Abstract
Background:
Genetic variants of TPMT and NUDT15 have been reported to predict the inter-patient variability in response and toxicity profiles of patients receiving thiopurine therapy. However, the clinical utility of TPMT genotyping in guiding thiopurine doses has been questionable, in part due to underlying differences in the prevalence of TPMT variants in both Caucasian and Asian populations. Several NUDT15 variants have been associated with thiopurine-induced leukopenia, particularly in Asian cohorts. So far, none have been reported in a multiethnic Asian population.
Aim:
To investigate the associations between TPMT and NUDT15 variants with thiopurine-induced myelotoxicity in 129 Asian inflammatory bowel disease patients.
Materials & methods:
Pyrosequencing was performed to screen for TPMT and NUDT15 variants. Intracellular steady-state metabolite concentrations were quantified using liquid chromatography–tandem mass spectrometry.
Results:
Significant declines in nadir white blood cell, absolute neutrophil count and platelet counts were observed with increasing copy numbers of the risk T allele at NUDT15 c.415C>T locus (overall p < 0.05) within 4, 8 and 12 weeks and 6 months after thiopurine initiation. Patients with low and intermediate NUDT15 activity, as inferred from haplotype pairs, had significantly higher risks of leukopenia (p = 0.000253) and neutropenia (p = 0.002) compared with patients with normal NUDT15 activity.
Conclusion:
These findings highlight the critical relevance of NUDT15 pharmacogenetics in predicting for thiopurine-induced myelotoxicity and confirm the lack of significance of TPMT variants in Asian inflammatory bowel disease patients.
Keywords: : IBD, NUDT15 polymorphisms, thiopurine-induced myelotoxicity, TPMT
Thiopurine drugs, namely azathioprine (AZA) and 6-mercaptopurine (6-MP), have been effectively used as immunomodulators in inducing clinical remission and reducing steroid dependence in patients with active inflammatory bowel disease (IBD) [1–3]. They have also been shown to be effective in maintaining remission in patients with quiescent disease and remain as the cornerstone of pharmacological management of IBD patients due to their effectiveness, safety and affordability; especially when compared with costly biologic therapies [1–3]. However, up to 30–40% of patients discontinue thiopurine therapy or have their treatments interrupted due to adverse effects, particularly myelosuppression [4,5].
Both AZA and 6-MP are pro-drugs that require bioactivation to exert their therapeutic effects. AZA is converted to 6-MP by glutathione S-transferase, which then undergoes complex metabolism involving three competing pathways that are catalyzed by TPMT, XO and HGPRT [6,7]. 6-MP metabolism via the multistep pathway of HGPRT results in the production of the pharmacologically active 6-thioguanine nucleotides (6-TGN), which are potentially myelotoxic at supratherapeutic levels exceeding 450 pmole/8 × 108 erythrocytes [6,8,9]. 6-MP methylation via the TPMT pathway leads to the formation of potentially hepatotoxic 6-methylmercaptopurine (6-MMPN) metabolites, while XO catalyzes its metabolism into inactive 6-thiouric acid [6,9].
The observed inter-patient variability in response and toxicity profiles have been postulated to be heavily influenced by polymorphisms in the genes encoding the enzymes involved in thiopurine metabolism. Indeed, pretreatment assessment of TPMT genotype status has been recommended in clinical practice guidelines to guide the initial thiopurine doses [10,11]. However, its utility in personalizing thiopurine treatment has remained controversial, particularly in multiethnic Asian population in Singapore where TPMT variant alleles have only been shown to occur at a low overall frequency of 2.5% [12]. Such low prevalence of TPMT variants does not explain the relatively high rates of thiopurine-induced myelotoxicity in Asian IBD patients.
Recently, Yang et al. reported a strong association between a non-synonymous polymorphism in the NUDT15 gene encoding p.Arg139Cys (rs116855232, c.415C>T) with thiopurine-induced leukopenia in Korean patients with Crohn's disease (CD) [13]. Two other studies by Kakuta et al. and Asada et al. also reported similar associations in Japanese IBD patients [14,15]. Moriyama et al. subsequently reported the presence of three additional variants in NUDT15 and their associations with thiopurine intolerance in children with acute lymphoblastic leukemia [16]. To date, the frequencies of these variants in Asian IBD patients in Singapore, and their predictive roles for thiopurine-induced myelotoxicity have not been reported. This study therefore sought to evaluate the associations between TPMT and NUDT15 genetic variants with thiopurine-induced myelotoxicity in Asian IBD patients.
Materials & methods
Study population & thiopurine treatment
Patients with confirmed diagnosis of CD and ulcerative colitis (UC) who were treated at the outpatient IBD clinic at the Singapore General Hospital and receiving thiopurine therapy were enrolled (n = 144), of whom 134 provided DNA samples.
Thiopurine doses were typically started at an AZA equivalent dose of 0.5–1.0 mg/kg/day in our institution and escalated up to 2.0–3.0 mg/kg/day as per guidelines at a rate of 0.5 mg/kg/day every 4–8 weeks, unless patients developed severe toxicities which warranted discontinuation of thiopurine therapy. This study was approved by the ethics review committee of the Singapore General Hospital. All recruited patients provided written informed consent to participate in the study.
