Abstract
Nivolumab is a monoclonal antibody that blocks the interaction between programmed cell death 1 (PD1) and programmed cell death 1-ligand 1 (PD-L1), resulting in enhanced antitumor activity by the immune system. Nivolumab is currently approved by the US Food and Drug Administration (FDA) for melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma, classical Hodgkin lymphoma, squamous cell carcinoma of the head and neck, and urothelial carcinoma. PD-L1 IHC 28-8 pharmDx is FDA-approved as a complementary diagnostic for immunohistochemical (IHC) detection of PD-L1 in non-squamous NSCLC and melanoma. We report validation of PD-L1 IHC 28-8 pharmDx for PD-L1 detection on formalin-fixed, paraffin-embedded human melanoma specimens using Autostainer Link 48. A prevalence assessment of 104 melanoma specimens indicated that PD-L1 was detected across the full expression level range (0% to 100% of tumor cells). Assay robustness and precision studies were conducted at Agilent Technologies, with additional reproducibility studies performed at 3 external laboratories. Precision studies evaluated at ≥1% and ≥5% expression levels revealed a range of average negative agreement from 89.5%, 95% CI (83.2, 93.6) to 100%, 95% CI (97.3, 100), and average positive agreement from 85.5%, 95% CI (77.6, 90.9) to 100%, 95% CI (97.9, 100). For external reproducibility, precise results were obtained. These results demonstrate PD-L1 IHC 28-8 pharmDx is a precise, robust, and reproducible assay for determining PD-L1 expression in melanoma. This is the first PD-L1 IHC test to receive FDA approval as a complementary diagnostic in melanoma patients whereby positive PD-L1 expression is correlated with the magnitude of nivolumab treatment effect.
Key Words: diagnostic, immunohistochemistry, melanoma, nivolumab, programmed cell death 1-ligand 1 (PD-L1)
Alandmark therapeutic innovation occurred in 1996 when Dana R. Leach1 proposed immune checkpoint blockade as a strategy to treat cancer. Under normal physiological conditions, immune checkpoints maintain self-tolerance and protect tissues from damage during infection by balancing the costimulatory and coinhibitory signals that regulate T-cell response.2 One of the major hallmarks of cancer is evading host immunity by dysregulation of these immune checkpoints.3 Cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), a receptor expressed on the surface of activated T cells, was one of the first immune checkpoint molecules to be investigated in oncology.1,2 Discovery of CTLA-4 led to the development of ipilimumab, a human monoclonal antibody that blocks CTLA-4.1,4 Ipilimumab is the first immune checkpoint inhibitor approved by the US Food and Drug Administration (FDA), with indications for treating patients with advanced melanoma.4
Programmed cell death 1 (PD-1) is another immune checkpoint receptor with important implications in cancer.2 Binding of PD-1 on activated T cells to its ligands, programmed cell death 1-ligand 1 (PD-L1) and programmed cell death 1-ligand 2 (PD-L2), on tumor cells can suppress antitumor immunity.2,5,6 PD-L1 is a transmembrane protein that is normally expressed on the cell surface of antigen presenting cells, but can also be expressed by a wide range of tumor cell types.7 PD-L1 expression can be induced on melanoma cells by immune stimulating cytokines produced by T cells as a mechanism of adaptive immune resistance.8 Therapeutically blocking the PD-1/PD-L1 interaction may enhance antitumor immunity.2 Nivolumab is a PD-1–blocking human monoclonal antibody that has demonstrated improved overall survival (OS) versus standard of care in patients with advanced melanoma and is approved by the FDA for this indication, as well as for metastatic non-small cell lung cancer (NSCLC), advanced renal cell carcinoma, classical Hodgkin lymphoma, recurrent or metastatic squamous cell carcinoma of the head and neck, and locally advanced or metastatic urothelial carcinoma.9
