Supplemental Digital Content is available in the text.
Keywords: brain edema, cell death, inflammation, neuroprotection
Abstract
Background and Purpose—
Intracerebral hemorrhage (ICH) is a devastating disease without effective treatment. As a key component of the innate immune system, the NOD-like receptor (NLR) family, NLRP3 (pyrin domain–containing protein 3) inflammasome, when activated after ICH, promotes neuroinflammation and brain edema. MCC950 is a potent, selective, small-molecule NLRP3 inhibitor that blocks NLRP3 activation at nanomolar concentrations. Here, we examined the effect of MCC950 on brain injury and inflammation in 2 models of ICH in mice.
Methods—
In mice with ICH induced by injection of autologous blood or bacterial collagenase, we determined the therapeutic potential of MCC950 and its mechanisms of neuroprotection.
Results—
MCC950 reduced IL-1β (interleukin-1β) production and attenuated neurodeficits and perihematomal brain edema after ICH induction by injection of either autologous blood or collagenase. In mice with autologous blood-induced ICH, the protection of MCC950 was associated with reduced leukocyte infiltration into the brain and microglial production of IL-6. MCC950 improved blood–brain barrier integrity and diminished cell death. Notably, the protective effect of MCC950 was abolished in mice depleted of either microglia or Gr-1+ myeloid cells.
Conclusions—
These results indicate that the NLRP3 inflammasome inhibitor, MCC950, attenuates brain injury and inflammation after ICH. Hence, NLRP3 inflammasome inhibition is a potential therapy for ICH that warrants further investigation.
Intracerebral hemorrhage (ICH) is a devastating, often fatal aftermath of stroke, but not generally amenable to treatment.1–5 Emerging evidence has demonstrated that a stroke-incited inflammatory cascade accelerates the formation of edema that surrounds hematomas, exacerbates the mass effect, and amplifies cell death.4–8 After the ictus of ICH, blood components, including leukocytes, enter the brain and activate resident immune cells such as microglia. Subsequently, the infiltrating leukocytes and activated microglia boost the local production of proinflammatory factors. Together with cell death products, these catastrophic events amplify blood–brain barrier (BBB) disruption and destroy surrounding tissues, contributing to the development of perihematomal edema and the aggravated mass effect.4–8 Therefore, brain inflammation has emerged as a major modifiable determinant now targeted for the development of novel ICH treatment.7
As a critical component of the innate immune response to tissue injury, activation of the NOD-like receptor (NLR) NLRP3 (pyrin domain–containing protein 3) inflammasome leads to the cleavage of pro–IL (interleukin)-1β and pro–IL-18, as well as the subsequent release of biologically active IL-1β, IL-18, and other soluble mediators of inflammation. Recent evidence indicates that the ICH-induced activation of NLRP3 inflammasome can amplify the inflammatory response by releasing IL-1β and promoting neutrophil infiltration, thereby exacerbating brain edema.9–11 Although previous studies provide mechanistic insights into NLRP3 as a promising target for restricting neuroinflammation and brain edema after ICH, they do not reflect clinical application because they involve either knockout models or use of pharmacological agents with limited potency and nonspecificity preceding ICH induction.9–11 For clinical translation of prior experimental outcomes, preclinical testing in relevant models is mandatory. However, this option has been precluded by the lack of selective, potent NLRP3 inflammasome inhibitors.
Now, we have devised a means of clinically relevant testing in an experimental ICH model by using MCC950, a selective, small-molecule NLRP3 inflammasome inhibitor with proven high potency in vitro and in vivo. We hypothesized that the pharmacological inhibition of NLRP3 inflammasome by administration of MCC950 would reduce inflammation and brain edema after ICH induced by the injection of autologous blood or collagenase.
Materials and Methods
Details of materials and experimental procedures are available from the online-only Data Supplement. This article adheres to the AHA Journals implementation of the Transparency and Openness Promotion Guidelines.
