Abstract
Background
Berberine, a herbal extract, has been reported to protect against inflammatory disorders. The adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) signaling pathway can be activated by berberine and inhibited by the synthetic, reversible AMP-competitive inhibitor, Compound C. The aim of this study was to investigate the effects of berberine on concanavalin A (Con A)-induced autoimmune hepatitis (AIH) in mice via the AMPK pathway.
Material/Methods
BALB/c mice were treated with berberine, with or without Compound C, followed by treatment with Con A. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured. Liver tissue histology was performed to evaluate hepatic injury and AIH. Cytokine levels in serum and hepatic tissue were measured by enzyme-linked immunoassay (ELISA) and used quantitative polymerase chain reaction (qPCR). Levels of phosphorylated acetyl coenzyme-A carboxylase (ACC), representing AMPK activation, were detected by Western blotting.
Results
Serum ALT and AST levels were significantly reduced by berberine (100 and 200 mg/kg/day) in mice with Con A-induced hepatitis. Berberine also reduced Con A-induced hepatocyte swelling, cell death, and infiltration of leukocytes. Serum levels of tumor necrosis factor (TNF)-alpha, interferon (IF)-gamma, interleukin (IL)-2, and IL-1beta were reduced by berberine pre-treatment; levels of serum IL-10, an anti-inflammatory cytokine, was elevated. These protective effects of berberine on Con-A-induced AIH were reversed by treatment with Compound C.
Conclusions
In a murine model of Con A-induced AIH, berberine treatment reduced hepatic injury via activation of the AMPK pathway. Further studies are recommended to determine the potential therapeutic role for berberine in AIH.
MeSH Keywords: AMP-Activated Protein Kinases; Berberine; Hepatitis, Autoimmune; Liver
Background
Clinically, hepatitis can be a severe disease and has a variety of causes, including alcohol, drugs, and viral infections, and autoimmune hepatitis (AIH) also occurs [1]. An established murine model of concanavalin A (Con A)-induced AIH has been well-characterized, in which T-cells mediate liver damage, resulting in changes that are similar to those that occur in human AIH [2]. This murine model is widely used to study the pathogenesis, microscopic morphological changes, and effects of potential treatments for AIH. Apoptosis and necrosis of hepatocytes, and leukocyte infiltration are considered to be key features of hepatitis, and in this murine model, activated T-cells infiltrate the liver tissue and induce the expression of pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, interferon (IF)-γ, interleukin (IL)-lβ, and IL-2 [3].
Berberine is an isoquinoline alkaloid (molecular formula, C20H18NO4), which can be extracted from many traditional Chinese medicinal plants, including Coptis chinensis and Phellodendron amurense, and has hypoglycemic, antibacterial, anti-inflammatory, hypolipidemic, anti-oxidant, and anti-tumor pharmacological properties [4]. Recent in vitro studies have been undertaken on the effects of berberine on inflammatory mediators and immune function, including using cultured immune cells (macrophages and splenic lymphocytes) [5]. In these in vitro studies, berberine has been shown to reduce the expression of TNF-α, IL-6, and IL-1β, inducible nitric oxide synthase (iNOS), monocyte chemotactic protein (MCP)-1, cyclooxygenase (COX)-2, and C-reactive protein (CRP) [5].
In 2013, Xie et al. reported that berberine could downregulate the expression of pro-inflammatory cytokines and acute response proteins in vivo in streptozocin (STZ)-induced diabetic mice [6]. In 2005, Xu et al. confirmed the immunosuppressive role of berberine, via inhibition of the activation and proliferation of T-cells [7]. Although Con A-induced liver injury is believed to be related to the T-cell mediated immune response, no previous studies have shown that berberine can alleviate this response.
Adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) is a key metabolic regulator for glucose, fat, and protein metabolism, and it is capable of maintaining cell homeostasis [8]. AMPK can also be activated by several factors such as adiponectin, statins, and metformin [8]. AMPK activation is primarily regulated by the ratio of AMP/ATP, and any factor that reduces ATP expression or increases ATP consumption, such as tissue ischemia, hypoxia, heat shock, and exercise, can activate AMPK [8,9]. Also, AMPK can be directly activated by upstream AMPK kinases, including serine/threonine protein kinase 11 (STK11) also known as LKB1, and by Ca2+/calmodulin-dependent protein kinase kinase (CaMKK), which activate AMPK by direct phosphorylation of the AMPKα subunit at Thr172 [9]. Activated AMPK can phosphorylate its downstream target proteins, such as acetyl coenzyme A carboxylase (ACC) and hydroxymethyl glutaryl CoA (HMG CoA) reductase, thereby regulating cellular biological activity [10]. Studies have also confirmed that berberine can inhibit mitochondrial respiratory chain complex I and increase the ratio of AMP/ATP, leading to AMPK activation [11]. It has also recently been shown that activated AMPK can resolve inflammation and suppress the immune response [12]. However, currently, there are no studies on the effect of the AMPK signaling pathway on Con A-induced liver injury.
The aim of this study was to investigate the effects of berberine on concanavalin A (Con A)-induced autoimmune hepatitis (AIH) in mice via the AMPK pathway.
Material and Methods
Antibodies and reagents
Primary antibodies to berberine, concanavalin A (Con A) and Compound C were obtained from Sigma-Aldrich (St. Louis, MO, USA). Primary mouse monoclonal antibodies to p38 acetyl coenzyme-A carboxylase (ACC) and phospho-p38 ACC were obtained from Cell Signaling Technologies (Beverly, MA, USA). The secondary anti-mouse horseradish peroxide (HRP)-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (CA, USA).
Animals
Male BALB/c mice (6–8 weeks old) (mean weight, 23±2 gm) were purchased from SPF Laboratory Animal Technology Co., Ltd. (Beijing, China), and housed under a natural light: dark cycle (12: 12 hrs) at 25°C, with food and water available ad libitum. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, from the US National Institutes of Health (NIH Publication No. 85–23, Revised 1996). The animal experimental protocol was approved by the Institutional Animal Care and Use Committee of Hebei University.
