Abstract
We report successful transvenous treatment of direct carotid–cavernous fistula in a patient with Ehlers–Danlos syndrome type IV using a novel triple-overlay embolization (TAILOREd) technique without the need for arterial puncture, which is known to be highly risky in this patient group. The TAILOREd technique allowed for successful treatment using preoperative MR angiography as a three-dimensional overlay roadmap combined with cone beam CT and live fluoroscopy, precluding the need for an arterial puncture.
Keywords: Fistula, Technique, Navigation, Intervention, Magnetic Resonance Angiography
Background
Ehlers–Danlos syndrome (EDS) type IV, also known as vascular EDS (vEDS), is a rare inherited collagen vascular disease representing 4% of all patients with EDS.1 vEDS results from COL3A1 mutations and type 3 collagen deficiency and is associated with high morbidity and mortality from spontaneous arterial dissections, aneurysms, and vessel and/or organ rupture.2 Direct carotid–cavernous fistulas (CCF), classified as Barrow type A, are the most common non-lethal central nervous system arterial vascular anomalies in vEDS.2 They are associated with progressive vision loss and intracerebral hemorrhage due to ophthalmic and cortical venous reflux and intracranial venous hypertension.
Previous reports have demonstrated the high risk of bleeding complications and arterial injury associated with catheter angiography and endovascular treatment of patients with vEDS with CCF, with mortality ranging from 12% to 59%.3 To minimize arterial vascular complications, several authors have suggested a transvenous route of cavernous sinus embolization in vEDS CCF.4 5 Despite this treatment strategy, arterial access is still commonly required in order to identify the precise anatomy of the cavernous sinus and to evaluate the fistulous venous drainage pattern.4
Non-invasive three-dimensional (3D) overlay roadmapping with MR angiography (MRA) prior to intervention may provide sufficient depiction of arterial and venous anatomy, precluding the need for direct arterial access.6 In this case report, we demonstrate how the combination of preoperative 3D MRA overlaid with intraoperative cone beam CT (CBCT) and fluoroscopy in addition to using intravenous digital subtraction angiography (IVDSA) provides the vascular anatomic information required for entirely transvenous embolization of a Barrow type A CCF in a patient with vEDS. We call this method the transvenous triple-overlay embolization (TAILOREd) technique.
Case presentation and investigations
A 32-year-old woman with genetically confirmed COL3A1 mutation and a family history of vEDS was referred to our neurovascular clinic with a 2-week history of new onset of headaches, left-sided pulsatile tinnitus, left eye ptosis and diplopia. Physical examination demonstrated disconjugate primary gaze with left exotropia, ptosis, mydriasis, and ocular bruit. Visual acuity was 20/30 in the left eye and 20/20 in the right, with normal intraocular pressure bilaterally.
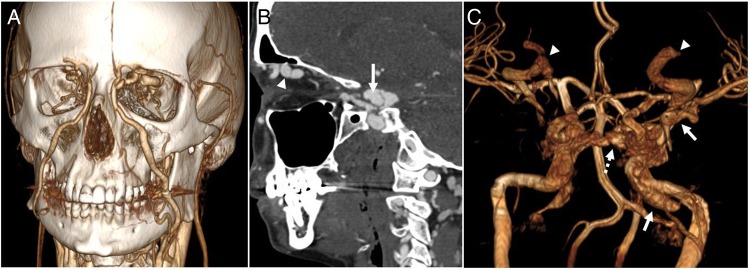
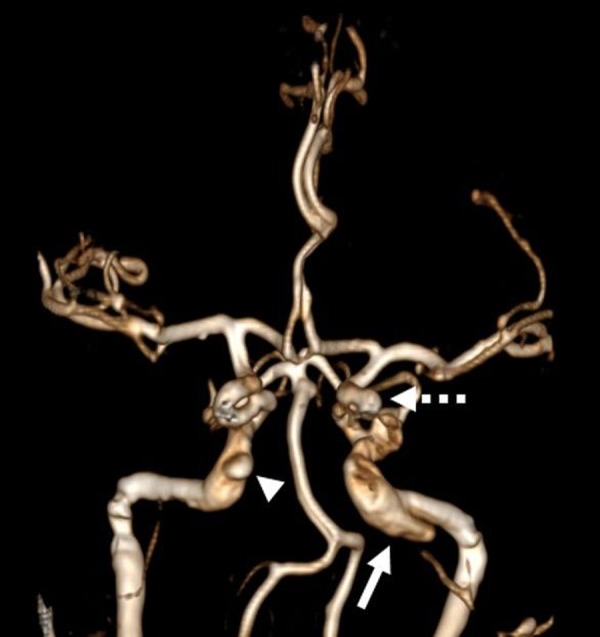
CT angiography (CTA) of the abdomen and pelvis prior to admission demonstrated abdominal aortic and renal artery aneurysms in addition to bilateral common iliac artery, renal artery, and visceral artery dissections (figure 1). CTA and contrast-enhanced MRA of the head and neck demonstrated a direct left CCF from a ruptured left cavernous carotid pseudoaneurysm, with dilated bilateral superior ophthalmic veins (figure 2). A separate unruptured pseudoaneurysm was noted involving the left distal petrous segment of the internal carotid artery (ICA).