Clinical data & assessment of myelotoxicity
Demographics and clinical data, including disease characteristics, details of thiopurine treatment and concomitant medications, as well as nadir (lowest) full blood counts within 4, 8 and 12 weeks and 6 months following initiation of thiopurine therapy were reviewed retrospectively from electronic medical records. Patients with missing data were not included in the study. The dose of 6-MP was adjusted to AZA equivalent doses by a factor of 2.08. Myelotoxicity was classified and graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events version 4.0. In addition to leukopenia, incidence of neutropenia and thrombocytopenia, which have not been previously investigated, were also evaluated.
Leukopenia was defined as white blood cell (WBC) count less than 3.0 × 109/l. Neutropenia was defined as absolute neutrophil count (ANC) less than 1.5 × 109/l. Thrombocytopenia was defined as platelet count less than 75 × 109/l. The thiopurine doses at which leukopenia occurred were determined at the time of lowest observed (nadir) WBC count. In addition, we also evaluated the rate of decrease in WBC, ANC and platelet counts from baseline (prior to thiopurine initiation).
Quantification of 6-TGN & 6-MMPN levels in erythrocytes by liquid chromatography–tandem mass spectrometry
Steady-state concentrations of 6-TGN and 6-MMPN metabolites in erythrocytes of patients who had been on stable doses of thiopurine for at least 4 weeks, were quantified using a validated liquid chromatography–tandem mass spectrometry method, which was adapted from Dervieux et al. with slight modifications [17]. Briefly, erythrocytes were harvested from whole blood (6 ml), washed twice with normal saline and stored at -80°C until analysis. Erythrocytes were lysed by extensive vortexing and diluted 1:1 with 0.02 M phosphate buffer. 8-bromoadenine (8-BA; 50 μl) was added to 50 μl of red blood cell (RBC) lysate as internal standard. A total of 375 μl of dithiothreitol (DTT; 25 mg/ml in pure water) was added to protect 6-TGN from oxidation. The metabolites 6-TGN and 6-MMPN were subsequently converted to 6-TG and 6-MMP derivatives by incubation with perchloric acid (70%) for 1 h at 100°C. Appropriately diluted hydrolyzed samples were subsequently injected into the Agilent 1290 UHPLC (Agilent Technologies, CA, USA) tandem QTRAP 5500 MS system (AB Sciex, MA, USA). Mobile phases A and B used were 2.5 mM ammonium acetate containing 0.1% formic acid and acetonitrile containing 0.1% formic acid, respectively. Chromatographic separation of the metabolites was achieved on an Atlantis T3 analytical column fitted with T3 guard column with an isocratic elution mode. The QTRAP 5500 mass spectrometer was used in positive ionization mode with 5500 V ionization, and the heater set at 450°C for the quantification of 6-TGN and 6-MMPN metabolites. Analyst software version 1.6.2 (AB Sciex) was used for the quantification of peak areas, with linear 1/x2-weighted regression.
Pharmacogenetics analyses of NUDT15 & TPMT variants
Peripheral blood samples (6 ml) were obtained from enrolled patients and genomic DNA was extracted using the DNeasy® Blood & Tissue kit according to manufacturer's protocol (Qiagen, Hilden, Germany). Genomic DNA was quantified using NanoDrop ND-1000 Spectrophotometer at 260 nm (DE, USA)
The following variants in the NUDT15 gene were genotyped: p.Arg139Cys (c.415C>T, rs116855232); p.Arg139His (c.416G>A, rs147390019); p.Val18Ile (c.52G>A, rs186364861); and p.Val18_Val19insGlyVal (c.36_37insGGAGTC, rs554405994). The TPMT variants genotyped were as follows: TPMT*2 (p.Ala80Pro, c.238G>C, rs1800462); TPMT*3A (presence of both *3B and *3C); TPMT*3B (p.Ala154Thr, c.460G>A, rs1800460); TPMT*3C (p.Tyr240Cys, c.719A>G, rs1142345); and TPMT*6 (p.Tyr180Phe, c.539A>T, rs75543815). Genotyping was performed using Pyrosequencing and results were validated against Sanger sequencing. Pyrosequencing and Sanger sequencing primers were designed with PyroMark® Assay Design software 2.0 (Qiagen) and PrimerQuest® Tool (IDT), respectively.
Linkage disequilibrium & statistical analysis
Conformity to Hardy–Weinberg equilibrium was determined using χ2 tests. Pairwise linkage disequilibrium (LD) analysis was conducted using Haploview software v4.2 (Broad Institute, MA, USA) and described by |D’| and r2. Haplotype phasing was conducted using PLINK v1.02 to assign haplotype status for each patient [18]. Normality of data was determined by visual inspection of skewness, z-values of kurtosis and D' Agostino–Pearson normality test. Linear mixed effects model was used to assess whether the associations between NUDT15 c.415C>T (rs116855232) and WBC, ANC and platelet counts were time varying or stable. Likelihood ratio test was used to test models with versus without interaction between NUDT15 c.415C>T (rs116855232) and time on these three outcome variables.
Logistic regression analyses assuming log-additive and dominant models were performed to determine the odds ratios (OR) of toxicities associated with the variant alleles in NUDT15 and TPMT as well as NUDT15 activity groups. p-values were two sided and considered statistically significant for values lower than 0.05. All plots were generated using GraphPad Prism® version 6.0 (CA, USA), and statistical analyses were performed on SPSS version 14.0 (SPSS, Inc., IL, USA) and STATA version 14.0 (StataCorp LP, TX, USA).
