Abstract
Thalassemia is a congenital blood disorder often requiring chronic blood transfusions and iron chelation therapy [1,2]. While advances in treatment have resulted in increased life expectancy [3], extended life spans have exposed previously unidentified issues, including bodily pain. The aim of this study was to examine the prevalence, severity, predictors, and effects of pain in 265 adults/adolescents and 103 children with thalassemia. Overall, 69% of adults/adolescents reported bodily pain on the SF-36v2 health survey, with 28% reporting at least moderate pain. Parents reported pain in 56% of children using the PF-28 child health questionnaire, with only 11% reporting pain fairly often. There were no significant differences in pain in children with thalassemia compared with the general population. In adults/adolescents, pain increased significantly with age (P = 0.005), more so than in the general population. This study highlights the fact that children and young adults with thalassemia experience pain comparable to the general population, whereas older adults (aged 35+) experience greater pain. Our findings show that increased pain is associated with decreased quality of life and increased anxiety and depression.
A recent review of the literature revealed no comprehensive assessments of pain in the thalassemia population, although some studies included specific types of pain, pain treatment, or pain as a component of general quality of life (QOL) [4–15]. The Thalassemia Clinical Research Network (TCRN) is an NIH/NHLBI-funded network composed of five core thalassemia centers in North America, one in London and their associated satellite sites. To date, the TCRN Low Bone Mass Cross-sectional Observational Study (LBMCOS) has published data on bone and joint pain, with 34% of participants reporting pain [12]. We expand on these findings using further data from the LBMCOS study, along with recently collected baseline data from the Thalassemia Longitudinal Cohort (TLC).
LBMCOS Study
Data were collected from 371 patients (aged 6–75, mean 22.9 ± 12.1 years, 49% male, 65% β-thal major). One hundred twenty-seven (34%) reported bone or joint pain in the last 30 days that was not related to a specific trauma or injury. These patients reported feeling pain on average 3 days a week, 7.5 hr per day, in their worst week that month. The most frequent site of bone/joint pain was the back (24%), followed by knees (15%) and head/neck (10%). Eight percent of participants experienced pain in their shoulders, hips, ankles, and/or legs. Up to 5% of participants reported pain in their hands, wrists, elbows, arms, feet, and/or ribs. Forty-four participants had received transfusions while experiencing pain and 34% reported that the transfusions helped reduce or eliminate the pain.
TLC Study
Data were collected from 265 adults/adolescents (29.4 ± 10.4 years, range 14–58, 52% female, 77% β-thal major) and 103 children (9.8 ± 2.6 years, range 5–14, 52% female, 71% β-thal major) (Table I). Overall, 69% of adults/adolescents reported bodily pain in the past 4 weeks, with 28% reporting at least moderate pain. Fifty percent reported that pain interfered with their work (both inside and outside of the home) in the last 4 weeks, with 25% reporting at least moderate interference. As expected, pain severity and interference were highly correlated (Spearman correlation = 0.82; P < 0.001). Parents reported pain in 56% of children, with 11% reporting pain fairly often in the last 4 weeks.
TABLE I.