The advancement of precision medicine has accelerated the necessity to codevelop “companion” or “complementary” in vitro diagnostics (IVDs) during drug clinical trials that may identify patients most likely to benefit from treatment, help monitor treatment response, and characterize patients at increased risk of treatment-related adverse events.10,11 A companion IVD is defined as required for the therapeutic product’s safe and effective use.10 In contrast, a complementary IVD is not required for treatment use, but provides clinically meaningful information that aids in the benefit-risk decision regarding treatment and is described in the treatment labeling.11,12 Both companion and complementary IVDs are held to the same assay performance standards. The FDA approved the validated PD-L1 IHC 28-8 pharmDx assay as a complementary diagnostic for second-line treatment of non-squamous NSCLC patients with nivolumab.12,13 The complementary designation for PD-L1 IHC 28-8 pharmDx is based on clinical data showing a survival advantage across non-squamous NSCLC patient populations, regardless of PD-L1 expression level.12
PD-L1 expression has been investigated as a potential biomarker of nivolumab efficacy in previously untreated advanced melanoma patients using PD-L1 IHC 28-8 pharmDx.14–16 In clinical trials, PD-L1 positivity was defined as PD-L1 complete circumferential and/or partial linear cell membrane expression at any intensity in at least 1% or 5% of tumor cells in a tissue section with a minimum of 100 tumor cells. The ≥1% and ≥5% PD-L1 positivity expression levels were based on preliminary evidence indicating a reasonable frequency of PD-L1 positivity that effectively divided the patient population, and were associated with a numerically higher objective response rate (ORR) among PD-L1 positive versus PD-L1 negative patients.13 In the phase I CheckMate 069 study, where nivolumab plus ipilimumab demonstrated greater ORR and progression-free survival (PFS) versus ipilimumab alone, PD-L1 expression was not associated with ORR.14 In the phase III CheckMate 066 study, where nivolumab provided significant improvements in OS, PFS, and ORR versus dacarbazine, PD-L1 status was not clinically useful, as patients demonstrated improved OS with nivolumab versus dacarbazine regardless of PD-L1 status.15 However, in the phase III CheckMate 067 study, where nivolumab alone or combined with ipilimumab resulted in significantly longer PFS than ipilimumab alone, patients with PD-L1 tumor expression showed improved PFS following nivolumab-plus-ipilimumab therapy, compared with nivolumab monotherapy.16 Patients with PD-L1–positive tumors showed similar prolongation in PFS with combination therapy and nivolumab monotherapy compared with ipilimumab monotherapy.16 These results suggest that melanoma patients with PD-L1–negative tumors may derive greater benefit from nivolumab combined with ipilimumab than from nivolumab alone. Findings from the CheckMate 067 study served as the basis for the FDA approval of PD-L1 IHC 28-8 pharmDx as a complementary IVD in melanoma.
The PD-L1 IHC 28-8 pharmDx assay was analytically validated for the detection of PD-L1 protein on formalin-fixed, paraffin-embedded (FFPE) human melanoma specimens using the Autostainer Link 48. Similar to companion diagnostics, PD-L1 IHC 28-8 pharmDx is a class III IVD complementary diagnostic device and requires premarket approval to verify the safety and effectiveness of the assay. The validated PD-L1 IHC 28-8 pharmDx is the only FDA-approved complementary diagnostics for use in patients with either non-squamous NSCLC or metastatic melanoma considering nivolumab treatment. We examined the robustness, precision, and reproducibility of PD-L1 IHC 28-8 pharmDx on the Autostainer Link 48 staining platform.
MATERIALS AND METHODS
Tissue Specimen Preparation and PD-L1 IHC 28-8 pharmDx Assay
All specimens used in these studies were prepared and PD-L1 IHC 28-8 pharmDx (Agilent Technologies, Santa Clara, CA; Dako Code SK005) staining was performed as previously described by Phillips et al, using Dako PT Link Pretreatment Module (Agilent Technologies; Dako Code PT100) and Autostainer Link 48 (Agilent Technologies; Dako Code AS480).13 All instructions on how to run the assay are found in the PD-L1 IHC 28-8 pharmDx instructions for use (IFU).17 Cell line control slides supplied with PD-L1 IHC 28-8 pharmDx were used as run controls as per the product IFU.17
Blinding and Randomization
For PT Link incubation time and temperature, target retrieval solution (TRS) pH, precision/repeatability, and external reproducibility studies, stained slides were blinded and randomized to avoid bias during scoring. These slides were evaluated in a random order according to a randomization key, generated using Minitab 17 or Microsoft Excel, and documented before the interpretation of staining.
