Abstract
Patient: Male, 80
Final Diagnosis: Unilateral complicated Herpetic Simplex Virus 1 Keratitis
Symptoms: Visual impairment
Medication: Anti-herpetic treatment • Anti-VEGF • Cyclosporine A • Matrix regeneration therapy
Clinical Procedure: Amniotic membrane and limbal stem cell transplantation
Specialty: Ophthalmology
Objective:
Management of emergency care
Background:
Keratitis caused by herpes simplex virus (HSV) can have detrimental effects on the cornea leading to loss of vision. Modern therapies can contribute to the prevention of anatomical and functional damage.
Case Report:
An 80-year-old male with complicated HSV-1 keratitis of the left eye (confirmed diagnosis after microbiological investigation) presented three months after antiviral treatment with corneal blurring, severe epitheliopathy, thinning of the stroma, and neovascularization. At the time he was referred, the visual acuity of his left eye was very low, as he could only count fingers at a one-foot distance. He was initially started on oral acyclovir (800 mg once daily) and topical poly-carboxymethyl glucose sulfate; afterwards he underwent amniotic membrane (AM) transplantation and localized treatment with anti-VEGF factors. One month after the AM transplantation there was an obvious improvement of the corneal surface. Ophthalmic suspension of cyclosporine-A 1% was also added to his treatment. After three months, a transplantation of stem cells (deriving from the sclerocorneal junction of his right eye) was carried out at the sclerocorneal junction, as the corneal damage and neovascularization was more severe at this anatomical area. Four months after the last surgery, his visual acuity was 1/10 (note, he had a history of an old vascular episode) and the cornea was sufficiently clear with no signs of epitheliopathy and almost complete subsidence of the neovascularization.
Conclusions:
Transplantation of AM and stem cells in combination with anti-VEGF factors and topical administration of cyclosporine-A 1% and poly-carboxymethyl glucose sulfate (a regenerative factor of corneal matrix) contributed substantially in the management of herpetic keratitis complications.
MeSH Keywords: Acyclovir; Allografts; Cyclosporine; Keratitis, Herpetic; Stem Cell Transplantation
Background
Herpes simplex virus (HSV) is a double-stranded DNA virus that belongs to a subfamily of the Herpesviridae family, known as Alphaherpesvirinae, which includes herpes simplex virus-1 (HSV-1), herpes simplex virus-2 (HSV-2), and varicella zoster virus [1]. HSV-1 and HSV-2 share many common features, but HSV-1 appears to have a more significant correlation with ocular diseases. HSV-1 is endemic all over the world and is transmitted mainly via direct contact with infected biological secretions (tears or saliva) or lesions [2]. It is very likely that its seroprevalence is affected by the level of exposure to these sources as well as other factors such as the age, poor hygiene, and socioeconomic class [2]. Ocular HSV-1 infection is correlated with a wide spectrum of ocular pathologies, including conjunctivitis, keratitis, iridocyclitis, and acute retinal necrosis [1]. HSV is a common etiology of corneal disease (i.e., keratitis) and one of the leading causes of infectious corneal blindness in developed nations [2]. The loss of vision is most often associated with stromal opacification and in more severe cases with corneal ulceration [1]. It is estimated that the incidence of HSV-related keratitis is approximately 1.5 million worldwide [1]. However, due to the absence of surveillance-based epidemiological studies, it is generally difficult to define the global impact of ocular HSV.
Case Report
An 80-year-old male was referred to our department due to a complicated and progressively worsening HSV-1 keratitis of his left eye (confirmed by polymerase chain reaction method) that initially presented as a dendritic ulcer of the corneal epithelium according to his medical report. He had been unsuccessfully treated with antiviral treatment for a period of three months. The patient himself mentioned an impairment of his vision over the course of time that was accompanied with an obvious deterioration of the clinical presentation,
On examination, Snellen visual acuity of the right eye was 10/10, but was significantly distorted in the left eye as it did not exceed finger counting at a one-foot distance. Slit-lamp biomicroscopy of the affected eye revealed intense corneal blurring, severe epitheliopathy, thinning of the stroma, and neovascularization (Figure 1).
Figure 1.
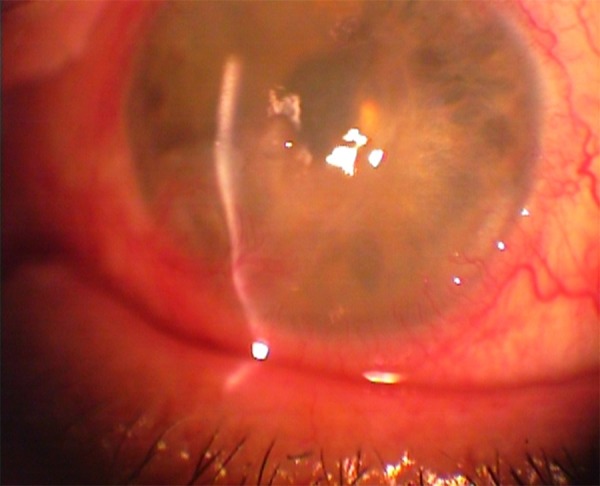
Initial presentation of patient’s left eye with corneal blurring, severe epitheliopathy, thinning of the stroma, and neovascularization.