Measurement of aminotransferases and cytokines
At 8 hour after Con A injection (20 mg/kg), blood samples were collected from the murine heart under anesthesia, and then centrifugated at 1000×g for 10 min to obtain serum samples. Serum aspartate aminotransferase (AST), and serum alanine aminotransferase (ALT) levels were measured using a 7170 fully automated biochemical analyzer (Hitachi, Japan). Levels of tumor necrosis factor (TNF(-α, interferon (IFN)-γ, interleukin (IL)-2, IL-1β, and IL-10 were measured using enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturer’s instructions (Abcam, Cambridge, UK).
Histology
A portion of mouse hepatic tissue was harvested at 8 hours after Con A injection, fixed in 4% paraformaldehyde for 24 hours, and embedded in paraffin wax. Sections (3 μm thick) were cut onto glass slides and stored at room temperature. The paraffin wax embedded tissue sections were then stained with hematoxylin and eosin (H&E) for histological evaluation of cell swelling, degeneration, apoptosis, and necrosis, using light microscopy.
RNA extraction and quantitative polymerase chain reaction (qPCR)
At 8 hours following Con A injection (20 mg/kg), a 2 mg sample of hepatic tissue was collected. Total RNA was extracted from hepatic tissue samples using RNA-Trip reagent (Applygen, Beijing, China). After quantification, RNA was reverse-transcribed to complementary DNA (cDNA) using a first strand synthesis kit (Thermo Scientific, Rockford, IL, USA). Amplification was performed in a total volume of 20 μl comprising the cDNA template, primers, 2×Taq PCR Master Mix (Solarbio, Beijing, China).
The standard PCR conditions were of 95 C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec, with a final dissociation stage. The samples were run on an MX3000P qPCR detection system (Stratagene, Santa Clara, CA, USA). The amounts of target genes were determined and normalized to the amount of β-actin. Primer sequences are listed in Table 1.
Table 1.
The sequences of primers used in this study.
| Designation | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| IL-1β | ggctcatctgggatcctctc | tcatcttttggggtccgtca |
| IL-2 | aggaacctgaaactccccag | aacactctgctccctaccac |
| IL-10 | ataactgcacccacttccca | gggcatcacttctaccaggt |
| INF-gamma | cggcctagctctgagacaat | tccttttgccagttcctcca |
| TNF-alpha | ccaccacgctcttctgtcta | cacttggtggtttgctacga |
| β-actin | ttgtgatggactccggagac | tgatgtcacgcacgatttcc |
Western blot analysis
Mouse hepatic tissue samples were lysed in lysis buffer consisting of 150 mM NaCl, 1% Triton X-100, 100 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml pepstatin A, 20 mM Na2P2O4 and 2 mM Na3VO4. Total protein was extracted and measured using a BCA protein assay kit (Pierce Biotechnology Inc., Rockford, IL, USA). Equal amounts of protein (40–80 μg) were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinyl difluoride (PVDF) membranes. Membranes were blocked in 5% (w/v) dried milk powder in Tris-buffered saline-Tween for at least 1 hour at room temperature, and then incubated with specific primary antibodies and appropriate HRP-conjugated secondary antibodies. The protein bands were demonstrated using an enhanced chemiluminescence (ECL) Western blot kit (Solarbio, Beijing, China). Band intensity was quantified using the Image J software, and protein expression were normalized to β-actin signals.
Statistical analysis
Data were expressed as mean ±SD from at least three independent experiments. Group comparisons were performed by one-way ANOVA and Dunnett’s multiple comparison tests using GraphPad Prism 3 (GraphPad, San Diego, CA, USA). P<0.05 was considered statistically significant.
Results
Berberine reduced concanavalin A (Con A)-induced immunologic hepatic injury
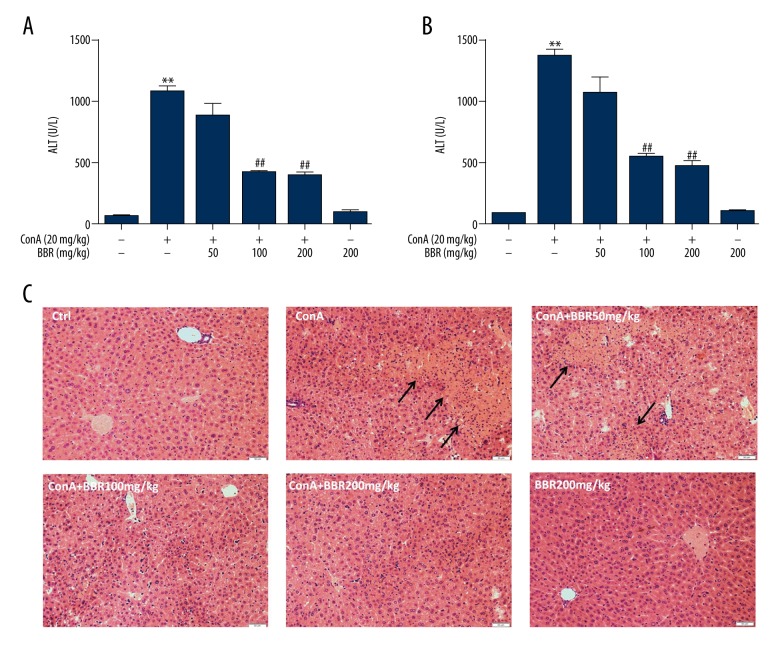
The levels of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were used as indicators of liver injury. After mice were gavaged (fed through the mouth via a feeding tube) with solvent or berberine (50, 100, and 200 mg/kg) for three days, the mice were intravenously injected with concanavalin A (Con A) (20 mg/kg). Serum levels of aminotransferases in mice were significantly increased at 8 hours after Con A treatment (P<0.01) (Figure 1A, 1B). Compared with the Con A group, aminotransferases in the low-dose berberine-treated group (50 mg/kg) showed a decreasing trend, which was not statistically significant (P>0.05). The aminotransferase levels in the medium-dose and high-dose berberine-treated groups (100 mg/kg and 200 mg/kg, respectively) were significantly reduced (P<0.01), but there was no significant difference between the two groups (Figure 1A, 1B) (P>0.05). Also, when compared with the control group, the aminotransferase levels did not change when high-dose berberine alone (200 mg/kg) was used to treat the mice (P>0.05) (Figure 1A, 1B). These results indicated that berberine had a protective effect on the elevation of aminotransferases induced by Con-A.
