Supplemental Digital Content is available in the text.
Keywords: carotid intima-media thickness, dyslipidemias, hydroxymethylglutaryl-CoA reductase inhibitors, pravastatin, ultrasonography
Abstract
Background and Purpose—
The effect of statins on progression of carotid intima–media complex thickness (IMT) has been shown exclusively in nonstroke Western patients. This study aimed to determine the effect of low-dose pravastatin on carotid IMT in Japanese patients with noncardioembolic ischemic stroke.
Methods—
This is a substudy of the J-STARS trial (Japan Statin Treatment Against Recurrent Stroke), a multicenter, randomized, open-label, parallel-group trial to examine whether pravastatin reduces stroke recurrence. Patients were randomized to receive pravastatin (10 mg daily, usual dose in Japan; pravastatin group) or not to receive any statins (control group). The primary outcome was IMT change of the common carotid artery for a 5-year observation period. IMT change was compared using mixed-effects models for repeated measures.
Results—
Of 864 patients registered in this substudy, 71 without baseline ultrasonography were excluded, and 388 were randomly assigned to the pravastatin group and 405 to the control group. Baseline characteristics were not significantly different, except National Institutes of Health Stroke Scale scores (median, 0 [interquartile range, 0–2] versus 1 [interquartile range, 0–2]; P=0.019) between the 2 groups. Baseline IMT (mean±SD) was 0.887±0.155 mm in the pravastatin group and 0.887±0.152 mm in the control group (P=0.99). The annual change in the IMT at 5-year visit was significantly reduced in the pravastatin group as compared with that in the control group (0.021±0.116 versus 0.040±0.118 mm; P=0.010).
Conclusions—
The usual Japanese dose of pravastatin significantly reduced the progression of carotid IMT at 5 years in patients with noncardioembolic stroke.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00361530.
Intima–media complex thickness (IMT) is a standard surrogate marker for atherosclerosis and a predictor of subsequent cardiovascular events independently of major vascular risk factors.1–5 This marker can be easily assessed by carotid B-mode ultrasound and shown as the hypoechoic region between the vascular lumen of the carotid artery and its adventitia, which seems hyperechoic, and corresponds to the intima and the media together, based on an a comparison study using ultrasound and pathological examination.5,6
Hyperlipidemia is a well-known major contributor to atherosclerosis that eventually can cause cardiovascular events and stroke. In Western countries, several studies involving the general population or patients with ischemic heart disease revealed that treatment with 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, referred to as statins, is associated with regression of IMT.4,7–10 A meta-analysis of 9 trials showed a strong correlation between reduction in low-density lipoprotein cholesterol (LDLC) and reduction in carotid IMT; each 10% reduction in LDLC was estimated to reduce carotid IMT by 0.73% per year.11 Although similar studies were done in Asia, based on a relatively small population,12–14 the effect of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors on IMT has not been well established in Asian countries.
The J-STARS trial (Japan Statin Treatment Against Recurrent Stroke) was performed in Japan as a multicenter, randomized, open-label, parallel-group trial on secondary stroke prevention to examine whether pravastatin at 10 mg/d, a traditional statin at its usual dose in Japan, reduces recurrence of stroke and the respective stroke subtypes in patients with hyperlipidemia and a history of noncardioembolic stroke (NCT00221104, University Hospital Medical Information Network C000000207).15,16 As an adjunct study, the J-STARS Echo Study was planned to determine the preventive effect of pravastatin on progression of carotid IMT and to explore pleiotropic effects of the traditional statin on the changes in carotid atherosclerosis assessed by ultrasound in patients with hyperlipidemia with a history of noncardioembolic ischemic stroke
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. The J-STARS Echo Study was a substudy of the J-STARS trial. The rationale and protocol of the J-STARS trial and this substudy are described elsewhere.15,17 The protocol and informed consent form for the present substudy were approved by the institutional review board of each center, and written informed consent was obtained from each patient together with the main trial. The J-STARS Echo Study was registered with ClinicalTrials.gov and the University Hospital Medical Information NetworkClinical Trials Registry (C000000212) separately from the J-STARS trial. Trial offices are cited in Table I in the online-only Data Supplement. Figure 1 shows a flowchart of the trial design.
Figure 1.