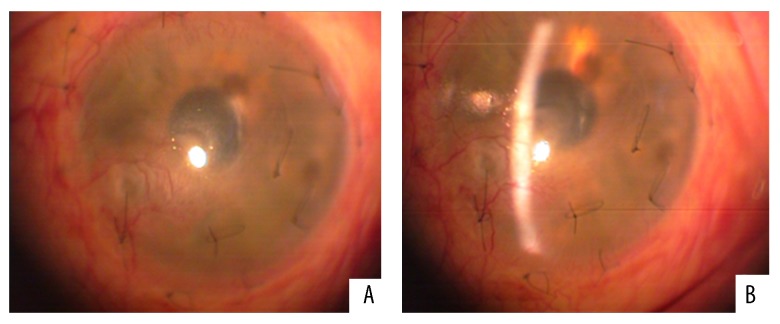
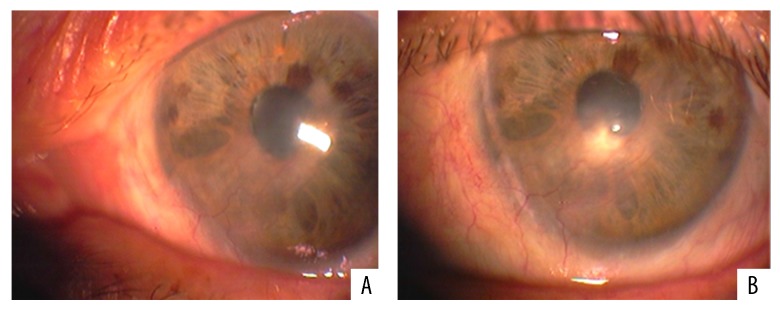
Our patient was immediately started on a therapeutic scheme of oral acyclovir (800 mg once daily), ganciclovir eye gel (four times a day) and topical poly-carboxymethyl glucose sulfate (Cacicol®, Théa Synapsis Ltd.). The latter is a regenerative factor of the corneal matrix and is applied weekly (or more frequently) contributing to the reconstruction of the ocular surface. The main target of this therapeutic approach was to eradicate the pathogen and control the corneal inflammatory status. As soon as the optimization of the ocular surface was achieved, amniotic membrane (AM) transplantation was carried out in order to restore the extensive corneal damage. In order to avoid the rejection of the transplanted tissue, this procedure was combined with localized treatment with anti-VEGF factors to reverse the neovascularization and use of ophthalmic suspension of cyclosporine-A 1% for topical immunomodification. Follow-up after one month showed apparent improvement and increased transparency of the cornea (Figure 2A, 2B). However, due to the persisting neovascularization and to some residual corneal lesions, three months after the AM transplantation an autologous transplantation of stem cells was also conducted in the patient’s left (affected) eye. The source of the stem cells was the sclerocorneal junction of the patient’s right (healthy) eye. The transplantation of the limbal epithelial stem cells was carried out at the inferior and nasal areas of the sclerocorneal junction of the affected eye, where the tissue damage and neovascularization were more prominent (Figure 3). After a course of four months, the cornea was sufficiently clear as there were no signs of epitheliopathy and neovascularization had almost completely subsided (Figure 4A, 4B). The Snellen visual acuity of the left eye did not exceed 1/10, but this was attributed to a past medical history of an old vascular episode of the retina after a regional septic infection secondary to a peritonsillar abscess.
Figure 2.
(A, B) Post-surgical follow-up (one month) after the amniotic membrane transplantation. There is obvious improvement of the corneal surface that is attributed to the adjunctive combined therapeutic approach with anti-herpetic treatment, anti-VEGF factors and Cyclosporine-A 1%.
Figure 3.
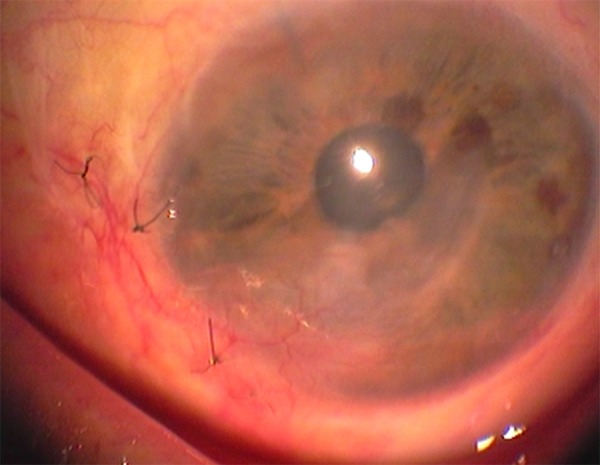
Follow-up three months after amniotic membrane transplantation. During this interval the transplantation of stem cells from the sclerocorneal junction of the right eye to the sclerocorneal junction of the left eye contributed in the management of the residual corneal lesions and the persisting neovascularization.