Interpretation of Staining
PD-L1 protein positivity was defined as complete circumferential or partial linear plasma membrane staining of tumor cells at any intensity. Cytoplasmic staining and melanin pigmentation, if present, were disregarded. Because melanin and DAB are similar in color, the presence of melanin can interfere with slide interpretation. For each slide stained with PD-L1 positive antibody, there was a sequential slide stained with Negative Control Reagent (NCR). Comparison to the NCR slide was useful for identifying melanin content and differentiating it from DAB chromogen. Any brown staining found on the NCR slide was recognized as nonspecific staining or melanin pigment, and therefore excluded when scoring the PD-L1 positive slide. Positively stained tumor-associated immune cells were excluded from the computation of the percentage scores. Studies were focused on agreement for PD-L1 positivity as defined at ≥1% and ≥5% tumor cell expression levels. Cases were classified as PD-L1 positive if percent positivity of tumor cells was scored as greater than or equal to each respective expression level, whereas cases were considered PD-L1 negative if percent positivity of tumor cells was scored below the expression level value. A minimum of 100 viable tumor cells must have been present to determine the percentage of stained cells. Nonspecific or background staining was recorded on a 0 to 3 intensity scale using 0.25 grade increments. Specimens stained with the NCR were required to have a score of 0 specific membrane staining and ≤1 nonspecific or background staining.
Prevalence Studies
Prevalence studies were conducted to evaluate the performance of PD-L1 IHC 28-8 pharmDx for detection of PD-L1 in a range of expression levels in FFPE human melanoma tissue specimens. The test set was comprised of 104 unique cases, including primary tumors and metastatic tumors, and stages I-IV disease.
Robustness Studies
Robustness testing was conducted to evaluate the staining performance of PD-L1 IHC 28-8 pharmDx under various laboratory conditions on FFPE human melanoma. Robustness testing included the following: tissue section thickness (4 μm and 5 μm); microscope slide type (FisherbrandTM SuperfrostTM Plus, FLEX IHC Microscope Slides, and Silanized Slides); PT Link incubation time and temperature (18 min, 20 min, 22 min; 95°C, 97°C, 99°C); TRS pH (pH 5.8, pH 6.1, pH 6.4); and TRS reuse (fresh TRS vs TRS used a total of 3 times over 5 days, with 24 slides treated per use).
Precision/Repeatability Studies
Studies were conducted to demonstrate precision when staining specimens with PD-L1 IHC 28-8 pharmDx. FFPE melanoma specimens were tested with the following precision conditions:
Inter-instrument: Single analyst operating 5 different instruments.
Inter-analyst: 6 different analysts operating a single instrument.
Inter-day: Single analyst operating a single instrument over 5 nonconsecutive days.
Intra-run: Single analyst operating a single run on a single instrument, testing replicates of each specimen.
Intra-lot: Multiple PD-L1 primary antibody vials from within one lot of assay reagents.
Inter-lot: 3 lots of assay reagents and TRS.
Precision testing was based on pair-wise comparisons of runs across the different precision conditions.
External Reproducibility Studies
Studies were also conducted at 3 external Clinical Laboratory Improvement Amendments (CLIA) certified clinical labs to demonstrate reproducibility when staining and evaluating melanoma specimens with PD-L1 IHC 28-8 pharmDx. Two separate studies were conducted to assess combined inter-site, intra-site, inter-observer, and intra-observer reproducibility for the ≥1% and ≥5% expression levels. The clinical labs were trained on the use of the assay, and 5 independent IHC runs were performed on 5 nonconsecutive days at each lab. Inter-site assessments consisted of 24 FFPE melanoma specimens at the ≥1% expression level and 18 FFPE melanoma specimens at the ≥5% expression level; blinded and randomized unstained tissue sections were supplied to each test site. Intra-observer and inter-observer assessments consisted of a single set of stained slides, prepared at Agilent Technologies and provided to the 3 study sites. A certified clinical pathologist at each site, who was previously trained on the interpretation of stained slides with our comprehensive pathologist training program, scored this blinded set (n=30) a total of 3 times. The slides were randomized and a washout period was imposed between the individual pathologist’s scoring sessions. A 2-week wash-out period was utilized to score specimens at the ≥1% expression level. Specimens at the ≥5% expression level were scored with a 4-day wash-out period.
RESULTS
Calculations for percent agreements were performed according to Clinical and Laboratory Standards Institute (CLSI) guidelines,18 using Average Negative Percent Agreement (ANA), Average Positive Percent Agreement (APA), Overall Percent Agreement (OA), and 95% confidence intervals (CI).
