Summary
Sex differences in behavior are widespread and often caused by hormonal differences between the sexes. In addition to hormones, the composition and numbers of the sex chromosomes also affect a variety of sex differences. In humans, X-chromosome genes are implicated in neurobehavioral disorders (i.e. fragile-X, autism). To investigate the role of X-chromosome genes in social behavior, we used a mouse model that has atypical sex chromosome configurations resembling Turner (45, XO) and Klinefelter syndromes (47, XXY). We examined a number of behaviors in juvenile mice. Mice with only one copy of most X-chromosome genes, regardless of gonadal sex, were less social in dyadic interaction and social preference tasks. In the elevated plus maze, mice with one X-chromosome spent less time in the distal ends of the open arms as compared to mice with two copies of X-chromosome genes. Using qRTPCR, we noted that amygdala from female mice with one X-chromosome had higher expression levels of vasopressin (Avp) as compared to mice in the other groups. Finally, in plasma from girls with Turner syndrome we detected reduced vasopressin (AVP) concentrations as compared to control patients. These novel findings link sex chromosome genes with social behavior via concentrations of AVP in brain, adding to our understanding of sex differences in neurobehavioral disorders.
Keywords: Turner syndrome, Klinefelter syndrome, Sexual differentiation, Sex differences, Social behavior, Anxiety, Social anxiety
1. Introduction
Sex differences in behavior have been attributed to differences in hormone levels between males and females (Arnold, 2009). In addition, sex chromosome complement correlates with differences in the brain and behavior in mice (reviewed in Cox et al., 2014). The basic mechanisms that cause sex differences are important to study and, more broadly, can also help us understand neurobehavioral diseases, many of which are sexually dimorphic in their incidence, severity, and/or timing of onset. Autism spectrum disorders (ASD) are one example of such gender dimorphism in human disease, as ASD is diagnosed five times more commonly in males than in females (Giarelli et al., 2010). Several sex chromosome genes, particularly on the X-chromosome, are implicated in neurobehavioral disorders (Raymond, 2006) and patients with Turner (45, XO) and Klinefelter syndrome (47, XXY), two of the most common sex chromosome aneuploidies, have a higher incidence of psychological dysfunction and autism than the general population (Lesniak-Karpiak et al., 2003; Russell et al., 2006; van Rijn et al., 2006).
Here we used a mouse model with atypical sex chromosome configurations to assess the contribution of X-chromosome gene copy number to juvenile social and anxiety behaviors. These mice (Eicher et al., 1991; Burgoyne et al., 1998; Cox et al., 2014) produce offspring with four sex chromosome genotypes: XY males, XXY males, XO females, and XX females (hereafter referred to as 1X males, 2X males, 1X females, and 2X females; Table 1). In order to control for the hormonal differences seen in adult mice, prepubertal mice were tested for social and anxiety behavior. The mRNA of candidate genes, including several that escape X-inactivation and which are typically expressed 1.5-fold higher in XX females as compare with XY males (Berletch et al., 2011), was quantified in the amygdala, a brain region involved in emotional, social and anxiety behaviors in rodents (Veenema and Neumann, 2008) and humans (Adolphs, 2010). Finally, to check for correlations between vasopressin and the XO genotype in young girls, we measured vasopressin (AVP) in plasma from Turner syndrome patients and controls.
Table 1.
Offspring produced and tested.
| Genotype | Model for | Gonadal sex | Copy of non-PAR X Genes | Copy of non-PAR Y Genes | Nomenclature |
|---|---|---|---|---|---|
| XY* | XY | Male | 1 | 1 | 1X male |
| XXY* | XXY (Klinefelter syndrome) | Male | 2 | 1 | 2X male |
| XY*X | XO (Turner syndrome) | Female | 1 | 0 | 1X female |
| XX | XX | Female | 2 | 0 | 2X female |
Details on the genotypes produced and tested in these studies. In the genotypes column, the first X-chromosome listed, by convention, is maternally inherited, while the second is inherited from the father. The offspring have either 1 or 2 copies of non-pseudoautosomal (non-PAR) X-chromosome genes, with gonadal males possessing a Y* or XY* chromosome.
2. Materials and methods
2.1. Animals
The Y* strain used for all studies originated from males with a mutation in the pseudoautosomal region of the Y-chromosome that resulted in a neocentromere and misalignment with the X-chromosome during meiosis. Mating these males with wild-type females produces offspring of both sexes with either 1 or 2 copies of X-chromosomes (Table 1) (Eicher et al., 1991; Burgoyne et al., 1998; Cox et al., 2014). Our Y* colony was established with XY* males (in the 6JEiJ substrain) and C57BL/6J (B6) females purchased from The Jackson Laboratory (Bar Harbor, ME; stock numbers 002021 and 000924), and maintained in the B6 sub-strain. To genotype the mice, RNA was isolated from tail clippings as previously described (Wolstenholme et al., 2013b) and reverse transcribed into cDNA. Xist mRNA was measured and normalized to Gapdh as an indication for the number of X chromosomes present for mice of each sex. The primers used for Xist genotyping were 5′-TAAGGACTACTTAACGGGCT-3′ (forward) and 5′-TACTCAGACATTCCCTGGCA-3′ (reverse), while the primers for Gapdh were 5′-ACCACAGTCCATGCCATCAC-3′ (forward) and 5′-TCCACCACCCTGTTGCTGTA-3′ (reverse).
Mice were bred and maintained at the University of Virginia School of Medicine, Jordan Hall Animal Facility, and all procedures were conducted in compliance with the University of Virginia Animal Use and Care Committee. Mice were maintained on a 12:12 light/dark cycle (lights off at 1800 EST) and food (Harlan Teklad no. 7912) and water were provided ad libitum. Mice were reared with both parents and left largely unhandled (excluding routine cage changes) until postnatal day (PN) 20. We chose to begin testing animals on PN21 to assure that the mice remained prepubertal throughout the testing series, thus avoiding the potential confounds of hormone differences. One cohort was used for the dyadic social interaction and preference tests, but separate cohorts were used for each of the other behavior tests, the physiological measurements, and the gene expression analysis. In total, 51 litters were used, with animals from at least 5 different litters used in each study to reduce litter effects.