Results
Patient demographics & clinical characteristics
Out of the 134 patients recruited, three patients had received thiopurine treatment for less than 6 months and were excluded from analysis. Two patients were not of Asian ethnicity and were also excluded. None of the enrolled patients had baseline laboratory abnormalities that may have interfered with the interpretation of study results. The demographics and clinical characteristics of recruited patients (n = 129) are listed in Table 1. The majority of the patients were male (n = 87, 67.4%), Chinese (n = 84, 65.1%) and were receiving thiopurine treatment for CD (n = 89, 69.0%). Most patients received AZA (n = 127, 98.4%) and were on concurrent 5-aminosalicylates (n = 83, 64.3%) and steroids (n = 87, 67.4%) at the time of thiopurine initiation.
Table 1. . Demographics and clinical characteristics (n = 129).
| Characteristics | n | % |
|---|---|---|
| Age | ||
| Median (range) | 42 (17–18) | |
| Gender | ||
| Male | 87 | 67.4 |
| Female | 42 | 32.6 |
| Ethnicity | ||
| Chinese | 84 | 65.1 |
| Malay | 12 | 9.3 |
| Indian | 31 | 24.0 |
| Others | 2 | 1.6 |
| Clinical indications of thiopurine therapy | ||
| Crohn's disease | 89 | 69.0 |
| Ulcerative colitis | 40 | 31.0 |
| Thiopurine drug received | ||
| Azathioprine | 127 | 98.4 |
| 6-mercaptopurine | 2 | 1.6 |
| Concurrent medications at thiopurine initiation | ||
| 5-aminosalicylates | 83 | 64.3 |
| Corticosteroids | 87 | 67.4 |
Genetic variations in NUDT15 & TPMT in Asian IBD patients
The frequencies of variants in the NUDT15 and TPMT genes are shown in Table 2. The overall variant allele frequency of NUDT15 c.415C>T (rs116855232) was 11% in this cohort of Asian IBD patients. There were no deviations from Hardy–Weinberg equilibrium (p = 0.321) either when individuals of Chinese, Indian and Malay ethnicity were analyzed jointly or separately. The frequencies of NUDT15 c.52G>A (rs186364861) and c.36_37insGGAGTC (rs554405994) were noticeably lower, with variant allele frequencies of 1 and 4%, respectively when compared with c.415C>T (Table 2). The variant c.416G>A (rs147390019) was nonpolymorphic in this cohort.
Table 2. . Genotype and allele frequencies of NUDT15 and TPMT variants.
| Gene | Variants | n | Genotype frequency (n [%]) | Allele frequency | |||
|---|---|---|---|---|---|---|---|
| CC | CT | TT | C | T | |||
| NUDT15 | c.415G>T (rs116855232) | ||||||
| All patients | 129 | 111 (86.0) | 16 (12.4) | 2 (1.6) | 0.89 | 0.11 | |
| Chinese | 84 | 70 (83.3) | 12 (14.3) | 2 (2.4) | 0.86 | 0.14 | |
| Malays | 12 | 11 (91.7) | 1 (8.3) | 0 (0) | 0.88 | 0.12 | |
| Indians | 31 | 29 (93.5) | 2 (6.5) | 0 (0) | 0.92 | 0.08 | |
| c.416G>A (rs147390019) | GG | GA | AA | G | A | ||
| All patients | 129 | 129 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Chinese | 84 | 84 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Malays | 12 | 12 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Indians | 31 | 31 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| c.52G>A (rs186364861) | GG | GA | AA | G | A | ||
| All patients | 129 | 127 (98.4) | 2 (1.6) | 0 (0) | 0.99 | 0.01 | |
| Chinese | 84 | 83 (98.8) | 1 (1.2) | 0 (0) | 0.99 | 0.01 | |
| Malays | 12 | 11 (91.7) | 1 (8.3) | 0 (0) | 0.96 | 0.04 | |
| Indians | 31 | 31 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| c.36_37insGGAGTC (rs554405994) | WT | HET | HV | WT | V | ||
| All patients | 129 | 118 (91.5) | 11 (8.5) | 0 (0) | 0.96 | 0.04 | |
| Chinese | 84 | 73 (86.9) | 11 (13.1) | 0 (0) | 0.93 | 0.07 | |
| Malays | 12 | 12 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Indians | 31 | 31 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| TPMT | *2 (rs1800462) | GG | GC | CC | G | C | |
| All patients | 129 | 129 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Chinese | 84 | 84 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Malays | 12 | 12 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Indians | 31 | 31 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| *3A (rs1800460 and rs1142345) | WT | HET | HV | WT | V | ||
| All patients | 129 | 129 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Chinese | 84 | 84 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Malays | 12 | 12 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Indians | 31 | 31 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| *3B (rs1800460) | GG | GA | AA | G | A | ||
| All patients | 129 | 129 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Chinese | 84 | 84 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Malays | 12 | 12 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Indians | 31 | 31 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| *3C (rs1142345) | AA | AG | GG | A | G | ||
| All patients | 129 | 127 (98.4) | 2 (1.6) | 0 (0) | 0.98 | 0.02 | |
| Chinese | 84 | 82 (97.6) | 2 (2.4) | 0 (0) | 0.97 | 0.03 | |
| Malays | 12 | 12 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Indians | 31 | 31 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| *6 (rs75543815) | AA | AT | TT | A | T | ||
| All patients | 129 | 128 (99.2) | 1 (0.8) | 0 (0) | 0.99 | 0.01 | |
| Chinese | 84 | 83 (98.8) | 1 (1.2) | 0 (0) | 0.98 | 0.02 | |
| Malays | 12 | 12 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
| Indians | 31 | 31 (100) | 0 (0) | 0 (0) | 1.00 | 0.00 | |
HET: Heterozygous; HV: Homovariant; V: Variant; WT: Wild-type.