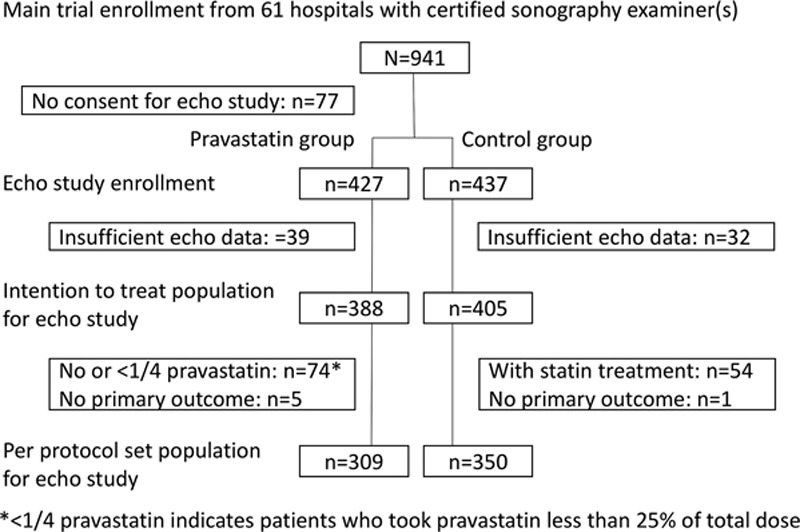
Flow chart of patient enrollment is shown.
Briefly, patients who met the following inclusion criteria for the main J-STARS trial and provided written consent to participate in this substudy were included. The inclusion criteria for the main trial were as follows: (1) age between 45 and 80 years of either sex; (2) clinical diagnosis of noncardioembolic ischemic stroke developing within the preceding month to 3 years; and (3) serum total cholesterol maintained between 4.65 and 6.21 mmol/L (180 and 240 mg/dL) without use of statins. Noncardioembolic ischemic stroke included atherothrombotic infarction, lacunar infarction, and infarction of undetermined cause. Stroke subtypes were diagnosed in accordance with the Treatment of Acute Stroke Trial criteria and defined in accordance with the National Institute of Neurological Disorders and Stroke classification.18,19 In addition to the exclusion criteria of the main trial described elsewhere,15 patients for whom carotid ultrasound would be technically problematic were ineligible. A full description of the inclusion and exclusion criteria is presented in Table II in the online-only Data Supplement. A web-based system randomly assigned participants to either the pravastatin or control group (1:1 allocation). Patients assigned to the pravastatin group received a daily 10 mg oral dose of pravastatin. Patients assigned to the control group received no statin treatment although other antihyperlipidemic drugs could be administered when necessary. Based on previous reports,9,10 we assumed that IMT increase for 5 years would be 0.02 mm for the pravastatin group and 0.04 mm for the control group with SD of 0.1, a total of 864 enrolled patients for this study exhibited a post hoc power of 83% under a 2-sided Student t test.
Carotid Ultrasound Examination and Image Measurement
Carotid ultrasonography was performed in each participating institute by well-trained physicians or laboratory technicians who were authorized as expert examiners for this study by the J-STARS Echo office. Examination with the ultrasound machine and a transducer with a frequency of ≥7.5 MHz was performed at the time of enrollment in the study and followed every year until the end of the follow-up period of the main trial at 5 years. The same method was applied in subsequent examinations. The details of ultrasound examination and image measurement are described elsewhere.17
Briefly, all ultrasound images were sent to the J-STARS Echo office. All measurements were done centrally using special IMT measurement software (IntimaScope, Mediacross, Tokyo, Japan) by a special technician (K.Y.) who was blinded to any clinical information of the subjects. IMT was measured on the distal wall in a continuous 2-cm section on the central side of the common carotid artery bifurcation. It automatically calculated the maximum and mean values of IMT in a 2-cm continuous section of the distal common carotid artery. Pivotal parameters for analysis were the mean IMT (right, left, and mean of right and left) and the maximum IMT (right, left, and thicker value between right and left) of the distal wall of the common carotid artery in the above-described 2-cm section. Regional plaques in the section were included in the measurement. The primary outcome was change in IMT for 5 years.
Statistical Analysis
The changes in mean and maximum IMTs from baseline were analyzed using mixed-effects models for repeated measures with baseline value, allocation group, visits at 1, 2, 3, 4, and 5 years, and interactions between groups and visits as fixed effects and the patient as the random effect. Baseline was defined as the time of enrollment. On the mixed-effects models for repeated measures model, the least square mean of the difference from baseline was estimated. The changes from baseline at each visit were compared between groups using the Wilcoxon rank-sum test. The primary analysis population was defined as all randomized patients according to the intention-to-treat principle. The above analysis for the per-protocol patient set was also performed as a sensitivity analysis. As a secondary analysis, the recurrence of cerebral vascular events (stroke plus transient ischemic attack, stroke, ischemic stroke, atherothrombotic infarction, and lacunar infarction) was examined, and the Cox proportional hazards model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) by adjusting the stratification factors at randomization in the main J-STARS trial: that is, subtype of stroke (atherothrombotic stroke versus others), elevated blood pressure (≥150/90 mm Hg versus not), and diabetes mellitus (presence versus absence).15 The significance level for statistical testing was defined as a 2-sided P value of 0.05. All analyses were performed using SAS, version 9.3 (Cary, NC). An interim analysis was not performed.