Animals
All animal experiments were approved by the Committee on the Ethics of Animal Experiments of Barrow Neurological Institute (Phoenix, AZ). All experiments were conducted in accordance with the National Institutes of Health (Bethesda, MD) Guide for the Care and Use of Laboratory Animals, and experiments were designed, performed, and reported according to the Animal Research: Reporting In Vivo Experiments guidelines (https://www.nc3rs.org.uk/arrive-guidelines). Male C57BL/6 mice, 8 to 10 weeks old (20–25 g), were purchased from the Charles River Laboratories (Wilmington, MA). Female mice were not used to avoid any influences of sex steroids. All mice were housed in animal facilities under a standardized light–dark cycle and had free access to food and water. All animal surgeries were performed under anesthesia. Animals were randomly divided and assigned to experimental groups using a random number list generated by Microsoft Excel 2013.
Induction of ICH in Mice
ICH was induced in mice by injection of autologous blood or bacterial collagenase, as we previously described.12–16 Details of ICH induction are given in the online-only Data Supplement.
Study Design and Drug Administration
A total of 302 male C57BL/6 mice (Charles River Laboratories), 8 to 10 weeks old, were used in this study. In autologous blood model, the mortality rate was 8.5% (20 of total 236) and exclusion rate was 16.7% (36 of total 236). In the collagenase model, the mortality rate was 13.3% (8 of total 60) and exclusion rate was 20% (12 of total 60). Six wild-type mice were used for in vitro experiments. MCC950 was dissolved in phosphate-buffered saline, as previously described.17 Mice were given MCC950 at a dose of 10 mg/kg by intraperitoneal injection at indicated time points after ICH. Mice that received an equal volume of vehicle (phosphate-buffered saline) only were designated as controls. For the microglia depletion experiment, PLX3397 (Selleckchem, Houston, TX) was given by oral gavage at 40 mg/kg for 21 days before ICH induction.16,18 Drug treatment was continued until the end of experiments. Subsequently, anti-mouse Gr-1 monoclonal antibody (MAb-RB6-8C5; BioXcell, West Lebanon, NH) was given by intraperitoneal injection 1 day before and 1 day after ICH surgery at 250 µg per mouse to deplete Gr-1+ myeloid cells in vivo.19,20 In mice subjected to microglia depletion, no mice were excluded. More than 90% of microglia were depleted in mice receiving PLX3397. In mice subjected to Gr-1+ cell depletion, the exclusion rate was 5.9% (1 of total 17 because of death after surgery). The depletion of Gr-1+ cells was verified by sampling circulating blood, and ≈90% of Gr-1+ cells were depleted in all mice included.
Behavioral Assessment
Behavioral assessment was conducted at days 1 and 3 after ICH using the modified Neurological Severity Score together with corner-turning test to comprehensively evaluate motor, sensory, reflex, and balance functions, as previously described.21–24 The range of scores for modified Neurological Severity Score is from 0 to 18, defined as follows: a score of 13 to 18 indicates severe injury, 7 to 12 indicates moderate injury, and 1 to 6 indicates mild injury. Mice were given 1 point if they failed to perform a task. The corner-turning test assesses sensorimotor and postural asymmetries. In this process, each mouse is allowed to proceed into a corner with an angle of 30° and then must turn right or left. Each mouse repeated this procedure for 10×, with at least 30 s between trials. The percentage of right turns is then calculated.
Magnetic Resonance Imaging
Lesion volume and perihematomal edema were quantified at day 3 after ICH using a 7-T small animal MRI, as previously described.13,16 T2-weighted image sequence and susceptibility-weighted image sequence were scanned to detect lesion volume and hematoma volume, respectively. Details are described in the online-only Data Supplement.
Brain Water Content Measurement
Water contents were measured at day 3 after ICH, as previously described.25 In brief, without perfusion, brains were removed from mice and divided into 3 parts: the ipsilateral, the contralateral, and the cerebellum. The brain samples were weighed immediately on an electronic balance to obtain wet weights, then dried for 24 hours at 100°C, and weighed again to obtain dry weights. The following formula was used to calculate the brain water content: (wet weight−dry weight)/wet weight×100%.
Flow Cytometry
To quantify cells expressing NLRP3, microglia (CD11b+CD45int), neutrophils (CD11b+CD45highLy6G+), monocyte/macrophages (CD11b+CD45highLy6C+), CD4+ T cells (CD45highCD3+CD4+), CD8+ T cells (CD45highCD3+CD8+), B cells (CD45highCD3−CD19+), NK cells (CD45highCD3−NK1.1+), cytokines produced by microglia, and cell apoptosis (Annexin V+) in the brain, we isolated cellular components from brain tissue to perform flow cytometry analysis as we previously described.23,26 Details appear in the online-only Data Supplement.
