Abstract
A 26-year-old female from India presented with progressive, unremitting low back pain for over 1 year. She had been treated unsuccessfully for left-sided sacroiliitis, pelvic floor dysfunction, ankylosing spondylitis and seronegative spondyloarthritis. MRI lumbar spine showed a Schmorl node with surrounding marrow oedema at L4, the relevance of which is not clear in literature. One year after initial presentation, a biopsy of this lesion revealed culture positive diagnosis of spinal tuberculosis. Despite advances in imaging, delayed diagnosis is not uncommon in spinal tuberculosis (TB). In our case, it was also attributed to an unknown early lesion: Schmorl node with surrounding oedema. Any association of this lesion with spinal TB has previously not been reported.
Keywords: Infections, Bone and joint infections, TB and other respiratory infections
Background
This case was an atypical presentation of tuberculous (TB) spondylitis in which diagnosis was delayed due to many factors. A Schmorl node1 is a herniation of the nucleus pulposus through cartilaginous and bony endplate into the body of an adjacent vertebra. Schmorl nodes are mostly benign and asymptomatic, but some result in severe pain to the patient. In our case, this lesion was identified as a precursor lesion to spinal TB, which has previously not been reported in literature. Despite medical advancements, delayed diagnosis continues to be a feature in spinal TB and our case explores such contributing factors. Spinal TB should be considered in the differential diagnosis of chronic back pain with or without constitutional symptoms, especially in young, otherwise healthy immigrants from countries with a high prevalence of TB.
Case presentation
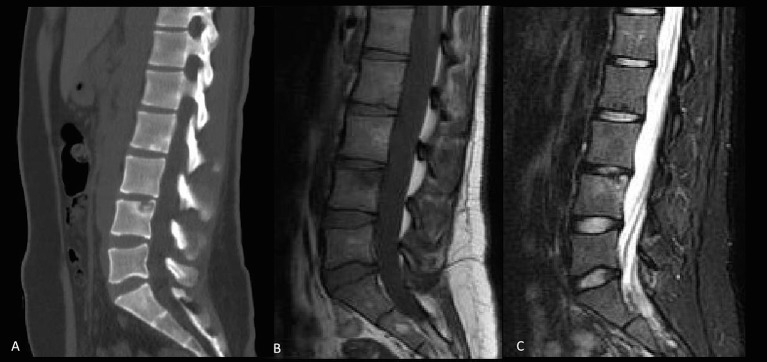
A 26-year-old female with no significant comorbidities presented to her primary care physician (PCP) with low back pain for 4 weeks characterised as moderate, aching in nature, with associated radiation down to bilateral lower limbs, worse with exertion and relieved with rest. She denied fever, chills, weight loss, fatigue, bowel or bladder involvement and had no history of trauma. She had returned from a 3-week trip to India 1 week prior to presentation and had been residing there since birth until immigrating to the USA 1.5 years ago. Examination revealed limb length discrepancy and mild left sacroiliac tenderness; no focal spinal tenderness or deformity was appreciated. Over 4–5 months, despite physical therapy (PT) and non-steroidal anti-inflammatory drugs, she reported worsening back pain with increased urinary frequency and constipation. Urinalysis and culture were negative for infection. MRI showed a 10 mm deep Schmorl node at L4, with surrounding marrow oedema, which was deemed incidental (figure 1). Physical medicine and rehabilitation was consulted and she was diagnosed with pelvic floor dysfunction. She underwent pelvic PT that resolved the bowel and bladder symptoms.
Figure 1.
Initial presentation: sagittal CT (A), sagittal T1 (B) and short tau inversion recovery (C) MRIs showing a Schmorl node at L4 level with mild surrounding marrow oedema.
The back pain gradually worsened about 9 months into her illness. Additionally, she reported pain in the small joints of hands with morning stiffness of >30 min. Examination showed limited motion in the lumbar spine. Small joints and tendons of the hands and feet were tender without erythema or swelling. Autoimmune tests were unrevealing except for an erythrocyte sedimentation rate (ESR) of 23 and speckled antinuclear antibodies (ANA) 50 (normal range 0–49 1/dilution). Repeat MRI showed questionable minimal bilateral sacroiliac oedema with worsening oedema around the previously noted Schmorl node. On the basis of Assessment of SpondyloArthritis International Society criteria, which accepted sacroiliitis on imaging plus inflammatory back pain, the rheumatology service diagnosed her with human leukocyte antigen (HLA) B27 negative ankylosing spondylitis. Prednisone 10 mg daily was started which improved her back pain, arthralgia and tendinitis. She pursued a second opinion from another rheumatologist. He treated her empirically for seronegative arthropathy of unknown cause with hydroxychloroquine and prednisone.