Figure 4.
(A, B) Four months after the last surgery, the cornea was sufficiently clear with no signs of epitheliopathy and almost complete subsidence of neovascularization.
Discussion
Concept of therapeutic approach and management
A proper classification of any disease prior to any therapeutic intervention is the cornerstone of a successful treatment. The main target of every disease classification system, especially for a severe or complicated clinical entity, should be to clearly define different forms of the disease to facilitate the clinician in treatment planning that directly follows an evidence-based algorithm.
HSV-related keratitis has various clinical presentations. Upon thorough examination, the ophthalmologist can distinguish the involvement of individual corneal layers (i.e., epithelium, stroma, and endothelium) and describe the manifestations of the ocular disease. Another crucial fact to consider is that different forms of the disease derive from distinct pathogenetic mechanisms. For instance, HSV stromal keratitis involves primarily immune mechanisms whereas HSV dendritic epithelial keratitis is attributed to direct infection of corneal epithelial cells.
Consequently, apart from the proper definition of the keratitis, comprehension of the pathogenesis will lead to an integral understanding of HSV keratitis and therefore to a more efficient management of the disease.
Highlights of treatment algorithm
Anti-herpetic treatment
Antiviral agents alone are the treatment of choice for HSV epithelial keratitis. Both topical and oral agents are available and can be incorporated in the therapeutic scheme. In regards to topical agents, both ganciclovir gel and trifluridine solution have been approved by FDA as their use is considered to be safe and highly effective [2]. Acyclovir ointment and trifluridine ointment appear to have similar efficacy [2], and the same efficacy was shown when acyclovir ointment was compared to ganciclovir gel [2]. Despite the fact that oral antivirals (acyclovir, valacyclovir, and famciclovir) are largely used in HSV keratitis, their use remains off label. They appear to be both safe and effective, but they do not have an official approval by FDA for this particular clinical entity [2]. Several studies have highlighted their effectiveness in the prevention of herpetic keratitis recurrence [3,4]. It is also importance to highlight the fact that topical corticosteroids must be avoided in the early stage of the herpetic epithelial keratitis.
The suggested treatment for HSV stromal keratitis includes an oral antiviral agent in combination with a topical corticosteroid agent for at least 10 weeks. The equilibrium between antivirals and corticosteroids should be imposed according to the presence or not of epithelial ulceration. It is noteworthy that HSV stromal keratitis with ulceration of the epithelium is more often encountered than stromal keratitis without ulceration, and is more commonly confused with microbial keratitis [2]. It is also significant to mention that individuals with renal insufficiency must be administered reduced doses of oral antivirals and longer intervals between administrations [2].
As concerns HSV endothelial keratitis, an oral antiviral agent in conjunction with a topical corticosteroid is again the preferred therapy [2]. This form of HSV keratitis is quite uncommon, but the mean healing time of patients is relatively shorter than those with HSV stromal keratitis, indicating that response to treatment is faster and prolonged therapy might not be necessary [2].
Amniotic membrane transplantation
Amniotic membrane (AM) transplantation can be regarded as one of the most important surgical developments in the field of ocular surface reconstruction. The first time that AM transplantation was used in ophthalmology was recorded approximately 70 years ago, by de Roth who was the first person that attempted ophthalmic surgery using fetal membranes in the eyes of patients with symblepharon [5]. However, preserved human AM transplantation was not incorporated in therapeutic approaches until after 1995 [5–7]. AM transplantation seems to be a very sophisticated method with promising results for a wide range of pathological entities of the corneal and conjunctival surface, such as persistent epithelial defects, corneal ulceration, acute chemical burns, limbal stem cell deficiency (LSCD), symblepharon, and many other medical conditions (Table 1) [8–18]. Thanks to its unique biological features, AM contributes to the rejuvenation and healing of the ocular surface with a wide diversity of mechanisms (Table 2) [8,9,19–33]. Especially after the introduction of modern techniques of preservation of AM, it contributed even more in the reconstructive surgery of the ocular surface [6]. It has been estimated that both preserved and fresh AM can lead to equally successful outcomes. However, it is important to take into account that fresh AM could also possibly act as a source of disease transmission [34].
Table 1.
Ophthalmological indications of amniotic membrane transplantation.
| Reconstruction of the corneal surface | Reconstruction of the conjunctival surface |
|---|---|
|
|
| Miscellaneous indications | |
| |
Table 2.
Amniotic membrane mechanisms that promote the healing and reconstruction of the ocular surface.