As previously reported in patients of east Asian descent, variations in the NUDT15 gene occur at much higher frequencies than TPMT variants in this cohort of Asian IBD patients. None of the patients were carriers of TPMT*2, TPMT*3A and TPMT*3B. Only two patients (1.6%) were heterozygous for TPMT*3C, with none being homozygous carrier of the variant allele. Heterozygosity for TPMT*6 was found only in one patient (0.8%), and the remaining patients were wild-type at this locus. Co-occurrence of variant alleles in both the NUDT15 and TPMT genes was not observed in this study cohort.
Associations of TPMT variants with thiopurine-induced myelotoxicity
Myelotoxicity was absent in all three patients who were heterozygous at the TPMT*3C and *6 loci, thereby further confirming the lack of predictive role of TPMT variants on thiopurine-induced myelosuppression as previously reported in Koreans and Japanese [19–21].
Effects of NUDT15 variants on nadir WBC, ANC & platelet counts
The pharmacogenetic influence of individual NUDT15 variants on thiopurine-induced myelotoxicity was first investigated by comparing the differences in nadir WBC, ANC and platelet counts among patients carrying different copy numbers of the NUDT15 variant alleles within 4, 8 and 12 weeks and 6 months of thiopurine therapy initiation. Statistically significant trends of declining nadir WBC and ANC counts were observed with increasing copy number of the NUDT15 c.415C>T (rs116855232) risk allele, suggesting strong allele-dose effects (Figure 1A & Table 3). These associations remained significant even after the exclusion of the three patients carrying TPMT variants from analysis (data not shown). The distribution in nadir platelet counts between the three genotype groups of NUDT15 c.415C>T (rs116855232) were significantly different within 8, 12 weeks and 6 months of thiopurine initiation (overall p < 0.05).
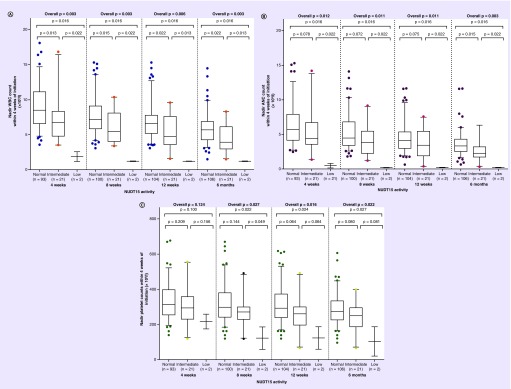
Figure 1. . Nadir white blood cell counts within 4, 8 and 12 weeks and 6 months according to NUDT15 activity group.
Nadir counts of (A) WBC, (B) ANC and (C) platelet counts within 4, 8, and 12 weeks and 6 months according to NUDT15 activity group.
ANC: Absolute neutrophil count; WBC: White blood cell.
Table 3. . Associations of NUDT15 variants with thiopurine-induced leukopenia and neutropenia.
| Genotype groups | Log-additive model | Dominant model | |||||
|---|---|---|---|---|---|---|---|
| Variants | Myelotoxicity | CC (n = 111) | CT (n = 16) | TT (n = 2) | p-value | OR (95% CI) | p-value |
| NUDT15 c.415C>T (rs116855232) | |||||||
| Leukopenia (WBC <3.0 × 109/l) | 3 (2.7%) | 5 (31.3%) | 2 (100%) | 0.002* | 22.9 (5.17–101.4) | 3.71 × 10-5* | |
| Grade 2 | 2 | 2 | 0 | ||||
| Grade 3 | 1 | 3 | 0 | ||||
| Grade 4 | 0 | 0 | 2 | ||||
| Neutropenia (ANC <1.5 × 109/l) | 4 (3.6%) | 4 (25.0%) | 2 (100%) | 0.018* | 13.4 (3.30–54.2) | 2.79 × 10–12.79 × 10-4* | |
| Grade 2 | 2 | 3 | 0 | ||||
| Grade 3 | 2 | 0 | 0 | ||||
| Grade 4 | 0 | 1 | 2 | ||||
| NUDT15 c.36_37insGGAGTC (rs554405994) | WT/WT (n = 118) | WT/Ins (n = 11) | Ins/Ins (n = 0) | ||||
| Leukopenia (WBC <3.0 × 109/l) | 7 (5.9%) | 3 (27.3%) | – | – | 5.95 (1.29–27.5) | 0.022* | |
| Grade 2 | 3 (2.5%) | 1 (9.1%) | |||||
| Grade 3 | 4 (3.4%) | 2 (18.2%) | |||||
| Grade 4 | 0 (0%) | 0 (0%) | |||||
| Neutropenia (ANC <1.5 × 109/l) | 8 (6.8%) | 2 (18.2%) | – | – | 3.06 (0.56–16.6) | 0.196 | |
| Grade 2 | 4 (3.4%) | 1 (9.1%) | |||||
| Grade 3 | 2 (1.7%) | 0 (0%) | |||||
| Grade 4 | 2 (1.7%) | 1 (9.1%) | |||||
*p < 0.05.
ANC: Absolute neutrophil count; OR: Odds ratio; WBC: White blood cell; WT: Wild-type.
There were no significant trends discernible in the nadir WBC, ANC and platelet counts of patients with regard to c.52G>A (rs186364861) and c.36_37insGGAGTC (rs554405994) within 4, 8 and 12 weeks and 6 months of thiopurine therapy initiation.
