Abstract
Background/Aims
Stem cell therapy has been applied to treat a variety of autoimmune diseases, including Crohn’s disease (CD), but few studies have examined the use of umbilical cord mesenchymal stem cells (UC-MSCs). This trial sought to investigate the efficacy and safety of UC-MSCs for the treatment of CD.
Methods
Eighty-two patients who had been diagnosed with CD and had received steroid maintenance therapy for more than 6 months were included in this study. Forty-one patients were randomly selected to receive a total of four peripheral intravenous infusions of 1×106 UC-MSCs/kg, with one infusion per week. Patients were followed up for 12 months. The Crohn’s disease activity index (CDAI), Harvey-Bradshaw index (HBI), and corticosteroid dosage were assessed.
Results
Twelve months after treatment, the CDAI, HBI, and corticosteroid dosage had decreased by 62.5±23.2, 3.4±1.2, and 4.2±0.84 mg/day, respectively, in the UC-MSC group and by 23.6±12.4, 1.2±0.58, and 1.2±0.35 mg/day, respectively, in the control group (p<0.01, p<0.05, and p<0.05 for UC-MSC vs control, respectively). Four patients developed a fever after cell infusion. No serious adverse events were observed.
Conclusions
UC-MSCs were effective in the treatment of CD and produced mild side effects.
Keywords: Umbilical cord, Mesenchymal stromal cells, Crohn disease, Cellular therapy, Autoimmune
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory bowel disease characterized by transmural inflammation, lymphocyte aggregation, and noncaseous granulomas.1 Patients with CD mainly present with abdominal pain, diarrhea, and intestinal obstruction, among other symptoms. The majority of patients eventually need surgery to remove the diseased bowel.2,3 However, the recurrence rate of CD can reach 75%.4
Although its etiology is unclear, most researchers believe that CD is associated with autoimmune response, and that inflammatory mediators such as cytokines and free radicals are involved in chronic inflammatory lesions.5
In recent years, stem cell transplantation has emerged as a promising treatment, which is characterized by regulating immunity, repairing injury, and controlling inflammation. Several studies have employed autologous stem cells or adipose-derived stem cells to treat CD and its complications, and have presented positive outcomes.6–11 Hematopoietic stem cell therapy usually requires myeloablative or nonmyeloablative conditioning, which increases the risk of infection; Collection of bone marrow (BM) in advance is necessary for BM mesenchymal stem cell (MSC) therapy, and the quantities and quality of cultured cells are unstable; Localized injection of adipose MSC in CD with complex anal fistula has shown promising outcomes. Intravenous infusion was utilized by the majority of the available trials due to, probably, ease of operation and the nature of CD, a systemic disorder. Thus far, the application of umbilical cord MSCs (UC-MSCs) in CD has rarely been reported. In this study, UC-MSCs were transplanted to treat patients with steroid-controlled CD. The alterations of patient conditions and corticosteroid dosage, and treatment-related adverse events were observed.
MATERIALS AND METHODS
1. Ethics statement
All patients signed an informed consent form. UCs were obtained from full-term neonates, and the mother of the donor signed an informed consent form. The study was approved by the Ethics Committee of Shaanxi Provincial People’s Hospital (IRB00006161), and complied with the Declaration of Helsinki and the standard ethical requirements of the local administrative department. This study is registered with the ClinicalTrials.gov (NCT02445547).
2. Patients
The study was a prospective, randomized, controlled, open-label clinical trial conducted between June 2012 and June 2015. The inclusion criteria were as follows: above 18 years of age, with moderate to severe CD (Crohn’s disease activity index [CDAI] between 220 and 450). All patients had received steroid maintenance therapy for more than 6 months before enrollment. Concomitant immunosuppressive agents (including azathioprine, 6-mercaptopurine or methotrexate) were allowed but the dosage was maintained unless steroid was discontinued. Anti-tumor necrosis factor (anti-TNF) therapy was not allowed within 3 months prior to the selection. Current treatment was continued after UC-MSC infusion. Patients were excluded for active tuberculosis, malignancy, and infection of human immunodeficiency virus, syphilis, hepatitis B virus, and hepatitis C virus. Patients underwent colonoscopy at enrollment to assess the degree of disease activity.