Prevalence Studies
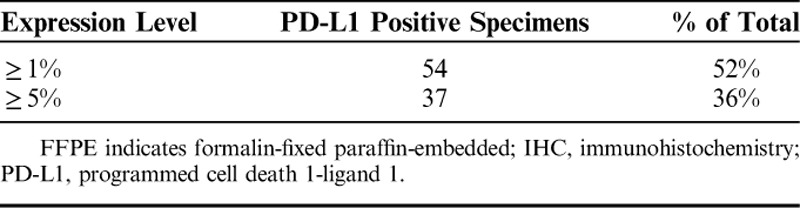
Sensitivity and specificity of the anti-human rabbit monoclonal 28-8 PD-L1 antibody were previously described and performed in cell lines, FFPE cell pellets, FFPE NSCLC tissue, and on a panel of normal FFPE tissues.13 To further assess PD-L1 IHC 28-8 pharmDx the prevalence of PD-L1 protein expression in FFPE melanoma tissue was evaluated. Prevalence testing was performed on a set of unique melanoma cases (n=104) which exhibited staining across the range of 0% to 100% positive tumor cells, and 0 to 3 staining intensity (Fig. 1). PD-L1 prevalence was 52% (n=54/104) of specimens expressing ≥1% PD-L1 and 36% (n=37/104) expressing ≥5% PD-L1 (Table 1).
FIGURE 1.
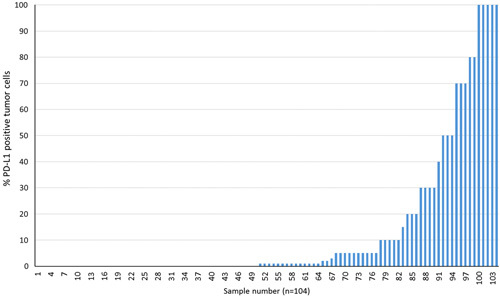
Prevalence of PD-L1 protein in FFPE Melanoma Specimens using PD-L1 IHC 28-8 pharmDx assay for detection (n=104 specimens). PD-L1 indicates programmed cell death 1-ligand 1; IHC, immunohistochemistry; FFPE indicates formalin-fixed paraffin-embedded.
TABLE 1.
PD-L1 Prevalence Using PD-L1 IHC 28-8 PharmDx Assay for PD-L1 Detection in FFPE Melanoma Specimens (n=104)
Robustness Studies
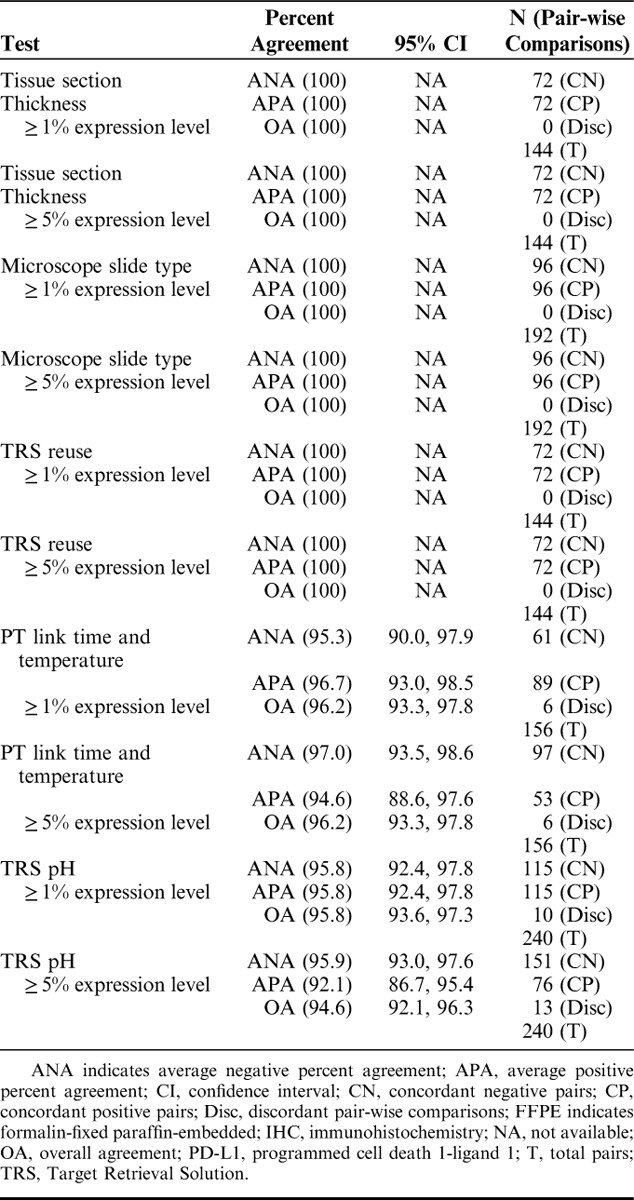
Robustness studies were performed to evaluate the staining performance of PD-L1 IHC 28-8 pharmDx under various laboratory conditions including: tissue section thickness, microscope slide type, PT Link incubation time and temperature, TRS pH, and TRS reuse. Slides were blinded and randomized for PT Link incubation time and temperature and TRS pH studies. Specific robustness study results are presented for the ≥1% and ≥5% expression levels in Table 2. ANA and APA for ≥1% and ≥5% expression levels for tissue section thickness, microscope