Figure 1.
Berberine reduced concanavalin A (Con A)-induced autoimmune hepatitis (AIH)-induced and immunological hepatic injury in mice. After the mice were gavaged (fed through the mouth via a tube) with solvent or berberine (50, 100, 200 mg/kg) for three days, they were intravenously injected with 20 mg/kg concanavalin A (Con A). Eight hours following injection, the serum and liver samples were collected. (A, B) Illustrate the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, respectively. C is a photomicrograph of the mouse liver tissue section stained with hematoxylin and eosin (H&E) (magnification, ×200). Data are expressed as mean ±SD (n=6, ** P<0.01 vs. control group, ## P<0.01 vs. Con A group).
Histochemical staining of mouse liver tissue sections with hematoxylin and eosin (H&E) showed the histological changes in the liver (Figure 1C). Histomorphology showed that liver tissue was normal in the untreated control mice and berberine-treated group. Stimulation with Con A resulted in damage to the liver tissue, seen histologically as hepatocyte necrosis and mononuclear cell infiltration. However, berberine administration reduced the injury induced by Con A, including cell swelling, necrosis, and mononuclear cell infiltration. These results indicated that berberine treatment was protective against Con A-induced hepatic injury.
Regulatory effects of berberine on pro-inflammatory cytokines in mice
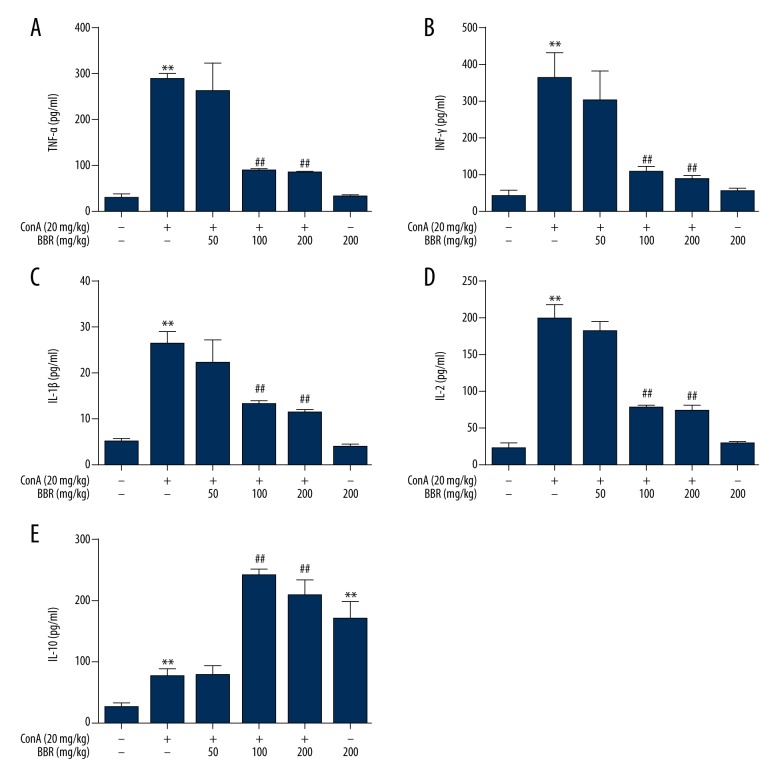
Con A-induced immune-mediated liver injury was related to the release of cytokines, as demonstrated by the findings from enzyme-linked immunosorbent assay (ELISA), which showed that when compared with the control group, the serum TNF-α, IFN-γ, IL-1β, IL-2, and IL-10 levels were elevated significantly at 8 hours following Con A injection (Figure 2) (P<0.01). The pretreatment with 100 and 200 mg/kg of berberine alleviated Con A-induced release of serum TNF-α, IFN-γ, IL-1β, and IL-2 (Figure 2A–2D) (P<0.01), while the serum IL-10 level was significantly elevated (Figure 2E) (P<0.01). There was no significant difference in the level of the serum cytokines between non-pretreated Con A-stimulated mice and Con A-stimulated mice pretreated with low-dose berberine (50 mg/kg). Compared with the control group, the levels of TNF-α, IFN-γ, IL-1β, and IL-2 were not significantly changed berberine treatment (200 mg/kg) alone (Figure 2A–2D) (P>0.05), while the IL-10 level was significantly elevated (Figure 2.E) (P<0.01).
Figure 2.
Effects of berberine on serum cytokines in concanavalin A (Con A)-stimulated mice. After the mice were gavaged (fed through the mouth via a tube) with solvent or berberine (50, 100, 200 mg/kg) for three days, they were injected intravenously with 20 mg/kg of Con A. Eight hours following injection, serum samples were obtained and used to detect TNF-α (A), IFN-γ (B), IL-1β (C), IL-2 (D) and IL-10 (E) with enzyme-linked immunosorbent assay (ELISA) (n=8). ** P<0.01 vs. the control group, ## P<0.01 vs. the Con A group.