Results
Participant Population
Among 123 participating sites in the main J-STARS Study, 61 hospitals (centers) had available certified sonographers. From the 61 centers, 941 patients were randomly allocated to the pravastatin or control group in the main J-STARS trial between March 2004 and February 2009. Of these patients, 864 (91.8%) simultaneously agreed to participate in the J-STARS Echo Study.16 Figure 1 shows the flow chart of patient enrollment. Finally, 427 and 437 patients were allocated to the pravastatin and control groups, respectively, and 388 and 405, respectively, were used for intention-to-treat analyses after excluding those without initial echographic data. Of these, 28 (7.2%) and 40 (9.9%) patients, respectively, received antihyperlipidemic drugs except statin, including fibrates (8 and 10, respectively), probucol (5 and 7, respectively), anion exchange resins (2 and 7, respectively), nicotinates (2 and 3, respectively), and others (11 and 19, respectively) during study period. Because of protocol violations (74 patients in the pravastatin group took no or <25% of total dose of pravastatin and 54 in the control group received statin treatment) or no data on primary outcomes (5 and 1, respectively), 309 patients in the pravastatin group and 350 control patients were used for sensitivity analyses as the per-protocol patient set.
The mean overall age of the studied patients was 66.4±8.3 years. A total of 33.2% of the patients were women. Ultrasound examination was done in 336 patients in the pravastatin group and 346 control patients, respectively, at 1 year; in 305 and 318 patients, respectively, at 2 years; in 268 and 281 patients, respectively, at 3 years; in 251 and 261 patients, respectively, at 4 years; and in 255 and 265 patients, respectively, at 5 years. Mean follow-up time was 4.4±1.4 years in both groups. Baseline characteristics and lipid profiles are shown in Table III in the online-only Data Supplement. There were no significant differences between the 2 groups, except initial National Institutes of Health Stroke Scale scores (median 0 [interquartile range, 0–2] versus 1 [interquartile range, 0–2]; P=0.019).
Effect of Pravastatin on Lipid Levels
Figure 2 shows baseline values of and annual change in total cholesterol, LDLC, triglycerides, and high-density lipoprotein cholesterol. Total cholesterol and LDLC were significantly reduced with 10 mg daily of pravastatin by 12% and 19%, respectively, at the 1-year follow-up and continuously thereafter. The average of differences for triglycerides until 5 years was significantly reduced with 10 mg daily of pravastatin(P=0.023). High-density lipoprotein cholesterol was not significantly different between the 2 groups.
Figure 2.
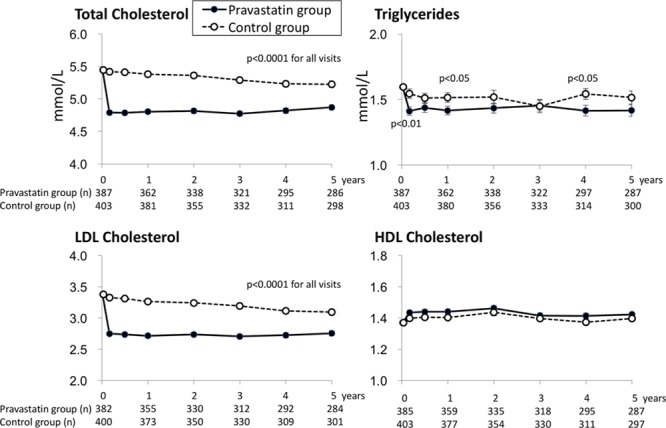
Temporal trends of total cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol by allocated groups for 5 years are shown.
Outcomes
Baseline mean IMT (mean±SD) was 0.887±0.155 mm in the pravastatin group and 0.887±0.152 mm in the control group (P=0.99). Trends of the mean IMT for 5 years are shown in Figure 3 and Table IV in the online-only Data Supplement. Each annual change in the mean IMT (mean±SD) for 5 years was 0.011±0.085, 0.023±0.114, 0.017±0.114, 0.018±0.118, and 0.021±0.116 mm in the pravastatin group and 0.008±0.074 mm (P=0.74), 0.020±0.087 mm (P=0.99), 0.017±0.097 mm (P=0.44), 0.030±0.113 mm (P=0.18), and 0.040±0.118 mm (P=0.010) in the control group. In the sensitivity analysis using per-protocol patient set with 309 patients in the pravastatin group and 350 in the control group, the changes were 0.012±0.084, 0.024±0.101, 0.018±0.108, 0.019±0.115, and 0.022±0.108 mm in the pravastatin group and 0.010±0.075 mm (P=0.69), 0.021±0.088 mm (P=0.89), 0.020±0.097 mm (P=0.49), 0.035±0.1138 mm (P=0.15), and 0.044±0.123 mm (P=0.016) in the control group.
Figure 3.