2.2. General behavior testing procedures
All behavior tests, except social recognition, were conducted in the dark, approximately 1 h after lights off (1900 EST), under red-light illumination, videotaped, and scored using Noldus Observer (5.0) software (Noldus, Leesburg, VA, USA). The social recognition task was scored live in the light (between 1000 and 1400 EST). All tests were scored by an observer (KHC), blind to both the sex and genotype of the test subjects.
2.3. Dyadic social interaction test
Mice were tested for social interactions using a protocol described previously (McFarlane et al., 2008; Cox and Rissman, 2011). Briefly, PN20 mice (N = 10–12 per group) from different litters were organized into same-sex, same-genotype pairs, with one member marked with black marker on its tail to allow us to discriminate between the two mice. Behavior data were recorded from each individual in the dyad. After habituation, mice were paired in a clean cage (standard homecage: 29.5 cm × 18 cm) containing clean bedding and recorded for 30 min. After testing, mice were weaned and housed with same sex littermates. Behaviors were grouped and scored as described previously (Cox and Rissman, 2011). We analyzed data from the last 20 of the 30 min encounters, after mice had adapted to the new environment and were more interactive.
2.4. Social preference testing
Social preference testing was performed using a paradigm similar to one previously described (Moy et al., 2004; Cox et al., 2010). On PN22, the same mice tested for social interaction (N = 12–20 per group) were moved to a testing room for habituation. Tests were conducted in a three-chambered box (76.2 cm × 26.67 cm × 17.78 cm), with 2 openings to the outer chambers, each containing a small “holding cell” (10.16 cm diam. × 13.97 cm). Mice habituated to the test box, with access to all three chambers, then were confined in the center chamber and a novel same-sex juvenile C57BL/6J mouse was randomly placed in one of the holding cells. The subject was released from the center and allowed to explore all three chambers for 10 min (part 1), then this process was repeated and a second same-sex juvenile C57BL/6 J mouse was placed in the other opposite chamber in the holding cell (part 2). Time spent in each of the three chambers, as well as the time spent investigating the holding cells, was recorded. In part 1 of the social preference task the amount of time the subject spent investigating an empty cage was subtracted from the time spent investigating a confined novel mouse to give each mouse a sociability score. In part 2 of the task, a preference score was calculated by subtracting the time spent investigating the familiar mouse from the time spent investigating the unfamiliar mouse.
2.5. Social recognition
A separate cohort of PN21 mice (N = 2–10 per group) was tested for social recognition in a clean, standard homecage (Tejada and Rissman, 2012). Mice were tested 9 times, 1 min per test, with an inter-trial interval of 9 min. The stimulus animal was placed into a “holding cell” for 1 min. In trials 1–8, the same ovariectomized adult mouse was used. Typically during this period habituation occurs and investigation of the female declines. On the 9th trial, dishabituation is measured by replacing the stimulus female with a novel ovariectomized female. For each trial, the time spent investigating the stimulus mouse was recorded.
2.6. Elevated plus maze
A third cohort of PN21 mice (N = 6–9 per group) was tested in the elevated plus maze (Med Associates ENV560A, each arm 35 cm × 7.5 cm) and recorded for 10 min as previously described (Cox et al., 2010). The total time spent in the closed and open arms and the numbers of crosses through the middle were scored. Time spent in the middle of the maze was calculated based on the total duration of the test less the time in the two arms. The open arms were further subdivided into proximal and distal portions of equal lengths (16.75 cm) by a line of tape 1.5 cm wide.
2.7. Social conditioned place preference
A fourth cohort of PN21 mice (N = 6–9 per group) was tested in this task, which was adapted from a published protocol (Panksepp and Lahvis, 2007). Mice were weaned into groups of 4 (2 males and 2 females) and assigned randomly to either woodchip or corncob bedding. Twenty-four hrs later, mice were individually housed on the other type of bedding. For the next 8 days, mice were rotated every 24 h between their group (with one type of bedding), and isolation (with the other bedding). On days 9 and 10, mice were habituated to a clean, empty conditioned place preference (CPP) box (Kudwa et al., 2005) for 20 min, each day. The CPP boxes consist of an external central compartment (15 × 6 × 13 cm) made of clear Plexiglas connected to two other compartments (15 × 15 × 15 cm). On day 11, mice were placed into the neutral center chamber of the CPP boxes, with one side containing woodchip bedding and the other corncob bedding. The distribution was randomized and balanced for each group. Mice were tested for 20 min and time spent on each side of the CPP chamber was recorded.
2.8. Brain collection and processing
Brains from PN21 mice, not used in any behavioral studies (N ≥ 6 per group) were removed and rapidly frozen on dry ice. Tissue was sectioned (120 μm) frozen in a cryostat onto glass superfrost+ slides, and the medial amygdala and paraventricular nucleus of the hypothalamus (PVN) were punched using anatomical guidelines (figures 40–48 for amygdala and figures 33–41 for PVN (Paxinos and Franklin, 2004)). For qRTPCR, RNA was isolated with RNeasy spin column purification kits and cDNA was synthesized using Quantitect Reverse Transcription Kits (both from Qiagen, Valencia, CA).
2.9. Quantitative real-time PCR
qRTPCR was performed in triplicate reactions using 5 ng of cDNA, and either Fast SYBR Green Master Mix and 100 nM primers, or Fast Taqman Master Mix and Taqman probes (Applied Biosystems) following manufacturers instructions. No reverse transcriptase (No RT) control reactions controlled for genomic DNA contamination. Oligonucleotide primers were designed using Primer-BLAST (NCBI) software. All primer pairs were verified as 90–110% efficient in standard curve reactions and amplified a single product determined by melt-curve analysis. Relative quantification of mRNA levels was measured using the ΔΔCt method on an Applied Biosystems (Grand Island, NY) StepOne Plus PCR machine, with Gapdh or B2m used as endogenous controls. Primer pairs for SYBR Realtime-PCR and Taqman probes are shown in Table 1S.
2.10. Human blood collection
Following IRB approval, 14 girls with Turner syndrome and 6 age-matched controls being seen in the pediatric endocrine clinic at Riley Hospital for Children were enrolled. Written informed consent was obtained from parents or legal guardians and assent from subjects ≥7 y.o. All procedures involving assay of human blood were approved by the University of Virginia Biosafety Committee. Subject’s ages ranged from 5.5 to 18 years. A majority of the Turner patients was receiving growth hormone therapy (N = 6), another group were on estradiol supplementation (N = 3) and two individuals were being treated with both GH and estradiol. Approximately 8 ml of blood were collected into vacutainer cell preparation tubes (Becton Dickenson, Franklin Lakes, NJ) and allowed to sit at room temperature for 30 min. Coagulated blood was spun for 30 min. at 3400 RPMs in a tabletop centrifuge (Vanguard V6500, Hamilton Bell, Montvale, NJ). After spinning, samples were immediately placed in a −20° C freezer, until shipment on dry ice to the University of Virginia.