Real-Time Polymerase Chain Reaction
At day 3 after ICH, total RNA was extracted from brain tissue in the ipsilateral hemisphere for real-time polymerase chain reaction analysis as described in the online-only Data Supplement.
Assessment of BBB Permeability
Evans Blue dye (Sigma, St. Louis, MO) extravasation assays were conducted at day 3 after ICH as a tracer to measure BBB permeability, as previously described.16,27
Western Blots
At day 3 after ICH, Western blots analyzed for NLRP3 inflammasome components and tight junction protein expression in the ipsilateral cerebral hemisphere, as we previously described.16 Details are given in the online-only Data Supplement.
Statistical Analysis
We determined each sample size by power analysis using a significance level of α=0.05 with 80% power to detect statistical differences. SAS 9.1 software (SAS Institute Inc, Cary, NC) was used for power analysis and sample size calculations. Data were analyzed by investigators blinded to experimental treatments. Data are shown as mean±SD. SPSS 19.0 software was used to compare differences between 2 groups where appropriate. One-way ANOVA followed by the Tukey post hoc test or 2-way ANOVA with multiple comparisons followed by Bonferroni post hoc test was used for comparison of multigroup data. P values <0.05 are considered significant.
Results
MCC950 Attenuates Brain Injury and Improves Long-Term Outcome After ICH
To determine whether the NLRP3 inflammasome inhibitor, MCC950, affects brain injury after ICH, we examined neurodeficits, lesion volume, and perihematomal edema in ICH mice receiving MCC950 or a phosphate-buffered saline vehicle. ICH was induced by injection of autologous blood or collagenase. Mice received MCC950 (10 mg/kg) or vehicle for 3 consecutive days starting immediately after ICH induction (Figure 1A). Neurological function was evaluated by using modified Neurological Severity Score and corner-turning tests at days 1 and 3 after ICH. Lesion volume, perihematomal edema, and brain water content were measured at day 3 after ICH. Compared with vehicle recipients, we found that MCC950-treated mice had significantly reduced neurodeficits, lesion volumes, and perihematomal edema after ICH (Figure 1B and 1C). MCC950 reduced brain water content after ICH (Figure I in the online-only Data Supplement). In addition, MCC950 reduces neurodeficits until day 28 after ICH induction (Figure 1D), suggesting that NLRP3 inflammasome inhibition can provide long-term benefit after ICH. Of note, the benefit of MCC950 to reduce neurodeficits and brain edema was restricted to within 24 hours after ICH (Figure II in the online-only Data Supplement).
Figure 1.
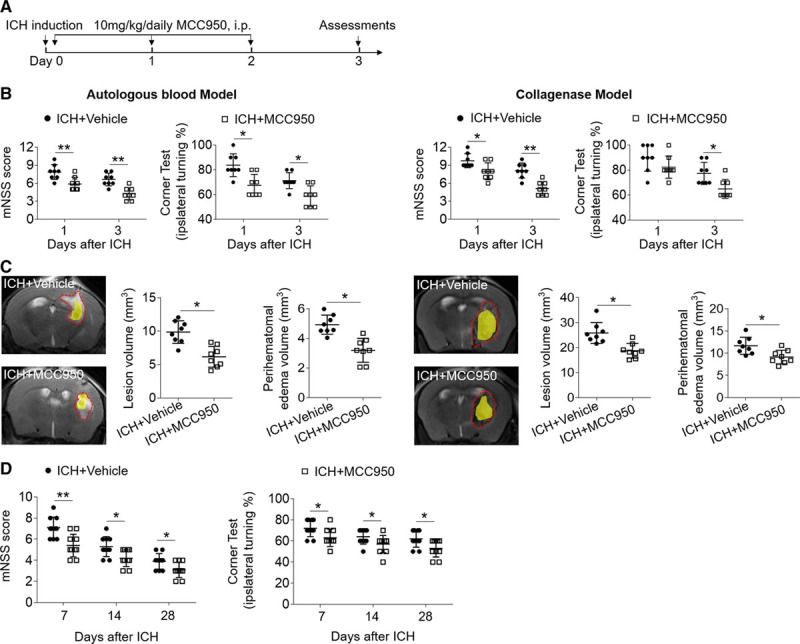