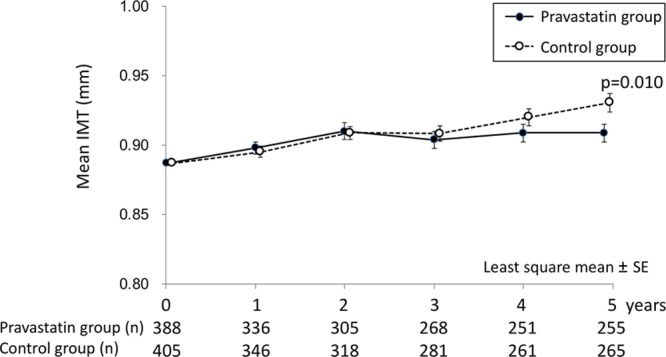
Temporal trends of mean intima–media thickness (IMT) by allocated groups for 5 years are shown. Each data indicate the least square mean of the difference from baseline.
Baseline maximum IMT (max IMT) was 1.195±0.331 mm in the pravastatin group and 1.182±0.314 mm in the control group (P=0.56). Annual max IMT values are shown in Figure 4. Each annual change in the max IMT for 5 years was 0.012±0.188, 0.010±0.270, 0.015±0.275, −0.005±0.265, and 0.014±0.252 mm in the pravastatin group and 0.006±0.148 mm (P=0.62), 0.018±0.184 mm (P=0.27), 0.020±0.189 mm (P=0.30), 0.034±0.236 mm (P=0.040), and 0.044±0.300 mm (P=0.40) in the control group. In the sensitivity analysis using per-protocol patient set, the changes were 0.017±0.190, 0.016±0.225, 0.017±0.263, 0.002±0.251, and 0.017±0.246 mm in the pravastatin group and 0.008±0.148 mm (P=0.78), 0.022±0.186 mm (P=0.26), 0.022±0.190 mm (P=0.45), 0.045±0.248 mm (P=0.043), and 0.051±0.313 mm (P=0.40) in the control group
Figure 4.
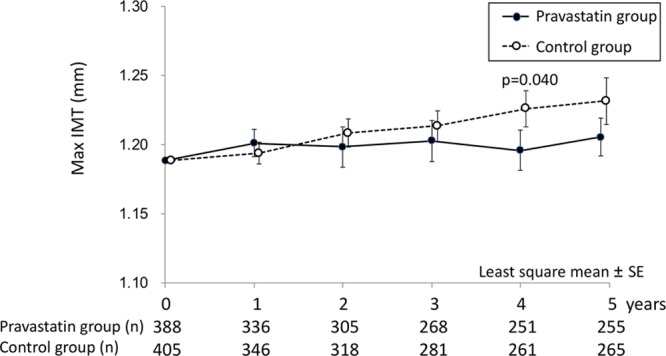
Temporal trends of maximum intima–media thickness (IMT) by allocated groups for 5 years are shown. Each data indicate the least square mean of the difference from baseline.
In the follow-up period, total stroke and transient ischemic attack (2.58 versus 2.89%/y; adjusted HR, 0.91; 95% CI, 0.61–1.35), total stroke (2.27 versus 2.66%/y; adjusted HR, 0.87; 95% CI, 0.58–1.33), ischemic stroke (2.09 versus 2.33%/y; adjusted HR, 0.92; 95% CI, 0.59–1.42), and lacunar infarction (1.20 versus 1.14%/y; adjusted HR, 1.07; 95% CI, 0.59–1.93) occurred similarly in the pravastatin and control groups, and atherothrombotic infarction tended to occur less frequently in the pravastatin group than in the control group (0.27 versus 0.71%/y; adjusted HR, 0.41; 95% CI, 0.15–1.15; Table V in the online-only Data Supplement). The results were similar with those on the main J-STARS trial.
Discussion
We conducted this study to determine the effect of 10 mg daily of pravastatin on carotid IMT in Japanese patients with hyperlipidemia and noncardioembolic ischemic stroke. A pravastatin dose of 10 mg/d significantly reduced total cholesterol by >10% and LDLC by >15% for 5 years. Mean IMT was not significantly different at baseline versus during the initial 4 years of observation, but the change from baseline reached a significant difference at 5 years. Thus, 10 mg daily of pravastatin could significantly reduce the progression of mean IMT after long-term treatment. Tendency toward a reduction in max IMT progression by 10 mg daily of pravastatin was also observed although the statistical difference was not constant probably because of the wide variation of max IMT value.
Little evidence has been available on the effect of statin on carotid IMT in patients with prior ischemic stroke or transient ischemic attack. This study enrolled >800 patients, and this is the largest study to assess the effect of a statin on carotid IMT in patients with noncardioembolic ischemic stroke. The main J-STARS trial (n=1578) showed that occurrence of atherothrombotic infarction was less frequent in the pravastatin group than in the control group (adjusted HR, 0.33; 95% CI, 0.15–0.74) although total stroke and transient ischemic attack occurred similarly in the pravastatin and control groups (adjusted HR, 0.97; 95 CI, 0.73–1.29).16 In this substudy, a similar trend was observed in the occurrence of atherothrombotic infarction.