Figure 1.

CT angiography (three-dimentional surface reconstruction) of the chest, abdomen, and pelvis showing large dissections and pseudoaneurysms involving the bilateral iliac, femoral, and renal arteries in addition to the descending aorta and visceral arteries, and left subclavian artery origin.
Figure 2.
(A) Three-dimensional surface reconstruction of a CT angiogram of the head and neck demonstrates dilated bilateral superior ophthalmic veins draining to the angular and facial veins. (B) Sagittal CT angiography demonstrates a pseudoaneurysm of the left internal carotid artery (arrow) which communicates with the left cavernous sinus consistent with a direct carotid–cavernous fistula. (C) Surface reconstruction of a pre-intervention MR angiogram shows abnormal opacification of the left cavernous sinus and tributaries including the intercavernous sinus (dotted arrow) to the contralateral cavernous sinus, left sphenoparietal sinus and left sylvian vein (arrow), and bilaterally dilated superior ophthalmic veins (arrowheads). Also note a large unruptured pseudoaneurysm at the left petrous segment of the internal carotid artery (arrow).
Conservative treatment was recommended given the patient's comorbidities; however, over the following 3 weeks the patient's headaches and pulsatile tinnitus worsened, at which point the patient elected to undergo transvenous embolization.
Treatment
The procedure was performed under general anesthesia in a biplane neurointerventional suite using an Allura Xper FD 20/20 angiographic unit and an XtraVision workstation (Philips Medical Systems, Eindhoven, Netherlands). The technique for the incorporation of pre-treatment imaging for 3D roadmap overlay has been previously described.6 7 Briefly, the pre-intervention MRA was imported to the XtraVision workstation and co-registered to the low-dose CBCT which was performed on the X-ray system, allowing the vasculature from both volumetric datasets to be overlaid on live fluoroscopy from any viewing angle and gantry configuration.
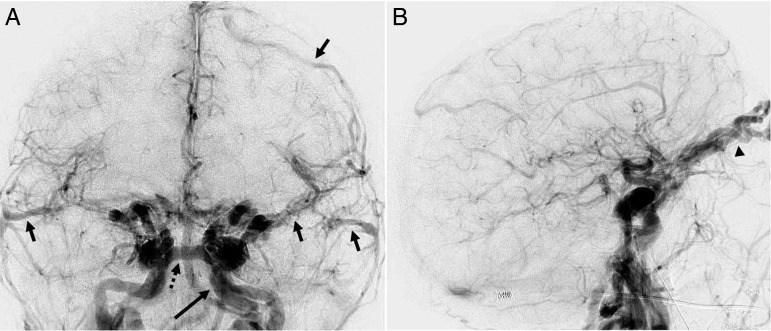
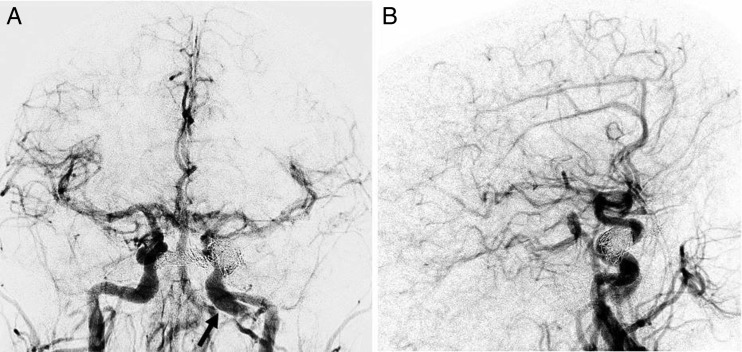
We then selectively cannulated the right common femoral vein using ultrasound guidance and placed a 55 cm 6 Fr sheath (Cordis, Fremont, USA) into the inferior vena cava. Biplane IVDSA was then performed using a 20 mL/s bolus injection of 40 mL iodinated contrast through the sheath (figure 3). This confirmed the left-sided direct CCF with brisk arteriovenous shunting within the left cavernous sinus tributaries including the presence of cortical venous reflux.