Baseline Demographics and Pain Dataa for the Thalassemia Clinical Research Network (TCRN) Thalassemia Longitudinal Cohort (TLC)
| Aged 14+ yearsb N = 265 |
Aged <14 yearsc N = 103 |
|
|---|---|---|
| Age (years) | 29.4 (10.4), 14.2–58.3 | 9.8 (2.6), 5.0–14.1 |
| Sex | ||
| Male | 128 (48.3%) | 49 (47.6%) |
| Female | 137 (51.7%) | 54 (52.4%) |
| Thalassemia diagnosis | ||
| B-thal transfused 8+ | 204 (77.0%) | 73 (70.9%) |
| B-thal transfused <8 | 24 (9.1%) | 3 (2.9%) |
| B-thal nontransfused | 2 (0.8%) | 2 (1.9%) |
| HbH | 0 (0.0%) | 4 (3.9%) |
| HbH CS | 4 (1.5%) | 3 (2.9%) |
| E-B-thal transfused 8+ | 21 (7.9%) | 10 (9.7%) |
| E-B-thal transfused <8 | 6 (2.3%) | 2 (1.9%) |
| E-B-thal nontransfused | 1 (0.4%) | 0 (0.0%) |
| alpha-thal | 2 (0.8%) | 3 (2.9%) |
| Other/missing | 1 (0.4%) | 3 (2.9%) |
| Country | ||
| US | 194 (73.2%) | 76 (73.8%) |
| Canada | 36 (13.6%) | 27 (26.2%) |
| UK | 35 (13.2%) | 0 (0.00%) |
| Hemoglobind (g/dL) | 10.0 (1.1), 5.0–12.9 | 9.5 (0.9), 6.4–11.2 |
| Bone mineral density z-scoree | ||
| Spine | −1.8 (1.7), −6.9–4.7 | −1.0 (1.3), −3.0–2.6 |
| Hip | −1.3 (1.2), −3.7–2.3 | −1.0 (1.3), −2.6–1.0 |
| Whole body | −0.5 (2.0), −3.7–3.5 | −1.5 (1.7), −3.2–1.5 |
| Pain severityf | ||
| None | 81 (30.6%) | |
| Very mild | 65 (24.5%) | |
| Mild | 46 (17.4%) | |
| Moderate | 40 (15.1%) | |
| Severe | 25 (9.4%) | |
| Very severe | 8 (3.0%) | |
| Pain interferenceg | ||
| Not at all | 133 (50.2%) | |
| A little bit | 65 (24.5%) | |
| Moderately | 29 (10.9%) | |
| Quite a bit | 34 (12.8%) | |
| Extremely | 4 (1.5%) | |
| Pain incidence in past 4 weeksh | ||
| None of the time | 45 (43.7%) | |
| Once or twice | 32 (31.1%) | |
| A few times | 15 (14.6%) | |
| Fairly often | 6 (5.8%) | |
| Very often, every/almost every day | 5 (4.9%) |
Mean (SD), range for continuous variables; N (%) for categorical variables.
Completed the self-report SF-36v2 quality of life health survey.
Completed the parent-report PF-28 child health questionnaire (CHQ).
Pretransfusion, for transfused patients only. N = 239 aged 14+ and N = 92 aged <14.
For age 14+, N = 222 for spine, 136 for hip, and 41 for whole body; for age <14, N = 36 for spine, 11 for hip, and 14 for whole body.
“How much bodily pain have you had during the past 4 weeks?” (SF36v2 question 8).
“During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)” (SF36v2 question 9).
“During the past 4 weeks, how often has your child had bodily pain or discomfort” (CHQ PF28 question 4.1).
Pain severity increases with age (Table II), but does not vary significantly with sex or thalassemia diagnosis. In analysis of the SF-36 bodily pain scale, which combines both pain severity and interference, age was a significant independent predictor of pain, with a decrease in quality of life due to pain with age (slope = −20.26 ± 0.09/year; P = 0.005). Quality of life due to pain in thalassemia declines greatly with age, compared with only a slight decline in the general population (Fig. 1A). Z-scores of pain in U.S. patients in relation to the normal population decrease significantly with age (P = 0.042), with a noticeable gap by age 35. In children, there were no significant differences (Fig. 1B); however, quality of life due to pain incidence decreased nonsignificantly with age in children (slope = −1.01 ± 0.93/year; P = 0.28). There was no association between pain and gender, thalassemia diagnosis, bone density, pretransfusion hemoglobin, vitamin D, or chelator choice in children.
TABLE II.