Mechanical
|
Promotion of epithelium regeneration (epithelialization)
|
Anti-fibrotic properties
|
Anti-inflammatory properties
|
Anti-angiogenic properties
|
Anti-microbial properties
|
The fetal membranes have two layers, the outer chorion and the amnion. The former is vascular and comes in contact with the uterine wall and the latter, which is avascular, is located inner to the chorion and contacts with amniotic fluid. Consequently, AM is defined as the innermost placental layer. Its thickness varies from 0.02 to 0.05 mm and characteristically consists of three distinct layers: the epithelium, the basement membrane, and the stroma [35]. AMs are obtained from donors undergoing elective caesarean section. The procedure takes place under strict aseptic conditions to restrict the possibilities of contamination. For the same reason placentas retrieved after vaginal delivery are not indicated for this purpose. Additionally, donors undergo a serological screening, including investigations for syphilis, hepatitis B and C and human immunodeficiency virus. It is also suggested that donors should repeat the serological testing after six months and before the AM is released for transplantation, as there is a “window period” for the transmission of communicable diseases [36].
For the procedure of transplantation, and more specifically the basic surgical techniques, the main aims include the curtailment of inflammation, reduction of pain, and augmentation of epithelialization so as to limit the possibilities of the transplant rejection [8]. Depending on the reason and the indication for AM transplantation, there are three surgical techniques that enable the application of AM on the ocular surface. These are the graft or inlay technique, patch or onlay technique, and the combined (inlay and onlay) technique [8,37]. Another technique that has been recently described is the use of AM as culture substrate and carrier that mainly facilitates the treatment of LSCD and defects of the outer eye surface [9,17,38].
AM transplantation plays a substantial role in the treatment of severe corneal ulceration due to herpetic stromal keratitis resistant to conventional therapeutic approach [39–43]. The anti-inflammatory potential of AM together with the modulation of stromal scarring is of great value in this particular clinical entity, enabling a successful penetrating keratoplasty if needed as a second step. Some of the most basic prerequisites are appropriate anti-herpetic and anti-inflammatory treatment, and the clinical decision regarding the timing of the surgery [41].
Management of corneal neovascularization with the use of anti-VEGF factors
One of the most crucial risk factors that can cause corneal opacification or corneal graft rejection is the development of corneal neovessels. Corneal neovascularization is a pathological condition characterized by the occurrence of neovessels, deriving from vascular structures that pre-exist at the limbus [44]. The invasion of these pathological vessels in the cornea results in the loss of the corneal immune privilege. This homeostatic status collapses comes more often than not as a result of several conditions, such as inflammation, infections, degenerative diseases, or trauma [45].
Several therapeutic approaches have been proposed for the management of corneal neovascularization. These include steroids, cauterization, physical therapy by keratectomy or argon laser, and topical injection of anti-VEGF factors (bevacizumab, ranibizumab) [46]. Although it is not easy to define with absolute certainty which one should be the most appropriate therapeutic modality, many recent studies have demonstrated that the intrastromal injection of bevacizumab might be an effective option [47–51]. The effectiveness might vary between patients and this can be attributed to several factors such the extension of the neovascularization, the condition of ranibizumab, and the possible delay between the onset of neovessels development and the initiation of treatment [47]. The use of bevacizumab seems to be a promising treatment as it is effective and generally well tolerated [48–51]. As for the form of administration, intrastromal injections ensure greater exposure and delivery of specific concentration of the substance [50].
Limbal stem cell transplantation
Reconstruction of the corneal surface after epithelial damage necessitates the migration and maturation of a specialized type of stem cells, which are detected in the limbus [52]. Several intrinsic and extrinsic insults can lead to LSCD affecting the delicate microenvironment of the corneal surface. In those cases, the regeneration of the epithelium is unsuccessful resulting in the extension of the conjunctival epithelium across the limbus, which causes neovascularization, severe epithelial defects, and chronicity of the inflammation. In eyes with sectoral LSCD, AM transplantation in combination with mechanical debridement of the conjunctival epithelium from the corneal surface, might be adequate [53,54]. However, in more severe cases with total LSCD, stem cell transplantation is considered a critical therapeutic option.
The cornerstone of successful limbal stem cell transplantation is the optimization of the ocular surface in order to establish control of the causative factors and any possible comorbid conditions. These factors include control of the ocular surface inflammatory status, eradication of associated pathogens, normal eyelid function, and adequately lubricated ocular surface. The main target is to establish an appropriate environment that will facilitate the regeneration of the existing stem cells and optimal conditions for the transplanted stem cells to reconcile [54]. These targets can be achieved by various methods such as improvement of lubrication (by use of scleral contact lenses, punctal occlusion, autologous serum tears, and salivary gland implants), reduction of mechanical irritation (reconstruction of symblephara and fornix with mucous membrane grafts), and correction of eyelid malposition for tear film stabilization [1].