Figure 3.
Biplane intravenous digital subtraction angiography in the frontal (A) and lateral projections (B) in the early arterial phase demonstrating marked arteriovenous shunting and early venous drainage to the left cavernous sinus via the intercavernous sinus (dotted arrow) and cortical venous reflux (arrows). A left petrous internal carotid artery pseudoaneurysm is incidentally noted (long arrow). Additional early venous drainage and venous hypertension is noted within the bilaterally dilated superior ophthalmic veins (arrowhead).
A 6 Fr Envoy (Codman, Massachusetts, USA) was advanced into the left jugular bulb over a 4 Fr Vert catheter (Cordis) and Glidewire (Terumo, New Jersey, USA). We then used the Glidewire to access the left cavernous sinus through the inferior petrosal sinus by probing medially and superiorly on unsubtracted fluoroscopy. After identifying the cavernous sinus, the Glidewire and Vert catheter were removed under a blank roadmap which identified a path to the cavernous sinus. A Synchro II microwire (Boston Scientific, Natick, USA) and SL-10 microcatheter (Boston Scientific) were introduced into the guide catheter and navigated into the cavernous sinus via this negative roadmap. All subsequent navigation of the microwire and microcatheter was performed using the triple-overlay information from the MRA, CBCT and live fluoroscopy to outline the cavernous sinus and carotid artery anatomy.
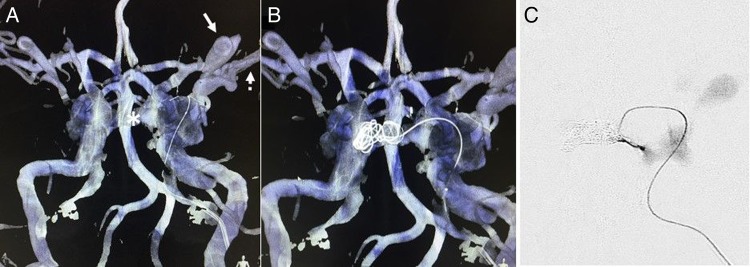
Five Target coils (Stryker, Kalamazoo, USA) were then deployed within the intercavernous sinus (figure 4 and video) to prevent venous reflux into the contralateral orbit and occlusion was confirmed with a microcatheter injection. The microcatheter was then navigated to the confluence of the ipsilateral superior and inferior ophthalmic veins for placement of five additional coils to reduce venous reflux into the ipsilateral eye. To protect the patient from cortical venous reflux, seven additional coils were deployed into the ipsilateral sphenoparietal sinus (figure 5). The microcatheter was then navigated to the cavernous sinus near the CCF itself and three coils were deployed. Final microcatheter runs demonstrated substantial venous stasis within the cavernous sinus indicating depressurization and occlusion of the fistula (figure 6E, F). Repeat IVDSA through the femoral venous sheath confirmed complete obliteration of the fistula (figure 7). The sheath was removed and hemostasis was achieved with manual compression. The patient was then extubated and observed in the neurological intensive care unit.
Figure 4.
The TAILOREd technique demonstrating overlay images of live fluoroscopy on the preoperative MR angiography (in blue) and cone beam CT (in white). (A) The microcatheter is navigated through the ipsilateral cavernous sinus into the contralateral cavernous sinus via the intercavernous sinus (asterisk). The ipsilateral superior ophthalmic vein can also clearly be seen on the three-dimensional roadmap (arrow) as well as the cortical venous reflux (dotted arrow). (B) Coils are placed within the contralateral cavernous sinus and intercavernous sinus. (C) Microcatheter injection confirms occlusion of the intercavernous sinus.
Figure 5.