MCC950 attenuates brain injury and improves long-term outcome after intracerebral hemorrhage (ICH). ICH was induced in C57BL/6 mice by injection of autologous blood or collagenase. A, Flow chart illustrates MCC950 administration and experimental design. Mice received daily intraperitoneal (IP) injections of MCC950 (10 mg/kg) or an equal volume of phosphate-buffered saline (PBS) vehicle for 3 consecutive days starting immediately after ICH induction. B, Neurological tests were performed to evaluate the motor, sensory, and balance functions in mice receiving vehicle or MCC950 at days 1 and 3 after injection of autologous blood (left) or collagenase (right). C, T2-weighted image (T2WI) sequences were scanned to assess lesion volume at day 3 after ICH induced by injection of autologous blood (left) or collagenase (right), as outlined in red. Susceptibility-weighted sequences were assessed for hematoma lesion volume, visible in yellow regions. Quantification of lesion volume and perihematomal edema in mice receiving MCC950 or vehicle at day 3 after ICH induced by injection of autologous blood (left) or collagenase (right). n=8 mice per group. D, Mice received vehicle or MCC950 at a dose of 10 mg/kg by intraperitoneal injection. The assessments of modified Neurological Severity Score (mNSS) score and corner test were performed at days 7, 14, and 28 after ICH induced by injection of collagenase. n=10 per group. Data are presented as mean±SD. *P<0.05, **P<0.01.
MCC950 Inhibits the Activation of NLRP3 Inflammasome Components and IL-1β Production After ICH
The effect of MCC950 on NLRP3 inflammasome activation and IL-1β production was examined in brain tissues of ICH mice. At day 3 after ICH, we found that MCC950 reduced the mRNA expression of NLRP3 inflammasome components (NLRP3/Caspase-1/ASC) and IL-1β (Figure 2A). In addition, the protein expression of NLRP3, caspase-1, and IL-1β in the brain was suppressed by MCC950 treatment (Figure 2B and 2C). Of interest, MCC950 does not affect lipopolysaccharides-induced production IL-1β and TNF-α (tumor necrosis factor-α) from splenocytes (Figure III in the online-only Data Supplement). These results demonstrate that MCC950 effectively inhibits the activation of NLRP3 inflammasome components and IL-1β production in the brain after ICH.
Figure 2.
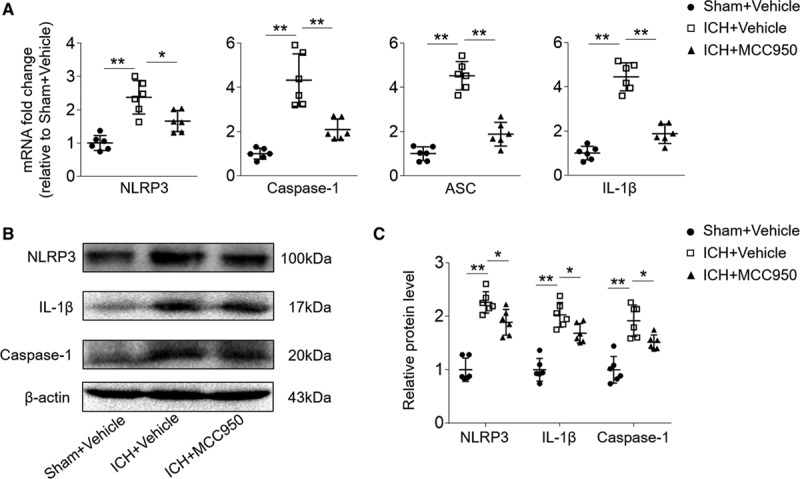
MCC950 inhibits NOD-like receptor (NLR) family, NLRP3 (pyrin domain-containing protein 3) inflammasome activation and IL (interleukin)-1β expression. Intracerebral hemorrhage (ICH) was induced in C57BL/6 mice by injection of autologous blood. Mice received daily intraperitoneal (IP) injections of MCC950 (10 mg/kg) or an equal volume of vehicle for 3 consecutive days starting immediately after ICH induction. At day 3 after ICH or sham surgery, brain tissues were harvested for the extraction of mRNA and protein. A, The mRNA expression levels of the NLRP3 inflammasome components and IL-1β after ICH. B, Western blot images show the expression of NLRP3, IL-1β, and caspase-1 in the brains of indicated groups of mice receiving sham plus vehicle, ICH plus vehicle, or ICH plus MCC950. C, Data points show the protein expression levels of NLRP3, IL-1β, and caspase-1 in the brains of indicated groups of mice receiving sham plus vehicle, ICH plus vehicle, or ICH plus MCC950. n=6 mice per group. Data are presented as mean±SD. *P<0.05, **P<0.01.
