Abstract
The noncanonical nuclear factor κB (NF-κB) pathway is a specific arm of NF-κB signaling that regulates important aspects of immune function. Activation of this pathway centers on the modulation of a pivotal signaling component: NF-κB–inducing kinase (NIK). Under normal conditions, NIK undergoes constitutive degradation, which keeps its abundance below the threshold required for its function, and signal-induced activation of the noncanonical NF-κB pathway is coupled with the stabilization and accumulation of NIK. A study now shows that signal-induced accumulation of NIK is subject to feedback control, which involves its phosphorylation by a downstream kinase, inhibitor of κB (IκB) kinase α (IKKα), and degradation. Thus, controlling the fate of NIK is emerging as a central mechanism in noncanonical NF-κB signaling.
The nuclear factor κB (NF-κB) family of transcription factors regulates diverse biological processes, including immune and inflammatory responses, cell growth and survival, and development (1, 2). Activation of NF-κB involves cascades of signaling events that are classified into canonical and non-canonical pathways (1, 2). Tight control of NF-κB signaling is vital, because deregulated activation of NF-κB contributes to diseases that range from chronic inflammation and autoimmunity to cancer (3–5). Whereas a number of inhibitory mechanisms have been reported for the canonical NF-κB pathway (2), we are just beginning to understand the regulation of noncanonical NF-κB signaling. Constitutive degradation of NF-κB–inducing kinase (NIK), a central player in the noncanonical NF-κB pathway, prevents basal activation of this pathway, and signal-induced stabilization of NIK may serve as a mechanism that initiates non-canonical NF-κB signaling (6). However, it has been unclear whether this pathway, similar to the canonical NF-κB pathway, is also subject to feedback regulation. Cheng and colleagues now report a negative-feedback mechanism that controls the magnitude and kinetics of signal-induced accumulation of NIK (7). This finding sheds new light on the regulation of NIK and further emphasizes a central role for the control of the fate of NIK in noncanonical NF-κB signaling.
NF-κB proteins are normally sequestered in the cytoplasm by inhibitors, including inhibitor of κB (IκB) proteins and related factors (1). The canonical pathway for the activation of NF-κB involves the phosphorylation and subsequent degradation of a prototypical IκB, IκBα, and the rapid nuclear translocation of different NF-κB members (1). In contrast, the non-canonical pathway activates a specific NF-κB member, RelB, which is sequestered by an IκB-like molecule, p100 (8, 9), which also functions as an NF-κB precursor protein because its N-terminal portion forms the NF-κB subunit p52, whereas its C-terminal portion functions as an IκB protein. Noncanonical NF-κB signaling induces the limited degradation of p100 in its C-terminal, IκB-like sequence, and this so-called processing both generates mature p52 and causes the nuclear translocation of the non-canonical NF-κB complex, p52-RelB (8, 9). The noncanonical pathway responds to signals elicited by a subset of tumor necrosis factor receptors (TNFRs), including B cell activation factor receptor (BAFFR), CD40, lymphotoxin β receptor (LTβR), and receptor activator of NF-κB (RANK). Consequently, the noncanonical pathway regulates specific biological processes, including lymphoid organ development, B cell maturation and survival, dendritic cell function, and bone metabolism (9).
A central signaling component of the noncanonical NF-κB pathway is NIK, which stimulates the processing of p100 by inducing its phosphorylation (10). This is achieved through the activation of a downstream kinase, IκB kinase α (IKKα) (11), which was originally identified as a component of a large kinase complex that also contains a homologous kinase, IKKβ, and a regulatory protein, IKKγ (also called NEMO) (1, 2). Whereas IKKβand IKKγare central components in the canonical NF-κB pathway, IKKα is essential for noncanonical NF-κB signaling (1, 2). IKKα physically interacts with NIK and serves as its primary substrate (12). NIK and IKKα appear to form a specific kinase complex in which NIK both activates IKKα and functions as an adaptor that recruits IKKα to p100 (13). The work by Razani et al. (7) suggests a previously un-characterized mechanism for the interplay between NIK and IKKα: IKKα also functions as a kinase that phosphorylates NIK. The IKKα-mediated phosphorylation of NIK targets it for degradation, which suggests a negative-feedback mechanism for the regulation of NIK (7).