slide type, and TRS reuse were 100% (95% CI, NA) (Table 2). ANA for the PT Link time and temperature for ≥1% expression level was 95.3%, 95% CI (90.0, 97.9) and APA was 96.7%, 95% CI (93.0, 98.5); for ≥5% expression level was ANA was 97.0%, 95% CI (93.5, 98.6), and APA was 94.6%, 95% CI (88.6, 97.6) (Table 2). These results indicate that, within the parameters tested, the assay demonstrated robust performance.
TABLE 2.
Robustness of the PD-L1 IHC 28-8 pharmDx Assay on FFPE Melanoma Specimens (n=16)
Precision/Repeatability Studies
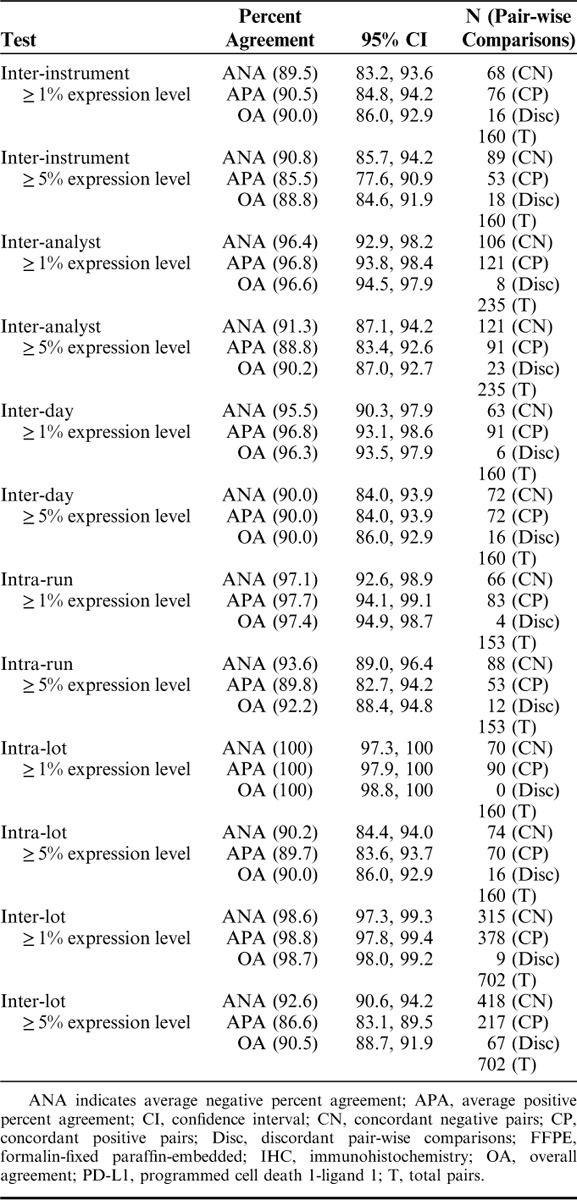
To demonstrate the ability of PD-L1 IHC 28-8 pharmDx to produce consistent and precise results in normal day to day testing, precision studies were performed including: inter-instrument, inter-analyst, inter-day, intra-run, intra-lot, and inter-lot. Specific precision study results are presented for the ≥1% and ≥5% expression levels in Table 3. ANA for inter-instrument studies at the ≥1% expression level was 89.5%, 95% CI (83.2, 93.6) and APA was 90.5%, 95% CI (84.8, 94.2); ANA for ≥5% expression level was 90.8%, 95% CI (85.7, 94.2) and APA was 85.5%, 95% CI (77.6, 90.9) (Table 3). ANA for inter-day at the ≥1% expression level was 95.5%, 95% CI (90.3, 97.9) and APA was 96.8%, 95% CI (93.1, 98.6); ANA and APA for ≥5% expression level were both 90.0%, 95% CI (84.0, 93.9) (Table 3). ANA for inter-lot for ≥1% expression level was 98.6%, 95% CI (97.3, 99.3) and APA was 98.8%, 95% CI (97.8, 99.4); ANA for ≥5% expression level was 92.6%, 95% CI (90.6, 94.2) and APA was 86.6%, 95% CI (83.1, 89.5) (Table 3). Overall, the above results indicate that PD-L1 IHC 28-8 pharmDx produces precise and repeatable results in normal day to day testing.