Several approaches are available for techniques that can be used for the ocular surface stem cell transplantation. A classification for these techniques has been proposed by the Cornea Society, based on the following factors: anatomic source of the transplanted tissue (conjunctival, keratolimbal, mucosal); autologous or allogeneic (cadaveric or living-related) source; and cell culture techniques [55]. In general, in unilateral LSCD cases, stem cells can be obtained from the contra-lateral eye, and in cases of individuals with bilateral involvement, ophthalmologists can use allogeneic tissue coupled with immunosuppressive therapy [1].
It appears that due to the more comprehensive and detailed knowledge of the limbal epithelial stem cell physiology, significant steps have been made in the prognosis of patients with LSCD over the last 30 years. At the moment, the use of autologous tissues is the method that ensures the best results. The main aim of the newer techniques is the expansion of cells either in vivo or in vitro so as to curtail the need for large limbal resection that could potentially harm the healthy eye in cases of unilateral involvement. Overall, limbal stem cell transplantation plays a key role in the improvement of vision in these patients.
Topical use of cyclosporine-A
Cyclosporine-A is an immunomodulatory drug that was initially used to prevent the rejection of transplanted organs or tissues. Systemic administration of cyclosporine has also been incorporated in the management of several autoimmune disorders, including those with ocular involvement [56]. Cyclosporine-A is hydrophobic, neutral, cyclic undecapeptide metabolite of the fungus Tolypocladium inflatum. Its main effect is the distortion of interleukin-2 (IL-2) expression by helper T-cells, inhibiting the proliferation of T-cells. In ophthalmology, it was originally employed to prevent experimental corneal allograft reaction, until it was later established as an efficient drug for individuals with non-infectious uveitis and various inflammatory diseases of the ocular surface. More specifically, the ophthalmic use (as suspension) of cyclosporine-A includes dry eye syndrome, vernal and atopic keratoconjunctivitis, non-infectious keratitis, Thygeson’s superficial punctate keratitis, ligneous conjunctivitis, lichen planus, superior limbic keratitis, and corneal opacities or subepithelial infiltrates related to keratitis (e.g., viral keratitis) [56]. There are several studies that justify the use of cyclosporine-A in cases with subepithelial infiltrates attributed to viral keratoconjunctivitis (mostly adenoviruses), especially in patients that require an alternative corticosteroid-sparing treatment or do not respond to other therapeutic modalities [57–59]. According to the current literature, the cyclosporine-A concentrations that have been topically used vary from 0.5–2% [60]. There are a few side effects reported (i.e., itching, redness, and burning sensation), but the majority of patients do not experience any of them. Overall, patients with keratoconjunctivitis appear to be satisfied as there is an improvement in vision, which is explained by the treatment of subepithelial infiltrates and the lack of recurrences [59,60]. There is supportive evidence that the ophthalmic suspension of cyclosporine-A can restrict the corneal immune reaction in various ocular surface diseases due to its immunomodulatory properties, resulting in enhancement of corneal thickening and epithelial regeneration, and regression of opacification and neovascularization [56,61].
Matrix regeneration therapy
One of the latest developments in the challenging field of the treatment of corneal neurotrophic ulcers is the topical application of matrix regeneration therapy (RGTA). This a dextran derivative polymer that mimics heparin sulfates and is the first product in ophthalmology based on matrix therapy. No local or systemic side effects have been reported [62–64]. RGTA is considered a promising regenerative therapy. It can be applied as an alternative or an additional regimen in cases where cor-neal healing does not improve, contributing to the control of inflammation and reduction of pain [65,66]. An experimental study conducted in the eyes of rabbits showed that RGTA was effective in the reduction of inflammatory signs and reinforcement of epithelization. Additionally, there was a more satisfying management of edema, fibrosis, and neovascularization [65]. According to a pilot study, RGTA favored the healing of corneal ulcers as well as chronic and severe corneal dystrophies. The same study suggested that an installation of a single drop on a weekly basis seemed to be insufficient, but a larger population is required to participate in a controlled trial to obtain more information [65].
Conclusions
HSV-1 can affect the cornea and potentially lead to severe lesions resulting even in blindness.
Both direct viral insult and corneal immunological reaction contribute to destruction of the cornea structure. Therefore, this complex pathophysiological procedure requires, in most cases, a combined treatment for disease management.
Although antiviral treatment is an essential part of herpes keratitis therapy, the use of various adjunctive drugs and surgical techniques is necessary to confront the disease evolution.
Timing and algorithms with regard to the use of those treatments is the key for the successful management of severe forms of HSV keratitis.
Surgical techniques, including AM transplantation and autologous stem cells transplantation, along with limbal and stromal injections of anti-VEGFs, and ophthalmic drops of immunosuppressive and regenerating drugs seem to impede most of the crucial steps of HSV keratitis pathophysiology, thus enhancing the restoration of anatomical and functional integrity of the cornea.
Footnotes
Conflict of interest
None.