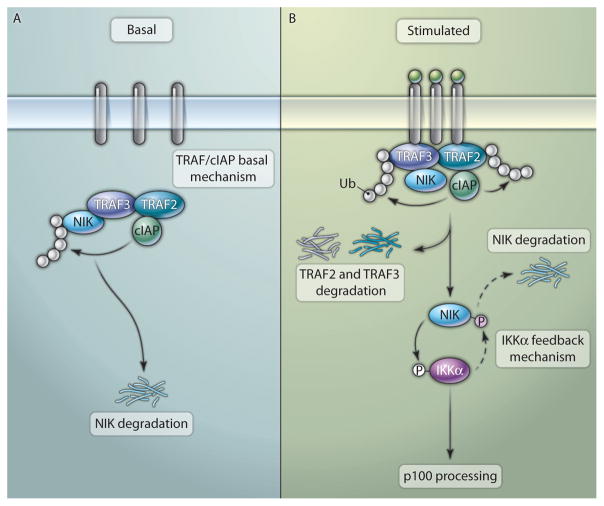
The degradation of NIK was first discovered as a mechanism that prevents basal activation of p100 processing (14). When cells are not exposed to stimuli, NIK undergoes constant degradation through a ubiquitin-dependent mechanism, which keeps NIK low in abundance. Signal-induced processing of p100 involves the stabilization of NIK and its accumulation through de novo synthesis (14), a finding that explains why stimulation of the phosphorylation of p100 and its processing require de novo protein synthesis (15, 16). Identification of tumor necrosis factor receptor–associated factor 3 (TRAF3) as a major NIK-binding protein led to the discovery that TRAF3 induces the constitutive degradation of NIK (14). A major step in noncanonical NF-κB signaling is the proteolytic elimination of TRAF3, a mechanism that mediates the stabilization of NIK (14). Accumulation of NIK appears to be sufficient to trigger noncanonical NF-κB signaling, because knockdown of TRAF3 by RNA interference or knockout of TRAF3 leads to the accumulation of NIK and the processing of p100 (14, 17). Although TRAF3 induces ubiquitination of NIK in cells, TRAF3 is not the ubiquitin ligase that targets NIK because purified TRAF3 does not catalyze the ubiquitination of NIK in vitro (14). Recent studies identified cytosolic inhibitor of apoptosis 1 (cIAP1) and cIAP2 as E3 ubiquitin ligases that mediate the degradation of NIK (18, 19). TRAF3 recruits cIAP1 and cIAP2 to NIK, a molecular event that also requires TRAF2 (Fig. 1) (20, 21). Thus, deficiency in either TRAF3 or TRAF2 causes the stabilization of NIK and the processing of p100 (17, 22, 23); degradation of cIAP1 and cIAP2 by pharmacological antagonists has similar consequences (18, 19). These findings establish a TRAF- and cIAP-dependent basal mechanism for the regulation of NIK that prevents its signal-independent accumulation (Fig. 1A). The importance of this basal mechanism is underscored by the finding that deficiencies in TRAF3, TRAF2, cIAP1, or cIAP2 are associated with aberrant activation of noncanonical NF-κB signaling and B cell malignancies (24, 25).
Fig. 1.
Basal and feedback mechanisms that regulate NIK activity. (A) Under unstimulated conditions, NIK is constantly targeted for ubiquitination and degradation by the redundant functions of the E3 ubiquitin ligases cIAP1 and cIAP2, which do not directly interact with NIK but are recruited to NIK through TRAF2 and TRAF3, which interact with cIAP1/2 and NIK, respectively. The basal degradation of NIK may keep its abundance below a threshold required for function, thus preventing signal-independent activation of the processing of p100. (B) In response to receptor-dependent signals, TRAF2 or TRAF3, or both, are recruited to the signaling receptor and are targeted for degradation by cIAP-mediated ubiquitination, which triggers the release and stabilization of NIK. NIK accumulates and activates IKKα, which triggers the processing of p100. Activated IKKα also phosphorylates NIK and induces its degradation, which prevents an overaccumulation of NIK in activated cells. The IKKα-mediated feedback mechanism controls the magnitude of the accumulation of NIK but may not be able to completely shut off noncanonical NF-κB signaling.