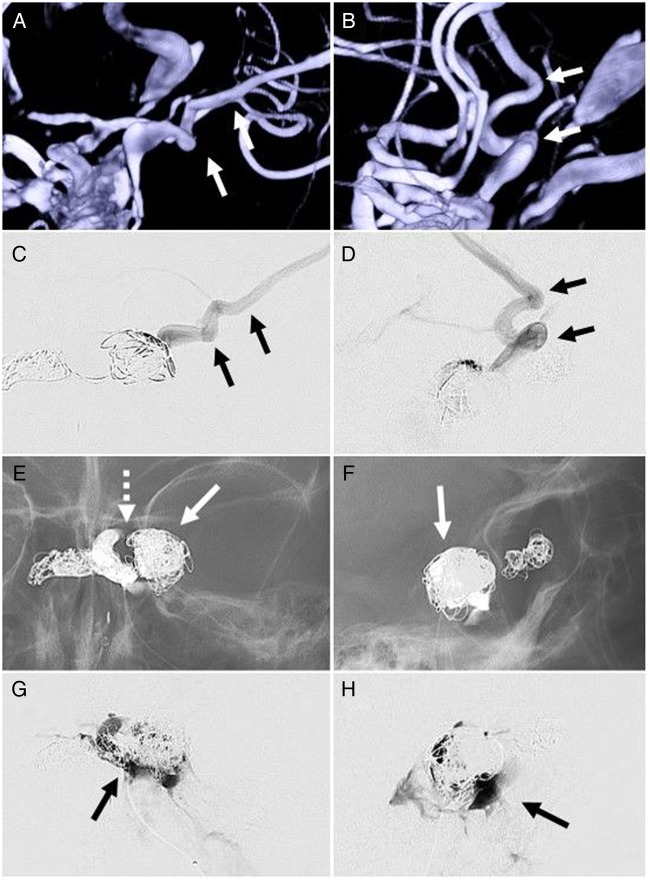
Anteroposterior (A and C) and lateral (B and D) three-dimensional roadmap and microcatheter runs, respectively, demonstrating catheterization of a venous pouch with direct posterior lateral cortical venous reflux through the sphenoparietal sinus and sylvian vein (arrows). This high-risk feature was embolized with coils (arrows), with additional coils deployed within the cavernous sinus adjacent to the fistula as shown on anteroposterior (E) and lateral fluoroscopy (F). The left internal carotid artery cavernous segment is noted as a gap within the coil mass (E, dotted arrow). Anteroposterior (G) and lateral (H) final microcatheter runs of the left cavernous sinus in the late phase of the injection demonstrate stasis of contrast in the cavernous sinus proper without ophthalmic or cortical vein opacification, suggesting occlusion of the carotid–cavernous fistula.
Figure 6.
Anteroposterior (A) and lateral (B) final post-embolization intravenous digital subtraction angiography confirms complete occlusion of the left direct carotid–cavernous fistula without evidence of early venous drainage. An unrelated chronic dissection and pseudoaneurysm of the left internal carotid artery is again seen (black arrow).
Figure 7.
Six-week follow-up contrast-enhanced MR angiography confirms persistent occlusion of the left direct carotid–cavernous fistula with residual left cavernous (dotted arrow) and petrous (arrow) segment internal carotid artery (ICA) pseudoaneurysms. A new contralateral pseudoaneurysm is noted at the distal petrous segment of the right ICA (arrowhead).
Video 1.
The TAILOREd technique: Overlay of live fluoroscopy on the pre-operative MR angiography (in blue) and cone-beam CT (in white) while deploying a coil under continuous fluoroscopy into the contralateral cavernous sinus and intercavernous sinus through a microcatheter through the ipsilateral cavernous sinus.
Outcome and follow-up
The patient was discharged home on post-procedure day 2 with significant improvement in left extraocular movements, reduction of headache and resolution of her pulsatile tinnitus. At 6-week follow-up, extraocular movements were normal and there was no ptosis, mydriasis or headache. Contrast-enhanced MRA at follow-up re-demonstrated a left cavernous ICA pseudoaneurysm without evidence of CCF (figure 7).
Discussion
This report demonstrates the successful endovascular treatment of a direct CCF in a patient with vEDS without the need for arterial puncture. This was made possible by the TAILOREd technique: overlaying the preoperative MRA on the intraoperative CBCT to use as a roadmap for real-time fluoroscopy and embolization. This permitted precise visualization of the arterial and venous sinus anatomy during the procedure at any viewing angle, enabling accurate microcatheter navigation and coil deployment while avoiding arterial complications. IVDSA is not used as part of the triple-overlay portion of the procedure and is primarily used to confirm successful embolization of the fistula.
We have previously published our experience with CCF including alternative transvenous access techniques.8 Although a transvenous approach to cavernous sinus embolization may be less effective than transarterial embolization in the treatment of direct CCF, there is substantial risk associated with arterial access in patients with vEDS using this approach with reported mortality rates of up to 58%.3 Several authors have recommended a transvenous treatment approach for CCF in patients with vEDS; however, arterial puncture is often required in order to delineate the vascular anatomy.4 Other case reports have described direct distal arterial access within the neck requiring surgical cut-down.9 In our case, severe arterial tortuosity within the carotid arteries as well as dissections and pseudoaneurysms within the femoral and iliac arteries and aorta (figure 1) underscored the potential complications of arterial access. A pure transvenous approach has been described,5 but this technique requires microcatheterization of the fistula directly which may be technically challenging and potentially increase the risk for arterial injury. Our completely transvenous technique was ideal as it does not require arterial access or surgical cut-down and avoids potentially serious arterial vascular complications.