| Pain severity: Adults
|
||||||
|---|---|---|---|---|---|---|
| None | Very mild | Mild | Moderate | Severe | Very severe | |
| Diagnosis | ||||||
| B-thal major | 62 (30.4%) | 53 (26.0%) | 32 (15.7%) | 34 (16.7%) | 17 (8.3%) | 6 (2.9%) |
| B-thal intermedia | 6 (23.1%) | 5 (19.2%) | 6 (23.1%) | 4 (15.4%) | 4 (15.4%) | 1 (3.9%) |
| Sex | ||||||
| Male | 40 (31.3%) | 40 (31.3%) | 17 (13.3%) | 18 (14.1%) | 11 (8.6%) | 2 (1.6%) |
| Female | 41 (29.9%) | 25 (18.3%) | 29 (21.2%) | 22 (16.1%) | 14 (10.2%) | 6 (4.4%) |
| Age | ||||||
| 14–17 years | 17 (47.2%) | 12 (33.3%) | 4 (11.1%) | 0 (0.0%) | 3 (8.3%) | 0 (0.0%) |
| 18–24 years | 25 (32.9%) | 20 (26.3%) | 15 (19.7%) | 9 (11.8%) | 7 (9.2%) | 0 (0.0%) |
| 25–34 years | 22 (31.0%) | 16 (22.5%) | 14 (19.7%) | 11 (15.5%) | 4 (5.6%) | 4 (5.6%) |
| 35–44 years | 14 (23.7%) | 15 (25.4%) | 6 (10.2%) | 13 (22.0%) | 9 (15.3%) | 2 (3.4%) |
| 45–54 years | 3 (15.0%) | 2 (10.0%) | 6 (30.0%) | 6 (30.0%) | 1 (5.0%) | 2 (10.0%) |
| 55–64 years | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | 0 (0.0%) |
|
| ||||||
| Pain severity: Children
|
||||||
| None of the time | Once or twice | A few times | Fairly often | Very often | ||
|
| ||||||
| Diagnosis | ||||||
| B-thal major | 30 (41.1%) | 25 (34.3%) | 12 (16.4%) | 4 (5.5%) | 2 (2.7%) | |
| B-thal intermedia | 1 (20.0%) | 2 (40.0%) | 1 (20.0%) | 1 (20.0%) | 0 (0.0%) | |
| Sex | ||||||
| Male | 20 (40.8%) | 15 (30.6%) | 9 (18.4%) | 3 (6.1%) | 2 (4.1%) | |
| Female | 25 (46.3%) | 17 (31.5%) | 6 (11.1%) | 3 (5.6%) | 3 (5.6%) | |
| Age | ||||||
| 5–7 years | 14 (48.3%) | 8 (27.6%) | 4 (13.8%) | 1 (3.5%) | 2 (6.9%) | |
| 8–10 years | 18 (50.0%) | 11 (30.6%) | 4 (11.1%) | 2 (5.6%) | 1 (2.8%) | |
| 11–12 years | 9 (40.9%) | 6 (27.3%) | 4 (18.2%) | 2 (9.1%) | 1 (4.6%) | |
| 13–14 years | 4 (25.0%) | 7 (43.8%) | 3 (18.8%) | 1 (6.3%) | 1 (6.3%) | |
Pain severity as self-reported on the SF-36v2 quality of life health survey by participants aged 14+ years.
Pain severity as parent-reported on the PF-28 child health questionnaire (CHQ) for participants <14 years.
Figure 1.
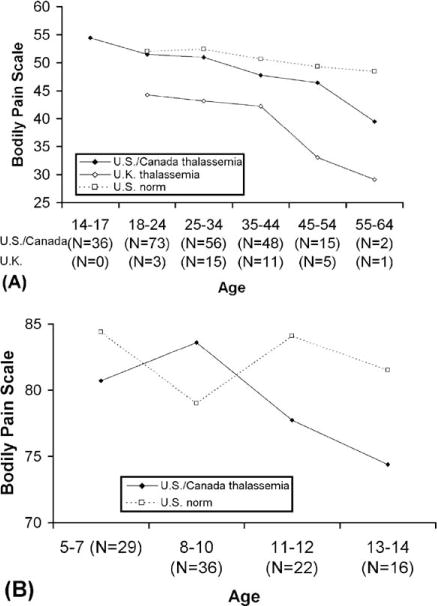
Bodily pain: thalassemia cohort by country versus U.S. norms. (A) Adults (Bodily pain (BP) scale of the SF-36v2 quality of life health survey compared with U.S. adult norms [16]. Higher scores indicate higher QOL (i.e., less pain). There are no normed data available for age <18.). (B) Children (Bodily pain (BP) scale of the PF-28 child health questionnaire (CHQ) compared with U.S. pediatric norms [17]. Higher scores indicate higher QOL (i.e., less pain).). As norms differ by culture, only thalassemia patients in North America are compared with U.S. norms; age trends are similar for patients in the U.K., but scores are consistently lower than in the North American group.
Clinically, in adults, lower bone density was marginally significantly correlated with increased pain (r = 0.29 for whole body, P = 0.065; r = 0.15 for hip, P = 0.079; r = 0.11 for spine, P = 0.095), as was use of bisphosphonates (P = 0.14). Pretransfusion hemoglobin was not correlated with pain (r = −20.06, P = 0.33). Hormone replacement therapy and 1,25 vitamin D were not associated with pain, but low 25 vitamin D was associated with increased pain (P = 0.010). There was a marginally significant effect of chelator, with higher pain associated with use of deferoxamine compared with deferasirox (P = 0.11), with a slight decrease in pain with increasing dose of deferasirox (P = 0.074).