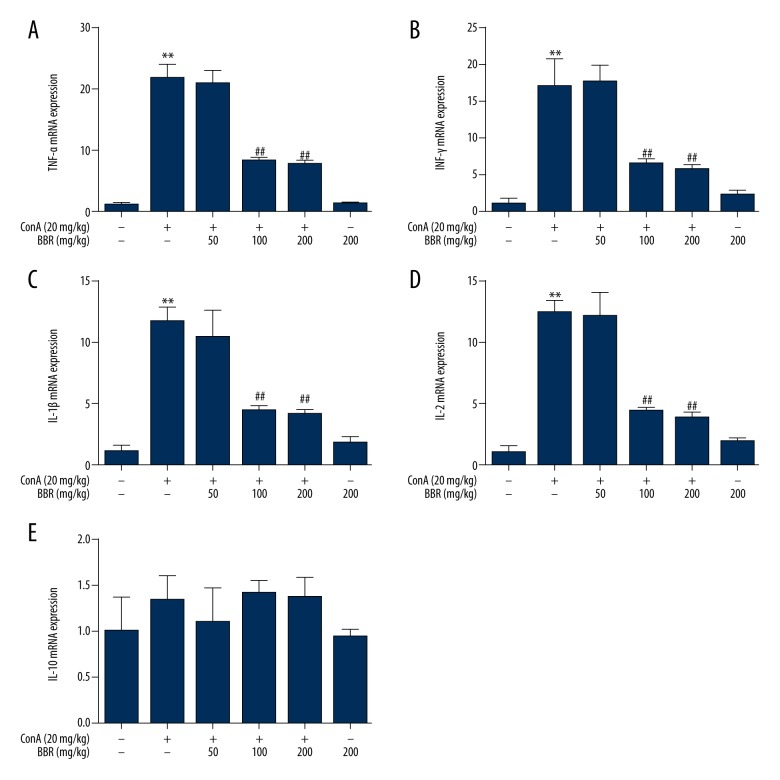
Quantitative polymerase chain reaction (qPCR) was used to detect cytokine mRNA expression levels in the mouse liver. Compared with the control group, the TNF-α, IFN-γ, IL-1β, and IL-2 mRNA levels in the Con A-treated group were significantly elevated (Figure 3A–3D) (P<0.01). The Con A-induced increase in the expression of pro-inflammatory cytokines was alleviated by berberine pretreatment (100 and 200 mg/kg) (Figure 3A–3D) (P<0.01). Compared with the control group, the cytokine levels did not significantly change following berberine treatment (200 mg/kg) alone (Figure 3) (P>0.05). These results indicated that the mRNA expression levels of IL-10 were not significantly changed by Con-A or berberine treatment.
Figure 3.
Effects of berberine on the expression of cytokines in the liver tissue of concanavalin A (Con A)-stimulated mice. After the mice were gavaged (fed through the mouth via a tube) with solvent or 50, 100, and 200 mg/kg berberine for three days, they were injected intravenously with 20 mg/kg of concanavalin A (Con A). At 8 hours after Con A injection, the TNF-α (A), IFN-γ (B), IL-1β (C), IL-2 (D) and IL-10 (E) mRNA levels in liver tissue were detected by quantitative polymerase chain reaction (qPCR) (n=6). ** P<0.01 vs. the control group, ## P<0.01 vs. the Con A group.
Berberine activated the adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) signaling pathway in mouse liver
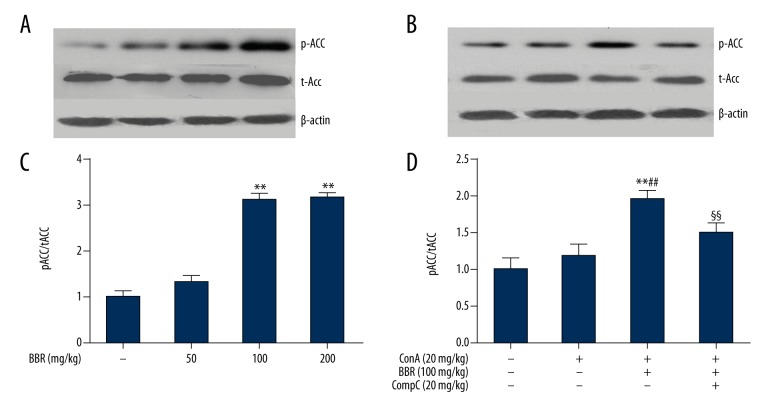
The activation of the signaling pathway of adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) was demonstrated by phosphorylation of acetyl coenzyme-A carboxylase (ACC), a target protein of AMPK [10]. The mice were treated for three days with 50, 100, and 200 mg/kg berberine, respectively. Western blot analysis was used to detect ACC phosphorylation. As shown in Figure 4A and 4C, the phosphorylated ACC/total ACC ratio increased significantly (P<0.01) after treatment with 100 and 200 mg/kg of berberine, indicating that berberine activated AMPK. There was no statistically significant difference in AMPK activation at berberine treatment doses between 100 and 200 mg/kg. Berberine (100 mg/kg) with or without Compound C (20 mg/kg), a specific inhibitor of AMPK, was administrated to mice for three days prior to Con A injection. As shown in Figure 4B and 4D, when compared with the Con A-treated group, berberine significantly increased the ratio of phosphorylated ACC to total ACC. These results indicated that berberine-activated AMPK was at least partially blocked by Compound C (P<0.01).
Figure 4.
Berberine activated adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) in mouse liver tissue. (A, C) Mice were treated with 50, 100, and 200 mg/kg of berberine for three days. After treatment with berberine for 8 hours, Western blot analysis was used to detect the phosphorylation of acetyl coenzyme-A carboxylase (ACC). (B, D) Con A-injected mice were pretreated with 100 mg/kg berberine, with or without the adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) inhibitor, Compound C (20 mg/kg) for three days. At 8 hours after Con A injection, ACC phosphorylation levels were detected by Western blot analysis. The western blot bands were semi-quantified with NIH Image J software and normalized with internal control, β-actin (C, D) (n=6). ** P<0.01 vs. the control group, ## P<0.01 vs. the Con A group, §§ P<0.01 vs. the Con A + berberine group.