MCC950 Reduces Leukocyte Infiltration and Production of Proinflammatory Factors by Microglia After ICH
Next, we determined the impact of MCC950 on brain inflammation after ICH. Using flow cytometry, we examined such cellular components as brain-infiltrating leukocytes and microglia in the brains of ICH mice (Figure 3A). At day 3 post-ICH, the numbers of brain-infiltrating leukocytes, neutrophils (CD11b+CD45highLy6G+), monocyte/macrophages (CD11b+CD45highLy6C+), CD4+ T cells (CD45high CD3+CD4+), and CD8+ T cells (CD45high CD3+CD8+) were reduced in ICH mice receiving MCC950 compared with those in vehicle-treated controls (Figure 3B and 3C). Of note, MCC950 reduced the infiltration of CD4+ T cells, CD8+ T cells and neutrophils until day 7 after ICH (Figure IV in the online-only Data Supplement). Further, microglia cell numbers decreased as did microglial expression of factor IL-6 (Figure 3D). In contrast, the microglial expression of IL-10 and TGF-β (transforming growth factor-β) was increased in ICH mice receiving MCC950 (Figure 3D). These results indicate that MCC950 can reduce brain inflammation and cause a shift in microglia phenotype toward an anti-inflammatory status.
Figure 3.
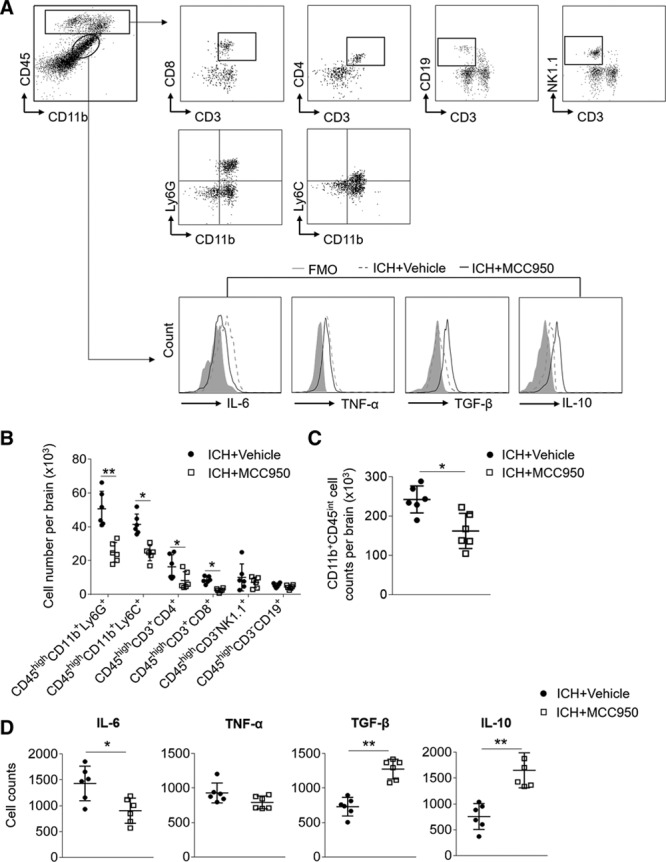
MCC950 reduces leukocyte infiltration and microglia production of proinflammatory factors after intracerebral hemorrhage (ICH). ICH was induced in C57BL/6 mice by injection of autologous blood. Mice received daily intraperitoneal (IP) injections of MCC950 (10 mg/kg) or an equal volume of vehicle for 3 consecutive days starting immediately after ICH induction. At day 3 after ICH, immune cells were isolated from brain tissues of ICH mice receiving MCC950 or vehicle. A, Gating strategy of brain-infiltrating immune cells including CD4+ T cells (CD3+ CD4+), CD8+ T cells (CD3+ CD8+), B cells (CD3−CD19+), NK cells (CD3−NK1.1+), monocyte/macrophages (CD11b+CD45highLy6C+), neutrophils (CD11b+CD45highLy6G+), and microglia (CD11b+CD45int) and their expression of IL (interleukin)-6, TNF-α (tumor necrosis factor-α), TGF-β (transforming growth factor-β), and IL-10. FMO, fluorescence minus one. B, Data points show counts of brain-infiltrating leukocytes in the brains of ICH mice receiving indicated treatment. C, Data points show counts of microglia in the brains of ICH mice receiving indicated treatment. D, Data points show the counts of microglia expressing IL-6, TNF-α, TGF-β, and IL-10 in the brains of ICH mice receiving indicated treatment. n=6 mice per group. Data are presented as mean±SD. *P<0.05, **P<0.01.