TABLE 3.
Precision/Repeatability of the PD-L1 IHC 28-8 pharmDx Assay on FFPE Melanoma Specimens (n=16)
External Reproducibility Studies
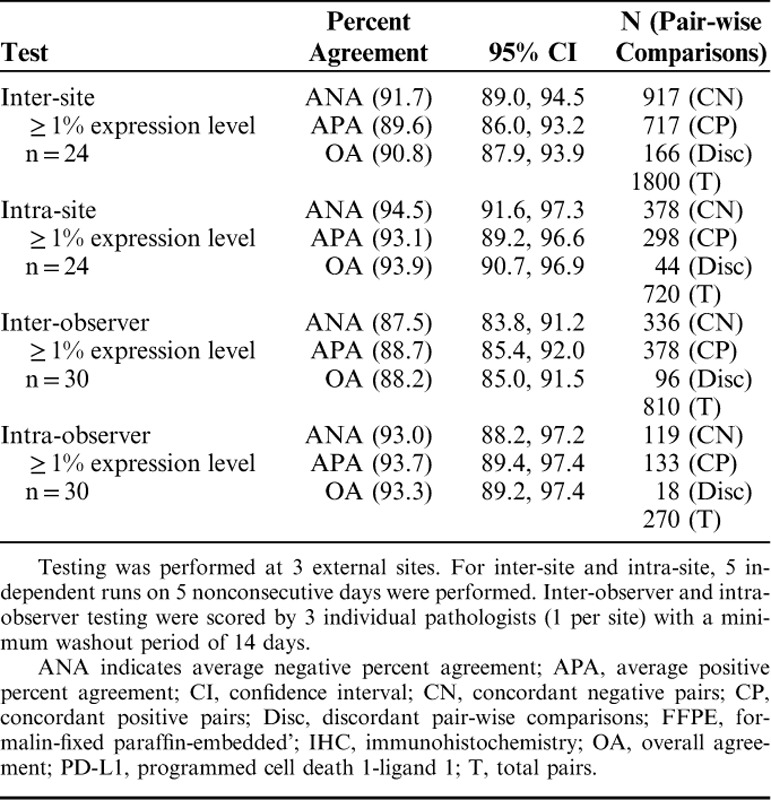
To evaluate the inter-site, intra-site, inter-observer, and intra-observer reproducibility of PD-L1 IHC 28-8 pharmDx, external reproducibility studies were performed at 3 independent CLIA-certified external clinical laboratories. External reproducibility results are presented for the ≥1% expression level in Table 4. At the ≥1% expression level for the inter-site studies, ANA was 91.7%, 95% CI (89.0, 94.5), and APA was 89.6%, 95% CI (86.0, 93.2) (Table 4). For inter-observer at the ≥1% expression level ANA was 87.5%, 95% CI (83.8, 91.2), and APA was 88.7%, 95% CI (85.4, 92.0) (Table 4).
TABLE 4.