Linear mixed effects analyses and likelihood-ratio tests showed no sign of time variation in the associations between NUDT15 c.415C>T (rs116855232) with WBC count (p = 0.931) and ANC (p = 0.916). However, the association between NUDT15 c.415C>T (rs116855232) and platelet counts was time dependent (p = 0.020; data not shown). At 4, 8 and 12 weeks and 6 months, patients carrying the T alleles had lower platelet count than those with the wild-type (CC) genotype at this locus; platelet counts: 38.2, 64.2, 74.5 and 71.3 units, respectively. Collectively, these findings highlight the significant role of this NUDT15 variant in not only predicting for leukopenia, but also neutropenia and thrombocytopenia in thiopurine-treated Asian IBD patients.
Associations of NUDT15 variants with thiopurine-induced myelotoxicity
NUDT15 c.415C>T (rs116855232) was significantly predictive of thiopurine-induced leukopenia in this cohort (n = 10; 7.8%), with the risk allele being associated with a 22.9-fold increased risk of grade 2 leukopenia and above (OR: 22.9; 95% CI: 5.17–101.4; p = 3.71 × 10-5; Table 3). Similarly, NUDT15 c.415C>T (rs116855232) was significantly associated with a 13.4-fold higher risk of grade 2 neutropenia and above (n = 10; 7.8%). Trends of faster onset of leukopenia and neutropenia were also observed with increasing copy numbers of the variant NUDT15 c.415C>T (rs116855232) allele (data not shown). Interestingly, NUDT15 c.36_37insGGAGTC (rs554405994) was also associated with significantly higher risks of leukopenia but not neutropenia. None of the patients carrying variant NUDT15 c.52G>A (rs186364861) allele had thiopurine-induced leukopenia or neutropenia.
Effects of NUDT15 variants on steady-state levels of 6-TGN & 6-MMPN
Higher concentrations of thiopurine active metabolites, particularly 6-TGN, have been widely associated with higher risks of myelotoxicity [8]. We therefore investigated the differences in steady-state levels of 6-TGN and 6-MMPN in patients carrying different copy numbers of the NUDT15 variant alleles in this cohort of Asian IBD patients. Unfortunately, thiopurine therapy was immediately withdrawn from the two patients carrying the homovariant (TT) genotype at the NUDT15 c.415C>T (rs116855232) locus due to the development of severe myelotoxicity. Since this preceded the monitoring of steady-state thiopurine metabolite levels in these patients, they were excluded from this analysis. No significant differences were observed in 6-TGN and 6-MMPN levels between patients who were wild-type and heterozygous for NUDT15 c.415C>T (rs116855232) after adjustments for thiopurine doses. Similarly, no significant associations were observed between the other two NUDT15 variants (c.36_37insGGAGTC [rs554405994] and c.52G>A [rs186364861]) with steady-state 6-TGN and 6-MMPN levels. There was a lack of association observed between WBC and ANC levels with carriers of TPMT normal-NUDT15 variants and TPMT intermediate-NUDT15 variants; most likely due to the low frequency of TPMT polymorphic variants in our Asian cohort.
Linkage disequilibrium analysis in Asian IBD patients
Since haplotype- and diplotype-based analyses have been reported to be more robust than genotype-based analyses and NUDT15 variants investigated in this study were found to be strongly linked [16], we subsequently performed pairwise linkage disequilibrium analysis on the NUDT15 variants present in this study cohort and identified a moderate linkage between NUDT15 c.415C>T (rs116855232) and c.36_37insGGAGTC (rs554405994) [|D′| = 0.69 and r2 = 0.25]. Haplotype phasing and assessment of the three NUDT15 variants led to the inference of five haplotypes: reference haplotype NUDT15*1 (90.3%), NUDT15*2 (3%), NUDT15*3 (4.7%), NUDT15*5 (0.8%) and NUDT15*6 (1.2%). The NUDT15*4 haplotype was absent in our cohort of IBD patients. Diplotype analysis revealed a total of six NUDT15 diplotype groups of which *1/*1 (82.2%), *1/*2 (6.2%) and *1/*3 (6.2%) comprised the most common diplotypes. Rare diplotypes *1/*5, *1/*6 and *3/*3 occurred at low frequencies of less than 3% each.
Effects of NUDT15 activity on nadir WBC, ANC & platelet counts
No significant differences were observed in the nadir WBC, ANC and platelet counts of patients with compound-heterozygous diplotypes (NUDT15 *1/*2, *1/*3, *1/*2 and *1/*6) within 4, 8 and 12 weeks and 6 months of thiopurine initiation (overall p > 0.05). The IBD patients in this study were then classified into three NUDT15 activity groups: carriers of the reference diplotype, NUDT15 *1/*1, were classified as having normal NUDT15 activity; patients harboring NUDT15 compound-heterozygous diplotypes (NUDT15 *1/*2, *1/*3, *1/*2 and *1/*6) were considered to have intermediate NUDT15 activity whereas carriers of compound-homovariant diplotype (NUDT15 *3/*3) were classified as having low NUDT15 activity.
Significant differences were observed in the nadir WBC and ANC of patients with different NUDT15 activities within 4, 8 and 12 weeks and 6 months of thiopurine initiation. Specifically, patients with intermediate and low NUDT15 activities had significantly lower nadir WBC and ANC compared with those with normal NUDT15 activity (Figure 1A & B). Significant trends were also observed in the nadir platelet counts of patients with normal NUDT15 activity and those with intermediate and low NUDT15 activity at 8, 12 weeks and 6 months of therapy initiation (Figure 1C). Similar to the findings of the single-variant analysis for NUDT15 c.415C>T (rs116855232) described earlier, strong gene-dose effects were observed in the diplotype-based association analyses.