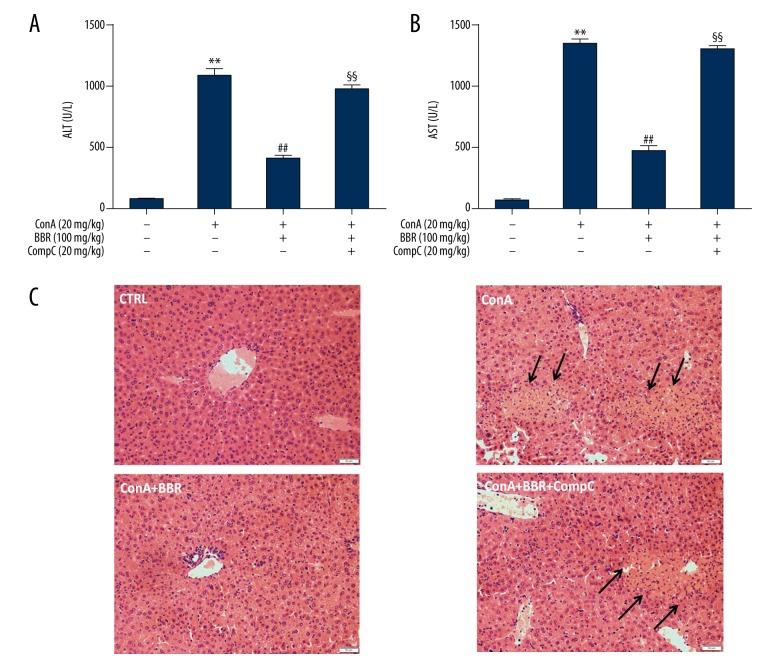
Compound C blocked the protective effect of berberine on liver injury
Berberine (100 mg/kg) with or without Compound C (20 mg/kg) were administrated to mice for three days; then the mice were injected with Con A. Berberine pretreatment effectively reduced the serum ALT and AST levels in Con A-stimulated mice. The ALT and AST levels in the berberine plus Compound C-treated group were significantly greater compared with those in the berberine-treated group (Figure 5A, 5B) (P<0.01). Histological changes were observed with routine hematoxylin and eosin (H&E) staining (Figure 5C). Liver cell necrosis and mononuclear cell infiltration were increased in the berberine plus Compound C group compared with the berberine group. These results indicated that AMPK activation might be involved in the protective effect of berberine on liver injury.
Figure 5.
Effects of Compound C on serum cytokines in berberine-treated mice. Concanavalin A (Con A)-injected mice were pretreated with 100 mg/kg berberine with or without 20 mg/kg Compound C for three days. At 8 hours after Con A injection, the serum and liver samples were collected. Serum alanine aminotransferase (ALT) (A) and aspartate aminotransferase (AST) (B) levels were detected with an enzyme-linked immunoassay (ELISA). C is a photomicrograph of the mouse liver tissue section stained with hematoxylin and eosin (H&E) (magnification, ×200), data are expressed as the mean ±SD (n=6). ** P<0.01 vs. the control group, ## P<0.01 vs. the Con A group, §§ P<0.01 vs. the Con A plus berberine group.
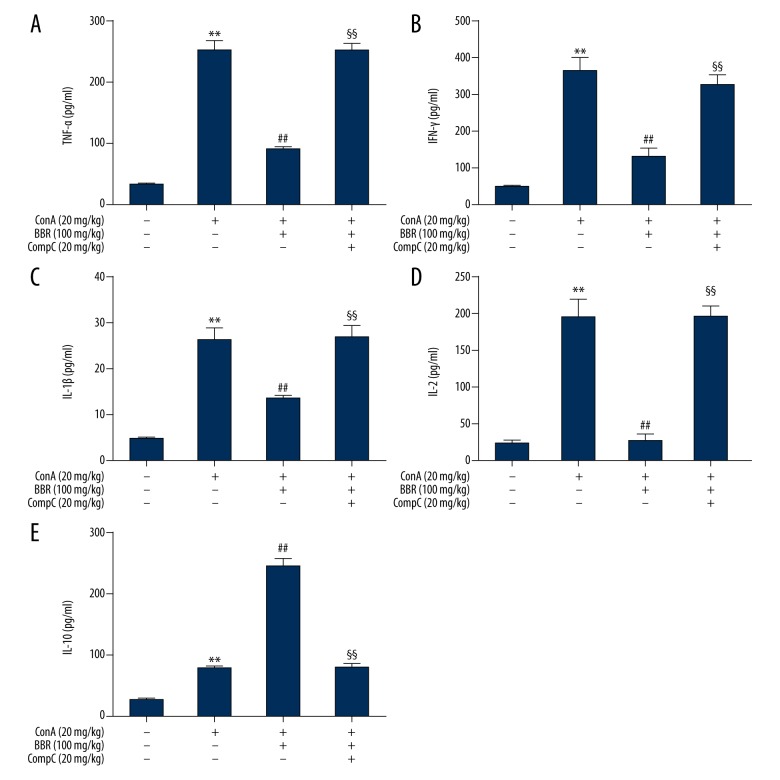
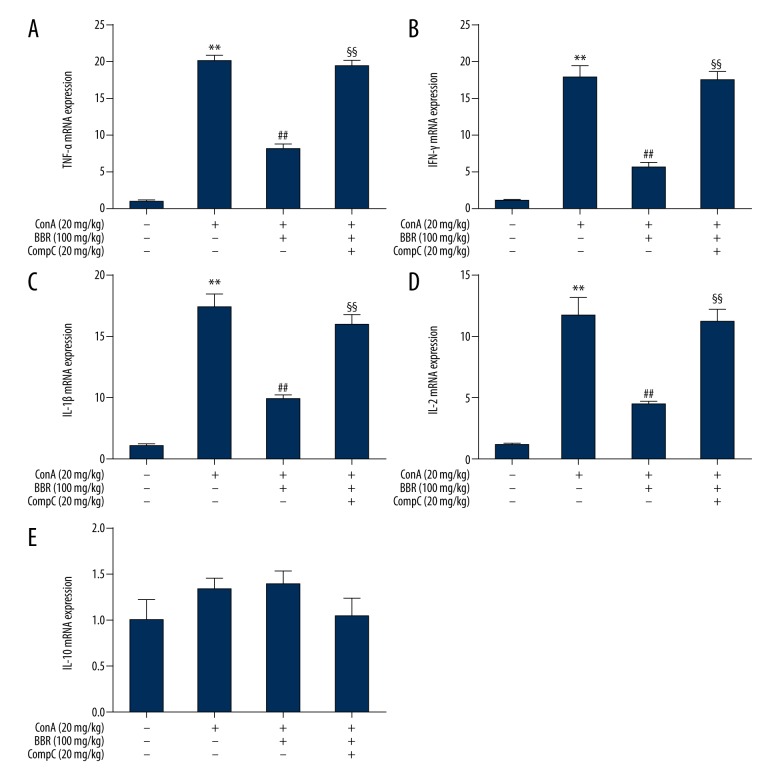
Compound C blocked berberine regulation of cytokine secretion
Berberine (100 mg/kg) treatment of mice, with or without Compound C (20 mg/kg) were administrated to mice for three days, followed by injection of Con A. Eight hours following injection, blood and liver samples were collected. Serum cytokine levels were measured by ELISA. When compared with the berberine-treated group, the TNF-α, IFN-γ, IL-1β, and IL-2 levels were significantly increased in the berberine plus Compound C-treated group (Figure 6A–6D) (P<0.01), while the IL-10 levels declined (Figure 6E) (P<0.01). The expression of cytokine mRNA in the liver, detected by qPCR showed that when compared with the berberine-treated group, the TNF-α, IFN-γ, IL-1β, and IL-2 levels in the berberine plus Compound C-treated group were significantly increased (Figure 7A–7D) (P<0.01). These results indicated that the mRNA level of IL-10 was not significantly changed by treatment with berberine and Compound C.