Intra-procedural overlay of preoperative non-invasive angiographic CT and MR imaging on CBCT and live fluoroscopy has been previously described for use in diagnostic and therapeutic interventional procedures in the head and neck, including percutaneous biopsy, sclerotherapy, tumor embolization, and carotid stenting.6 7 However, it has not been described for transvenous cavernous sinus embolization. The overlay has a high fidelity to fluoroscopy and angiography, with needle biopsy studies demonstrating accuracy between 1.3 and 3.0 mm.6 Reported radiation doses for low-dose and normal CBCT are approximately 0.8 and 1.6 mSv, respectively.10
The TAILOREd technique allows precise visualization of arterial, venous, and extravascular anatomy and also reduces overall iodinated contrast dose administration and procedural radiation exposure due to the reduced need for arterial angiography.6 Although a transvenous approach is preferable in patients with vEDS, serious complications from the transvenous approach have been described, and vigilance to minimize venous injury at the time of puncture and while obtaining access to the jugular vein remains necessary.
Learning points.
Patients with Ehlers–Danlos syndrome type IV are at high risk for arterial dissections and aneurysms and commonly present with direct carotid–cavernous fistula.
Conventional catheter angiography is associated with a high risk of arterial vascular complications including access site complications and great vessel dissection.
The transvenous triple overlay embolization (TAILOREd) technique uses pre-treatment MR angiography combined with cone beam CT and live fluoroscopy to guide transvenous embolization without need for an arterial puncture.
Footnotes
Contributors: All authors contributed to the planning and study design, writing and editing the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Schievink WI, Michels VV, Piepgras DG. Neurovascular manifestations of heritable connective tissue disorders. A review. Stroke 1994;25:889–903. 10.1161/01.STR.25.4.889 [DOI] [PubMed] [Google Scholar]
- 2.Pepin M, Schwarze U, Superti-Furga A, et al. Clinical and genetic features of Ehlers–Danlos syndrome type IV, the vascular type. N Engl J Med 2000;342:673–80. 10.1056/NEJM200003093421001 [DOI] [PubMed] [Google Scholar]
- 3.Schievink WI, Piepgras DG, Earnest F, et al. Spontaneous carotid–cavernous fistulae in Ehlers–Danlos syndrome type IV. Case report. J Neurosurg 1991;74:991–8. 10.3171/jns.1991.74.6.0991 [DOI] [PubMed] [Google Scholar]
- 4.Linfante I, Lin E, Knott E, et al. Endovascular repair of direct carotid–cavernous fistula in Ehlers–Danlos type IV. J Neurointerv Surg 2015;7:e3 10.1136/neurintsurg-2013-010990.rep [DOI] [PubMed] [Google Scholar]
- 5.Van Overmeire O, De Keukeleire K, Van Langenhove P, et al. Carotid–cavernous fistula in Ehlers–Danlos syndrome by pure transvenous approach. Interv Neuroradiol 2006;12:45–51. 10.1177/159101990601200109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levitt MR, Vaidya SS, Su DK, et al. The “triple-overlay” technique for percutaneous diagnosis and treatment of lesions of the head and neck: combined three-dimensional guidance with magnetic resonance imaging, cone-beam computed tomography, and fluoroscopy. World Neurosurg 2013;79:509–14. 10.1016/j.wneu.2012.03.031 [DOI] [PubMed] [Google Scholar]
- 7.Levitt MR, Ghodke BV, Cooke DL, et al. Endovascular procedures with CTA and MRA roadmapping. J Neuroimaging 2011;21:259–62. 10.1111/j.1552-6569.2010.00507.x [DOI] [PubMed] [Google Scholar]
- 8.Morton RP, Tariq F, Levitt MR, et al. Radiographic and clinical outcomes in cavernous carotid fistula with special focus on alternative transvenous access techniques. J Clin Neurosci 2015;22:859–64. 10.1016/j.jocn.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 9.Hollands JK, Santarius T, Kirkpatrick PJ, et al. Treatment of a direct carotid–cavernous fistula in a patient with type IV Ehlers–Danlos syndrome: a novel approach. Neuroradiology 2006;48:491–4. 10.1007/s00234-006-0084-1 [DOI] [PubMed] [Google Scholar]
- 10.Sanchez RM, Vano E, Fernández JM, et al. Brain radiation doses to patients in an interventional neuroradiology laboratory. AJNR Am J Neuroradiol 2014;35:1276–80. 10.3174/ajnr.A3884 [DOI] [PMC free article] [PubMed] [Google Scholar]