2.11. Vasopressin and oxytocin enzyme-linked immunosorbant assay (ELISA)
ELISAs for vasopressin and oxytocin were performed in triplicate using Arg8 Vasopressin and Oxytocin ELISA kits (Enzo Life Sciences, Farmingdale, NY) using supplied standards and following kit instructions. Assay sensitivities were 4.10–1000 pg/ml for vasopressin and 15.6–1000 pg/ml for oxytocin. Intra-assay CVs were 3.82 ± 0.36% for vasopressin and 4.05 ± 0.42% for the oxytocin assay (mean ± SEM).
2.12. Statistical analysis
All data were analyzed using NCSS Software (2000). For behavioral data, we excluded data points greater than two standard deviations from the mean. This resulted in dropping 85 out of 2086 data points, only 4% of all observations. Except for the social recognition task, which was analyzed by repeated measures ANOVA, we used two-way ANOVAs to assess the contributions of gonadal sex and X-chromosome number. To assess significant main effects and interactions, paired comparisons were conducted using Fisher’s LSD multiple comparison tests. In the dyadic and social preference tests, we also used the more conservative Bonferroni Multiple Comparisons to examine interactions within main effects. Expression of Y-chromosome genes was compared using two-tailed Student’s t-tests. Steroid hormone data and log-transformed AVP/OXT blood concentrations were analyzed using Mann–Whitney tests.
3. Results
3.1. Mice with one X-chromosome show altered social behaviors
In a dyadic social interaction task, juvenile mice with one X-chromosome (1X mice) displayed more nonsocial behavior (Fig. 1A, F(1, 42) = 13.10, p < 0.001) than 2X mice. Specifically, 1X mice explored alone, (F(1, 41) = 7.53, p < 0.01) and sat by themselves (F(1, 40) = 7.63, p < 0.01) for more time than 2X mice (Table 2S). Conversely, 2X mice participated in more social behaviors than 1X mice (Fig. 1A, F(1, 42) = 10.07, p < 0.003), participating in more side-by-side sitting (Table 2S, F(1, 42) = 6.98, p < 0.02). Planned comparisons revealed that 1X females spent more time engaged in nonsocial behaviors and less time involved in social interactions than 2X females, whereas 1X and 2X males did not differ in these measures (Fig. 1A). In addition, 1X females spent significantly more time sitting and sleeping alone than all other groups (Table 2S). No significant interactions between sex and genotype were found for these behaviors. In addition to differences in social contact, 1X mice investigated their partners more frequently than 2X mice (Fig. 1B, F(1, 40) = 6.90, p < 0.02), approaching F(1, 40) = 8.00, p < 0.01) and pushing past their partners (F(1, 42) = 7.03, p < 0.02) more frequently than 2X mice (Table 3S). Interestingly, despite spending less time in close contact with partners, when compared with 2X mice, 1X mice more frequently initiated contact (Fig. 1B, F(1, 41) = 6.48, p < 0.02) with their partners, with a trend for 1X mice to nose-nose sniff with their partners more often than the 2X pairs (Table 3S, F(1, 41) = 3.72, p = 0.06). There were no effects of gonadal sex on any of the behaviors measured in this task.
Fig. 1.
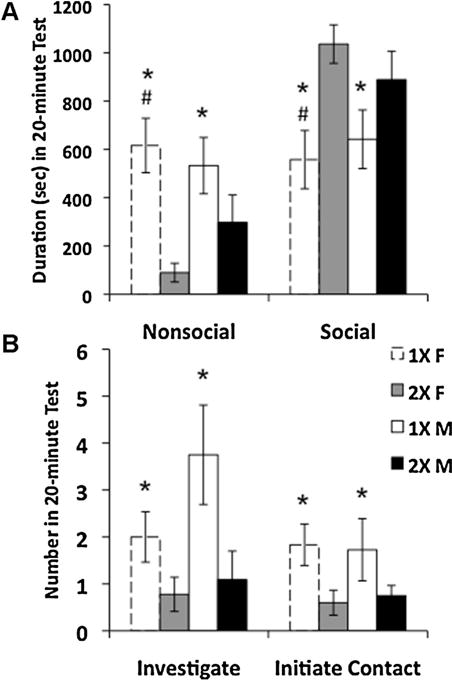
Juvenile mice with one X-chromosome spent less time engaged in social behaviors in a dyadic social interaction test as compared with mice with two X-chromosomes. Data shown are mean ± SEM. (A) Time spent engaged in nonsocial and social behaviors. (B) Number of investigative and play soliciting behav-iors. 1X females, N = 10 (white histograms with dashed lines); 2X females, N = 12 (gray histograms); 1X males, N = 12 (white histograms); 2X males, N = 12 (black histograms). * Main effect of genotype, p < 0.003; # 1X females different from 2X females, p < 0.05.
3.2. X-Chromosome number affects preference for an unfamiliar mouse
The dyadic social interaction task measures the behaviors of two unrestrained mice. Here we assessed behavioral interactions with a stimulus animal (same age and sex) that was physically separated from the subject and constrained. We found a significant gonadal sex difference, with females displaying less of a preference for the novel mouse than males (Table 4S, (F(1, 53) = 6.11, p < 0.02), spending more time, instead, investigating the empty chamber (F(1, 53) = 8.55, p < 0.01).
The second part of the social preference task assesses interest in a novel versus familiar mouse (i.e. the mouse present in the first part of the task). Here, the preference score revealed a significant main effect of genotype (Fig. 2A, F(1, 59) = 5.15, p < 0.04). Post-hoc comparisons revealed that 2X males showed more of a preference for the novel stimulus mouse than did the 1X males or 1X females. There was no significant difference in overall activity levels between groups as measured by the number of crosses from chamber to chamber in the preference task (data not shown).
Fig. 2.