Figure 6.
Effects of Compound C on serum cytokines in berberine-treated mice. Concanavalin A (Con A)-injected mice were pretreated with 100 mg/kg berberine with or without 20 mg/kg of Compound C for three days. At 8 hours after Con A injection, the serum TNF-α (A), IFN-γ (B), IL-1β (C), IL-2 (D), and IL-10 (E) levels were detected with an enzyme-linked immunoassay (ELISA) (n=6). ** P<0.01 vs. the control group, ## P<0.01 vs. the Con A group, §§ P<0.01 vs. the Con A plus berberine group.
Figure 7.
Effect of Compound C on liver cytokine expression in berberine-treated mice. Mice injected with concanavalin A (Con A)- were pretreated with 100 mg/kg berberine with or without 20 mg/kg Compound C for three days. At 8 hours after Con A injection, the TNF-α (A), IFN-γ (B), IL-1β (C), IL-2 (D), and IL-10 (E) mRNA levels in liver tissue were detected by quantitative polymerase chain reaction (qPCR) (n = 6). ** P<0.01 vs. the control group, ## P<0.01 vs. the Con A group, §§ P<0.01 vs. the Con A plus berberine group.
Discussion
The findings from this study, using a murine model of autoimmune hepatitis (AIH), have demonstrated the immunosuppressive effects of berberine on the inflammatory response induced by concanavalin A (Con A) and the inhibitory effects the synthetic, reversible AMP-competitive inhibitor, Compound C. By inhibiting the activation and differentiation of immune cells, berberine may be studied further in the treatment of several immune disorders such as autoimmune neuritis, myocarditis, encephalomyelitis, ocular Behçet’s disease and inflammatory bowel disease [13–17]. Because studies on the effects of berberine on Con A-induced hepatitis had not yet been undertaken, the aim of this study was to investigate the effects of berberine on Con A-induced AIH in mice via AMPK signaling pathway.
Previous studies have shown that immunoregulatory cytokines play an important role in Con A-induced liver injury. Tumor necrosis factor (TNF)-α and interferon (IFN)-γ directly promote hepatocyte apoptosis, and function in the initiation of Con A-induced injury [18]. Lavon et al. reported that TNF-α and IFN-γ expression levels significantly increased one hour following Con A injection [18]. Following the binding of TNF-α to TNFR-1, this complex activates three important intracellular signaling pathways via TRADD and FADD, namely, caspase-8, nuclear factor-kappa B (NF-κB), and c-Jun N-terminal kinase (JNK), eventually leading to cell injury [20]. IFN-γ promotes liver cell injury via several different pathways [21]. Mice that overexpress IFN-γ may develop chronic hepatitis, as IFN-γ triggers STAT1 activation, and STAT1 induces apoptosis [22]. Also, TNF promotes the expression of major histocompatibility complex (MHC) class I antigens in T-cells, enhances T-cell proliferation, and promotes the secretion of multiple interleukins [22]. Interleukins can be produced by Con A-activated lymphocytes, which can be used as an important indicator of T-cell and NK cell functions [22,23]. Shen et al. reported that IL-2 and IL-1β levels started to increase at three hours after injection of Con A, and peaked at six hours [23]. IL-2 and IL-1β are pro-inflammatory factors that synergistically work with each other to stimulate T-cell proliferation, activate CD8+ T-cells to cytotoxic T-lymphocytes.
In the present study, pro-inflammatory cytokines were shown to be induced in Con A-treated mice, leading to hepatocyte damage. The findings of the present study demonstrated that the levels of TNF-α, IFN-γ, IL-2, and IL-1β in the liver and serum of mice were significantly increased after Con A injection. Berberine was shown to reduce Con A-induced cytokine expression and release, which was reflected in the reduction of ALT and AST. These results indicate that berberine can protect against Con A-induced liver damage by suppressing pro-inflammatory cytokine expression and release. Unlike the pro-inflammatory cytokines described above, IL-10 can inhibit inflammation and protect hepatocytes. In support of the findings of this present study, He et al. reported that serum IL-10 expression levels were significantly increased in a mouse model of Con A-induced immune liver injury [24].
Di Marco et al. reported that the TNF-α, IFN-γ, and IL-12 levels in mice injected with Con A were 47%, 47%, and 80% lower than those in the control group, respectively following injection of recombinant IL-10 [25]. Injection of IL-10 neutralizing antibody increased Con A-induced liver injury [25]. The present study demonstrated that the serum IL-10 levels significantly increased following Con A injection, which may be considered to be a feedback mechanism to suppress liver inflammation. Compared with the Con A injection group, berberine pretreatment further increased the levels of IL-10. These results suggested that berberine can, at least partially, inhibit liver inflammation and reduce liver injury by upregulating IL-10 expression. However, compared with the control group, IL-10 levels were not significantly altered in the livers of Con A-treated mice, and berberine did not significantly increase the hepatic IL-10 levels. These results are supported by the findings from the study by Sass et al., which demonstrated that serum IL-10 was mainly expressed in the spleen rather than the liver [26].
In addition to the regulation of cellular metabolism, published evidence supports that adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) exerts anti-inflammatory and immunosuppressive effects. AMPK signaling was found to be involved in controlling immune-related diseases such as autoimmune encephalomyelitis, arthritis, and inflammatory bowel disease [27,28]. AMPK is highly expressed in several immune cell types, including macrophages, lymphocytes and dendritic cells [28]. After AMPK activation, by agents such as metformin and the AMP analog 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), T-cell mediated immune responses can be suppressed, as shown by inhibition of T-cell differentiation and reduction of cytokines and chemokines [29–31]. Based on previous reports, it is possible that AMPK activation can play a role in inhibiting or suppressing Con A-induced hepatitis.