The signal-induced degradation of TRAF3 has persistent kinetics (14). However, Cheng and colleagues noticed that NIK did not continuously accumulate upon receptor ligation in the context of the persistent degradation of TRAF3 and an increase in the abundance of NIK mRNA (7). This phenomenon prompted the authors to examine whether a feedback mechanism existed to prevent the unchecked accumulation of NIK in response to receptor ligation. If this were the case, the fate of NIK should be regulated by a downstream signaling factor(s). Indeed, the authors detected a substantial increase in the steady-state abundance of NIK in IKKα-deficient cells (7). Similarly, NIK accumulated in cells derived from alymphoplasia (aly) mice that have a mutant NIK protein that is incapable of binding to or activating IKKα (26). Further studies revealed that IKKα phosphorylated NIK at three putative serine residues located at the C terminus of NIK. Mutation of all of these serines created a NIK mutant (NIK 3SA) that displayed increased stability compared to that of wild-type NIK (7). These results suggested a feedback mechanism for the degradation of NIK that involved IKKα-mediated phosphorylation of NIK (Fig. 1B).
What is the relationship between the IKKα- and the TRAF- and cIAP-mediated degradation of NIK? Are these coupled or separate mechanisms of action? If the former, one would expect to see no further increase in the abundance of NIK upon disruption of the TRAF-cIAP complex in IKKα-deficient or aly cells. Cheng and colleagues addressed this question by inducing the degradation of TRAF3 and the cIAP proteins with an agonistic antibody against LT?R and an antagonist of cIAP. Treatment of IKKα-deficient and aly fibroblasts with these reagents led to further accumulation of NIK, thus suggesting that two separate mechanisms were involved in the regulation of NIK stability (7). Why, then, is there a requirement for two different mechanisms to control the abundance of NIK? The work by Razani et al. suggests a feedback regulatory function for IKKα. In response to stimulation of LTβR, NIK continuously accumulated in IKKα-deficient cells, whereas the abundance of NIK remained constant after an initial increase in wild-type cells (7). Although it seems that the IKKα-dependent feedback mechanism controls the magnitude of NIK accumulation, it cannot completely shut off noncanonical NF-κB signaling in the absence of the TRAF- and cIAP-dependent mechanism. This would explain why genetic mutations in components of the TRAF-cIAP pathway lead to deregulated non-canonical NF-κB signaling and the development of lymphoma even in the presence of wild-type IKKα.
The study by Cheng and colleagues (7) will stimulate further investigation of the degradation of NIK and its central role in the regulation of noncanonical NF-κB signaling. Several intriguing questions are raised for future studies. First, how does IKKα-mediated phosphorylation of NIK trigger its degradation? Inducible degradation of proteins typically involves their ubiquitination-mediated targeting to the 26S proteasome. However, IKKα did not induce detectable ubiquitination of NIK in transfected human embryonic kidney 293T cells. Is this because of the lack of a NIK-ubiquitination component in these cells, or is it due to the involvement of a ubiquitination-independent mechanism in the proteolysis of NIK? Second, does IKKα mediate crosstalk between the canonical and non-canonical NF-κB pathways? When increased in abundance, NIK not only induces the processing of p100 but also activates canonical NF-κB signaling (27, 28). Because a deficiency in IKKα causes the accumulation of NIK, it is conceivable that IKKα may provide a link between the canonical and noncanonical NF-κB signaling pathways. In this regard, loss of IKKα promotes the Toll-like receptor (TLR)–mediated production of proinflammatory cytokines (29, 30), raising the question of whether it involves the deregulation of NIK. Third, is NIK a constitutively active kinase? That the accumulation of NIK is sufficient to trigger the processing of p100 suggests the possibility that NIK is a constitutively active kinase, whose activity is controlled by its stability. Although NIK activity is dependent on phosphorylation at its T loop (31), this could be an autophosphorylation event. However, it remains possible that inducible phosphorylation of NIK may also contribute to its activation under certain conditions. Better understanding of the regulation of NIK is important for the rational design of therapies to treat human diseases associated with deregulated noncanonical NF-κB signaling.