In adults and adolescents, bodily pain is significantly correlated with decreased quality of life in all domains measured by the SF-36 (physical functioning, role-physical, general health, vitality, social functioning, role-emotional, and mental health). Pain shows a higher correlation with the physical component summary (r = 0.78, P < 0.001) than the mental component summary (r = 0.33, P < 0.001) (Fig. 2A). Increased bodily pain is also significantly correlated with increased anxiety and depression (r = −0.37 for anxiety, r = −0.36 for depression, P < 0.001 for both) (Fig. 2B). In children, the bodily pain scale is significantly correlated with the physical functioning, mental health, general health, and family cohesion scales, but not the role-emotional behavior, role-physical behavior, self esteem, parental impact-emotional, parent impact-time, or family activities scales. Pain was significantly correlated with the physical summary score (r = 0.53, P < 0.001), but not the psychosocial summary score (r = 0.12, P = 0.24) (Fig. 2C).
Figure 2.
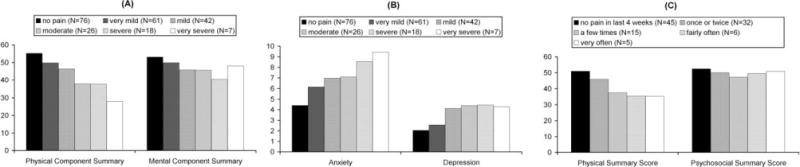
Quality of Life by pain severity (excludes patients from the U.K. because of significant country effects on these scales; however, U.K. patients show similar trends). (A) PCS and MCS summary scales of the SF-36 in adults/adolescents (Physical Component Summary and Mental Component Summary scales of the SF-36v2 health survey administered to participants aged 14+ years. Higher scores indicate higher QOL.). (B) Anxiety and Depression in adults/adolescents (Hospital Anxiety and Depression Scale (HADS) administered to participants aged 141 years. Higher scores indicate higher levels of anxiety and depression.). (C) PhS and PsS summary scales of the CHQ in children (Physical Summary and Psychosocial Summary scales of the PF-28 child health questionnaire (CHQ) for participants <14 years. Higher scores indicate higher QOL.).
This study had several advantages compared with previous studies of pain in thalassemia. First, we were able to assess bodily pain in a large sample of adults and children of varying ages with thalassemia. Second, because we used validated and well-studied quality of life instruments, we were able to compare our findings to the general U.S. population of varying ages. We were also able to study the impact of bodily pain on quality of life and mood using these validated instruments.
This study identified 69% of adults/adolescents reporting bodily pain in the past 4 weeks, which is consistent with the 62% reporting any pain in the Scalone study [18]. This study also found 28% of adults/adolescents experiencing at least moderate pain. This is consistent with the Pakbaz [9] study, which found moderate pain in 21% of transfusion-dependent and independent patients, though unlike this study, found decreased prevalence of severe pain in transfusion-independent patients. The high prevalence of pain in the thal intermedia patients in this study is likely due to the eligibility criteria requiring patients who need at least annual monitoring for end-organ injury. This study also expanded upon the Vogiatzi [12] study, which found bone and/or joint pain in 34% of participants, compared with our finding of any bodily pain in 68% of participants. Thus, further study of causes and locations of pain in addition to bone/joint pain are warranted.
Parents reported frequent pain in only 11% of children. This is in comparison to impaired QOL due to pain/discomfort in 64% of children in the Shaligram [10] study. This sharp difference is likely due to differences in quality of life instruments. The EQ5D used by Shaligram asks parents (as a proxy) to assess their child’s pain or discomfort (none, moderate, or extreme), whereas the CHQ asks how often does the child have bodily pain or discomfort. Importantly, our CHQ PF28 data found some pain in 58% of children.