External Reproducibility of the PD-L1 IHC 28-8 pharmDx Assay for ≥1% Expression Level on FFPE Melanoma Specimens
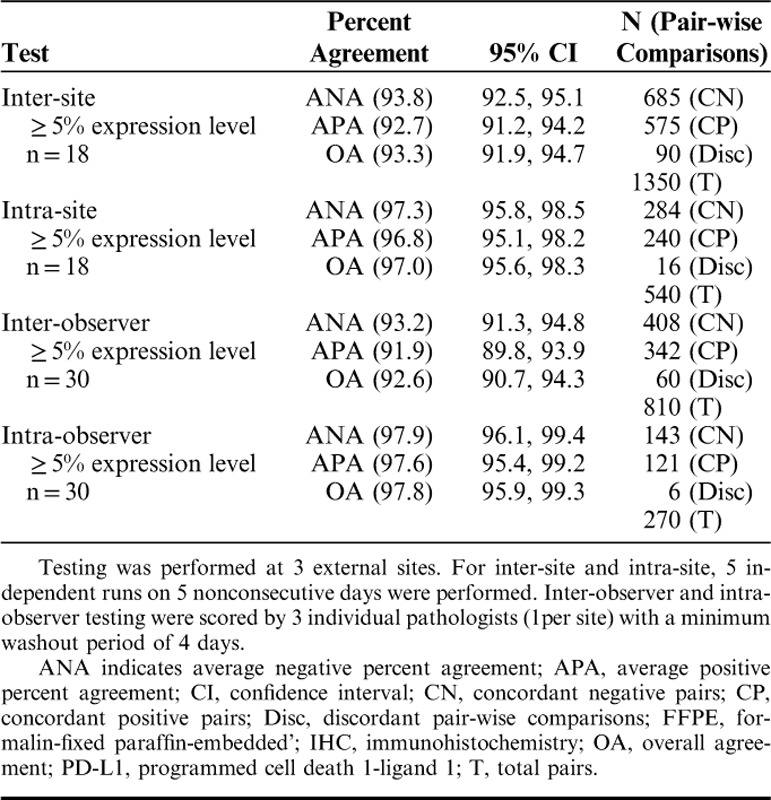
Examining agreements at the ≥5% expression level, shown in Table 5, inter-site was 93.8%, 95% CI (92.5, 95.1), and APA was 92.7%, 95% CI (91.2, 94.2) (Table 5). ANA for inter-observer was 93.2%, 95% CI (91.3, 94.8), and APA was 91.9%, 95% CI (89.8, 93.9) (Table 5). The above studies indicate that PD-L1 IHC 28-8 pharmDx is reproducible for the detection of PD-L1 in FFPE melanoma specimens when using the Autostainer Link 48 platform as prescribed by the product IFU.
TABLE 5.
External Reproducibility of the PD-L1 IHC 28-8 pharmDx Assay for ≥5% Expression Level
DISCUSSION
A number of FDA-approved PD-L1 IHC IVD assays are currently available using different primary antibodies, assay platforms, scoring algorithms, and expression levels. These have been utilized in numerous clinical trials to detect PD-L1 expression as an indicator of clinical efficacy for several immune checkpoint inhibitors for NSCLC, as well as other tumor types.13,19,20 However, PD-L1 IHC 28-8 pharmDx is the only FDA-approved complementary diagnostic for the detection of PD-L1 in FFPE non-squamous NSCLC and melanoma specimens using Autostainer Link 48. There are a number of variables that can potentially influence the robustness, precision, and reproducibility of PD-L1 IHC 28-8 pharmDx. Investigating how some of these variables affect product performance is essential to achieve premarket approval as a Class III IVD.
Previously published studies on the assay included information on the generation of the 28-8 antibody clone and antibody sensitivity and specificity testing.13 Assay robustness, precision, and reproducibility testing was performed in non-squamous NSCLC.13 Our validation testing using melanoma specimens was designed to investigate some variables that could impact the performance of PD-L1 IHC 28-8 pharmDx in this tumor indication.
Assessment of the prevalence of PD-L1 expression indicated detection across the full range of PD-L1 expression, from 0% to 100%. The distribution of PD-L1 expression across specimens evaluated indicated 52% of specimens exhibited ≥1% PD-L1 expression and 36% of specimens exhibited ≥5% PD-L1 expression.
Robustness testing was performed to evaluate: tissue section thickness, microscope slide type, PT Link incubation time and temperature, TRS pH, and TRS reuse. Within the range of parameters tested, we did not observe alteration in the staining quality with PD-L1 IHC 28-8 pharmDx. Variable conditions between clinical laboratories are an inherent component of day to day, site to site, and observer to observer patient diagnostic testing. However, clear parameters must be established to ensure the safety and efficacy of class III IVD devices. Further, the assay showed high analytical precision during internal precision testing, while external reproducibility demonstrated that the assay was reproducible across a wide dynamic range of variables that can affect performance. In addition, established scoring guidelines and a comprehensive pathologist training program aided in the achieved assay performance.