Many studies have suggested pleiotropic effects of statins, including atheromatous plaque stabilization and anti-inflammation, which could play a role for the suppression of atherothrombotic infarction.20–23 This substudy supports that the protective effect of a statin against IMT progression is one of related prevention mechanisms of atherothrombotic infarction. The prevention of carotid atherosclerosis by pravastatin seems independent from the reduction of LDLC, LDLC/high-density lipoprotein cholesterol, or high-sensitivity C-reactive protein because there were no significant associations between the IMT change and the change of LDLC, LDLC/high-density lipoprotein cholesterol, or log-transformed high-sensitivity C-reactive protein both in the pravastatin and control groups (Figures I–III in the online-only Data Supplement).
In the 1990s, Furberg et al24 revealed that 20 to 40 mg daily of lovastatin significantly reduced carotid max IMT for 3 years in 919 patients without symptomatic cardiovascular disease. The PLAC-II (Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries) study showed that 20 to 40 mg daily of pravastatin resulted in a significant 35% reduction in IMT progression and a nonsignificant 12% reduction in progression of the mean max IMT in the common carotid in 151 coronary patients.7 The KAPS (Kuopio Atherosclerosis Prevention Study) also revealed that 40 mg per day of pravastatin significantly reduced carotid IMT progression in 447 hypercholesterolemic men from a primary prevention cohort.8 The LIPID (Long-Term Intervention with Pravastatin in Ischemic Disease) Atherosclerosis Substudy showed that treatment with 40 mg daily of pravastatin significantly reduced the progression of carotid atherosclerosis in 522 patients with a history of myocardial infarction or unstable angina.10 A systematic review and meta-analysis showed that LDLC reduction by a statin and carotid IMT change reduction strongly correlated, and each 10% reduction in LDLC was estimated to reduce carotid IMT change by 0.73% per year.11 However, most previous reports on this topic were from Western countries, and there have been scarce data in patients with prior ischemic stroke or transient ischemic attack. Our study revealed the effect of 10 mg daily of pravastatin to reduce the progression of carotid IMT in >800 Japanese patients with noncardioembolic ischemic stroke.
According to the previous reports, the significant reduction in IMT progression occurred 1 or 2 years after statin initiation.8,10,24 In this study, trend separation of mean IMT between the pravastatin and placebo groups started at 3 years after enrollment (Figure 3). Therefore, the significant effect of 10 mg daily of pravastatin on carotid IMT may occur somewhat later than with a higher dose of pravastatin or with other statins although the trend separation of atherothrombotic infarction reduction started at 1 year after enrollment in the main J-STARS trial.16 The early reduction of atherothrombotic infarction might be because of the improvement of endothelial function by pravastatin that seemed to occur earlier than IMT change.
Recently, several studies on this topic have been reported from Asian countries. Fang et al25 reported a meta-analysis on atorvastatin treatment for carotid IMT in Chinese patients with type 2 diabetes mellitus. They showed that treatment, especially with high-dose atorvastatin, significantly reduced carotid IMT in Chinese patients without ischemic stroke.25 Yamada et al13 showed that atorvastatin significantly reduced relative lipid volume of carotid plaques involving 40 patients without cerebral ischemic events. The FAST (Fukuoka Atherosclerosis Trial) revealed that pravastatin significantly reduced common carotid IMT by 13.9% for 2 years in asymptomatic hypercholesterolemic patients.12 Deguchi et al14 reported that rosuvastatin did not reduce max IMT or plaque score for 12 months in 24 dyslipidemic patients with cerebral infarction. However, there are still scarce and inconclusive data in patients with noncardioembolic ischemic stroke. Our study reveals that statin treatment with 10 mg daily of pravastatin could reduce the progression of carotid IMT after long-term treatment in a large Asian cohort with a history of noncardioembolic ischemic stroke. Asians are known to have a high incidence of both ischemic and hemorrhagic strokes,26 and intracranial atherosclerosis is relatively common in Asians.27 Thus, a reduction in IMT progression by statins seems either directly or indirectly important in the reduction of atherothrombotic infarction in Asian patients.
Nohara et al28 reported that rosuvastatin significantly reduced progression of carotid IMT at 12 months compared with pravastatin (10 mg daily) in Japanese patients with hypercholesterolemia. However, it is yet uncertain whether strong statin or higher dose pravastatin is more effective and safer to prevent IMT progression than pravastatin in patients with prior ischemic stroke. Japanese stroke physicians still commonly use pravastatin (10 mg daily) because we may have a concern of increased hemorrhagic stroke risk with strong statin or higher dose pravastatin therapy. Further studies are warranted in this regard.
The study drug in this trial, pravastatin, is not a strong, but rather, a traditional statin. In addition, the daily dose of 10 mg is lower than that in the previous trials on statin therapy and carotid IMT in Western countries (generally 40 mg daily).8–10 According to dose finding studies in Japanese patients with hyperlipidemia, 10 mg daily of pravastatin significantly reduced total cholesterol and LDLC.29,30 Our data also showed that 10 mg daily of pravastatin significantly reduced total cholesterol by >10% and LDLC level by >15%, which could contribute to a reduction in the progression of carotid mean IMT in Asian patients with noncardioembolic ischemic stroke.