3. UC-MSC preparation
A piece of human UC (5 cm) from a full-term newborn (blood type O) was harvested at the time of delivery in the Department of Obstetrics and Gynecology, Shaanxi Provincial People’s Hospital. The mesenchymal tissue (in Wharton’s jelly) was diced into cubes of about 0.1 cm3 and centrifuged at 250 g for 5 minutes. After removal of the supernatant fraction, the precipitate (mesenchymal tissue) was washed with serum-free Dulbecco’s Modified Eagle Medium (DMEM; HyClone, Logan, UT, USA) and centrifuged at 250 g for 5 minutes. The mesenchymal tissue was treated with 0.1% collagenase (Sigma, St Louis, MO, USA) at 37°C overnight, washed twice, and further digested with 0.25% trypsin (Gibco, Grand Island, NY, USA) at 37°C for 60 minutes. Fetal bovine serum (HyClone) was added to the mesenchymal tissue to neutralize the excess trypsin. Cells were plated in DMEM supplemented with 10% fetal bovine serum (HyClone), 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mmol/L L-glutamine (Gibco) at a density of 1×106 cells/mL. The medium was renewed every 2 to 3 days and suspended cells were discarded. After reaching 80% to 90% confluence, cells were harvested with 0.25% trypsin and 0.02% EDTA and replated at 1:3 under the same condition. When the number of cells was enough for infusion, confluent MSC in flasks were washed with isotonic normal saline, incubated with medium 199 (Gibco) for 60 minutes, and detached with trypsin-EDTA. MSCs were resuspended in medium 199+1% human serum albumin, and cells were cryopreserved using a rate controlled freezer at a final concentration of 10% DMSO (Sigma) and 5% human serum albumin. On the day of infusion cryopreserved units were thawed at the bedside in a 37°C water bath, and transported to the clinical team for infusion. Cells were phenotypically characterized by flow cytometry and their differentiation potential evaluated following the 2006 International Society of Cellular Therapy’s criteria. Bacteria, mycoplasma, and fungi contamination testing and endotoxin test were performed before each batch of cells was released.
4. Study procedure
Patients in the UC-MSC group received a UC-MSC infusion of 1×106 cells/kg once a week, four times in total. For preoperative prophylaxis of thrombosis, 2,500 IU of low-molecular-weight heparin were administered by subcutaneous injection once a day, for a total of 3 days. Patients were followed up at 3, 6, 9, and 12 months after cell infusion. At each follow-up visit, CDAI, Harvey-Bradshaw index (HBI), corticosteroid dosage, and adverse events were assessed. Additionally, blood cell analysis was conducted, and liver and renal function, coagulation function, and D-dimer were tested. At 12 months, patients were reexamined by colonoscopy, and mucosal inflammation was assessed using the CD endoscopic index of severity (CDEIS). Fistula healing was defined as absence of discharge and <2 cm of fluid collection-the latter determined by magnetic resonance imaging.
5. Statistical analysis
Data were analyzed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Computer-generated block randomization was used to assign each participant to one of the study groups. Data are presented as mean values±standard deviation or median values (range). For baseline data, count data were compared using chi-square, nonparametric test or Fisher test and measurement data were analyzed using an independent samples t-test; differences in data before and after treatment were compared with repeated measures analysis of variance. p<0.05 was considered significant. Power and sample size considerations assume a 30% decrease of steroid dose at 1 year after infusion from an average 12.5 mg per day and 10% patient loss to follow-up. The Student t-test indicated two independent groups of 40 patients each as having adequate power to detect this assumed difference (type I error=0.05, 90% power).
RESULTS
A flow chart of the study is shown in Fig. 1. In the UC-MSC group, there were 24 men and 17 women, with an average age of 34.3 years (range, 21 to 44 years). In the control group, there were 26 men and 15 women, with an average age of 32.7 years (range, 20 to 41 years). The baseline and demographic data of the patients are summarized in Table 1. Four patients dropped out in the UC-MSC group and three dropped out in the control group both due to nonadherence.