Statement
All the examinations carried out for the particular individual were provided for free by the Hellenic National Health Care System.
References:
- 1.Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57(5):448–62. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White ML, Chodosh J. Herpes simplex virus keratitis; A Treatment Guideline. 2014. Massachusetts Eye and Ear Infirmary Department of Ophthalmology, Harvard Medical School. Reviewed and endorsed by the Ocular Microbiology and Immunology Group;
- 3.Miserocchi E, Modorati G, Galli L, Rama P. Efficacy of valacyclovir vs. acyclovir for the prevention of recurrent herpes simplex virus eye disease: A pilot study. Am J Ophthalmol. 2007;144(4):547–51. doi: 10.1016/j.ajo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Chong DY, Johnson MW, Huynh TH, et al. Vitreous penetration of orally administered famciclovir. Am J Ophthalmol. 2009;148(1):38–42. doi: 10.1016/j.ajo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 5.de Rotth A. Plastic repair of conjunctival defects with fetal membrane. Arch Ophthalmol. 1940;23:522–25. [Google Scholar]
- 6.Meller D, Pauklin M, Thomasen Henning, et al. Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int. 2011;108(14):243–48. doi: 10.3238/arztebl.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473–84. [PubMed] [Google Scholar]
- 8.Seitz B. Amniotic membrane transplantation. An indispensable therapy option for persistent corneal epithelial defects. Ophthalmologe. 2007;104:1075–79. doi: 10.1007/s00347-007-1661-3. [DOI] [PubMed] [Google Scholar]
- 9.Shortt AJ, Secker GA, Notara MD, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: A review of techniques and clinical results. Surv Ophthalmol. 2007;52:483–502. doi: 10.1016/j.survophthal.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Meller D, Pires RT, Mack RJ, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–89. doi: 10.1016/s0161-6420(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 11.Solomon A, Meller D, Prabhasawat P, et al. Amniotic membrane grafts for non-traumatic corneal perforations, descemetoceles, and deep ulcers. Ophthalmology. 2002;109:694–703. doi: 10.1016/s0161-6420(01)01032-6. [DOI] [PubMed] [Google Scholar]
- 12.Paridaens D, Beekhuis H, van Den Bosch W, et al. Amniotic membrane transplantation in the management of conjunctival malignant melanoma and primary acquired melanosis with atypia. Br J Ophthalmol. 2001;85:658–61. doi: 10.1136/bjo.85.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espana EM, Prabhasawat P, Grueterich M, et al. Amniotic membrane transplantation for reconstruction after excision of large ocular surface neoplasias. Br J Ophthalmol. 2002;86:640–45. doi: 10.1136/bjo.86.6.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kheirkhah A, Johnson DA, Paranjpe DR, et al. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol. 2008;126:1059–66. doi: 10.1001/archopht.126.8.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letko E, Stechschulte SU, Kenyon KR, et al. Amniotic membrane inlay and overlay grafting for corneal epithelial defects and stromal ulcers. Arch Ophthalmol. 2001;119:659–63. doi: 10.1001/archopht.119.5.659. [DOI] [PubMed] [Google Scholar]
- 16.Kim HK, Park HS. Fibrin glue-assisted augmented amniotic membrane transplantation for the treatment of large noninfectious corneal perforations. Cornea. 2009;28:170–76. doi: 10.1097/ICO.0b013e3181861c54. [DOI] [PubMed] [Google Scholar]
- 17.Prabhasawat P, Ekpo P, Uiprasertkul M, et al. Efficacy of cultivated corneal epithelial stem cells for ocular surface reconstruction. Clin Ophthalmol. 2012;6:1483–92. doi: 10.2147/OPTH.S33951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palamar M, Kaya E, Egrilmez S, et al. Amniotic membrane transplantation in surgical management of ocular surface neoplasias: Long-term results. Eye. 2014;28:1131–35. doi: 10.1038/eye.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HS, Kim JC. Effect of amniotic fluid in corneal sensitivity and nerve regeneration after eximer laser ablation. Cornea. 1996;15:517–24. [PubMed] [Google Scholar]
- 20.Meller D, Pires RT, Tseng SC. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463–71. doi: 10.1136/bjo.86.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meller D, Tseng SC. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. 1999;40:878–86. [PubMed] [Google Scholar]