She also reported subjective weight loss and fatigue. Infectious aetiologies like brucellosis, chikungunya and HIV were negative. TB was deemed unlikely given documented negative purified protein derivative (PPD) on preimmigration check-up, and second negative PPD about 2 months into her back pain (as a part of pre-employment physical). Moreover, lack of conclusive findings on MRI and clear chest X-ray argued against TB. However, an interferon gamma-release assay (QuantiFERON TB gold in tube) was done and noted to be positive. The repeat MRI still showed the Schmorl node with marrow oedema, and it was thought to be a benign source of back pain. With such a presentation, the positive quantiferon was attributed to latent pulmonary infection. When the back pain escalated with nocturnal fevers up to 102°F, there was concern for osteomyelitis. By then, the patient was bedridden and unable to walk from pain. Steroids and hydroxychloroquine were discontinued.
Investigations
At this 12-month mark, fluoroscopy-guided biopsy of Schmorl node was done to evaluate for osteomyelitis. The sample revealed rare acid-fast bacilli on fluorochrome stain and Mycobacterium tuberculosis DNA by real-time PCR. Microbiological evaluation was notable for rare acid-fast bacilli on smear with ultimate growth of fully drug-susceptible M. tuberculosis. Blood cultures were negative.
Differential diagnosis
Initially, the patient was thought to have musculoskeletal causes of back pain most likely due to lumbar disc herniation. At that time, alarm symptoms of fever, weight loss, fatigue, bowel and bladder dysfunction were not present. MRI was pursued when the patient exhibited bladder symptoms, which did not show any evidence of nerve compression. She underwent pelvic PT for presumed diagnosis of pelvic floor dysfunction. Urinalysis, including testing for organisms like Chlamydia and Neisseria gonorrhoeae' was negative. Later, when she reported small joint arthralgia and tendinitis, rheumatology was consulted. Given her age group and constitutional symptoms, systemic lupus erythematous (SLE) and ankylosing spondylitis were considered. Pelvic MRI showed very subtle, questionable oedema in sacroiliac joints. For the Schmorl node with marrow oedema, Anderson lesion (which is found in ankylosing spondylitis) and osteoid osteoma were considered in the differential. She was treated symptomatically for seronegative spondyloarthritis, including ankylosing spondylitis. Infectious causes of back pain like chikungunya, Brucella were considered, but she tested negative for them. Finally, a quantiferon test was performed for workup of TB. Around 12 months into her illness, a fluoroscopy-guided biopsy of the suspicious Schmorl node was pursued leading to a diagnosis of spinal tuberculosis.
Treatment
Standard four-drug antimycobacterial therapy with isoniazid, rifampin, ethambutol and pyrazinamide was initiated according to Centers for Disease Control and Prevention (CDC) guidelines. Despite the lack of chest radiographic findings or respiratory symptoms, one of three sputum samples at 24-hour intervals was positive. Smear conversion was achieved after 2 weeks of daily therapy, and thrice-weekly antimycobacterial therapy was initiated. Within 1 week, she had significant clinical worsening with progressive back pain requiring hospitalisation and raised inflammatory markers (ESR 130 units). MRI lumbar spine revealed compression fracture of the L4 vertebra with epidural abscess causing moderate spinal canal stenosis (figure 2). The patient suffered clinical worsening every time her therapy was switched from daily therapy to a thrice-weekly regimen. Ultimately, she was put back on a daily antimycobacterial regimen given the temporal correlation of her worsening with thrice weekly therapy.