MCC950 Preserves BBB Integrity and Reduces Cell Death After ICH
BBB disruption after ICH contributes to vasogenic edema and the expansion of perihematomal edema.28 To measure the effect of MCC950 on BBB disruption after ICH, we examined Evans Blue dye extravasation and the expression of tight junction proteins. At day 3 after ICH, MCC950 significantly ameliorated leakage of the dye (Figure 4A), denoting a reduction of BBB permeability. MCC950 also preserved expression of the tight junction proteins (claudin-5 and ZO-1; Figure 4B). In addition, MCC950 reduced numbers of Annexin V–expressing cells in the brain at day 3 after ICH, suggesting a decrease in cell death (Figure 4C). Together, these results demonstrate that MCC950 preserves BBB integrity and reduces cell death after ICH.
Figure 4.
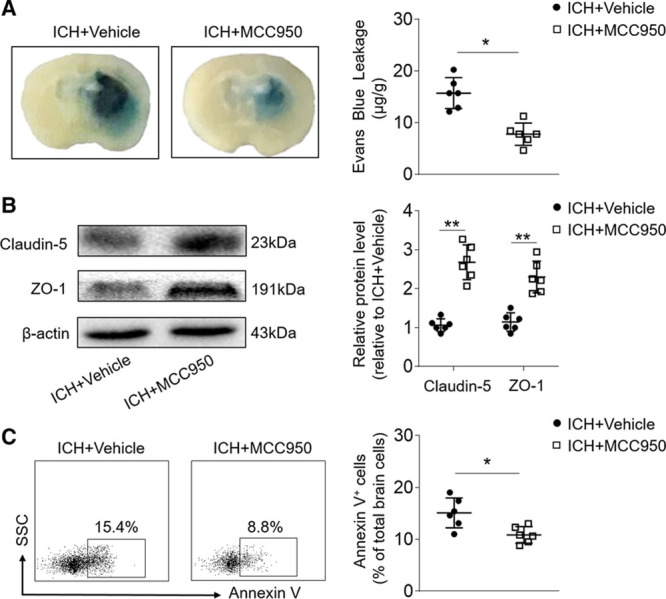
MCC950 treatment preserves blood–brain barrier (BBB) integrity and reduces cell death after intracerebral hemorrhage (ICH). ICH was induced in C57BL/6 mice by injection of autologous blood. Mice received daily intraperitoneal (IP) injections of MCC950 (10 mg/kg) or an equal volume of vehicle for 3 consecutive days starting immediately after ICH induction. At day 3 after ICH, brain tissues were prepared for measurements of BBB integrity and cell death. A, Histology images show that MCC950 decreased Evens Blue dye leakage compared with vehicle (phosphate-buffered saline [PBS]) treatment. B, Western blot images show expression of the tight junction proteins, claudin-5, and ZO-1, in the brains of ICH mice receiving MCC950 or vehicle. C, Flow cytometry plots and summarized results show percentages of Annexin V+ cells in the brains of ICH mice receiving MCC950 or vehicle. n=6 mice per group. Data are presented as mean±SD. *P<0.05, **P<0.01.