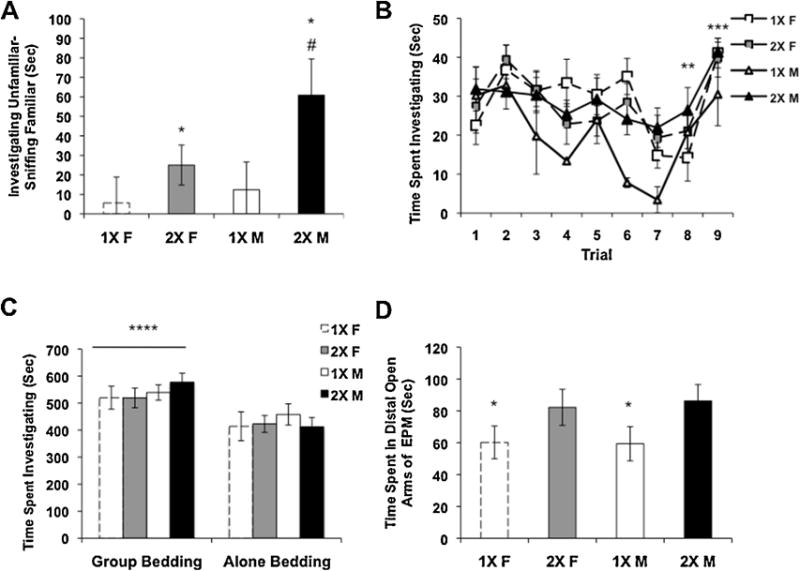
Social behaviors in three different tests and activity in the elevated plus maze. Means ± SEM are graphed. (A) Preference for an unfamiliar mouse as compared with a familiar mouse in the social preference task, 1X females, N = 10; 2X females, N = 20; 1X males, N = 11; 2X males, N = 15. (B) Time spent investigating an adult female mouse in the social recognition task, in trial nine a novel female is presented, 1X females, N = 6; 2X females, N = 8; 1X males, N = 2; 2X males, N = 10. (C) Time investigating bedding associated with social housing versus bedding associated with solitary housing in a conditioned place preference task, 1X females, N = 8; 2X females, N = 7; 1X males, N = 9; 2X males, N = 6. (D) Elevated plus maze, time in the distal portion of the open arms, 1X females, N = 8; 2X females, N = 9; 1X males, N = 6; 2X males, N = 7. * 1X mice different from 2X mice, p < 0.04, # 1X males different from 2X males and 1X females p < 0.05, ** trial 8 is different from trials 1–3, *** trial 9, during which a novel female was placed in the test cage, is significantly different from trials 7 and 8 for all groups, p < 0.0001. **** All mice had a preference for bedding associated with group housing, p < 0.002.
3.3. All mice display social recognition of a novel mouse
In both the dyadic task and the social preference task, 1X mice had social deficits; therefore, we next assessed social recognition. In this task, there were no effects of sex or genotype, but there was a main effect of trial (F(1, 225) = 6.48, p < 0.0001). When tested for multiple comparisons, the amount of sniffing on trial 8 was significantly different from trials 1, 2, and 3, suggesting that all mice at least partially habituated to the stimulus mouse. All animals also displayed more investigation in trial 9 versus 8 demonstrating dishabituation, and indicating normal social recognition (Fig. 2B). The 1X male group was small in number, leading to the possibility that if we had tested more mice we may have uncovered differences in habituation. However, at this age we have not seen sex differences in C57 mice in this task previously (Wolstenholme et al., 2013a), suggesting that 1X males would be similar to 2X females, and thus not different from the other groups.
3.4. All mice display social conditioned place preference
All mice were able to recognize a novel mouse in the social recognition task, however this task still includes interactions with another mouse. To ask if lower levels of social interaction in mice with one X-chromosome might be caused by social avoidance we used the CPP task. All mice, regardless of genotype or sex, developed a preference for bedding associated with a group as compared to bedding in which they had been housed alone (Fig. 2C, p < 0.002). These results show that the mice prefer bedding associated with group housing.
3.5. Anxiety-like behavior is related to number of X-chromosomes
The last hypothesis that we investigated was that the 1X mice displayed less social behavior in the interaction tasks due to elevated anxiety. In the EPM, 1X mice spent significantly less time in the distal portion of the open arms (Fig. 2D, Table 4S, F(1, 26) = 4.94, p < 0.04) as compared with mice that had two X-chromosomes. Because this EPM is designed for adult mice, and therefore has longer and wider arms than necessary to accommodate a juvenile, we used the time spent in the distal portion of the open arms as the most robust measure of reduced anxiety. There was no main effect of sex, but there was a significant interaction between sex and genotype for the amount of time spent in the middle of the EPM, with 1X males spending more time than 1X females. (Table 4S, F(1, 26) = 5.59, p < 0.03). The number of crosses through the middle of the EPM were equivalent in the groups which suggests that overall activity levels did not differ between them (data not shown).
3.6. Differences in gene expression in the amygdala
Using a candidate gene approach, we quantified expression of a number of autosomal genes known to be involved in social and anxiety behaviors, including vasopressin and its receptors, oxytocin and its receptor, serotonin and glucocorticoid receptors, and estrogen receptor alpha. In addition, we examined the expression of several X-chromosome genes that either escape inactivation or are associated with behaviors (Table 5S). There were several genes affected by X-chromosome complement. 2X mice of both sexes showed increased expression of the four X-chromosome genes we measured, all of which escape X-inactivation on the inactive X-chromosome [Ddx3x (F(1, 18) = 17.32, p < 0.0006), Eif2s3x (F(1, 15) = 32.63, p < 0.00005), Kdm6a (F(1, 19) = 14.00, p < 0.002), Kdm5c (F(1, 20) = 9.15, p < 0.07), Fig. 3A]. In addition, 2X males had increased expression of the Y-paralog, Ddx3y, as compared to 1X males (Fig. 3B, p < 0.04). There were no differences in the expression of Mecp2, or Maoa, which do not escape X-inactivation (Table 5S).
Fig. 3.