Fig. 1.
Study flow chart.
Table 1.
Participants’ Baseline and Demographic Data
| Characteristic | Stem cell group (n=41) | Control group (n=41) | p-value |
|---|---|---|---|
| Age, yr | 34.3 (21–44) | 32.7 (20–41) | 0.72 |
| Sex, male/female | 24/17 | 26/15 | 0.65 |
| Medical history of CD, yr | 7 (2–15) | 8 (3–14) | 0.66 |
| BMI, kg/m2 | 22.5±2.6 | 23.1±3.2 | 0.58 |
| Current smoker/smoking history | 2/10 | 1/8 | 0.55/0.59 |
| Montreal classification | |||
| Age at diagnosis, yr | 0.99 | ||
| A1: below 16 | 2 | 1 | |
| A2: between 17 and 40 | 38 | 40 | |
| A3: above 40 | 1 | 0 | |
| Location | 0.73 | ||
| L1: ileal | 14 | 17 | |
| L2: colonic | 18 | 14 | |
| L3: ileocolonic | 9 | 10 | |
| L4: isolated upper disease* | 6 | 9 | 0.39 |
| Behavior | 0.24 | ||
| B1: nonstricturing, nonpenetrating | 23 | 17 | |
| B2: stricturing | 10 | 14 | |
| B3: penetrating | 8 | 10 | |
| p: perianal disease modifier* | 6 | 7 | 0.76 |
| Operation history | 12 | 9 | 0.45 |
| CDAI | 281.5±75.2 | 293.2±68.4 | 0.48 |
| HBI | 12.7±3.2 | 11.9±3.5 | 0.55 |
| CDEIS | 9.2±1.5 | 8.7±2.3 | 0.63 |
| Steroid dosage, mg | 13.6±2.8 | 12.8±2.5 | 0.57 |
| Concomitant medication | |||
| AZA | 7 | 8 | 0.78 |
| MTX | 5 | 7 | 0.53 |
| 6MP | 6 | 10 | 0.27 |
| Antibiotics | 12 | 8 | 0.30 |
| Prior anti-TNF therapy | 13 | 9 | 0.32 |
| Infliximab | 4 | 3 | 0.69 |
| Adalimumab | 9 | 6 | 0.39 |
Data are presented as median (range) or mean±SD.
CD, Crohn’s disease; BMI, body mass index; CDAI, Crohn’s disease activity index; HBI, Harvey-Bradshaw index; CDEIS, Crohn’s disease endoscopic index of severity; AZA, azathioprine; MTX, methotrexate; 6MP, 6-mercaptopurine; TNF, tumor necrosis factor.
Indicates a modifier that can be added to other classifications when present as a concomitant symptom.
1. Efficacy
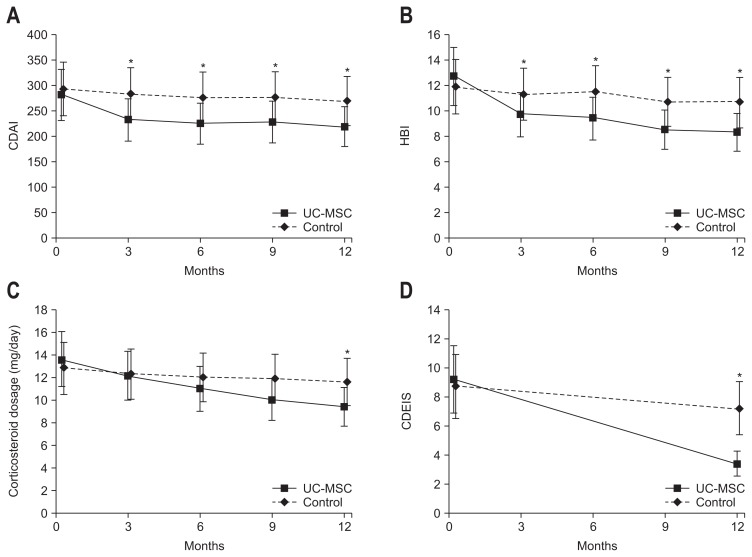
