Abstract
Eosinophilic myocarditis (EM) is a rare myocardial disease that results from various eosinophilic diseases, such as idiopathic hypereosinophilic syndrome, helminth infection, medications and vasculitis. Patients with EM may present with different severities, ranging from mild symptoms to a life-threatening condition. Diagnosis of EM is a challenge and requires an extensive workup, including endomyocardial biopsy. Treatment options are limited because EM is rare and there is a lack of randomised controlled trials. We report a case of EM that presented as cardiac tamponade, which was initially treated with high-dose prednisone and immunosuppressant medications without significant improvement. Mepolizumab (anti-interleukin (IL)-5 antibody) was then applied, leading to an increased ejection fraction and stabilised cardiac function. This case report shows, for the first time, that mepolizumab has novel effects in treating EM. Our findings suggest that mepolizumab can be used as a steroid-sparing agent for treating EM.
Keywords: Cardiovascular medicine, Immunology
Background
Eosinophilic myocarditis (EM), first reported by Löffler in 1936,1 is a rare myocardial entity, which comprises myocardial and vascular damage due to eosinophilic infiltration and degranulation. Since Löffler’s report,1 a growing number of cases of EM have been reported. Currently, the first-line treatment for EM is steroids. Immunosuppressants have been used as steroid-sparing agents, but they do not always show a therapeutic effect in EM and may even produce severe side effects. We report here a case of EM, which was initially treated with steroids and immunosuppressants. The patient had become dependent on a high dose of steroids, despite using steroid-sparing agents. Finally, mepolizumab (an anti-interleukin (IL)-5 monoclonal antibody) was applied and showed a stabilising effect on cardiac function. The patient was able to reduce the intake of steroids.
Case presentation
A 60-year-old Caucasian man was admitted to our hospital with a 2-week history of progressive shortness of breath. He reported a mild non-productive cough that started at the same time. He denied any chest pain. He did not have any fever, chills, night sweats or recent travel. He also had no history of smoking, alcohol or recreational drug use. He was previously diagnosed with asthma and mild obstructive sleep apnoea and had surgery for nasal polyps in the past. He was not taking any medications. A clinical examination showed a respiratory rate of 22 breaths/minute, a heart rate of 110 beats/minute, blood pressure was 90/55 mm Hg and body temperature was 98.6 °F. Lung auscultation showed diffuse bilateral crackles and deep heart sounds. The remainder of the physical exam was within normal limits. Initial blood tests of the leucocyte count and differential showed the following: white blood cells: 12.2×109/L; eosinophils: 39% (0%–5%); neutrophils: 46% (38%–56%); lymphocytes: 11% (28%–42%); and absolute eosinophil count: 7.8×109/L (0–0.5×109/L). Haemoglobin was 14 g/dL, haematocrit was 41% and the platelet count was 287×109/L. Liver and kidney function, urinalysis and cardiac troponin levels were within normal values. ECG suggested sinus tachycardia with low-voltage QRS.
A chest X-ray showed widening of the cardiac silhouette with mild interstitial pulmonary oedema. An echocardiogram suggested large pericardial effusion with tamponade physiology and an ejection fraction (EF) of 30% (figure 1). He underwent urgent pericardiocentesis and pericardial window. Pericardial fluid analysis showed numerous eosinophils and a negative culture for bacteria and fungi.
Figure 1.
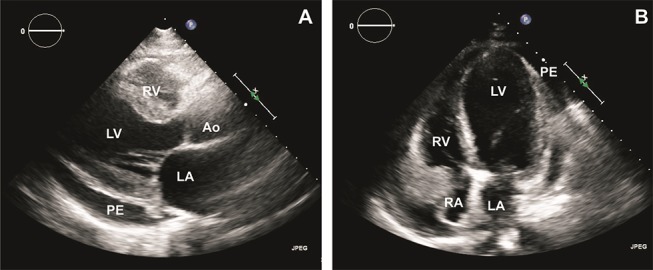
An echocardiogram on initial presentation shows pericardial effusion (PE) with an EF of 30%. (A) Parasternal long axis view. (B) Apical four-chamber view. Ao, aorta; LA, left atrium; EF, ejection fraction; LV, left ventricle; PE, pericardial effusion; RA, right atrium; RV, right ventricle.