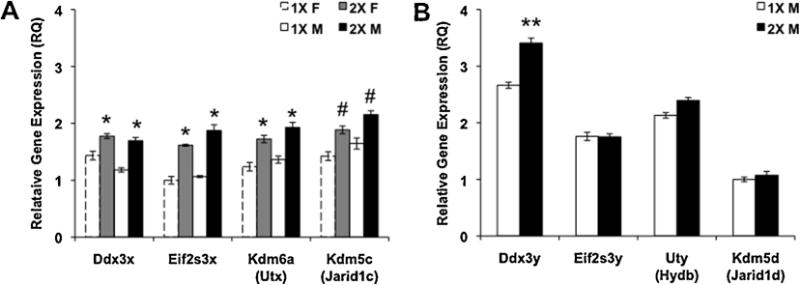
Expression of sex chromosome genes in the amygdala. Mean ± SEM relative gene expression. (A) Some of the X-chromosome genes that escape X-inactivation. 1X females, N = 6; 2X females, N = 7; 1X males, N = 8; 2X males, N = 7. (B) Gene expression of Y-chromosome paralogs in the amygdala. N = 8 per group. * Significant difference between 2X and 1X, p < 0.002, # trend for a difference between 1X and 2X, p < 0.07; ** significant difference between 1X and 2X males, p < 0.04.
In addition to X-chromosome effects, gonadal sex influenced expression of steroid sulfatase (Sts); females had higher expression than did males (F(1, 25) = 4.25, p < 0.05). However, this finding is attributable to the Y* mouse model itself, rather than a general impact of gonadal sex, because Y* males of both genotypes lack the Sts gene due to the aberrant recombination of their sex chromosomes. Two trends were also found for gonadal sex effects on gene expression: both the vasopressin receptor 1a (V1ar; F(1, 21) = 3.06, p = 0.095) and the oxytocin receptor (Oxtr; F(1, 22) = 3.35, p = 0.08) genes tended to be higher in male amygdalae (Table 5S).
Only one of the autosomal genes that we measured was affected by X-chromosome dosage. There was an interaction between sex and genotype for the expression of Avp, 1X females had more Avp expression than all other groups (Fig. 4A, F(1, 22) = 5.61, p < 0.03). There was no effect of X-chromosome dosage on Oxt gene expression, (Fig. 4B). In contrast to the amygdala, there were no significant differences in either Avp or Oxt gene expression in the PVN (Fig. 4C and D).
Fig. 4.
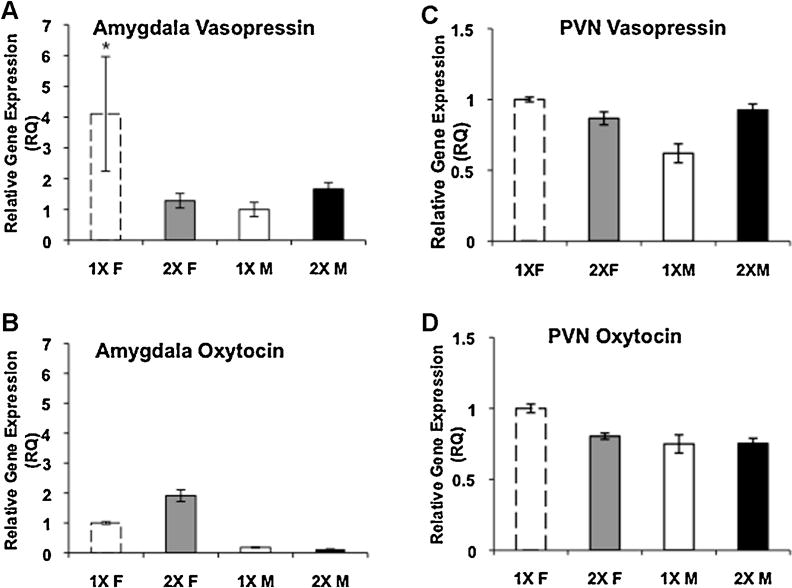
X-Chromosome dosage and neuropeptide gene expression in the amygdala and paraventricular hypothalamus (PVN). Mean ± SEM relative gene expression. (A) Vasopressin mRNA in the amygdala. 1X females, N = 5; 2X females, N = 7; 1X males, N = 7; 2X males, N = 7. (B) Oxytocin gene expression in the amygdala. 1X females, N = 5; 2X females, N = 5; 1X males, N = 4; 2X males, N = 4. (C) Vasopressin expression in the PVN N = 6 per group. (D) Oxytocin mRNA in the PVN N = 6 per group. 1X females (white histograms with dashed lines); 2X females (gray histograms); 1X males (white histograms); 2X males (black histograms). * 1X females significantly different from all other groups, p < 0.03.
3.7. Plasma AVP in Turner syndrome patients are lower than in normals
Our data from 1X mice suggested dysregulation of the Avp gene. We tested this hypothesis in patients with Turner syndrome. Enzyme-linked immunosorbant assays (ELISAs) for OXT and AVP in plasma from Turner syndrome and control patients showed a small, but significant, difference in AVP (but not OXT) levels (p < 0.05; Fig. 5A and B). The majority of the Turner patients were receiving either estradiol and/or growth hormone supplement. Because these treatments could alter Avp expression, we asked if AVP plasma levels were different between the Turner syndrome patients receiving estradiol treatment (N = 5, 2 of which were also receiving GH), GH treatment alone (N = 6), or no treatment (N = 3). In addition, one of the control patients was receiving estradiol. Although numbers were small for each subgroup, no differences were found in AVP levels between treatments (data not shown).
Fig. 5.
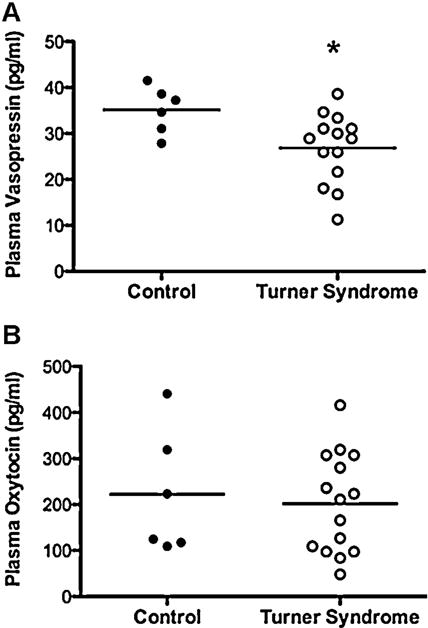
Plasma levels of vasopressin (AVP) and oxytocin (OXT) in controls and patients with Turner syndrome. Mean levels are denoted with horizontal bars. Each dot represents an individual. (A) Levels of vasopressin (pg/ml) in plasma. (B) Concentrations of oxytocin (pg/ml) in plasma. TS, N = 14; Cont, N = 6. * Significant difference p < 0.05.