All patients in the UC-MSC group completed the full course of UC-MSC infusions. The third and fourth infusions were delayed by one week in one patient for personal reasons. In the UC-MSC group, the pretreatment CDAI, HBI, and corticosteroid dosage were 281.5±75.2, 12.7±3.2 and 13.6±2.8 mg/day, respectively; 12 months after treatment, the CDAI, HBI, and corticosteroid dosage were 219.0±67.6, 9.3±2.9, and 9.4±3.1, respectively, representing respective decreases of 62.5±23.2, 3.4±1.2, and 4.2±0.84 mg/day. In the control group, the CDAI, HBI, and corticosteroid dosage were 293.2±68.4, 11.9±3.5, and 12.8±2.5, respectively; 12 months after treatment, the CDAI, HBI, and corticosteroid dosage were 269.6±62.5, 10.7±3.3, and 11.6±2.4, respectively, representing respective decreases of 23.6±12.4, 1.2±0.58, and 1.2±0.35 mg/day (p<0.01, p<0.05, and p<0.05; UC-MSC vs control; respectively) (Fig. 2A–C). No patient achieved complete remission (CDAI<150). Colonoscopy showed that the CDEIS in the UC-MSC group was 9.2±1.5 and 3.4±1.2 before treatment and at the 12-month follow-up, respectively (Fig. 3). The values were 8.7±2.3 at the start of the study and 7.2±1.9 at the 12-month follow-up in the control group, which indicates significant differences in the two groups (p<0.01, UC-MSC vs control) (Fig. 2D). Concomitant anal fistula was improved in six patients in the UC-MSC group, while it was not improved in seven patients in the control group.
Fig. 2.
(A) Crohn’s disease activity index (CDAI), (B) Harvey-Bradshaw index (HBI), (C) corticosteroid dosage, and (D) CD endoscopic index of severity (CDEIS) before treatment and at 3, 6, 9, and 12 months after treatment.
*Indicates a significant difference between the umbilical cord mesenchymal stem cell (UC-MSC) and control groups.
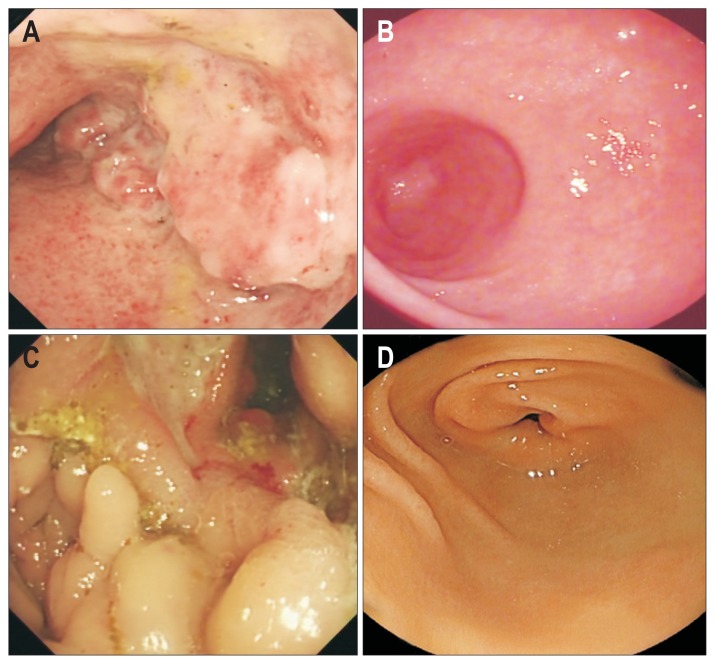
Fig. 3.
Colonoscopy showed remarkable mucosal recovery at 12 months compared with baseline findings. (A, B) Panels depict images for patient 14 and (C, D) depict images for patient 23.