Among medical devices, class III IVD devices, such as PD-L1 IHC 28-8 pharmDx, require the highest levels of review by the FDA, and consistent product performance is essential to achieve premarket approval. The studies described herein demonstrate the robustness, precision, and reproducibility of PD-L1 IHC 28-8 pharmDx in detecting PD-L1 expression, including the selected ≥1% and ≥5% expression levels in melanoma specimens. The FDA-approved PD-L1 IHC 28-8 pharmDx has the following approved claims: (1) PD-L1 expression in non-squamous NSCLC may be associated with enhanced survival from nivolumab, and (2) positive PD-L1 status in melanoma is correlated with the magnitude of the treatment effect on PFS from nivolumab. The assay has been further clinically validated in nivolumab phase I-III clinical trials examining multiple indications with distinct histologies.21,22 In addition, numerous ongoing clinical trials for nivolumab are utilizing PD-L1 IHC 28-8 pharmDx to investigate PD-L1 expression in a variety of tumor types.
ACKNOWLEDGMENTS
The authors thank Kate Sojka for critical review and editing of the manuscript; Andrew Segal for critical review of the manuscript; Dako histology team for procurement of tissues and preparation of cut sections for this study, especially Lenka Van Alphen; Tissue samples were provided by the Cooperative Human Tissue Network which is funded by the National Cancer Institute. Other investigators may have received specimens from the same subjects; tissue samples supplied by Asterand Bioscience; Dako operations team for the production of the reagents for this assay; and Grace Kim, Erika Klohe, and Sam An for project management support.
Footnotes
Present address: Molly M. Millett, PhD, Terumo BCT, Lakewood, CO; Xiaoling Zhang, PhD, MedImmune-A member of the AstraZeneca Group, Gaithersburg, MD; Pauline Simmons, MS, Thermo Fisher, Fremont, CA; Josette William, MD, PhD, NeoGenomics Laboratories, Fort Myers, FL; H. David Inzunza, MD, Halozyme Therapeutics Inc., San Diego, CA; Henrik Winther, DVM, PhD, Immunovia AB, Medicon Village, Lund, Sweden.
Supported by Bristol-Myers Squibb.
J.C. and J.N.: are employed by and own stock in Bristol-Myers Squibb. R.C., G.C., and J.C.: are employed by Agilent Technologies. T.P., M.J., I.R.S., and E.O.: are employed by and own stock in Agilent Technologies. The remaining authors declare that they have nothing to disclose.
REFERENCES
- 1.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 4.YERVOY® (ipilimumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2017. Available at: https://packageinserts.bms.com/pi/pi_yervoy.pdf. [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- 7.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.OPDIVO® (nivolumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017. Available at: https://packageinserts.bms.com/pi/pi_opdivo.pdf. [Google Scholar]
- 10.US Food and Drug Administration. In Vitro Companion Diagnostic Devices. Guidance for Industry and Food and Drug Administration Staff. Issued: August 6, 2014. Available at: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm262327.pdf. Accessed November 16, 2016.
- 11.Beaver JA, Tzou A, Blumenthal GM, et al. An FDA perspective on the regulatory implications of complex signatures to predict response to targeted therapies. Clin Cancer Res. 2016;23:1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazandjian D, Suzman DL, Blumenthal G, et al. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21:634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips T, Simmons P, Inzunza HD, et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol. 2015;23:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 16.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PD-L1 IHC 28-8 pharmDx [package insert]. Santa Clara, CA: Agilent Technologies; 2017. Available at: http://www.agilent.com/en-us/products/pharmdx/pd-l1-ihc-28-8-pharmdx/pd-l1-ihc-28-8-pharmdx-for-autostainer-link-48-1. [Google Scholar]
- 18.CLSI. Quality Assurance for Design Control and Implementation of Immunohistochemistry Assays; Approved Guideline-Second Edition CLSI document I/LA28-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. [Google Scholar]
- 19.Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheel AH, Dietel M, Heukamp LC, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol. 2016;29:1165–1172. [DOI] [PubMed] [Google Scholar]
- 21.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]