4. Discussion
Here we report that, regardless of gonadal sex or numbers of sex chromosomes, 1X juvenile mice display less social behavior than 2X mice. Social behaviors are inherently complex, and can also be influenced by environmental factors such as litter composition (Yang et al., 2011), or the testing environment (Cox and Rissman, 2011); therefore, we assessed various aspects of social behavior. Our results indicate that reduced social interest in 1X mice may be due to increased anxiety levels, as measured by the reduced time spent by 2X mice in the distal portion of the open arms of the EPM. The distal portion is the most open part of the maze and least “secure” since it is furthest from the middle.
These results in juveniles are consistent with the limited data on social behavior in adult mice in this same line, and other models of sex chromosome aneuploidy. Adult male XXY mice which are true aneuploids (from a different cross than the one used here) showed more social interest in a social preference task as compared to XY males (Liu et al., 2010), and adult 1X and XO females both spent less time in the open arms of an EPM compared to 2X females (Isles et al., 2004). Genetic sex also affects the expression of genes involved in anxiety and depressive behaviors (Seney et al., 2013). Combined with our present results in juvenile mice, these findings suggest that the increased anxiety-like behavior seen in 1X mice develops prior to puberty and is stable into adulthood. While we cannot rule out the possibility that gonadal steroid levels could differ in utero between 1X and 2X mice (particularly in males), observations from our lab indicate that steroid hormone levels do not differ in 2X versus 1X juvenile (PN21) mice, suggesting that activational hormones are not responsible for behavioral differences (Bonthuis and Rissman, 2013).
Using the four-core genotypes (FCG) mouse, another model for investigating the roles of sex chromosomes, we found an interaction between gonadal sex and chromosome complement on juvenile social behavior in the dyadic social interaction task (Cox and Rissman, 2011). In that study, XX females were less social than XY females and XX males, but did not differ statistically from XY males in any behavioral measure. Because the FCG cross produces mice of both gonadal sexes with either XX or XY genotypes, it is not possible to assess whether sex chromosome effects are caused by the presence of 2 X-chromosomes, or the absence of a Y-chromosome (reviewed in (Cox et al., 2014). Moreover, in addition to the sex chromosome differences, while both the FCG and Y* mouse models are backcrossed into the B6 background strain, there are other genetic differences between the two strains (Eicher et al., 1991). One obvious source of behavioral differences is that the two strains have Y-chromosomes from different origins (reviewed in (Cox et al., 2014)). This difference has been shown to affect offspring behavior (Maxson et al., 1979; Nelson et al., 2010).
As expected, 2X mice had increased expression of several X-chromosome genes known to escape inactivation. Two such genes, Kdm5c and Kdm6a, encode histone-modifying enzymes: Kdm5c (also known as Jarid1c and Smcx) encodes a histone lysine demethylase that removes the activating methylation from histone 3, lysine 4, therefore KDM5C is largely thought of as a transcriptional repressor. In contrast, Kdm6a (also known as Utx) encodes a histone demethylase that preferentially removes repressive methylation from histone 3, lysine 27, and is a transcriptional activator (Mosammaparast and Shi, 2010). The expression of these genes in amygdala are consistent with data collected from the hippocampus, hypothalamus, and cerebellum, in which Kdm5c (Xu et al., 2008a), and Kdm6a (Xu et al., 2008b) are more highly expressed in 2X mice than in 1X mice.
Considerable evidence shows that expression of the Y-paralogs may not compensate for the lack of a second X-chromosome (Xu et al., 2008a, 2008b). While we did not directly compare the expression of X and Y-paralogs from males in a single PCR experiment, there was similar expression of most of the Y genes we measured in 1X and 2X males. The one exception to this finding was that 2X males had increased expression of Ddx3y, a gene encoding dead-box polypeptide that is involved in spermatogenesis in the testes. This finding is counterintuitive; if paralogs are transcribed equally we would have predicted that 1X males would have increased expression of Y-paralog genes in order to compensate for reduced expression from their single copy of the X-paralog. However, it is possible that the increased expression in the 2X males is in response to having 2 copies of the X-paralog of this gene, although we would have expected a similar result for the other Y-paralogs if this were the case. Still, each of these Y-paralogs could be regulated differently. The role of Ddx3y in the brain is unknown, but its expression has been noted in various brain regions (http://mouse.brain-map.org).
The most interesting gene expression finding was that 1X females had higher Avp mRNA as compared to all other groups. This is consistent with data in adult FCG mice showing that XY females have increased density of AVP immunoreactivity in the lateral septum compared to XX females (Gatewood et al., 2006). There were no gonadal sex differences in Avp gene expression in the amygdala of juvenile mice in this study, which is consistent with data from gonadectomized adult mice which have no circulating levels of gonadal androgens and suggests that in mice the Avp dimorphism in the amygdala is primarily dependent upon activational effects of steroid hormones (Rood et al., 2013). Vasopressin influences social behaviors, including anxiety and social recognition (Caldwell et al., 2008), and, in rats, systemic injections of vasopressin increase generalized anxiety (Bowen and McGregor, 2014). Mice lacking the vasopressin receptor 1a gene (V1ar) have impairments in social recognition, reduced anxiety behavior (Bielsky et al., 2004), and impaired social interactions (Egashira et al., 2007). Interestingly, we did not see any sex chromosome effects on vasopressin receptor expression, but we did note a trend for a sex difference (M > F) in expression of V1ar. It is unclear why Avp levels were not elevated in 1X males, who, like the 1X females, displayed a difference in social behaviors. While the data from our candidate gene approach show that the loss of an X-chromosome in 1X females has drastic effects on Avp gene expression, there are likely other genes involved in the regulation of these behaviors and also causing sex differences in regulation. For example, because the 1X males have a Y-chromosome, whereas the 1X females do not, there could be a role of the Y-paralogs in regulating Avp.