Associations of NUDT15 activity with thiopurine-induced myelotoxicity
Logistic regression analyses showed that patients possessing intermediate and low NUDT15 activity were associated with a 15-fold higher risk of leukopenia (95% CI: 3.52–64.1; p = 0.000253) compared with patients with normal NUDT15 activity. Similarly, with regard to the risk of developing neutropenia, IBD patients with low and intermediate NUDT15 activities were also significantly associated with nine-fold higher risks compared with patients harboring normal NUDT15 activity (95% CI: 2.30–35.3; p = 0.002; Table 4).
Table 4. . Associations of NUDT15 activity with thiopurine-induced leukopenia and neutropenia.
| Myelotoxicity | NUDT15 activity groups | Log-additive model | Dominant model | ||||
|---|---|---|---|---|---|---|---|
| Normal (n = 106) | Intermediate (n = 21) | Low (n = 2) | p-value | OR (95% CI) | p-value | ||
| Leukopenia (WBC < 3.0 × 109/l): | 3 (2.8%) | 5 (23.8%) | 2 (100%) | 0.010* | 15.0 (3.52–64.1) | 0.000253* | |
| – Grade 2 | 2 | 2 | 0 | ||||
| – Grade 3 | 1 | 3 | 2 | ||||
| – Grade 4 | 0 | 0 | 0 | ||||
| Neutropenia (ANC < 1.5 × 109/l): | 3 (2.8%) | 4 (19.0%) | 2 (100%) | 0.059 | 9.0 (2.30–35.3) | 0.002* | |
| – Grade 2 | 2 | 3 | 0 | ||||
| – Grade 3 | 1 | 0 | 0 | ||||
| – Grade 4 | 0 | 1 | 2 | ||||
*p < 0.05.
ANC: Absolute neutrophil count; OR: Odds ratio; WBC: White blood cell.
Discussion
Routine screening of TPMT genotype status prior to initiation of thiopurine therapy has been recommended in several clinical practice guidelines to reduce the risks of myelosuppression [10,22]. However, the low frequencies of these variants in Asians have greatly diminished its relevance and clinical utility in predicting myelosuppression in this ethnic group. In line with earlier findings in other Asian populations, the results of this study confirm that variations in the TPMT gene are rare and do not constitute a significant genetic factor that contribute to the development of thiopurine-induced myelotoxicity in Asian IBD patients.
Importantly, the significant associations between NUDT15 c.415C<T (rs116855232) with thiopurine-induced leukopenia previously identified in Taiwanese, Koreans and Japanese IBD and acute lymphoblastic leukemia patients [13,14,23] were further confirmed in this cohort of multiethnic Asian IBD patients. Specifically, we observed significant trends of declining nadir WBC counts and more rapid decrease in WBC counts with increasing copy number of the risk T allele (rs116855232), and significantly higher risks of leukopenia in patients carrying the variant NUDT15 allele at the c.415C>T (rs116855232) locus.
Notably, majority of the infectious complications related to thiopurine therapy have been noted to occur in the absence of leukopenia and risks of thiopurine-related infections have been noted to increase as a consequence of neutropenia [1,24]. Our results also indicate for the first time that the variant T allele at the c.415C<T locus (rs116855232) is predictive of thiopurine-induced neutropenia as well as thrombocytopenia. Taken together, these findings clearly highlight the greater influence of the NUDT15 c.415C>T variant (rs116855232) on hematological toxicities compared with the low penetrant TPMT variants and supports the clinical utility of genotyping for this variant in assessing the risks of thiopurine-induced myelotoxicity prior to treatment initiation in Asian IBD patients.
With regard to the chronological aspects of thiopurine-induced myelosuppression, we performed the analyses with nadir WBC, ANC and platelet counts within 4, 8 and 12 weeks and 6 months of thiopurine initiation as thiopurine-induced myelosuppression has been shown to occur frequently within the first weeks or months of treatment [25]. Indeed, significant trends of lower nadir WBC, ANC and platelet counts with increasing copy of the variant T allele at the c.415C>T (rs116855232) locus were observed across all the four time intervals, further emphasizing the consistency of these observations across the different durations of treatment. The onsets to leukopenia and neutropenia were also observed to be faster with increasing copy number of the variant T allele, where patients who were carriers of two homozygous variant alleles for NUDT15 c.415C>T (rs116855232) developed thiopurine-induced leukopenia and neutropenia within only 4–8 weeks of treatment initiation (data not shown). Taking into account the strong gene-dose effects observed in the present study and others, optimization of thiopurine dosages based on patients’ NUDT15 genotype status should certainly be considered in future studies aiming to personalize thiopurine therapy.
In addition to the NUDT15 c.415C>T (rs116855232) variant, which has been more widely investigated, we also investigated the frequencies of other NUDT15 variants (c.52G>A, c.36_37insGGAGTC and c.416G>A) and their influence on the incidence of thiopurine-induced myelotoxicity in our cohort. Despite the consistently lower trends of nadir WBC, ANC and platelet counts in patients who were heterozygous at the c.52G>A and c.36_37insGGAGTC loci, statistical significance was not achieved, which may be due in part to the low frequencies of these two functional variants in this study cohort.