The J-STARS trial also has other substudies, including those focusing on high-sensitivity C-reactive protein (ClinicalTrials.gov NCT00361699, University Hospital Medical Information Network C000000211),31 development of dementia, and genetic aspects (University Hospital Medical Information Network C000008055). We will further investigate relationships of carotid IMT with the above topics of interest during statin treatment.
One limitation of this trial is that all of the enrolled patients in the main J-STARS trial were not randomized for the J-STARS Echo study. However, baseline characteristics of the remaining patients in the main J-STARS trial were almost similar to those in the J-STARS Echo Study (Table VI in the online-only Data Supplement). Therefore, the results can be extrapolated to the entire population in the main J-STARS trial. The relatively high rate of protocol violations to stop pravastatin or start statin in the control group is another limitation because this could weaken the IMT change difference (statin effect) between 2 groups in the intention-to-treat analyses. The open-label design and a relatively high dropout rate (34%) for ultrasound examination at 5 years were other limitations. The dropout rate was not insubstantial and could affect the results. However, our further analysis only using 255 patients in the pravastatin group and 265 patients in the control group who underwent carotid ultrasonography examination at 5 years showed a similar reduction of mean IMT change by pravastatin (Table VII in the online-only Data Supplement). Although patients without initial echographic data (n=71) had some different baseline characteristics from those in the intention-to-treat analyses (n=793) and the results of the latter may not be extrapolated to the former, there were no differences between patients who had follow-up at 5 years (n=520) and those without (n=344; Tables VIII and IX in the online-only Data Supplement).
Conclusions
The J-STARS Echo Study revealed that the usual Japanese dose (10 mg daily) of pravastatin significantly reduced the progression of carotid mean IMT in Japanese patients with noncardioembolic stroke.
Acknowledgments
We thank the patients and their families involved in this study, and we also appreciate the other study participants, physicians, supporting medical staff, and coworkers for their assistance in the preparation and execution of this study. Also, we thank Hideki Kono and Yoko Nakagawa (Foundation for Biomedical Research and Innovation Translational Research Informatics Center) for their statistical analysis support, Drs Tatsuo Kohriyama and Setsuro Ibayashi for their valuable advice, and Kie Yamaguchi (Clinical Research Staff at National Cerebral and Cardiovascular Center) for ultrasound assessments.
Sources of Funding
This study was supported through a grant from the Ministry of Health, Labour and Welfare of Japan and in part by an Intramural Research Fund (28-4-1) for Cardiovascular Diseases of National Cerebral and Cardiovascular Center. This study was also conducted in collaboration with Hiroshima University Medical School and the Foundation for Biomedical Research and Innovation.
Disclosures
Dr Koga reports honoraria from Daiichi Sankyo Co, Ltd, Bayer Healthcare, Boehringer Ingelhaeim GmbH, AstraZeneca, and Pfizer Inc. Dr Toyoda reports honararia from Daiichi Sankyo Co, Ltd, Bayer Healthcare, Boehringer Ingelheim GmbH, and Bristol-Myers Squibb. Dr Minematsu reports honoraria from Bayer Healthcare, Otsuka Pharmaceuticals, Boehringer Ingelheim GmbH, AstraZeneca, Pfizer Inc, Mitsubishi Tanabe Pharma Cooperation, Japan Stryker, Kowa, Nihon Medi-Physics Co, Ltd, Bristol-Myers Squibb, Sawai Pharmaceutical Co, Ltd, Sumitomo Dainippon Pharma Co, Ltd, Medico’s Hirata Inc, Daiichi Sankyo Co, Ltd, Astellas Pharma Inc, Kyowa Hakko Kirin Pharma, Sanofi S.A., MSD, Eisai Co, Ltd, Nippon Chemiphar, and Towa Pharmaceutical Co, Ltd. Dr Yasaka reports grants and lecture fees from Daiichi Sankyo Co, Ltd. Dr Hosomi reports honoraria from Mochida Pharmaceutical Co, Ltd. Dr Kagimura is employed at the Translational Research Informatics Center which had contract of data center with J-STARS (Japan Statin Treatment Against Recurrent Stroke) office and received data center fee. Dr Origasa reports remuneration from Daiichi Sankyo Co, Ltd. Dr Maruyama reports speaker fees from Daiichi Sankyo, Otsuka Pharmaceutical, Eisai, Nihon Pharmaceutical, Takeda Pharmaceutical, Boehringer Ingelheim, Sumitomo Dainippon Pharma, Mitsubishi Tanabe Pharma, Pfizer, Sanofi, Bayer, Kyowa Hakko Kirin, and Fuji Film, and Grants-in-Aid for Scientific Research (JP26293211), and research support from Eisai, Pfizer, Takeda Pharmaceutical, Otsuka Pharmaceutical, Nihon Pharmaceutical, Shionogi, Teijin Pharma, Fuji Film, Boehringer Ingelheim, Nihon Medi-Physics, Bayer, MSD, Daiichi Sankyo, Kyowa Hakko Kirin, Novartis, Mitsubishi Tanabe Pharma. Dr Kitagawa reports grants and honoraria from Daiichi Sankyo Co, Ltd, Bayer Healthcare, Otsuka Pharmaceuticals, Boehringer Ingelheim GmbH, AstraZeneca, and Sumitomo Dainippon Pharma Co, Ltd, and honoraria from Sanofi K.K., Eisai Co, Ltd, and Kyowa Hakko Kirin Pharma. Dr Uchiyama reports honoraria from Daiichi Sankyo Co, Ltd, Astellas Pharma Inc, and Kowa Hakko Kirin. Dr Matsumoto reports grants from Takeda Pharmaceutical Co, Ltd, Sanofi K.K., Mochida Pharmaceutical Co, Ltd, Otsuka Pharmaceutical, and Daiichi Sankyo Co, Ltd, and honoraria from Sanofi K.K., Bayer Healthcare, and Daiichi Sankyo Co, Ltd. The other authors report no conflicts.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.117.018387/-/DC1.