Avp gene expression could be regulated by various mechanisms, including DNA methylation and/or histone modifications. In rats, expression of the Nuclear receptor corepressor (Ncor) and Methyl CpG Binding Protein 2 (Mecp2) genes during early postnatal development is important for the organization of sex differences in juvenile play behavior (Kurian et al., 2008; Jessen et al., 2010). The vasopressin system (Szot and Dorsa, 1993; Forbes-Lorman et al., 2012), and Mecp2 mRNA are both sexually dimorphic in the rat amygdala during development (Kurian et al., 2007). Some histone modifications are sexually dimorphic in the cortex and hippocampus during mouse development, and may also contribute to sex differences in the brain (Tsai et al., 2009). Preliminary chromatin immunoprecipitation data from 1X and 2X females suggest that the histone methylation marks H3K4me3 and H3K27me3 are present on the Avp gene promoter in amygdala tissues, and enrichment of these marks does not differ based on X-chromosome number (data not shown). It must be noted, however, that Kdm5c/Kdm6a may have histone-methylation independent roles as chromatin remodelers, so it is also possible that the differential expression of these genes between 1X and 2X mice could influence gene expression in many other ways (Mann et al., 2012).
As shown in data from rats (Forbes-Lorman et al., 2012) and mice (Murgatroyd et al., 2009), MECP2 may also be involved in regulating the expression of the Avp gene in mouse amygdala. Our own preliminary data (not shown) also suggests that MECP2 binds the Avp promoter in amygdala tissues. While we did not measure DNA methylation on the Avp promoter, it can be differentially methylated (Auger et al., 2011), which would likely lead to differential binding of MECP2 to the Avp promoter. MECP2 can act as a transcriptional activator by recruiting transcription factors such as CREB to gene promoters (Chahrour et al., 2008), but, Mecp2 does not escape X-inactivation, and we did not note X-chromosome mediated differences in Mecp2 gene expression in the amydala during this time period. Nonetheless, there might be another, X-chromosome dependent mechanism to regulate MECP2 protein function, as opposed to Mecp2 gene transcription that would, in turn, regulate Avp expression. The role of these transcriptional regulators and vasopressin expression would be an exciting area of future research.
The data described could bear relevance to several neurobehavioral disorders in humans, including attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). Both of these diseases are more commonly diagnosed in males than in females (Giarelli et al., 2010), but the underlying causes of these sex differences are unclear. ADHD and ASD are also more prevalent in girls with Turner syndrome than normal girls (Lesniak-Karpiak et al., 2003; Russell et al., 2006). Interestingly, our human data, albeit from a small population, suggest dysregulation of AVP in Turner syndrome patients. It is somewhat paradoxical that we noted more Avp mRNA in the amygdala of the XO mouse but lower levels of AVP in plasma of the Turner patients. The neurons in the PVN of the hypothalamus produce the AVP found in blood, and in this region we did not find Avp mRNA differences in mice. There could be differences in Avp regulation in the PVN between mice and humans, or it may be that the mRNA levels were too variable in the mice to detect differences. Nonetheless, while only correlative, our data suggest that increased AVP in 1X females could underlie differences in social behavior and anxiety that are seen in Turner syndrome patients and could also contribute to other, physiological differences seen in Turner syndrome, such as hypertension (Mortensen et al., 2012) and risk of diabetes (Trolle et al., 2012), since vasopressin is critical for the regulation of vasoconstriction and water conservation.
In summary, we have shown that X-chromosome dosage influences social behavior in juvenile mice, and that AVP, both centrally and peripherally, may be influenced by X-chromosome dosage. The exact mechanism for this regulation is unclear, but MECP2 may possibly be a key player in the regulation of Avp gene expression. Future studies should further investigate the link between X-chromosome genes and autosomal gene expression, including Avp, as these genes may influence sex differences in behaviors.
Supplementary Material
Acknowledgments
The authors would like to thank Aileen Ryalls and Savera Shetty for technical assistance, as well as Drs. Mazhar Adli and Patrick Grant for providing technical advice and reagents. This work was supported by NIH R01 grants MH057759 and NS055218. KHC was supported by NIH TG32 HD007323.
Role of the funding source
This work was supported by NIH R01 grants MH057759 and NS055218. These grants were awarded to EFR. KHC was a Ph.D. student and her stipend was supported by NIH TG32 HD007323.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2014.10.010.
Footnotes
Conflict of interest
None declared.
References
- Adolphs R. What does the amygdala contribute to social cognition? Ann NY Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. The organizational–activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger CJ, Coss D, Auger AP, Forbes-Lorman RM. Epigenetic control of vasopressin expression is maintained by steroid hormones in the adult male rat brain. Proc Natl Acad Sci USA. 2011;108:4242–4247. doi: 10.1073/pnas.1100314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berletch JB, Yang F, Xu J, Carrel L, Disteche CM. Genes that escape from X inactivation. Hum Genet. 2011;130:237–245. doi: 10.1007/s00439-011-1011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bonthuis PJ, Rissman EF. Neural growth hormone implicated in body weight sex differences. Endocrinology. 2013;154(10):3826–3835. doi: 10.1210/en.2013-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, McGregor IS. Oxytocin and vasopressin modulate the social response to threat: a preclinical study. Int J Neuropsychopharmacol. 2014;17(10):1621–1633. doi: 10.1017/S1461145714000388. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ, Ashworth A. The Y* rearrangement in mice: new insights into a perplexing PAR. Cytogenet Cell Genet. 1998;80:37–40. doi: 10.1159/000014954. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav. 2010;58:754–761. doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Rissman EF. Sex differences in juvenile mouse social behavior are influenced by sex chromosomes and social context. Genes Brain Behav. 2011;10:465–472. doi: 10.1111/j.1601-183X.2011.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Bonthuis PJ, Rissman EF. Mouse model systems to study sex chromosome genes and behavior: relevance to humans. Front Neuroendocrinol. 2014;35(4):405–419. doi: 10.1016/j.yfrne.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira N, Tanoue A, Matsuda T, Koushi E, Harada S, Takano Y, Tsujimoto G, Mishima K, Iwasaki K, Fujiwara M. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res. 2007;178:123–127. doi: 10.1016/j.bbr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Hale DW, Hunt PA, Lee BK, Tucker PK, King TR, Eppig JT, Washburn LL. The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet Cell Genet. 1991;57:221–230. doi: 10.1159/000133152. [DOI] [PubMed] [Google Scholar]