Consistent with the Vogiatzi [12] study of bone/joint pain, we found increased prevalence of pain with age. Young adults with thalassemia experienced pain comparable to the general population, whereas older adults (aged 351) experienced greater pain. This may explain why impaired QOL due to bodily pain has not been consistently observed [13–15,18]. Given the association of pain with low vitamin D level, care should be taken to ensure adequate vitamin D intake. Additionally, there was a trend toward increased pain with lower bone density and for patients on bisphsphonates, suggestive of a possible relationship in a subset of patients. Since there have been no previous studies of pain in thalassemia, the possible sources of pain are not clearly apparent. Some possible mechanisms of pain in thalassemia include the following: compression fractures, pathologic fractures, and impingement on nerve roots by extra medullary hematopoietic masses. While case studies of these pathophysiologic mechanisms have been discussed in standard textbooks, there has been no systematic review of the prevalence within this population. Patients with osteopenia and osteoporosis often complain of pain secondary to fractures, particularly back pain associated with compression fractures of the vertebrae.
Thalassemia patients as they age frequently complain of “throbbing back pain” in the week before transfusion. Practitioners generally assume that this is due to bone marrow “pressure” from increased marrow activity which is subsequently suppressed when the patient is transfused. This assumption is supported by our finding that 34% of participants reported improvement of pain with transfusion. Marrow expansion could also be a source of pain.
As expected, pain impacts negatively on quality of life, affecting both physical functioning and mental health, including increased reports of anxiety and depression. Increased pain was observed with use of deferoxamine compared with deferasirox, which may be due to the route of administration. This association may also reflect patient/physician preferences in chelator choice and is complicated by the fact that many patients have switched chelators in recent years, some multiple times.
As responses to the instruments used may vary by culture, evidenced by the large difference observed between patients in North America and the U.K., comparison across cultures is not possible. Another study limitation is that we have only assessed pain at one time point, although longitudinal data collection is ongoing. Furthermore, this study was not adequately powered to detect differences in levels of pain by thalassemia diagnosis, as the majority of participants were β-thal major. Given the emergence of pain as an issue in older thalassemia patients, further longitudinal study of both transfused and nontransfused patients would be advantageous to discern timing, location, and causes of pain in thalassemia.
Methods
The TCRN LBMCOS and TLC protocols were approved by the TCRN Data and Safety Monitoring Board and by the ethical review boards of all TCRN institutions. Informed consent, and assent in the case of a minor, was obtained.
The LMBCOS study recruited TCRN patients of all thalassemia syndromes, age 6 and older, excluding pregnancy and pre-existing medical conditions known to require chronic systemic administration of steroids. Data on bone and joint pain was collected by patient interview.
Eligibility for the TLC study included patients of all thalassemia syndromes who required at least annual monitoring for end-organ injury related to thalassemia. This manuscript reports baseline data from this ongoing study. Patients with a prior successful hematopoietic stem cell transplant (N = 12) were excluded from analysis. At baseline, participants aged 14 and older were asked to complete the SF-36v2 health survey [16] and the hospital anxiety and depression scale (HADS) [19]. Parents of children <14 years at baseline were asked to complete the PF-28 child health questionnaire (CHQ) [17]. The SF-36v2 and CHQ both include core pain assessments. For all participants, current medications, chelation regimen, most recent pretransfusion hemoglobin, and bone mineral density assessed by dual-energy X-ray absorptiometry (DXA) were recorded from chart review. Urine and serum samples from each participant were stored at −80°C and analyzed as a batch at a central facility. 25-hydroxyvitamin D was measured by competitive radioimmunoassay following extraction and 1,25-dihydroxyvitamin D by column chromatography and radioimmunoassay.
Statistical Analysis
Frequencies were calculated for all pain measures, both overall and by age groups, sex, and thalassemia diagnosis. Age groups were chosen to be consistent with those reported in the SF-36v2 and CHQ manuals [16,17] for the normal population. Betathal major was defined as β-thalassemia requiring at least eight transfusions in the last year, whereas β-thal intermedia were homozygous or compound heterozygous patients requiring fewer than eight transfusions in the last year.
The bodily pain scales of the SF-36 and CHQ were scored and responses compared with the “normed” general adult and pediatric populations [16,17] by computation of z-scores based on normed means and standard deviations. Regression analysis of z-scores on age, controlling for gender, were used to assess differences between pain in U.S. thalassemia patients and U.S. norms over age.
Correlation analysis and linear and ordinal logistic regression were used to model predictors of pain. Predictors significant in initial analysis, controlling for age, sex, and country, were entered into multivariate models. Potential predictors included age, sex, country, thalassemia diagnosis, regular transfusion (y/n), bone density, pretransfusion hemoglobin level, vitamin D level, hormone replacement therapy, bisphosphonate use, and chelator choice/dose. Partial correlation, controlling for age and sex, was used to assess the effect of pain (bodily pain scale of the SF-36 and CHQ) on quality of life (all other SF-36 and CHQ scales) and anxiety and depression (HADS scales).
