There are currently two fundamental questions facing cancer biologists, the first being the cell-of-origin of cancer, i.e. did the tumor in a specific patient arise from the transformation of stem cells or more differentiated progeny? Understanding the cellular etiology of cancer may help interpret the clinical manifestation of tumor phenotypes (e.g. those observed by pathologists) and stratify patients into appropriate treatment groups. The second question is whether cancer cells in a clinical tumor are functionally distinct and lineage related. The answer to this question holds clear clinical significance because, although the current targeted therapy and precision medicine emphasize corrupted molecular targets, it is the cancer cells that are being treated. If cancer cells are heterogeneous in expressing the therapeutic target(s), those that do not express the target(s) will not respond well or at all. In the past two decades, research has demonstrated that in most, if not all, human cancers, there exists a dynamically evolving population of cancer cells that possess at least some inherent properties of normal stem cells. These stem-like cancer cells, operationally dubbed as cancer stem cells (CSCs), are endowed with high tumor-regenerating and tumor-propagating capacities and, in many cases, have been shown to self-renew and generate more differentiated non-CSC progeny (Kreso & Dick, 2014). Recent lineage tracing studies in genetically-engineered mouse models (GEMMs) have provided further evidence for CSCs (Chen et al. 2012, Driessens et al. 2012, Schepers et al. 2012).
This thematic issue of ‘Stem Cells and Cancer’ is dedicated to breast and prostate cancers, which share many similarities (Risbriger et al. 2010). The mammary and prostatic glands are both organized primarily as two-layered, pseudostratified glandular structures with a differentiated luminal layer expressing steroid hormone receptors (ER, PR, AR, etc) and a basal or myoepithelial (for breast) cell layer expressing transcription factor p63 but not steroid hormone receptors. The tumors in both organs present mainly as a luminal phenotype in that most tumor cells express steroid hormone receptors (Fig. 1). Consequently, inhibition of steroid hormone signaling via blocking receptor function and hormone synthesis represents the major clinical intervention in both cancers. In this issue, five leading groups in the field discuss the cells of origin and CSCs in breast and prostate cancers, expounding on the potential clinical relevance.
Figure. 1.
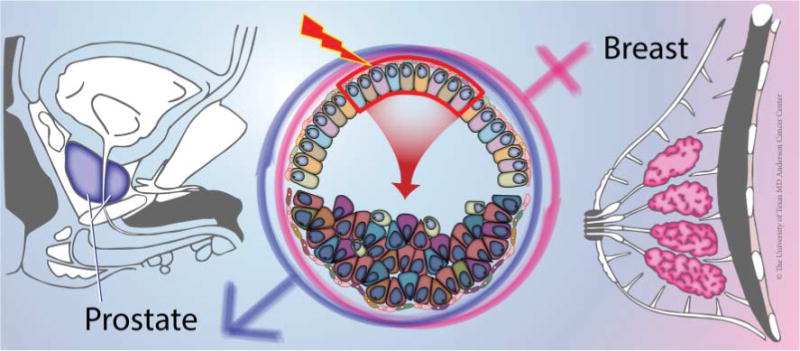
Both human prostate and breast are susceptible to tumorigenesis. The prostatic and mammary glands are two-layered secretory structures and recent lineage tracing studies in both organs suggest that luminal cells are preferred targets of tumorigenic transformation (middle, boxed area). The pile of cells below the arrow indicate disorganized cells in early hyperplastic lesions and different shapes, colors, and sizes signify tumor cell heterogeneity. We acknowledge Dr K Rycaj and Ms J Holcombe and Ms C Brown at the University of Texas MD Anderson Cancer Center in helping to make this figure.
Sreekumar et al. (2015) present a comprehensive, up-to-date, overview on the complex hierarchy of murine mammary stem cells (MaSCs). Multiple populations of adult MaSCs have been reported including CD29hi CD24+/CD49hi, s-SHIP+, label-retaining cells, CD1d+, Procr+, Axin2+, CD61+ and Lgr5+ cells. Lineage tracing studies show that the basal cell layer harbors both unipotent progenitors (K5+, K14+, αSMA+) and bipotent (some K5+, Procr+, and Axin2+) stem cells whereas luminal progenitor cells are unipotent as the K8+ luminal cells have never been observed to give rise to basal cells in vivo in the normal mammary gland. Basal MaSCs and most luminal progenitor cells are ERα−/PR−. Interestingly, in a basal-like breast cancer model induced by Brca1 loss, luminal progenitors are likely the cell of origin for the tumors, suggesting that luminal cells may be reprogrammed to undergo basal-like transdifferentiation by oncogenic insults.
Breast cancer is the first human solid tumor in which evidence for CSCs was presented (Al-Hajj et al. 2003). By now, multiple populations of breast cancer stem cells (BCSCs) have been reported including CD44+CD24− and ALDHhi populations as well as side population (SP) and label (e.g. PKH26) retaining cells. Simôes et al. (2015) focus their discussions on the role of steroid hormones in regulating BCSCs. As most of the reported BCSC populations, like their normal counterparts, are ER− and PR−, both estrogen and progesterone influence BCSC abundance and activities via indirect mechanisms. Depending on the experimental context, estrogen may either promote or diminish BCSC activity. Nevertheless, antiestrogen therapies seem to primarily target ER+ breast cancer cells leading to enrichment of dormant ER− BCSCs, which could become ‘reactivated’ to mediate endocrine therapy resistance and metastasis. Unlike estrogen, progesterone seems to clearly promote the expansion of BCSCs, suggesting that anti-progesterone drugs may find clinical use in targeting BCSCs. Although the underlying mechanisms remain obscure, the BCSC-promoting effects of progesterone are likely to be mediated via paracrine/juxtacrine signaling (Simôes et al. 2015).