- Forbes-Lorman RM, Rautio JJ, Kurian JR, Auger AP, Auger CJ. Neonatal MeCP2 is important for the organization of sex differences in vasopressin expression. Epigenetics. 2012;7:230–238. doi: 10.4161/epi.7.3.19265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarelli E, Wiggins LD, Rice CE, Levy SE, Kirby RS, Pinto-Martin J, Mandell D. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. 2010;3:107–116. doi: 10.1016/j.dhjo.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles AR, Davies W, Burrmann D, Burgoyne PS, Wilkinson LS. Effects on fear reactivity in XO mice are due to haploinsufficiency of a non-PAR X gene: implications for emotional function in Turner’s syndrome. Hum Mol Genet. 2004;13:1849–1855. doi: 10.1093/hmg/ddh203. [DOI] [PubMed] [Google Scholar]
- Jessen HM, Kolodkin MH, Bychowski ME, Auger CJ, Auger AP. The nuclear receptor corepressor has organizational effects within the developing amygdala on juvenile social play and anxiety-like behavior. Endocrinology. 2010;151:1212–1220. doi: 10.1210/en.2009-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Dominguez-Salazar E, Cabrera DM, Sibley DR, Rissman EF. Dopamine D5 receptor modulates male and female sexual behavior in mice. Psychopharmacology (Berl) 2005;180:206–214. doi: 10.1007/s00213-005-2150-5. [DOI] [PubMed] [Google Scholar]
- Kurian JR, Forbes-Lorman RM, Auger AP. Sex difference in mecp2 expression during a critical period of rat brain development. Epigenetics. 2007;2:173–178. doi: 10.4161/epi.2.3.4841. [DOI] [PubMed] [Google Scholar]
- Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP. Mecp2 organizes juvenile social behavior in a sex-specific manner. J Neurosci. 2008;28:7137–7142. doi: 10.1523/JNEUROSCI.1345-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak-Karpiak K, Mazzocco MM, Ross JL. Behavioral assessment of social anxiety in females with Turner or fragile X syndrome. J Autism Dev Disord. 2003;33:55–67. doi: 10.1023/a:1022230504787. [DOI] [PubMed] [Google Scholar]
- Liu PY, Erkkila K, Lue Y, Jentsch JD, Schwarcz MD, Abuyounes D, Hikim AS, Wang C, Lee PW, Swerdloff RS. Genetic, hormonal, and metabolomic influences on social behavior and sex preference of XXY mice. Am J Physiol Endocrinol Metab. 2010;299:E446–E455. doi: 10.1152/ajpendo.00085.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KM, Ward JM, Yew CC, Kovochich A, Dawson DW, Black MA, Brett BT, Sheetz TE, Dupuy AJ, Chang DK, Biankin AV, Waddell N, Kassahn KS, Grimmond SM, Rust AG, Adams DJ, Jenkins NA, Copeland NG. Sleeping Beauty mutagenesis reveals cooperating mutations and pathways in pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 2012;109:5934–5941. doi: 10.1073/pnas.1202490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson SC, Ginsburg BE, Trattner A. Interaction of Y-chromosomal and autosomal gene(s) in the development of intermale aggression in mice. Behav Genet. 1979;9:219–226. doi: 10.1007/BF01071302. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Mortensen KH, Andersen NH, Gravholt CH. Cardiovascular phenotype in Turner syndrome–integrating cardiology, genetics, and endocrinology. Endocr Rev. 2012;33:677–714. doi: 10.1210/er.2011-1059. [DOI] [PubMed] [Google Scholar]
- Mosammaparast N, Shi Y. Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu Rev Biochem. 2010;79:155–179. doi: 10.1146/annurev.biochem.78.070907.103946. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Nelson VR, Spiezio SH, Nadeau JH. Transgenerational genetic effects of the paternal Y chromosome on daughters’ phenotypes. Epigenomics. 2010;2:513–521. doi: 10.2217/epi.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 2004. [Google Scholar]
- Raymond FL. X linked mental retardation: a clinical guide. J Med Genet. 2006;43:193–200. doi: 10.1136/jmg.2005.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Stott RT, You S, Smith CJ, Woodbury ME, De Vries GJ. Site of origin of and sex differences in the vasopressin innervation of the mouse (Mus musculus) brain. J Comp Neurol. 2013;521:2321–2358. doi: 10.1002/cne.23288. [DOI] [PubMed] [Google Scholar]
- Russell HF, Wallis D, Mazzocco MM, Moshang T, Zackai E, Zinn AR, Ross JL, Muenke M. Increased prevalence of ADHD in Turner syndrome with no evidence of imprinting effects. J Pediatr Psychol. 2006;31:945–955. doi: 10.1093/jpepsy/jsj106. [DOI] [PubMed] [Google Scholar]
- Seney ML, Chang LC, Oh H, Wang X, Tseng GC, Lewis DA, Sibille E. The role of genetic sex in affect regulation and expression of GABA-related genes across species. Front Psychiatry. 2013;4:104. doi: 10.3389/fpsyt.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, Dorsa DM. Expression of vasopressin mRNA in extrahypothalamic nuclei of the homozygous Brattleboro rat is not modulated by testosterone. Neuroendocrinology. 1993;58:381–387. doi: 10.1159/000126567. [DOI] [PubMed] [Google Scholar]
- Tejada LD, Rissman EF. Sex differences in social investigation: effects of androgen receptors, hormones and test partner. J Neuroendocrinol. 2012 doi: 10.1111/j.1365-2826.2012.02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trolle C, Mortensen KH, Hjerrild BE, Cleemann L, Gravholt CH. Clinical care of adult Turner syndrome–new aspects. Pediatr Endocrinol Rev. 2012;9(Suppl. 2):739–749. [PubMed] [Google Scholar]
- Tsai HW, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47–53. doi: 10.4161/epi.4.1.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S, Aleman A, Swaab H, Kahn R. Klinefelter’s syndrome (karyotype 47,XXY) and schizophrenia-spectrum pathology. Br J Psychiatry. 2006;189:459–460. doi: 10.1192/bjp.bp.105.008961. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013a;64:833–839. doi: 10.1016/j.yhbeh.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Bekiranov S. Sexual differentiation in the developing mouse brain: contributions of sex chromosome genes. Genes Brain Behav. 2013b;12:166–180. doi: 10.1111/gbb.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS One. 2008a;3:e2553. doi: 10.1371/journal.pone.0002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Deng X, Watkins R, Disteche CM. Sex-specific differences in expression of histone demethylases Utx and Uty in mouse brain and neurons. J Neurosci. 2008b;28:4521–4527. doi: 10.1523/JNEUROSCI.5382-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