Benefit of MCCC950 Against ICH Involves Microglia and Gr-1+ Myeloid Cells
Because microglia are the predominant cell subset expressing NLRP3 inflammasome after ICH (Figure V in the online-only Data Supplement), we sought to understand to what extent microglia may contribute to the protective effect of MCC950. The survival of microglia depends on signaling through CSF1R (colony-stimulating factor 1 receptor); therefore, we used a CSF1R inhibitor, PLX3397, to deplete microglia before ICH induction (Figure 5A).29 Consistent with our previously described PLX3397 treatment, the present result was a loss of >90% microglia (CD11b+CD45int) from mice undergoing ICH.12 Of interest, we found that the benefit of MCC950 treatment was abolished in ICH mice receiving PLX3397 (Figure 5B and 5C), suggesting that microglia participate in the beneficial effect of MCC950 after ICH.
Figure 5.
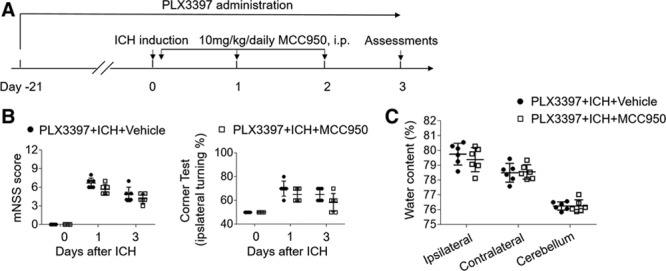
Microglia contribute to the benefit of MCC950 after intracerebral hemorrhage (ICH). A, Schematic diagram illustrates drug administration and experimental design. C57BL/6 mice received oral gavage of PLX3397 (40 mg/kg) for 21 days and continuously until the experiment ended. ICH was induced in PLX3397-treated mice by injection of autologous blood. Thereafter, these mice received daily injections of MCC950 (10 mg/kg, intraperitoneal [IP]) or an equal volume of vehicle for 3 consecutive days starting immediately after ICH induction. At day 3 after ICH, neurodeficits and brain water content were assessed. B, Quantification of modified Neurological Severity Score (mNSS) and corner-turning test scores in indicated groups of ICH mice. C, Quantification of brain water content in indicated groups of ICH mice. n=6 per group. Data are presented as mean±SD.
Because NLRP3 inflammasome was clearly expressed in Gr-1+ myeloid cells, consisting mainly of neutrophils and monocytes that are known contributors to BBB disruption and brain inflammation,30,31 we therefore determined whether Gr-1+ myeloid cells also add to the benefit of MCC950. An anti–Gr-1+ mAb (RB6-8C5) was used to deplete Gr-1+ myeloid cells from mice before ICH induction (Figure 6A). We monitored the counts of Gr-1+ cells in circulating blood at 24 hours before the first injection of anti–Gr-1 mAb and 48 hours after the second injection of anti–Gr-1 mAb (Figure 6B). Consistent with a previous report,32 anti–Gr-1+ mAb treatment depleted ≈90% of Gr-1+ myeloid cells (Figure 6B), that is, mainly neutrophils and monocytes. Of note, the depletion of Gr-1+ myeloid cells diminished the beneficial effects of MCC950 after ICH (Figure 6C and 6D), suggesting that the protection of MCC950 also involves Gr-1+ myeloid cells.
Figure 6.
