1136/bmj.c4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Palepu A, Cornelisse PG, Yip B, O’Shaughnessy MV, Montaner JS, … Hogg RS. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280(6):547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. 2013 Retrieved from https://http://www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf. [PubMed]
- Suryaprasad AG, White JZ, Xu F, Eichler BA, Hamilton J, Patel A, … Holmberg SD. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis. 2014;59(10):1411–1419. doi: 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- Tjagvad C, Skurtveit S, Linnet K, Andersen LV, Christoffersen DJ, Clausen T. Methadone-Related Overdose Deaths in a Liberal Opioid Maintenance Treatment Programme. Eur Addict Res. 2016;22(5):249–258. doi: 10.1159/000446429. [DOI] [PubMed] [Google Scholar]
- Tyndall MW, Currie S, Spittal P, Li K, Wood E, O’Shaughnessy MV, Schechter MT. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17(6):887–893. doi: 10.1097/01.aids.0000050859.71999.ae. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. New York: World drug report. 2014 Retrieved from https://http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf.
- Walsh SL, June HL, Schuh KJ, Preston KL, Bigelow GE, Stitzer ML. Effects of buprenorphine and methadone in methadone-maintained subjects. Psychopharmacology (Berl) 1995;119(3):268–276. doi: 10.1007/BF02246290. [DOI] [PubMed] [Google Scholar]
- Webster LR, Cochella S, Dasgupta N, Fakata KL, Fine PG, Fishman SM, … Wakeland W. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(Suppl 2):S26–35. doi: 10.1111/j.1526-4637.2011.01134.x. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Lea T, Sheridan J. Prevalence of diversion and injection of methadone and buprenorphine among clients receiving opioid treatment at community pharmacies in New South Wales, Australia. Int J Drug Policy. 2008;19(6):450–458. doi: 10.1016/j.drugpo.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Wood E, Kerr T, Marshall BD, Li K, Zhang R, Hogg RS, … Montaner JS. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E, Stoltz JA, Montaner JS, Kerr T. Evaluating methamphetamine use and risks of injection initiation among street youth: the ARYS study. Harm Reduct J. 2006;3:18. doi: 10.1186/1477-7517-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E, Tyndall MW, Spittal PM, Li K, Kerr T, Hogg RS, … Schechter MT. Unsafe injection practices in a cohort of injection drug users in Vancouver: Could safer injecting rooms help? Canadian Medical Association Journal. 2001;165(4):405–410. [PMC free article] [PubMed] [Google Scholar]
- Zibbell JE, Hart-Malloy R, Barry J, Fan L, Flanigan C. Risk factors for HCV infection among young adults in rural New York who inject prescription opioid analgesics. Am J Public Health. 2014;104(11):2226–2232. doi: 10.2105/AJPH.2014.302142. [DOI] [PMC free article] [PubMed] [Google Scholar]