Acknowledgments
Contract grant sponsor: NIH-NHLBI Cooperative Agreement; Contract grant number: U01 HL065238
Footnotes
This is publication number 12 of the Thalassemia Clinical Research Network (TCRN). The following institutions and researchers contributed to the Thalassemia Clinical Research Network Thalassemia Longitudinal Cohort data reported in this article.
Children’s Hospital, Boston (N = 38): Ellis Neufeld, MD, PhD, Principal Investigator, Jennifer Braunstein, NP, Research Nurse, Amber Smith, Study Coordinator, Latoya Lashley, Study Coordinator; Satellite: University of Texas Southwestern Medical Center at Dallas (N = 12), Charles Quinn, MD, MS, Principal Investigator, Deborah Boger, RN, MSN, PNP, Study Coordinator, Leah Adix, Study Coordinator, Sandra Richardson, Study Coordinator; Children’s Healthcare of Atlanta (N = 16), Jeanne Boudreaux, MD, Principal Investigator, Leann Hassen, Study Coordinator; Baylor College of Medicine (N = 6), Brigitta Mueller, MD, Principal Investigator, Bogden Dino, Study Coordinator. Weill Medical College of Cornell University (N = 59): Patricia Giardina, MD, Principal Investigator, Elizabeth Evans, Study Coordinator; Satellite: Winthrop University Hospital (N = 6), Mark Weinblatt, MD, Principal Investigator, Linda Skelly, Study Coordinator. The Children’s Hospital of Philadelphia (N = 59): Janet Kwiatkowski, MD, Principal Investigator, Marie Martin, RN, Research Nurse, Owen Beams, Study Coordinator; Satellite: Children’s Memorial Hospital, Chicago, IL (N = 39), Alexis Thompson, MD, Principal Investigator, Janice Beatty, RN, Research Nurse, Tiffany Drinkwater, Study Coordinator. Children’s Hospital at Oakland (N = 52): Elliott Vichinsky, MD, Principal Investigator, Dru Foote, NP, Research Nurse, Nancy Sweeters, Study Coordinator, Olivia Vega, Study Coordinator; Satellites: Children’s Hospital of Los Angeles (N = 12), Thomas Coates, MD, Principal Investigator, Susan Carson, RN, Research Nurse, Eun Ha Pang, Study Coordinator, Rachna Khanna, Study Coordinator; Stanford Hospital (N = 5), Michael Jeng, MD, Principal Investigator, Kokil Bakshi, Clinical Research Associate; Children’s and Women’s Health Center of British Columbia (N = 4), John Wu, Principal Investigator, Heather McCartney, RN, Research Nurse, Colleen Fitzgerald, Study Coordinator, Stephanie Badour, Study Coordinator. Toronto General Hospital, Toronto, Ontario, Canada (N = 5): Nancy F. Olivieri, MD, Principal Investigator, Vivek Thayalasuthan, Study Coordinator; Satellite: Hospital for Sick Children (N = 64), Isaac Odame, MD, Principal Investigator, Manuela Merelles-Pulcini, RN, Study Coordinator. University College London (N = 15), John Porter, MD, Principal Investigator, Cindy Bhagwandin, Study Coordinator; Satellite: Whittington Hospital (N = 24), Farrukh Shah, MD, Principal Investigator. NHLBI oversight, Kathryn Hassell, MD. Data Coordinating Center: New England Research Institutes, Sonja McKinlay, PhD, Principal Investigator, Lisa Virzi, RN, MS, MBA, Project Director, Felicia Trachtenberg, PhD, Senior Statistician.
Conflict of interest: Nothing to report.