It is also plausible that the functional consequences of these two variants on NUDT15 activity may be less pronounced as compared with that of NUDT15 c.415C>T (rs116855232), as suggested by the functional studies conducted by Moriyama et al. [16], which showed markedly lower nucleoside diphosphatase activity for the NUDT15 c.415C>T gene product compared with the other two variants in Escherichia coli. Recently, Carter et al. [26] characterized the crystal structure and biochemical activities of the NUDIX family proteins, which includes NUDT15. The findings of Carter et al. [26] suggest that the structural change resulting from NUDT15 c.415C>T (rs116855232) variation would occur at the base of the substrate-binding pocket of the NUDT15 monomer. They also postulated that the arginine to cysteine substitution (p.Arg139Cys) may result in the formation of disulfide bond with an adjacent cysteine residue (Cys140), which may in turn disrupt the conformation of NUDT15 active site. It is possible that the structural disruptions resulting from other variations in the NUDT15 gene may not occur at sites that are critical to NUDT15 enzymatic activity. However, further studies are essential to fully elucidate the functional consequences of these variants.
Nevertheless, the predictive roles of these less-commonly occurring NUDT15 variants require further validation in a larger cohort of thiopurine-treated patients. Notably, the results of our diplotype-based association analyses revealed significantly lower nadir WBC, ANC and platelet counts and significantly higher risks of myelotoxicity, particularly leukopenia and neutropenia, in patients with low and intermediate NUDT15 activity as compared with those with normal NUDT15 activity who were wild-type at all loci. Interestingly, comparison of the goodness-of-fit of the regression models showed that the model based on NUDT15 c.415C>T (rs116855232) alone was better than NUDT15 activity group in predicting thiopurine-induced leukopenia and neutropenia. Although the differences in goodness-of-fit indices between the two models were marginal, the receiver operating characteristic areas were significantly different between the two models, with the model based on NUDT15 c.415C>T (rs116855232) alone showing consistently better performance in predicting thiopurine-induced leukopenia and neutropenia (data not shown). It is therefore highly plausible that the contributory effect of the NUDT15 c.415C>T variant is probably greater than the other three variants in the NUDT15 gene toward development of thiopurine-induced leukopenia and neutropenia. These findings were also supported by the findings of the functional studies done by Moriyama et al., which showed the greatest loss in NUDT15 activity with the NUDT15 c.415C>T and NUDT15 c.415C>T plus c.36_37insGGAGTC variants [16]. Given the significant practical advantage of determining NUDT15 c.415C>T genotype status over NUDT15 activity group, which incorporates the genotype information of three other NUDT15 variants, it is possible that genotyping for NUDT15 c.415C>T alone may be sufficient in predicting for thiopurine-induced myelotoxicity.
Moriyama et al. also compared the intracellular levels of thioguanine incorporated into DNA in NUDT15-knockdown and control human lymphoid cells and demonstrated the accumulation of active metabolites in NUDT15-knockdown cells [16], which indicate the role of NUDT15 in inactivating thiopurine metabolites and leading to the postulation that NUDT15 modulates thiopurine-induced myelosuppression via accumulation of 6-TGN. In contrast, however, we found no significant differences in the 6-TGN levels of patients with wild-type genotype and those who were heterozygous at the c.415C>T (rs116855232) locus (data not shown). In accordance with the findings of Asada et al. [15], thiopurine-induced leukopenia appears to be mediated by NUDT15 variants, independent of 6-TGN concentrations. However, further investigations with a larger cohort size and which include the steady-state levels of patients with homovariant (TT) genotype are necessary to confirm these findings.
Conclusion
In conclusion, pharmacogenetic variants in NUDT15 were found to be significantly associated with the development of thiopurine-induced myelotoxicity in Asian IBD patients. The evidence that NUDT15 variants confer higher risks of thiopurine-induced leukopenia also supports the compelling need to genotype this pharmacogenetic marker prior to initiation of thiopurine; specifically c.415C>T (rs116855232), which was associated with a 22.9-fold higher risk of leukopenia in our cohort. This study also confirmed the lack of utility of TPMT variants as well as the low penetrant NUDT15 variants (c.52G>A and c.36_37insGGAGTC) compared with NUDT15 c.415C>T in predicting thiopurine-induced myelosuppression.
Summary points.
Our results revealed a strong dose-allele effect amongst patient carriers of NUDT15 c.415C>T allele (rs116855232) on decreasing nadir WBC and ANC counts for 4, 8 and 12 weeks and 6 months upon thiopurine initiation.
Significant associations between NUDT15 c.415C>T risk alleles and grade 2 leukopenia (OR = 22.9, p = 3.71 × 10-5) and neutropenia (OR = 13.4, p = 2.79 × 10-4) were also observed.
No significant observations were found between TPMT and NUDT15 variants on steady-state levels of thiopurine-metabolites 6-thioguanine nucleotide (6-TGN) and 6-mercaptomercaptopurine (6-MMPN).
Patients with intermediate (NUDT15 *1/*2, *1/*3, *1/*2 and *1/*6) and low NUDT15 (NUDT15 *3/*3) activities were found to have lower WBC and ANC counts compared to those with normal NUDT15 activity (NUDT15 *1/*1).
The intermediate and low NUDT15 activity groups were also associated with increased risks in leukopenia (OR = 15.0, p = 0.000253) and neutropenia (OR = 9.0, p = 0.002).
No significant associations were observed with other NUDT15 variants (c.52G>A, rs186364861; c.36_37insGGAGTC, rs5544005994), due to the low penetrance of these variants in our Asian population.