References
- 1.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 2.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 3.Crouse JR, 3rd, Craven TE, Hagaman AP, Bond MG. Association of coronary disease with segment-specific intimal-medial thickening of the extracranial carotid artery. Circulation. 1995;92:1141–1147. doi: 10.1161/01.cir.92.5.1141. [DOI] [PubMed] [Google Scholar]
- 4.Probstfield JL, Margitic SE, Byington RP, Espeland MA, Furberg CD. Results of the primary outcome measure and clinical events from the Asymptomatic Carotid Artery Progression Study. Am J Cardiol. 1995;76:47C–53C. doi: 10.1016/s0002-9149(99)80470-6. [DOI] [PubMed] [Google Scholar]
- 5.Nezu T, Hosomi N, Aoki S, Matsumoto M. Carotid intima-media thickness for atherosclerosis. J Atheroscler Thromb. 2016;23:18–31. doi: 10.5551/jat.31989. doi: 10.5551/jat.31989. [DOI] [PubMed] [Google Scholar]
- 6.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 7.Crouse JR, 3rd, Byington RP, Bond MG, Espeland MA, Craven TE, Sprinkle JW, et al. Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries (PLAC-II). Am J Cardiol. 1995;75:455–459. doi: 10.1016/s0002-9149(99)80580-3. [DOI] [PubMed] [Google Scholar]
- 8.Salonen R, Nyyssönen K, Porkkala E, Rummukainen J, Belder R, Park JS, et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation. 1995;92:1758–1764. doi: 10.1161/01.cir.92.7.1758. [DOI] [PubMed] [Google Scholar]
- 9.Mercuri M, Bond MG, Sirtori CR, Veglia F, Crepaldi G, Feruglio FS, et al. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic Mediterranean population: the Carotid Atherosclerosis Italian Ultrasound Study. Am J Med. 1996;101:627–634. doi: 10.1016/s0002-9343(96)00333-6. [DOI] [PubMed] [Google Scholar]
- 10.MacMahon S, Sharpe N, Gamble G, Hart H, Scott J, Simes J, et al. Effects of lowering average of below-average cholesterol levels on the progression of carotid atherosclerosis: results of the LIPID Atherosclerosis Substudy. LIPID Trial Research Group. Circulation. 1998;97:1784–1790. doi: 10.1161/01.cir.97.18.1784. [DOI] [PubMed] [Google Scholar]
- 11.Amarenco P, Labreuche J, Lavallée P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke. 2004;35:2902–2909. doi: 10.1161/01.STR.0000147965.52712.fa. doi: 10.1161/01.STR.0000147965.52712.fa. [DOI] [PubMed] [Google Scholar]
- 12.Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, et al. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST). J Am Coll Cardiol. 2002;39:610–616. doi: 10.1016/s0735-1097(01)01783-1. [DOI] [PubMed] [Google Scholar]
- 13.Yamada K, Yoshimura S, Kawasaki M, Enomoto Y, Asano T, Minatoguchi S, et al. Effects of atorvastatin on carotid atherosclerotic plaques: a randomized trial for quantitative tissue characterization of carotid atherosclerotic plaques with integrated backscatter ultrasound. Cerebrovasc Dis. 2009;28:417–424. doi: 10.1159/000235746. doi: 10.1159/000235746. [DOI] [PubMed] [Google Scholar]
- 14.Deguchi I, Horiuchi Y, Hayashi T, Sehara Y, Kato Y, Ohe Y, et al. Effects of rosuvastatin on serum lipids and arteriosclerosis in dyslipidemic patients with cerebral infarction. J Stroke Cerebrovasc Dis. 2014;23:2007–2011. doi: 10.1016/j.jstrokecerebrovasdis.2014.02.028. doi: 10.1016/j.jstrokecerebrovasdis.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Nagai Y, Kohriyama T, Origasa H, Minematsu K, Yokota C, Uchiyama S, et al. J-STARS Investigators. Rationale, design, and baseline features of a randomized controlled trial to assess the effects of statin for the secondary prevention of stroke: the Japan Statin Treatment Against Recurrent Stroke (J-STARS). Int J Stroke. 2014;9:232–239. doi: 10.1111/ijs.12099. doi: 10.1111/ijs.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosomi N, Nagai Y, Kohriyama T, Ohtsuki T, Aoki S, Nezu T, et al. J-STARS Collaborators. The Japan Statin Treatment Against Recurrent Stroke (J-STARS): a multicenter, randomized, open-label, parallel-group study. EBioMedicine. 