Consequently, the patient underwent right and left heart catheterisation, which showed no significant coronary artery disease. An endomyocardial biopsy showed eosinophil-rich infiltrates without vasculitis (figure 2). Further blood tests showed the following. Antinuclear antibody was 1:320 with a speckled pattern. Anti-Smith antibodies, ribonucleoprotein, anti-SSA (Sjögren's syndrome type A) antibodies, anti-SSB (Sjögren's syndrome type B) antibodies, antineutrophil cytoplasmic antibodies, myeloperoxidase antibodies and antiproteinase-3 antibodies were all negative. Serum protein electrophoresis and urine protein electrophoresis with immunofixation were normal. A stool parasite workup was negative. QuantiFERON, a hepatitis B panel, and hepatitis C antibodies were also negative. The patient underwent further investigation, and a bone marrow biopsy showed a mild increase in eosinophils (8%; normal range: 0%–5%). There was no evidence of Fip1-Like 1 (FIP1L1) and platelet-derived growth factor receptor A (PDGFRA) gene mutations. Cardiac MRI showed endomyocardial infiltration. Diagnosis of EM was made based on the endomyocardial biopsy and investigational results.
Figure 2.
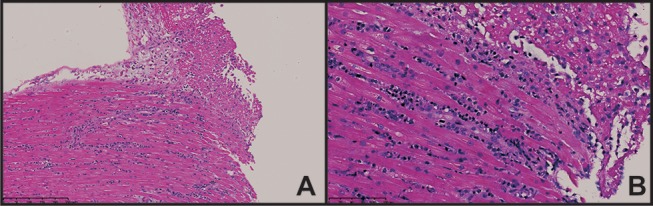
A biopsy shows two fragments of endomyocardium each with moderate interstitial infiltrate dominated by eosinophils and lymphocytes. There was single-cell necrosis, interstitial oedema and early endocardial thrombus formation. There was no evidence of vasculitis, granuloma, viral inclusions or organisms. (A) H&E-stained section at 150× with interstitial eosinophils and lymphocytes. Fibrin deposition and early endocardial thrombus are present. (B) Same section as (A), but at a power of 300×.
Treatment
The patient was initially treated with intravenous methylprednisolone 500 mg/day for 3 days and was then switched to oral prednisone 60 mg/day. After stabilisation and discharge, azathioprine was started at a dose of 2 mg/kg. On discharge, the EF was 30%. The patient experienced multiple episodes of recurrent worsening shortness of breath with a decrease in EF to less than 30% with every attempt to taper prednisone less than 30 mg/day. Azathioprine was stopped, and mycophenolate mofetil (MMF) was started as a steroid-sparing agent and titrated to a dose of 3 mg/day. However, the patient remained dependent on a high dose of prednisone (30 mg/day). Therefore, MMF was stopped, and rituximab was initiated. The patient was induced by intravenous rituximab 375 mg/m2 once a week for four doses, and then he received one maintenance dose after 6 months. Prednisone was decreased to 20 mg/day. However, he was admitted to our institute 3 days after he received the maintenance dose with a worsened EF to less than 20% (figure 3). He was treated with a high dose of prednisone and discharged with an EF of 30%. Mepolizumab was started as the final option. The patient received 100 mg of mepolizumab subcutaneously every 4 weeks. He also started enalapril 2.5 mg/day and carvedilol 3.125 mg/day after he was stabilised at the first admission. Enalapril was later increased to 5 mg/day and carvedilol to 6.25 mg/day, and he was maintained on the same doses throughout the course of his treatment with mepolizumab. After 7 months of treatment with mepolizumab, prednisone was decreased to 15 mg/day, and the EF was maintained at 35%–40% (figure 4).
Figure 3.
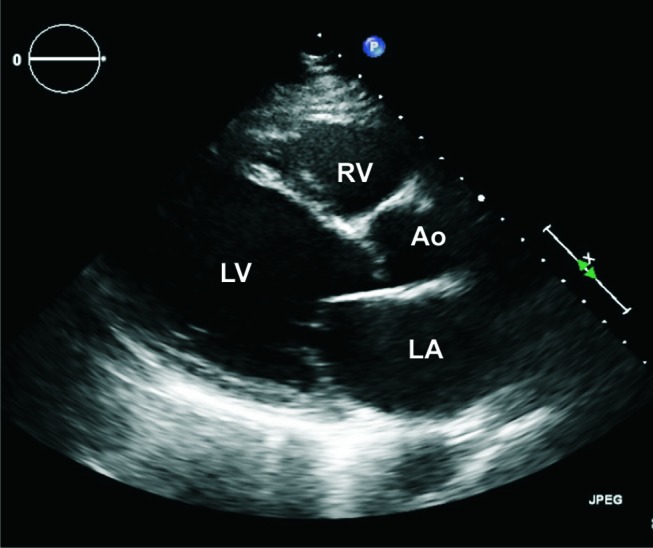
Echocardiogram at relapse with a decrease in EF to less than 20%. Ao, aorta; LA, left atrium; EF, ejection fraction; LV, left ventricle; RV, right ventricle.