- 22.Keene DR, Sakai LY, Lunstrum GP. Type VII collagen forms an extended network of anchoring fibrils. J Cell Biol. 1987;104:611–21. doi: 10.1083/jcb.104.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonnenberg A, Calafat J, Janssen H, et al. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell basement membrane adhesion. J Cell Biol. 1991;113:907–17. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terranova VP, Lyall RM. Chemotaxis of human gingival epithelial cells to laminin. A mechanism for epithelial cell apical migration. J Periodonto. 1986;57:311–17. doi: 10.1902/jop.1986.57.5.311. [DOI] [PubMed] [Google Scholar]
- 25.Guo M, Grinnell F. Basement membrane and human epidermal differentiation in vitro. J Invest Dermatol. 1989;93:372–78. [PubMed] [Google Scholar]
- 26.Kurpakus MA, Stock EL, Jones JC. The role of the basement membrane in differential expression of keratin proteins in epithelial cells. Dev Biol. 1992;150:243–55. doi: 10.1016/0012-1606(92)90239-d. [DOI] [PubMed] [Google Scholar]
- 27.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: Basement membrane induces tissue specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–95. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci USA. 1996;93:3509–13. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SB, Li DQ, Tan DT, et al. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20:325–34. [PubMed] [Google Scholar]
- 30.Solomon A, Rosenblatt M, Monroy D, et al. Suppression of Interleukin 1 alpha and Interleukin 1 beta in the human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol. 2001;85:444–49. doi: 10.1136/bjo.85.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao Y, Ma DH, Hwang DG, et al. Identification of antiangiogenic and anti-inflammatory proteins in human amniotic membrane. Cornea. 2000;19:348–52. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Robson MC, Krizek TJ. The effect of human amniotic membranes on the bacteria population of infected rat burns. Ann Surg. 1973;177:144–49. doi: 10.1097/00000658-197302000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adinolfi M, Akle CA, McColl I, et al. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature. 1982;295:325–27. doi: 10.1038/295325a0. [DOI] [PubMed] [Google Scholar]
- 34.Adds PJ, Hunt CJ, Dart JK. Amniotic membrane grafts, “fresh” or frozen? A clinical and in vitro comparison. Br J Ophthalmol. 2001;85:905–7. doi: 10.1136/bjo.85.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhotra C, Jain AK. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J Transplant. 2014;4(2):111–21. doi: 10.5500/wjt.v4.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dua HS, Azuara-Blanco A. Amniotic membrane transplantation. Br J Ophthalmol. 1999;83:748–52. doi: 10.1136/bjo.83.6.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sippel KC, Ma JJ, Foster CS. Amniotic membrane surgery. Curr Opin Ophthalmol. 2001;12:269–81. doi: 10.1097/00055735-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 39.Altay Y, Tamer S, Burcu A, Balta Ö. Amniotic membrane transplantation in bacterial and herpetic stromal keratitis. Turk J Med Sci. 2016;46(2):457–62. doi: 10.3906/sag-1501-6. [DOI] [PubMed] [Google Scholar]
- 40.Meller D, Thomasen H, Steuhl K. Amniotic membrane transplantation in herpetic corneal infections. Klin Monbl Augenheilkd. 2010;227(5):393–99. doi: 10.1055/s-0029-1245339. [DOI] [PubMed] [Google Scholar]
- 41.Spelsberg H, Reichelt JA. Amniotic membrane transplantation in proven ulcerative herpetic keratitis: Successful anti-inflammatory treatment in time. Klin Monbl Augenheilkd. 2008;225(1):75–79. doi: 10.1055/s-2007-963779. [DOI] [PubMed] [Google Scholar]
- 42.Shi W, Chen M, Xie L. Amniotic membrane transplantation combined with antiviral and steroid therapy for herpes necrotizing stromal keratitis. Ophthalmology. 2007;114(8):1476–81. doi: 10.1016/j.ophtha.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 43.Muraine M, Descargues G, Franck O, et al. Amniotic membrane graft in ocular surface disease. Prospective study with 31 cases. J Fr Ophtalmol. 2001;24(8):798–812. [PubMed] [Google Scholar]
- 44.Benyaddoun Y, Casse G, Forte R, et al. Neovascularisation corneenne: Aspects ‘epidemiologiques, physiopathologiques et cliniques. Journal Francais d’Ophtalmologie. 2013;36:627–39. doi: 10.1016/j.jfo.2013.03.002. [in French] [DOI] [PubMed] [Google Scholar]
- 45.Pillai CT, Dua HS, Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci. 2000;41:2148–53. [PubMed] [Google Scholar]