Our study demonstrates the predictive role of NUDT15 variant c.415C>T on leukopenia risk in a multiethnic Asian population and confirm the lack of clinical utility in TPMT genotyping in our cohort.
Footnotes
Financial & competing interests disclosure
This study was supported by the Health Services Development Programme (HSDP) grant 13/X03 (Ministry of Health, Singapore). The study sponsor had no role in the study design, collection, analysis and interpretation of data. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Lichtenstein GR, Abreu MT, Cohen R, Tremaine W American Gastroenterological Association. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130(3):935–939. doi: 10.1053/j.gastro.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 2.Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: current management. J. Crohns Colitis. 2010;4(1):28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Travis SPL, Stange EF, Lémann M, et al. European evidence-based Consensus on the management of ulcerative colitis: current management. J. Crohns Colitis. 2008;2(1):24–62. doi: 10.1016/j.crohns.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Chaparro M, Ordás I, Cabré E, et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm. Bowel Dis. 2013;19(7):1404–1410. doi: 10.1097/MIB.0b013e318281f28f. [DOI] [PubMed] [Google Scholar]
- 5.Yamada S, Yoshino T, Matsuura M, et al. Efficacy and safety of long-term thiopurine maintenance treatment in Japanese patients with ulcerative colitis. Intest. Res. 2015;13(3):250–258. doi: 10.5217/ir.2015.13.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaza G, Cheok M, Krynetskaia N, et al. Thiopurine pathway. Pharmacogenet. Genomics. 2010;20(9):573–574. doi: 10.1097/FPC.0b013e328334338f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur. J. Clin. Pharmacol. 2008;64(8):753–767. doi: 10.1007/s00228-008-0478-6. [DOI] [PubMed] [Google Scholar]
- 8.Gearry RB, Barclay ML. Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2005;20(8):1149–1157. doi: 10.1111/j.1440-1746.2005.03832.x. [DOI] [PubMed] [Google Scholar]
- 9.Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118(4):705–713. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 10.Relling MV, Gardner EE, Sandborn WJ, et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin. Pharmacol. Ther. 2013;93(4):324–325. doi: 10.1038/clpt.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Relling MV, Gardner EE, Sandborn WJ, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin. Pharmacol. Ther. 2011;89(3):387–391. doi: 10.1038/clpt.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kham SKY, Tan PL, Tay AHN, Heng CK, Yeoh AEJ, Quah T-C. Thiopurine methyltransferase polymorphisms in a multiracial Asian population and children with acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2002;24(5):353–359. doi: 10.1097/00043426-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Yang S-K, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat. Genet. 2014;46(9):1017–1020. doi: 10.1038/ng.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakuta Y, Naito T, Onodera M, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016;16(3):280–285. doi: 10.1038/tpj.2015.43. [DOI] [PubMed] [Google Scholar]
- 15.Asada A, Nishida A, Shioya M, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J. Gastroenterol. 2016;51(1):22–29. doi: 10.1007/s00535-015-1142-4. [DOI] [PubMed] [Google Scholar]
- 16.Moriyama T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat. Genet. 2016;48(4):367–373. doi: 10.1038/ng.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dervieux T, Meyer G, Barham R, et al. Liquid chromatography-tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6-mercaptopurine therapy. Clin. Chem. 2005;51(11):2074–2084. doi: 10.1373/clinchem.2005.050831. [DOI] [PubMed] [Google Scholar]
- 18.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Cheon JH, Hong SS, et al. Influences of thiopurine methyltransferase genotype and activity on thiopurine-induced leukopenia in Korean patients with inflammatory bowel disease: a retrospective cohort study. J. Clin. Gastroenterol. 2010;44(10):e242–e248. doi: 10.1097/MCG.0b013e3181d6baf5. [DOI] [PubMed] [Google Scholar]
- 20.Kim MJ, Choe YH. Monitoring and safety of azathioprine therapy in inflammatory bowel disease. Pediatr. Gastroenterol. Hepatol. Nutr. 2013;16(2):65–70. doi: 10.5223/pghn.2013.16.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takatsu N, Matsui T, Murakami Y, et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2009;24(7):1258–1264. doi: 10.1111/j.1440-1746.2009.05917.x. [DOI] [PubMed] [Google Scholar]
- 22.Burnett HF, Tanoshima R, Chandranipapongse W, Madadi P, Ito S, Ungar WJ. Testing for thiopurine methyltransferase status for safe and effective thiopurine administration: a systematic review of clinical guidance documents. Pharmacogenomics J. 2014;14(6):493–502. doi: 10.1038/tpj.2014.47. [DOI] [PubMed] [Google Scholar]
- 23.Liang D-C, Yang C-P, Liu H-C, et al. NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia. Pharmacogenomics J. 2016;16(6):536–539. doi: 10.1038/tpj.2015.75. [DOI] [PubMed] [Google Scholar]
- 24.Gisbert JP, Gomollón F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am. J. Gastroenterol. 2008;103(7):1783–1800. doi: 10.1111/j.1572-0241.2008.01848.x. [DOI] [PubMed] [Google Scholar]
- 25.Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann. Intern. Med. 1989;111(8):641–649. doi: 10.7326/0003-4819-111-8-641. [DOI] [PubMed] [Google Scholar]
- 26.Carter M, Jemth A-S, Hagenkort A, et al. Crystal structure, biochemical and cellular activities demonstrate separate functions of MTH1 and MTH2. Nat. Commun. 2015;6:7871. doi: 10.1038/ncomms8871. [DOI] [PMC free article] [PubMed] [Google Scholar]