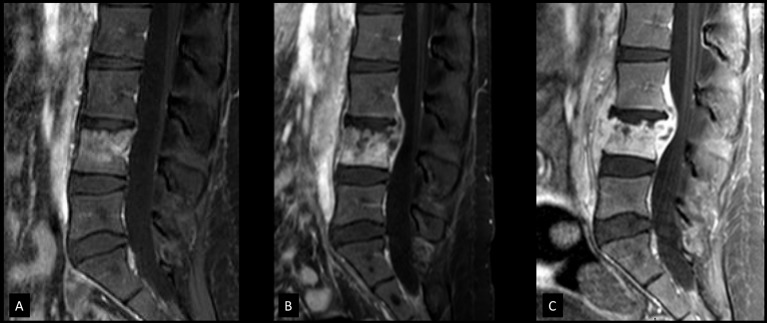
Figure 2.
Contrast-enhanced T1-weighted sagittal MRIs showing progression of disease. (A) MRI obtained 4 weeks prior to biopsy. Enhancement of L4 vertebral body was seen but due to presence of Schmorl node which may present with enhancement, infection was thought to be less likely but follow-up recommended. (B) MRI obtained at the first episode of clinical worsening, 6 days after start of thrice-weekly antimycobacterial therapy. The image shows loss of vertebral body height and new epidural abscess formation. Daily antimycobacterial therapy was initiated after biopsy but the patient was switched to thrice-weekly therapy on day 16 after smear conversion. Clinical worsening occurred 6 days into thrice-weekly therapy (day 22 of first initiating antimycobacterial treatment) as shown here. The patient was switched back to daily therapy for a week, and subsequently again back to thrice-weekly therapy. (C) MRI obtained at the second episode of clinical worsening, 12 days into the second round of thrice-weekly therapy (day 41 of first initiating antimycobacterial treatment). Image shows increased loss of height of vertebral body and increased component of epidural abscess. Patient was switched back to and continued on daily therapy after this event.
Outcome and follow-up
After discharge from the hospital, the patient developed grade 2/5 weakness in her left thigh with sensory loss in the L4/L5 dermatome. The left patellar reflex was absent. Despite orthopaedic recommendation, the patient declined operative intervention. Amikacin and levofloxacin were added for 3 months. Therapeutic drug monitoring was performed given concern regarding limited penetration of antitubercular drugs in bone, and she was noted to have significantly subtherapeutic rifampin levels, leading to dose adjustment from 600 mg to 900 mg once daily. Over 4–5 months, she reported substantial improvement in back pain with medical management and was able to ambulate. The neurological symptoms resolved with return of appetite and weight gain. Limb length discrepancy was eventually considered to be an incidental finding. Small joint pain was speculated to be reactive arthritis (Poncet disease) but no further workup was done for this condition as it resolved after initiation of anti-TB therapy.
Discussion
Despite advances in TB diagnostics with the advent of more widely available culture and nucleic acid amplification methods, delay in diagnosis is extremely common with spinal tuberculosis.2 The duration of symptoms in spinal tuberculosis range up to 36 months, with a median time of about 11 months before diagnosis is made. A sentinel study among patients in UK noted that 33% of spinal patients with TB required five or more assessments before a referral for investigation was made.3 The causes associated with chronic back pain are many, and there is a significant population in whom an aetiology is not identified. More than 85% of patients presenting with low back pain to their PCP have non-specific pain in which the aetiology remains unknown. Also, the absence of constitutional symptoms is not uncommon in a patient with spinal TB.4 A low proportion of patients are noted to have concomitant pulmonary TB. Discordance between PPD test and quantiferon may occur,5 and a negative PPD should not be relied on, especially in cases with a high clinical suspicion.
On imaging, classic tuberculous spondylitis presents with relative preservation of the disc, as is seen in our case. It is proposed to be due to lack of proteolytic enzymes' production by the bacilli as opposed to more common causes of spinal osteomyelitis such as staphylococcus.6 Schmorl nodes are common, mostly innocuous and typically asymptomatic findings on MRI. They occur from an area of weakness in the cartilaginous endplate, which causes extrusion of the nucleus pulposus of the intervertebral disc into adjacent vertebra. The weakness can be congenital, traumatic, or rarely, acquired from neoplasms or infections. Schmorl nodes are seldom implicated as a cause of low back pain.7
Early radiological signs of spinal TB are not well defined. In a study of 14 patients with Schmorl nodes, one patient who failed to respond to conservative management had spinal tuberculosis on biopsy.8 A study of Schmorl nodes on autopsy specimens found them to be inversely related to tuberculosis. However, the statistical significance of such a conclusion was not reported.9 In our case, the Schmorl node seemed to be the site of infection because it was a singular lesion with the oedema centred around it, with worsening of oedema on subsequent imaging. The same lesion was targeted for fluoroscopic biopsy, which eventually revealed the diagnosis. The imaging progression of osteomyelitis postepidural abscess formation was also centred around that lesion with progression to involve the rest of the vertebral body.