- 46.Stevenson W, Cheng SF, Dastjerdi MH, et al. Corneal neovascularization and the utility of topical VEGF inhibition: Ranibizumab (Lucentis) vs. bevacizumab (Avastin) Ocul Surf. 2012;10(2):67–83. doi: 10.1016/j.jtos.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belghmaidi S, Hajji I, Baali M, et al. Intrastromal injection of bevacizumab in the management of corneal neovascularization: About 25 eyes. J Ophthalmol. 2016;2016:6084270. doi: 10.1155/2016/6084270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vieira AC, Höfling-Lima AL, Gomes JÁ, et al. Intrastromal injection of bevacizumab in patients with corneal neovascularization. Arq Bras Oftalmol. 2012;75(4):277–79. doi: 10.1590/s0004-27492012000400012. [DOI] [PubMed] [Google Scholar]
- 49.Hashemian MN, Zare MA, Rahimi F, Mohammadpour M. Deep intrastromal bevacizumab injection for management of corneal stromal vascularization after deep anterior lamellar keratoplasty, a novel technique. Cornea. 2011;30(2):215–18. doi: 10.1097/ICO.0b013e3181e291a6. [DOI] [PubMed] [Google Scholar]
- 50.Yeung SN, Lichtinger A, Kim P, et al. Combined use of subconjunctival and intracorneal bevacizumab injection for corneal neovascularization. Cornea. 2011;30(10):1110–14. doi: 10.1097/ICO.0b013e31821379aa. [DOI] [PubMed] [Google Scholar]
- 51.Mohammadpour M. Deep intrastromal injection of bevacizumab for the management of corneal neovascularization. Cornea. 2013;32(1):109–10. doi: 10.1097/ICO.0b013e318262e872. [DOI] [PubMed] [Google Scholar]
- 52.Trounson A, McDonald C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell. 2015;17(1):11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Duan R, de Vries RD, Osterhaus AD, et al. Acyclovir-resistant HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008;198:659–63. doi: 10.1086/590668. [DOI] [PubMed] [Google Scholar]
- 54.Dixit R, Tiwari V, Shukla D. Herpes simplex virus type 1 induces filopodia in differentiated P19 neural cells to facilitate viral spread. Neurosci Lett. 2008;440:113–18. doi: 10.1016/j.neulet.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaufman HE, Azcuy AM, Varnell ED, et al. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46:241–47. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tatlipinar S, Akpek EK. Topical ciclosporin in the treatment of ocular surface disorders. Br J Ophthalmol. 2005;89:1363–67. doi: 10.1136/bjo.2005.070888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levinger E, Slomovic A, Sansanayudh W, et al. Topical treatment with 1% cyclosporine for subepithelial infiltrates secondary to adenoviral keratoconjunctivitis. Cornea. 2010;29(6):638–40. doi: 10.1097/ICO.0b013e3181c33034. [DOI] [PubMed] [Google Scholar]
- 58.Jeng BH, Holsclaw DS. Cyclosporine A 1% eye drops for the treatment of subepithelial infiltrates after adenoviral keratoconjunctivitis. Cornea. 2011;30(9):958–61. doi: 10.1097/ICO.0b013e31820cd607. [DOI] [PubMed] [Google Scholar]
- 59.Kauss Hornecker M, Charles Weber S, Brandely Piat ML, et al. [Cyclosporine eye drops: A 4-year retrospective study (2009–2013)] J Fr Ophtalmol. 2015;38(8):700–8. doi: 10.1016/j.jfo.2015.02.008. [in French] [DOI] [PubMed] [Google Scholar]
- 60.Okumus S, Coskun E, Tatar MG, et al. Cyclosporine a 0.05% eye drops for the treatment of subepithelial infiltrates after epidemic keratoconjunctivitis. BMC Ophthalmol. 2012;12:42. doi: 10.1186/1471-2415-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sonmez B, Beden U, Erkan D. Regression of severe corneal stromal neovascularization with topical cyclosporine 0.05% after penetrating keratoplasty for fungal corneal ulcer. Int Ophthalmol. 2009;29(2):123–25. doi: 10.1007/s10792-007-9180-4. [DOI] [PubMed] [Google Scholar]
- 62.Arvola RP, Robciuc A, Holopainen JM. Matrix regeneration therapy: A case series of corneal neurotrophic ulcers. Cornea. 2016;35(4):451–55. doi: 10.1097/ICO.0000000000000759. [DOI] [PubMed] [Google Scholar]
- 63.Kymionis GD, Liakopoulos DA, Mikropoulos DG. Re: Arvola RP, Robciuc A, Holopainen JM: Matrix regeneration therapy: A case series of corneal neurotrophic ulcers. Cornea. 2016;35(9):e28. doi: 10.1097/ICO.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 64.Brignole-Baudouin F, Warnet JM, Barritault D, Baudouin C. RGTA-based matrix therapy in severe experimental corneal lesions: safety and efficacy studies. J Fr Ophtalmol. 2013;36(9):740–47. doi: 10.1016/j.jfo.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Chebbi CK, Kichenin K, Amar N, et al. Pilot study of a new matrix therapy agent (RGTA OTR4120) in treatment-resistant corneal ulcers and corneal dystrophy. J Fr Ophtalmol. 2008;31(5):465–71. doi: 10.1016/s0181-5512(08)72462-8. [DOI] [PubMed] [Google Scholar]
- 66.Aifa A, Gueudry J, Portmann A, et al. Topical treatment with a new matrix therapy agent (RGTA) for the treatment of corneal neurotrophic ulcers. Invest Ophthalmol Vis Sci. 2012;53(13):8181–85. doi: 10.1167/iovs.12-10476. [DOI] [PubMed] [Google Scholar]