2015;2:1071–1078. doi: 10.1016/j.ebiom.2015.08.006. doi: 10.1016/j.ebiom.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyoda K, Minematsu K, Yasaka M, Nagai Y, Hosomi N, Origasa H, et al. J-STARS Investigators. The Japan Statin Treatment Against Recurrent Stroke (J-STARS) Echo Study: rationale and trial protocol. J Stroke Cerebrovasc Dis. 2017;26:595–599. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.113. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.113. [DOI] [PubMed] [Google Scholar]
- 18.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 19.Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke. 1990;21:637–676.. doi: 10.1161/01.str.21.4.637. [DOI] [PubMed] [Google Scholar]
- 20.Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 2001;103:926–933. doi: 10.1161/01.cir.103.7.926. [DOI] [PubMed] [Google Scholar]
- 21.Crouse JR, 3rd, Raichlen JS, Riley WA, Evans GW, Palmer MK, O’Leary DH, et al. METEOR Study Group. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297:1344–1353. doi: 10.1001/jama.297.12.1344. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 22.Uchiyama S, Nakaya N, Mizuno K, Ohashi Y, Tajima N, Kushiro T, et al. MEGA Study Group. Risk factors for stroke and lipid-lowering effect of pravastatin on the risk of stroke in Japanese patients with hypercholesterolemia: analysis of data from the MEGA Study, a large randomized controlled trial. J Neurol Sci. 2009;284:72–76. doi: 10.1016/j.jns.2009.04.002. doi: 10.1016/j.jns.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Albert MA, Danielson E, Rifai N, Ridker PM PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 24.Furberg CD, Adams HP, Jr, Applegate WB, Byington RP, Espeland MA, Hartwell T, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation. 1994;90:1679–1687. doi: 10.1161/01.cir.90.4.1679. [DOI] [PubMed] [Google Scholar]
- 25.Fang N, Han W, Gong D, Zou Chen, Fan Y. Atorvastatin treatment for carotid intima-media thickness in Chinese patients with type 2 diabetes: a meta-analysis. Medicine (Baltimore) 2015;94:e1920. doi: 10.1097/MD.0000000000001920. doi: 10.1097/MD.0000000000001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–e281.. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013;12:1106–1114. doi: 10.1016/S1474-4422(13)70195-9. doi: 10.1016/S1474-4422(13)70195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nohara R, Daida H, Hata M, Kaku K, Kawamori R, Kishimoto J, et al. Justification for Atherosclerosis Regression Treatment (JART) Investigators. Effect of intensive lipid-lowering therapy with rosuvastatin on progression of carotid intima-media thickness in Japanese patients: Justification for Atherosclerosis Regression Treatment (JART) study. Circ J. 2012;76:221–229. doi: 10.1253/circj.cj-11-0887. [DOI] [PubMed] [Google Scholar]
- 29.Saito Y, Goto Y, Nakaya N, Hata Y, Homma Y, Naito C, et al. Dose-dependent hypolipidemic effect of an inhibitor of HMG-CoA reductase, pravastatin (CS-514), in hypercholesterolemic subjects. A double blind test. Atherosclerosis. 1988;72:205–211. doi: 10.1016/0021-9150(88)90082-2. [DOI] [PubMed] [Google Scholar]
- 30.Yoshino G, Kazumi T, Iwai M, Iwatani I, Matsuba K, Kasama T, et al. Effects of CS-514 on plasma lipids and lipoprotein composition in hypercholesterolemic subjects. Atherosclerosis. 1988;71:95–101. doi: 10.1016/0021-9150(88)90133-5. [DOI] [PubMed] [Google Scholar]
- 31.Kitagawa K, Hosomi N, Nagai Y, Kagimura T, Ohtsuki T, Origasa H, et al. J-STARS Investigators. Reduction in high-sensitivity c-reactive protein levels in patients with ischemic stroke by statin treatment: Hs-CRP sub-study in J-STARS. J Atheroscler Thromb. 2017;24:1039–1047. doi: 10.5551/jat.39354. doi: 10.5551/jat.39354. [DOI] [PMC free article] [PubMed] [Google Scholar]