2. Safety
In the UC-MSC group, four patients experienced fever after UC-MSC infusion, which was relieved after symptomatic treatment. Additionally, seven patients had nine episodes of upper respiratory tract infection, occurring within 6 months postinfusion. No serious adverse events were observed. None of the patients experienced dyspnea after infusion. Coagulation panel tests and D-dimer tests were negative for thrombosis. Blood cell count and liver and renal function showed no significant differences in either group at the 12-month follow-up point compared with baseline (data not shown).
DISCUSSION
In this study, after UC-MSC infusion, steroid dosage significantly decreased and the patients’ conditions were also improved significantly. This indicates that UC-MSCs can attenuate immune malfunction in patients with CD. However, most of the patients still used steroid at 12 months, which seemed not superior to the efficacy of TNF blocker. A possible explanation was that the UC-MSC therapy had a distinctive role because a proportion of patients who had poorly responded to TNF blocker had an evident decrease in steroid dosage. Despite of this fact, it has to be admitted that the UC-MSC therapy has moderate immunomodulatory effects.12 We suggest that the mechanisms of UC-MSC efficacy in CD be elucidated to facilitate the precise selection of patient for the cell treatment in the future.
A number of groups are currently using stem cells to treat CD and investigating complications associated with the treatment.9,13,14 Duijvestein et al.15 showed that autologous BM-derived MSCs alleviated the condition of patients with CD, with mild adverse effects. However, the above study was a phase I clinical trial that could not fully verify the efficacy of BM-derived stem cells; furthermore, qualities of autologous BM stem cells vary due to individual discrepancy, resulting in heterogeneity in therapeutic effects and culture results. In the study by Forbes et al.,16 allogeneic BM-MSCs were infused for four times, achieving favorable improvements of CDAI with only one serious adverse event probably not caused by MSCs. The present study had a larger scale and employed a novel type of cells. Additionally, six patients with anal fistula showed remarkable improvement.
The mechanisms of alleviating CD by UC-MSCs remain obscure. It was suggested that downregulation of proinflammatory cytokines including TNF-α, interleukin-2 and vascular endothelial growth factor played a beneficial role.13,14 The possibility to dampen Th17 cells and increase the apoptotic rate of effector T-cells, while favoring the expansion of both T-cell and macrophage population with regulatory function was of biological relevance.17
The therapeutic processes using BM MSCs were more complicated. Patients should undergo BM collection in advance and wait for the culture of BM MSCs, of which the duration and cell quantities were varying.18 The source of UC-MSCs in the current study was from one donor, which had the advantages of homogeneity, constant cell number, and good comparability of efficacy.
Controversy remains over the safety of the clinical application of stem cells. Several cases of stem cell infusion-related deaths, including cases in Thailand, Romania, and South Korea, have been published in Nature.19 For example, one patient died of pulmonary embolism after infusion of adipose-derived MSCs. Our study demonstrated that UC-MSCs were safe in the treatment of CD. Four patients had fever, which might result from an allergic reaction to residual culture media. The patients did not show coagulation dysfunction or thrombosis, which might be associated with thrombosis prophylaxis using low-molecular-weight heparin. Several patients developed upper respiratory tract infection postoperatively; the incidence rate was higher in the UC-MSC group than in the control group, but not significantly. Clinical trials of stems cell therapy in other diseases also showed that stem cells can regulate or even suppress immunity.20 Therefore, we inferred that the infections of the patients were associated with immunosuppression by stem cells.
This study suggested that the approach of peripheral infusion of UC-MSCs was convenient and safe. However, the disadvantages were little distribution of UC-MSCs in the intestinal tissue and a risk of cells being retained in the pulmonary capillaries. Clinical trials investigating other diseases have utilized arterial infusion to increase local stem cell concentration.18,21 In the future, it is necessary to compare the efficacy of interventional infusion into the inferior mesenteric artery to that of peripheral infusion.
The study was an open-label trial conducted at a single center; and it did not examine indicators of immune status or intestinal histopathology in patients. Therefore, the mechanism by which stem cell therapy affects CD remains unclear.