Figure 4.
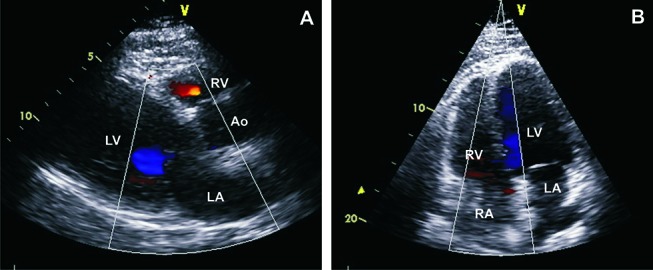
Echocardiogram after 6 months of mepolizumab treatment shows an EF of 35%–45%. (A) Parasternal view. (B) Apical four-chamber view. Ao, aorta; EF, ejection fraction; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Outcome and follow-up
Clinically, the patient’s EF increased to 35%–45% and remained stable since mepolizumab was started, without any admission or experiencing worsening shortness of breath. He has received seven doses until the time of submission of the case, with good tolerability. No side effects have been observed.
Discussion
EM is a rare myocardial disease, which can be potentially fatal. EM is an eosinophilic-associated disease (EAD), which can occur in the setting of different aetiologies. Allergy or hypersensitivity to various drugs has been reported as a cause of EAD. The most commonly reported medications for causing EAD are amphotericin b, ampicillin, clozapine, anti-inflammatory drugs, acetazolamide, hydrochlorothiazide, captopriland digoxin.2 Eosinophilia is associated with parasite infections, such as Trypanosoma, Toxoplasma, Trichinella and Echinococcus.3
EM may also occur in patients with vasculitis, such as eosinophilic granulomatosis with polyangiitis, previously known as Churg-Strauss syndrome.4 In our case, endomyocardial pathology did not show any evidence of vasculitis. Neoplastic and haematological disorders, such as myeloid leukaemia, myelodysplastic syndrome, polycythaemia vera and solid tumours, are associated with eosinophilia.5 Idiopathic hypereosinophilic syndrome (HES) is defined as a blood eosinophil count >1500/μL for ≥6 months and eosinophilia-related organ involvement or dysfunction without an identifiable secondary cause of eosinophilia.6 A diagnosis of EM is required to investigate of any of the above-mentioned eosinophilia-related causes. A bone marrow biopsy is necessary to evaluate FIP1L1 and PDGFRA gene mutations or any evidence of secondary HES. Cardiac MRI is helpful for diagnosis of EM. However, a myocardial biopsy is the gold standard and mandatory for identifying the underlying aetiology of EM, such as virus-positive myocarditis, and should not be delayed. Additionally, an endomyocardial biopsy (EMB) is the only tool for making a pathological diagnosis of myocarditis.
Eosinophils are produced in the bone marrow and follow the classic pattern of granulocyte differentiation. They contain many cytoplasmic granules, which contain hydrolases, cationic protein and basic protein. The most important cationic proteins are major basic protein, eosinophilic cationic protein, eosinophil-derived neurotoxin and eosinophilic peroxidase. These proteins can induce cellular apoptosis and necrosis when degranulation of eosinophils occurs.7 Eosinophils acquire IL-5 receptor α on their surface at the early stage during eosinophilopoiesis, differentiation and maturation, mainly under the influence of IL-5. Granulocyte macrophage colony-stimulating factor and IL-3 also promote eosinophil maturation from myeloid precursors.8 Diny et al9 showed that cytokines from the Th2 pathway (IL-4 and IL-13) can activate fibroblasts and macrophages in cardiomyocytes, which will further elevate eotaxin (CCL11 and CCL24) expression. Therefore, CCR3 expressed in eosinophils will accumulate in cardiomyocytes, which leads to damage of heart tissue. Recent literature has indicated that eosinophilic infiltration and degranulation are associated with myocardial necrosis.7 Moreover, eosinophils bind to thrombomodulin, causing impaired formation of the thrombomodulin–thrombin complex. Eosinophils also store tissue factor, which is the main anticoagulation factor, leading to a hypercoagulated state when eosinophils invade the heart.7 Furthermore, fibrosis has been observed in the endocardium. This is due to transforming growth factor-β-mediated and IL-1-mediated eosinophilic activation in the endocardium.7
IL-5 plays an important role in development eosinophils. Mepolizumab is an anti-IL-5 recombinant humanised monoclonal antibody. Mepolizumab stops the interaction between IL-5 and its receptor on eosinophils and their progenitors.10 Mepolizumab has been investigated as a therapeutic and steroid-sparing medication in patients with HES or asthma.10 11 A randomised, double-blind, placebo-controlled, multicentre study investigated the effect of mepolizumab on patients with HES who were negative for FIP1L1–PDGFRA gene mutations.12 Mepolizumab significantly stabilised the eosinophil count, and the steroid dose could be decreased.12 Another large retrospective study compared conventional and novel therapy (anti-IL-5) in patients with HES.13 Interestingly, this study showed that 80% of patients responded to mepolizumab treatment. ACE inhibitors and beta-blockers have benefits in patients with heart failure. However, in our case, there was a relapse while the patient was on enalapril (5 mg/day) and carvedilol (6.25 mg/day). Additionally, the dose of these medications did not change while the patient was administered mepolizumab, suggesting a positive effect on cardiac function.