Schmorl nodes are hypothesised to be the long-term result of displacement of intervertebral disk material through cracks in an otherwise normal endplate after axial loading trauma. Rarely, the predisposing causes for endplate weakness might include disk space infection, discitis, neoplasms or any other processes that may weaken the endplate or the underlying bone.10 In our case, it is unclear if it developed secondary to weakening of the bone from infection versus a pre-existing Schmorl node acting as a nidus. Only after rapid progression of symptoms were the more classical radiological features of abscess formation and fracture noted. The prior addition of steroids also contributed to this rapid progression.
Spinal tuberculosis must be considered in the differential diagnosis of chronic back pain with or without constitutional symptoms, especially in young, otherwise healthy patients, particularly among immigrants from countries with a high prevalence of TB.
Learning points.
Delayed diagnosis continues to remain a feature in spinal tuberculosis. High suspicion should be maintained in immigrants from endemic countries presenting with chronic back pain.
Schmorl node with marrow oedema could represent a focus of active infection. If an aetiology is not identified despite all investigations, a bone biopsy is essential to expedite diagnosis and prevent complications.
Antitubercular agents might have limited penetration in bone. A daily antimycobacterial regimen with drug-level monitoring is preferable to thrice-weekly regimen in such cases.
Discordance between tuberculin skin test (TST) and quantiferon test is not uncommon. Negative TSTs should not be considered conclusive in suspicious cases.
Glucocorticoids should be used carefully in empiric treatment of chronic back pain in which a rheumatological aetiology is uncertain.
Footnotes
Contributors: AP wrote and edited the manuscript. NM compiled the imaging studies and provided editorial feedback on the manuscript content. RMH edited and provided feedback on the infectious disease content of the manuscript. AD edited and provided feedback on the content of the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kyere KA, Than KD, Wang AC, et al. Schmorl’s nodes. European Spine Journal 2012;21:2115–21. 10.1007/s00586-012-2325-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker GF. Failure of early recognition of skeletal tuberculosis. Br Med J 1968;1:682–3. 10.1136/bmj.1.5593.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cormican L, Hammal R, Messenger J, et al. Current difficulties in the diagnosis and management of spinal tuberculosis. Postgrad Med J 2006;82:46–51. 10.1136/pgmj.2005.032862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotil K, Alan MS, Bilge T. Medical management of pott disease in the thoracic and lumbar spine: a prospective clinical study. J Neurosurg Spine 2007;6:222–8. 10.3171/spi.2007.6.3.222 [DOI] [PubMed] [Google Scholar]
- 5.Ghassemieh BJ, Attia EF, Koelle DM, et al. Latent tuberculosis infection test agreement in the National Health and Nutrition Examination Survey. Am J Respir Crit Care Med 2016;194:493–500. 10.1164/rccm.201508-1560OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross JS, Moore KR. Diagnostic Imaging: Spine 3rd Edition: Elsevier Health Sciences, 2015:1–1224. [Google Scholar]
- 7.Teraguchi M, Yoshimura N, Hashizume H, et al. The association of combination of disc degeneration, end plate signal change, and Schmorl node with low back pain in a large population study: the Wakayama Spine Study. Spine J 2015;15:622–8. 10.1016/j.spinee.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 8.Parasnis R, Talawadekar G, Sancheti KH, et al. Intraosseous disc prolapse: a diagnostic puzzle. Indian J Orthop 2006;40:168 10.4103/0019-5413.34485 [DOI] [Google Scholar]
- 9.Rothschild BM, Ho J, Masharawi Y. Macroscopic anatomy of the vertebral endplate: quid significat? Anthropol Anz 2014;71:191–217. 10.1127/0003-5548/2014/0365 [DOI] [PubMed] [Google Scholar]
- 10.Wagner AL, Murtagh FR, Arrington JA, et al. Relationship of Schmorl’s nodes to vertebral body endplate fractures and acute endplate disk extrusions. AJNR Am J Neuroradiol 2000;21:276–81. [PMC free article] [PubMed] [Google Scholar]