Acknowledgments
Funding: Work performed in the author’s laboratory is supported by NIH grants AI064639, AI057555, and GM084459.
References and Notes
- 1.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 3.Brown KD, Claudio E, Siebenlist U. The roles of the classical and alternative nuclear factor-kappaB pathways: Potential implications for autoimmunity and rheumatoid arthritis. Arthritis Res Ther. 2008;10:212. doi: 10.1186/ar2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: Implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 6.Sun SC, Ley SC. New insights into NF-kappaB regulation and function. Trends Immunol. 2008;29:469–478. doi: 10.1016/j.it.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Razani B, Zarnegar B, Ytterberg AJ, Shiba T, Dempsey PW, Ware CF, Loo JA, Cheng G. Negative feedback in noncanonical NF-κB signaling modulates NIK stability through IKKα-mediated phosphorylation. Sci Sig. 2010;3:ra41. doi: 10.1126/scisignal.2000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejardin E. The alternative NF-kappaB pathway from biochemistry to biology: Pitfalls and promises for future drug development. Biochem Pharmacol. 2006;72:1161–1179. doi: 10.1016/j.bcp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 11.Senftleben U, Cao Y, Xiao G, Greten FR, Krähn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 12.Ling L, Cao Z, Goeddel DV. NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao G, Fong A, Sun SC. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J Biol Chem. 2004;279:30099–30105. doi: 10.1074/jbc.M401428200. [DOI] [PubMed] [Google Scholar]
- 14.Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 15.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 16.Liang C, Zhang M, Sun SC. beta-TrCP binding and processing of NF-kappaB2/p100 involve its phosphorylation at serines 866 and 870. Cell Signal. 2006;18:1309–1317. doi: 10.1016/j.cellsig.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 17.He JQ, Zarnegar B, Oganesyan G, Saha SK, Yamazaki S, Doyle SE, Dempsey PW, Cheng G. Rescue of TRAF3-null mice by p100 NF-kappa B deficiency. J Exp Med. 2006;203:2413–2418. doi: 10.1084/jem.20061166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNF-alpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 20.Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, Korneluk RG, Cheng G. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, Dave S, Hurt EM, Tan B, Zhao H, Stephens O, Santra M, Williams DR, Dang L, Barlogie B, Shaughnessy JDJ, Jr, Kuehl WM, Staudt LM. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, Braggio E, Henry T, Zhu YX, Fogle H, Price-Troska T, Ahmann G, Mancini C, Brents LA, Kumar S, Greipp P, Dispenzieri A, Bryant B, Mulligan G, Bruhn L, Barrett M, Valdez R, Trent J, Stewart AK, Carpten J, Bergsagel PL. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushima A, Kaisho T, Rennert PD, Nakano H, Kurosawa K, Uchida D, Takeda K, Akira S, Matsumoto M. Essential role of nuclear factor (NF)-kappaB-inducing kinase and inhibitor of kappaB (IkappaB) kinase alpha in NF-kappaB activation through lymphotoxin beta receptor, but not through tumor necrosis factor receptor I. J Exp Med. 2001;193:631–636. doi: 10.1084/jem.193.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 28.Zarnegar B, Yamazaki S, He JQ, Cheng G. Control of canonical NF-kappaB activation through the NIK-IKK complex pathway. Proc Natl Acad Sci USA. 2008;105:3503–3508. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Lu Q, Bottero V, Estepa G, Morrison L, Mercurio F, Verma IM. Enhanced NF-kappaB activation and cellular function in macrophages lacking IkappaB kinase 1 (IKK1) Proc Natl Acad Sci USA. 2005;102:12425–12430. doi: 10.1073/pnas.0505997102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin X, Mu Y, Cunningham ETJ, Jr, Marcu KB, Geleziunas R, Greene WC. Molecular determinants of NF-kappaB-inducing kinase action. Mol Cell Biol. 1998;18:5899–5907. doi: 10.1128/mcb.18.10.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]