The prostate, like the breast, is a hormonally regulated glandular organ with androgens mainly regulating the luminal epithelial cells. Strand & Goldstein (2015) review the literature pertaining to the relationship between basal and luminal cells as well as their involvement in both benign prostatic diseases (BPH) and prostate cancer. Although proliferation of basal/stem cells may contribute to BPH, primary prostate cancer notably shows a decrease or loss of basal cells. Transplantation assays identify a population of basal cells (i.e. CD49hi Trop2+) as the cell of origin for human prostate cancer whereas lineage-tracing studies implicate murine luminal cells as the preferred target of tumorigenic transformation. The reason underlying this discrepancy remains unclear. Shibata & Shen (2015) focus on stem cells and CSCs in GEMMs of prostate cancer. They present an up-to-date overview on various populations of murine prostate stem/progenitor cells and their potential lineage relationship during development, adult homeostasis, and regeneration. Importantly, GEMM studies have revealed the presence of castration-resistant stem/progenitor cells in the normal prostate that retain stem cell properties upon androgen deprivation. Finally, they discuss several CSC populations (e.g. LSChigh, CD166high, CD133+) reported in various GEM models of prostate cancer. Along these lines, they discussed limitations and caveats associated with current approaches in assaying CSCs using cell cultures and transplantations.
Multiple populations of CSCs in human prostate cancer (i.e. PCSCs) have also been reported (Rybak et al. 2014, Liu et al. 2015). Deng & Tang (2015) dissect the relationship between AR and PCSCs. They present abundant evidence for heterogeneity in AR expression in untreated primary, treatment-failed and castration-resistant, and metastatic tumors. The prominent AR heterogeneity raises the possibility that the AR− prostate cancer cells will not be responsive to the clinical antiandrogen therapies and may thus represent a cellular source of therapy resistance. Interestingly, nearly all reported PCSCs populations in untreated cultures, xenografts, and patient tumors are AR−, similar to the ER− phenotype of BCSCs. These AR− PCSCs have been associated with therapy resistance and disease recurrence. On the other hand, as most castration-resistant clinical tumors clearly harbor AR+ cancer cells and clones, the authors offered a provoking hypothesis that PCSCs in some recurrent tumor clones might be AR+.
Collectively, papers in this issue provide an up-to-date review on breast and prostate tumorigenesis from a stem cell perspective and offer fresh insight on not only the etiology of the two prevalent cancers but also developing novel therapeutics targeting heterogeneous cancer cell populations.
Acknowledgments
Funding
Work in the author’s lab was supported, in part, by grants from NIH (NCI R01-CA155693), DOD (W81XWH-13-1-0352 and W81XWH-14-1-0575), CPRIT (RP120380), and MDACC Center for Cancer Epigenetics.
Footnotes
This editorial accompanies a thematic review section on Stem Cells and Cancer. The guest editor for this section was Dean Tang, The University of Texas MD Anderson Cancer Center, Smithville, TX, USA.
Declaration of interest
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of this editorial.
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. PNAS. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Tang DG. Androgen receptor and prostate cancer stem cells: biological mechanisms and clinical implications. Endocrine-Related Cancer. 2015;22:T209–T220. doi: 10.1530/ERC-15-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Liu X, Chen X, Chen X, Rycaj K, Chao HP, Deng Q, Jeter C, Liu C, Honorio S, Li H, et al. Systematic dissection of phenotypic, functional, and tumorigenic heterogeneity of human prostate cancer cells. Oncotarget. 2015;6:23959–23986. doi: 10.18632/oncotarget.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbriger GP, Davis ID, Birrell SN, Tilley WD. Breast and prostate cancer: more similar than different. Nature Reviews Cancer. 2010;10:205–212. doi: 10.1038/nrc2795. [DOI] [PubMed] [Google Scholar]
- Rybak AP, Bristow RG, Kapoor A. Prostate cancer stem cells: deciphering the origins and pathways involved in prostate tumorigenesis and aggression. Oncotarget. 2014;6:1900–1919. doi: 10.18632/oncotarget.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- Shibata M, Shen MM. Stem cells in genetically-engineered models of prostate cancer. Endocrine-Related Cancer. 2015;22:T199–T208. doi: 10.1530/ERC-15-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simôes BM, Alferez D, Howell S, Clarke RB. The role of steroid hormones in breast cancer. Endocrine-Related Cancer. 2015;22:T177–T186. doi: 10.1530/ERC-15-0350. [DOI] [PubMed] [Google Scholar]
- Sreekumar A, Roarty K, Rosen JM. The mammary stem cell hierarchy: a looking glass into heterogeneous breat cancer landscapes. Endocrine-Related Cancer. 2015;22:T161–T176. doi: 10.1530/ERC-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand DW, Goldstein AS. The many ways to make a luminal cell and a prostate cancer cell. Endocrine-Related Cancer. 2015;22:T187–T197. doi: 10.1530/ERC-15-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]