The findings of the present study also showed that berberine activated AMPK and that levels of phosphorylated acetyl coenzyme-A carboxylase (ACC), representing AMPK activation, were associated with an increased ratio of phospho-ACC/total ACC. The AMPK signaling pathway was activated by berberine and inhibited by the synthetic, reversible AMP-competitive inhibitor, Compound C. Compound C exacerbated Con A-induced liver damage and increased the levels of proinflammatory cytokines relative to Con A-stimulated mice pretreated with berberine.
The regulation of cytokines by AMPK is known to be associated with the activity of some transcriptional factors, as upon AMPK activation, NF-κB and JNK pathways are inhibited, which mediates the expression and release of cytokines induced by inflammatory stimuli [32,33]. Also, the immunosuppressive effect of AMPK appears to be associated with its regulation of cell metabolism [34]. Activated immune cells utilize aerobic glycolysis, while their inactivated or anti-inflammatory counterparts tend to utilize oxidative metabolism [31]. AMPK activation markedly promotes lipid oxidation, in addition to reducing cytokine release, AMPK activation restricts cytokine-induced inflammation and damage by inhibiting the related signaling pathways, for example, AMPK activation blocks IFN-γ-induced expression and transcriptional activity of STAT1 [31].
Con A-induced mouse hepatitis is a well-defined animal model for studying autoimmune hepatitis (AIH) [35]. In this model, intravenously injecting animals with Con A results in systemic immune activation and causes liver cell damage, which is similar to the pathological characteristics of human AIH. The clinical manifestations of AIH include elevated serum aminotransferases, specific autoantibodies in the serum, and hypergammaglobulinemia, which may eventually develop into cirrhosis. Currently, the pathogenesis of AIH and the exact cause triggering its occurrence are not fully understood. Although the role of MHC antigens in AIH have been extensively studied, the effect of the different changes in the MHC allele remains unclear, but the role of the MHC enhances the immunogenicity of the antigen and stimulates a strong T-cell response [36]. At the early stage of AIH, it is mainly CD4+ cell activation, followed by CD8+ cytotoxic T-cells, with activated T-cells and macrophages releasing a variety of cytokines, including IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, TNF-α, and IFN-γ [37]. Uncontrolled inflammation in AIH leads to chronic persistent liver damage [38]. Therefore, disease progression in AIH could be delayed, and liver damage could be reduced by controlling the inflammatory response. Currently, glucocorticoids and immunosuppressive agents are commonly used to treat AIH. However, these drugs are associated with adverse effects, which limit their long-term use. Clinical studies have confirmed the safety and efficacy of berberine [39]. This drug has few severe adverse effects, and, so far, no studies have reported any liver injuries associated with berberine use, which is also supported by the results of this study. In this study, there were no differences in the levels of aminotransferases in the liver tissue and serum between the untreated mice and the mice treated with berberine alone.
Conclusions
In a murine model of concanavalin A (Con A)-induced autoimmune hepatitis (AIH), berberine treatment reduced hepatic injury via activation of the adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) signaling pathway. Further studies are recommended to determine the potential therapeutic role for berberine in AIH.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the National Natural Science Foundation of China (81470834), the Natural Science Foundation of Hebei Province (H2013201178) and the Affiliated Hospital of Hebei University Grant (2016Q001)
References
- 1.Fei M, Xie Q, Zou Y, et al. Alpha-lipoic acid protects mice against concanavalin A-induced hepatitis by modulating cytokine secretion and reducing reactive oxygen species generation. Int Immunopharmacol. 2016;35:53–60. doi: 10.1016/j.intimp.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Wang HX, Liu M, Weng SY, et al. Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World J Gastroenterol. 2012;18:119–25. doi: 10.3748/wjg.v18.i2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xue J, Chen F, Wang J, et al. Emodin protects against concanavalin A-induced hepatitis in mice through inhibiting activation of the p38 MAPK-NF-κB signaling pathway. Cell Physiol Biochem. 2015;35:1557–70. doi: 10.1159/000373971. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava S, Srivastava M, Misra A, et al. A review on biological and chemical diversity in Berberis (Berberidaceae) EXCLI J. 2015;14:247–67. doi: 10.17179/excli2014-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai Q, Zhang D, Yu H, et al. Berberine restricts Coxsackievirus B Type 3 replication via inhibition of c-Jun N-terminal kinase (JNK) and p38 MAPK activation in vitro. Med Sci Monit. 2017;23:1448–55. doi: 10.12659/MSM.899804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie X, Chang X, Chen L, et al. Berberine ameliorates experimental diabetes-induced renal inflammation and fibronectin by inhibiting the activation of RhoA/ROCK signaling. Mol Cell Endocrinol. 2013;381:56–65. doi: 10.1016/j.mce.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Xu L, Liu Y, He X. Inhibitory effects of berberine on the activation and cell cycle progression of human peripheral lymphocytes. Cell Mol Immunol. 2005;2:295–300. [PubMed] [Google Scholar]