In conclusion, UC-MSC therapy can significantly and safely improve disease condition in patients with CD receiving a stable steroid dose. The long-term effect of UC-MSC therapy remains to be investigated.
ACKNOWLEDGEMENTS
Author contributions: J.Z. contributed to performing the study and writing the manuscript. S.L. contributed to performing the study, collecting and analyzing the data, and writing the manuscript. X.L. contributed to analyzing the data and writing the manuscript. B.S. contributed to performing the study. L.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
See editorial on page 5.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn’s disease: what is the actual risk? Gut. 2011;60:1178–1181. doi: 10.1136/gut.2010.234617. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB. Crohn’s disease: step up or top down therapy. Best Pract Res Clin Gastroenterol. 2003;17:131–137. doi: 10.1053/bega.2003.0361. [DOI] [PubMed] [Google Scholar]
- 3.Shaffer VO, Wexner SD. Surgical management of Crohn’s disease. Langenbecks Arch Surg. 2013;398:13–27. doi: 10.1007/s00423-012-0919-7. [DOI] [PubMed] [Google Scholar]
- 4.Fornaro R, Frascio M, Stabilini C, et al. Crohn’s disease surgery: problems of postoperative recurrence. Chir Ital. 2008;60:761–781. [PubMed] [Google Scholar]
- 5.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 6.Wainstein C, Quera R, Kronberg U, et al. Mesenchymal stem cells and platelet-rich plasma in the treatment of patients with perineal Crohn’s disease. Int J Colorectal Dis. 2016;31:725–726. doi: 10.1007/s00384-015-2221-y. [DOI] [PubMed] [Google Scholar]
- 7.Jauregui-Amezaga A, Rovira M, López A, et al. Long-lasting remission induced by syngeneic haematopoietic stem cell transplantation in a patient with refractory Crohn’s disease. J Crohns Colitis. 2016;10:1122–1124. doi: 10.1093/ecco-jcc/jjw062. [DOI] [PubMed] [Google Scholar]
- 8.Panés J, García-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 9.Jauregui-Amezaga A, Rovira M, Marín P, et al. Improving safety of autologous haematopoietic stem cell transplantation in patients with Crohn’s disease. Gut. 2016;65:1456–1462. doi: 10.1136/gutjnl-2015-309836. [DOI] [PubMed] [Google Scholar]
- 10.Hawkey CJ, Allez M, Clark MM, et al. Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. JAMA. 2015;314:2524–2534. doi: 10.1001/jama.2015.16700. [DOI] [PubMed] [Google Scholar]
- 11.Mayer L, Pandak WM, Melmed GY, et al. Safety and tolerability of human placenta-derived cells (PDA001) in treatment-resistant Crohn’s disease: a phase 1 study. Inflamm Bowel Dis. 2013;19:754–760. doi: 10.1097/MIB.0b013e31827f27df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai J, Wu Z, Xu X, et al. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care. 2016;39:149–157. doi: 10.2337/dc15-0171. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Olmo D, Garcia-Arranz M, Herreros D. Expanded adipose-derived stem cells for the treatment of complex perianal fistula including Crohn’s disease. Expert Opin Biol Ther. 2008;8:1417–1423. doi: 10.1517/14712598.8.9.1417. [DOI] [PubMed] [Google Scholar]
- 14.González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 16.Forbes GM, Sturm MJ, Leong RW, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai J, Wu Z, Huang L, et al. Cotransplantation of bone marrow mononuclear cells and umbilical cord mesenchymal stem cells in avascular necrosis of the femoral head. Transplant Proc. 2014;46:151–155. doi: 10.1016/j.transproceed.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Cyranoski D. Korean deaths spark inquiry. Nature. 2010;468:485. doi: 10.1038/468485a. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z, Cai J, Chen J, et al. Autologous bone marrow mononuclear cell infusion and hyperbaric oxygen therapy in type 2 diabetes mellitus: an open-label, randomized controlled clinical trial. Cytotherapy. 2014;16:258–265. doi: 10.1016/j.jcyt.2013.10.004. [DOI] [PubMed] [Google Scholar]