In agreement with previous reports, the findings in our case show that mepolizumab is effective in improving cardiac function and stabilising the underlying disease. Mepolizumab could also be used as a supplementary medication to steroid treatment in EM. More importantly, use of mepolizumab could lower the dose of steroids for preventing steroid-induced side effects.
Learning points.
To the best of our knowledge, this case is the first report of the treatment effect of mepolizumab in eosinophilic myocarditis (EM).
EM is rare and difficult to diagnose, and an extensive workup, including an endomyocardial biopsy, is often required.
Ruling out secondary and treatable causes of eosinophilia in patients with EM is important.
Footnotes
Contributors: TS was involved in writing and editing of the case report. DMJ was involved in pathological study and providing images for the purpose of case report. YH was involved in direct patient care, data collection, and writing and editing of case report.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Löffler W. Endocarditis parietalis fibroplastica mit Bluteosinophilie: ein eigenartiges Krankheitsbild. Schweiz Med Wochenschr 1936;18:817–20. [PubMed] [Google Scholar]
- 2.Taliercio CP, Olney BA, Lie JT. Myocarditis related to drug hypersensitivity. Mayo Clin Proc 1985;60:463–8. 10.1016/S0025-6196(12)60870-2 [DOI] [PubMed] [Google Scholar]
- 3.Baandrup U. Eosinophilic myocarditis. Herz 2012;37:849–52. 10.1007/s00059-012-3701-2 [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg M, Lorenz HM, Gassler N, et al. Rapid progressive eosinophilic cardiomyopathy in a patient with Churg-Strauss syndrome (CSS). Clin Res Cardiol 2006;95:289–94. 10.1007/s00392-006-0364-0 [DOI] [PubMed] [Google Scholar]
- 5.Gotlib J. Molecular classification and pathogenesis of eosinophilic disorders: 2005 update. Acta Haematol 2005;114:7–25. 10.1159/000085559 [DOI] [PubMed] [Google Scholar]
- 6.Chusid MJ, Dale DC, West BC, et al. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine 1975;54:1–27. [PubMed] [Google Scholar]
- 7.Kuchynka P, Palecek T, Masek M, et al. Current diagnostic and therapeutic aspects of eosinophilic myocarditis. Biomed Res Int 2016;2016:1–6. 10.1155/2016/2829583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uhm TG, Kim BS, Chung IY. Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol Res 2012;4:68–79. 10.4168/aair.2012.4.2.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diny NL, Hou X, Barin JG, et al. Macrophages and cardiac fibroblasts are the main producers of eotaxins and regulate eosinophil trafficking to the heart. Eur J Immunol 2016;46:2749–60. 10.1002/eji.201646557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Büttner C, Lun A, Splettstoesser T, et al. Monoclonal anti-interleukin-5 treatment suppresses eosinophil but not T-cell functions. Eur Respir J 2003;21:799–803. 10.1183/09031936.03.00027302 [DOI] [PubMed] [Google Scholar]
- 11.Menzies-Gow A, Flood-Page P, Sehmi R, et al. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol 2003;111:714–9. 10.1067/mai.2003.1382 [DOI] [PubMed] [Google Scholar]
- 12.Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med 2008;358:1215–28. 10.1056/NEJMoa070812 [DOI] [PubMed] [Google Scholar]
- 13.Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol 2009;124:1319–25. 10.1016/j.jaci.2009.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]