- 8.Li J, Zhong L, Wang F, Zhu H. Dissecting the role of AMP-activated protein kinase in human diseases. Acta Pharm Sin B. 2017;7:249–59. doi: 10.1016/j.apsb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marín-Aguilar F, Pavillard LE, Giampieri F, et al. Adenosine monophosphate (AMP)-activated protein kinase: A new target for nutraceutical compounds. Int J Mol Sci. 2017;18:288. doi: 10.3390/ijms18020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Zhong L, Wang F, Zhu H. Dissecting the role of AMP-activated protein kinase in human diseases. Acta Pharm Sin B. 2017;7:249–59. doi: 10.1016/j.apsb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Y, Liu S, Ma Q, et al. Berberine enhances the AMPK activation and autophagy and mitigates high glucose-induced apoptosis of mouse podocytes. Eur J Pharmacol. 2017;794:106–14. doi: 10.1016/j.ejphar.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 12.Dandapani M, Hardie DG. AMPK: Opposing the metabolic changes in both tumour cells and inflammatory cells? Biochem Soc Trans. 2013;41:687–93. doi: 10.1042/BST20120351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Wang Q, Xie M, et al. Berberine exerts an anti-inflammatory role in ocular Behçet’s disease. Mol Med Rep. 2017;15:97–102. doi: 10.3892/mmr.2016.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habtemariam S. Berberine and inflammatory bowel disease: A concise review. Pharmacol Res. 2016;113:592–99. doi: 10.1016/j.phrs.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Li XL, Zhang M, et al. Berberine ameliorates experimental autoimmune neuritis by suppressing both cellular and humoral immunity. Scand J Immunol. 2014;79:12–19. doi: 10.1111/sji.12123. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Zhang X, Ye L, Yuan H. Protective mechanisms of berberine against experimental autoimmune myocarditis in a rat model. Biomed Pharmacother. 2016;79:222–30. doi: 10.1016/j.biopha.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Qin X, Guo BT, Wan B, et al. Regulation of Th1 and Th17 cell differentiation and amelioration of experimental autoimmune encephalomyelitis by natural product compound berberine. J Immunol. 2010;185:1855–63. doi: 10.4049/jimmunol.0903853. [DOI] [PubMed] [Google Scholar]
- 18.Streetz KL, Wüstefeld T, Klein C, et al. Mediators of inflammation and acute phase response in the liver. Cell Mol Biol. 47:661–73. 200. [PubMed] [Google Scholar]
- 19.Lavon I, Sheinin T, Meilin S, et al. A novel synthetic cannabinoid derivative inhibits inflammatory liver damage via negative cytokine regulation. Mol Pharmacol. 2003;64:1334–41. doi: 10.1124/mol.64.6.1334. [DOI] [PubMed] [Google Scholar]
- 20.Ksontini R, Colagiovanni DB, Josephs MD, et al. Disparate roles for TNF-alpha and Fas ligand in concanavalin A-induced hepatitis. J Immunol. 1998;160:4082–89. [PubMed] [Google Scholar]
- 21.Toyonaga T, Hino O, Sugai S, et al. Chronic active hepatitis in transgenic mice expressing interferon-gamma in the liver. Proc Natl Acad Sci USA. 1994;91:614–18. doi: 10.1073/pnas.91.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin YE, Kitagawa M, Kuida K, et al. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17:5328–37. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen M, Lu J, Cheng P, et al. Ethyl pyruvate pretreatment attenuates concanavalin A-induced autoimmune hepatitis in mice. PLoS One. 2014;9:e87977. doi: 10.1371/journal.pone.0087977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He R, Wang L, Zhu J, et al. Methane-rich saline protects against concanavalin A-induced autoimmune hepatitis in mice through anti-inflammatory and anti-oxidative pathways. Biochem Biophys Res Commun. 2016;470:22–28. doi: 10.1016/j.bbrc.2015.12.080. [DOI] [PubMed] [Google Scholar]
- 25.Di Marco R, Xiang M, Zaccone P, et al. Concanavalin A-induced hepatitis in mice is prevented by interleukin (IL)-10 and exacerbated by endogenous IL-10 deficiency. Autoimmunity. 1999;31:75–83. doi: 10.3109/08916939908994050. [DOI] [PubMed] [Google Scholar]
- 26.Sass G, Heinlein S, Agli A, et al. Cytokine expression in three mouse models of experimental hepatitis. Cytokine. 2002;19:115–20. doi: 10.1006/cyto.2002.1948. [DOI] [PubMed] [Google Scholar]
- 27.Guma M, Wang Y, Viollet B, Liu-Bryan R. AMPK activation by A-769662 controls IL-6 expression in inflammatory arthritis. PLoS One. 2015;10:e0140452. doi: 10.1371/journal.pone.0140452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonioli L, Colucci R, Pellegrini C, et al. The AMPK enzyme-complex: from the regulation of cellular energy homeostasis to a possible new molecular target in the management of chronic inflammatory disorders. Expert Opin Ther Targets. 2016;20:179–91. doi: 10.1517/14728222.2016.1086752. [DOI] [PubMed] [Google Scholar]
- 29.Bai A, Ma AG, Yong M, et al. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol. 2010;80:1708–17. doi: 10.1016/j.bcp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Nath N, Khan M, Paintlia MK, et al. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol. 2009;182:8005–14. doi: 10.4049/jimmunol.0803563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meares GP, Qin H, Liu Y, et al. AMP-activated protein kinase restricts IFN-g signaling. J Immunol. 2013;190:372–80. doi: 10.4049/jimmunol.1202390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiang CF, Chao TT, Su YF, et al. Metformin-treated cancer cells modulate macrophage polarization through AMPK-NF-κB signaling. Oncotarget. 2017;8:20706–18. doi: 10.18632/oncotarget.14982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeong GS, Lee DS, Li B, et al. Anti-inflammatory effects of lindenenyl acetate via heme oxygenase-1 and AMPK in human periodontal ligament cells. Eur J Pharmacol. 2011;670:295–303. doi: 10.1016/j.ejphar.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Dandapani M, Hardie DG. AMPK: Opposing the metabolic changes in both tumour cells and inflammatory cells? Biochem Soc Trans. 2013;41:687–93. doi: 10.1042/BST20120351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang HX, Liu M, Weng SY, et al. Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World J Gastroenterol. 2012;18:119–25. doi: 10.3748/wjg.v18.i2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira LC, Porta G, Marin ML, et al. Autoimmune hepatitis, HLA and extended haplotypes. Autoimmun Rev. 2011;10:189–93. doi: 10.1016/j.autrev.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 37.Vergani D, Mieli-Vergani G. Aetiopathogenesis of autoimmune hepatitis. World J Gastroenterol. 2008;14:3306–12. doi: 10.3748/wjg.14.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liberal R, Grant CR. Cirrhosis and autoimmune liver disease: Current understanding. World J Hepatol. 2016;8:1157–68. doi: 10.4254/wjh.v8.i28.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caliceti C, Rizzo P, Cicero AF. Potential benefits of berberine in the management of perimenopausal syndrome. Oxid Med Cell Longev. 2015;2015:723093. doi: 10.1155/2015/723093. [DOI] [PMC free article] [PubMed] [Google Scholar]