References
- 1.Forget B, Cohen A. Thalassemia syndromes. In: Hoffman R, Benz E, Shattil S, editors. Hematology: Basic Principles and Practices. 4th. Philadelphia, PA: Elsevier, Churchill, Livingstone; 2005. pp. 557–598. [Google Scholar]
- 2.Cohen AR, Galanello R, Pennell DJ, et al. Thalassemia. Hematology Am Soc Hematol Educ Program. 2004:14–34. doi: 10.1182/asheducation-2004.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Modell B, Khan M, Darlison M, et al. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:42. doi: 10.1186/1532-429X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desigan S, Hall-Craggs MA, Ho CP, et al. Degenerative disc disease as a cause of back pain in the thalassaemic population: A case-control study using MRI and plain radiographs. Skeletal Radiol. 2006;35:95–102. doi: 10.1007/s00256-005-0020-1. [DOI] [PubMed] [Google Scholar]
- 5.Fucharoen S, Ketvichit P, Pootrakul P, et al. Clinical manifestation of beta-thalassemia/hemoglobin E disease. J Pediatr Hematol Oncol. 2000;22:552–557. doi: 10.1097/00043426-200011000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Munn RK, Kramer CA, Arnold SM. Spinal cord compression due to extramedullary hematopoiesis in beta-thalassemia intermedia. Int J Radiat Oncol Biol Phys. 1998;42:607–609. doi: 10.1016/s0360-3016(98)00245-4. [DOI] [PubMed] [Google Scholar]
- 7.Onur O, Sivri A, Gumruk F, Altay C. Beta thalassaemia: A report of 20 children. Clin Rheumatol. 1999;18:42–44. doi: 10.1007/s100670050050. [DOI] [PubMed] [Google Scholar]
- 8.Otrock ZK, Azar ST, Shamseddeen WA, et al. Intravenous zoledronic acid treatment in thalassemia-induced osteoporosis: Results of a phase II clinical trial. Ann Hematol. 2006;85:605–609. doi: 10.1007/s00277-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 9.Pakbaz Z, Treadwell M, Yamashita R, et al. Quality of life in patients with thalassemia intermedia compared to thalassemia major. Ann NY Acad Sci. 2005;1054:457–461. doi: 10.1196/annals.1345.059. [DOI] [PubMed] [Google Scholar]
- 10.Shaligram D, Girimaji SC, Chaturvedi SK. Psychological problems and quality of life in children with thalassemia. Indian J Pediatr. 2007;74:727–730. doi: 10.1007/s12098-007-0127-6. [DOI] [PubMed] [Google Scholar]
- 11.Vichinsky EP. The morbidity of bone disease in thalassemia. Ann NY Acad Sci. 1998;850:344–348. doi: 10.1111/j.1749-6632.1998.tb10491.x. [DOI] [PubMed] [Google Scholar]
- 12.Vogiatzi MG, Macklin EA, Fung EB, et al. Bone disease in thalassemia: A frequent and still unresolved problem. J Bone Miner Res. 2009;24:543–557. doi: 10.1359/jbmr.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messina G, Colombo E, Cassinerio E, et al. Psychosocial aspects and psychiatric disorders in young adult with thalassemia major. Intern Emerg Med. 2008;3:339–343. doi: 10.1007/s11739-008-0166-7. [DOI] [PubMed] [Google Scholar]
- 14.Payne KA, Desrosiers MP, Caro JJ, et al. Clinical and economic burden of infused iron chelation therapy in the United States. Transfusion. 2007;47:1820–1829. doi: 10.1111/j.1537-2995.2007.01398.x. [DOI] [PubMed] [Google Scholar]
- 15.Payne KA, Rofail D, Baladi JF, et al. Iron chelation therapy: Clinical effectiveness, economic burden and quality of life in patients with iron overload. Adv Ther. 2008;25:725–742. doi: 10.1007/s12325-008-0085-z. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE, Kosinski M, Bjorner BB, et al., editors. User’s Manual for the SF-36v2 Health Survey. 2nd. Lincoln, RI: Quality Metric Incorporated; 2007. [Google Scholar]
- 17.Landgraf JM, Abetz L, Ware JE, editors. Child Health Questionnaire (CHQ): A User’s Manual, Third Printing. Lincoln, RI: Quality Metric Incorporated; 2006. [Google Scholar]
- 18.Scalone L, Mantovani LG, Krol M, et al. Costs, quality of life, treatment satisfaction and compliance in patients with beta-thalassemia major undergoing iron chelation therapy: The ITHACA study. Curr Med Res Opin. 2008;24:1905–1917. doi: 10.1185/03007990802160834. [DOI] [PubMed] [Google Scholar]
- 19.Snaith R, Zigmond A, editors. The Hospital Anxiety and Depression Scale Manual. London, UK: nferNelson; 1994. [Google Scholar]