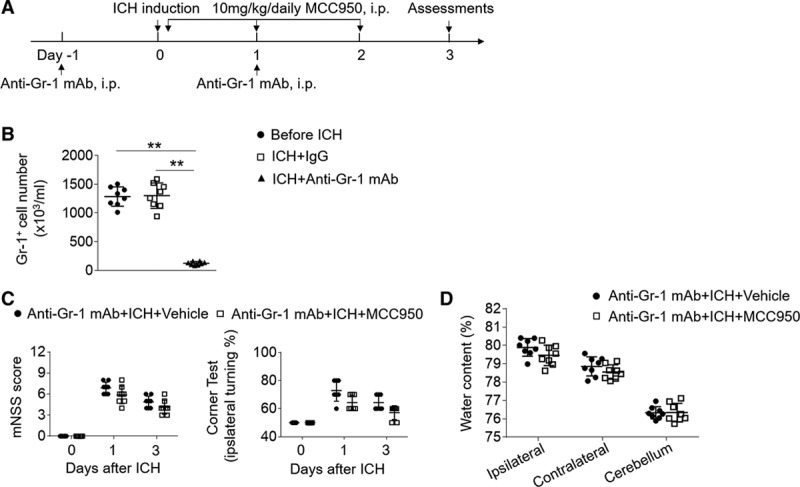
Gr-1+ myeloid cells contribute to the benefit of MCC950 after intracerebral hemorrhage (ICH). A, Schematic diagram illustrates drug administration and experimental design. C57BL/6 mice received anti–Gr-1 mAb 1 day before and 1 day after ICH surgery. ICH was induced by injection of autologous blood. Thereafter, these mice received daily injections of MCC950 (10 mg/kg, intraperitoneal [IP]) or an equal volume of vehicle for 3 consecutive days starting immediately after ICH induction. At day 3 after ICH, neurodeficits and brain water content were assessed. B, Data points show the counts of circulating Gr-1+ cells in mice before ICH induction, or mice receiving anti–Gr-1 mAb or IgG control at day 3 after ICH. C, Summarized results of modified Neurological Severity Score (mNSS) and corner-turning test scores in ICH mice receiving indicated treatments. D, Data points show brain water content in ICH mice receiving indicated treatments. n=8 mice per group. Data are presented as mean±SD.
Discussion
This study provides the first evidence that the NLRP3 inflammasome inhibitor, MCC950, attenuates hemorrhagic brain injury. MCC950 significantly reduced neurodeficits and perihematomal edema in 2 mouse models of ICH induced by injection of autologous blood or bacterial collagenase. MCC950 treatment was sufficient to reduce the resulting leukocyte infiltration, microglial production of proinflammatory factors, BBB disruption, and cell death after ICH. In addition, the benefit of MCC950 protection from ICH was diminished in mice subjected to depletion of microglia or Gr-1+ myeloid cells (neutrophils and monocytes). Together, these results suggest that MCC950 has potential therapeutic value for reducing ICH injury.
Mechanistic studies have shown an essential role for the NLRP3 inflammasome in brain injury and neuroinflammation after ICH.9–11 The activation of NLRP3 inflammasome facilitates caspase-1 activation and IL-1β processing, leading to the amplification of the inflammatory response, which culminates in the expansion of perihematomal edema, and thereby exacerbating hemorrhagic brain injury.9–11 As a small molecule, the NLRP3 inflammasome inhibitor, MCC950, was recently found to selectively inhibit NLRP3 inflammasome formation and reduce pyroptosis and IL-1β signaling.17 In the present study, our results confirmed the ability of MCC950 to effectively inhibit the activation of NLRP3 inflammasome components and IL-1β production in experimental ICH. In advancing previous findings,9–11 our study for the first time provides efficacy data depicting pharmacological inhibition by NLRP3 inflammasome in the setting of ICH in vivo.
Other than MCC950, several small molecules inhibit the NLRP3 inflammasome. For example, glyburide inhibited IL-1β production at micromolar concentrations in response to the activation of NLRP3 and was effective in reducing edema formation.33 Purinergic 2X7 receptor antagonist (blue brilliant G) also diminished NLRP3 inflammasome activation and lessened hemorrhagic brain injury.34 However, because of the limited potency and nonspecific nature of those agents, the extent to which pharmacologically selective targeting by NLRP3 inflammasome can impact ICH injury is still unclear. However, results from this study provide new evidence that selective NLRP3 inflammasome inhibition offers the benefit of reducing ICH injury and improving long-term outcome, which is an essential step for bringing NLRP3 inflammasome-targeted therapies from the bench into clinical application.
Reportedly, NLRP3 might present certain advantages over the use of biological inhibitors of IL-1β.17 Because MCC950 does not block the major antimicrobial inflammasome NLRC4 or NLRP1, specific targeting of NLRP3 will not result in the complete blockade of IL-1β in vivo, and antimicrobial responses may remain intact. In support of this view, we found that MCC950 does not affect lipopolysaccharides-induced production IL-1β and TNF-α from splenocytes. Considering the immunosuppression that follows stroke,35–37 MCC950 may have the advantage of less immunosuppressive effects than biologics such as canakinumab (anti–IL-1β monoclonal antibody), which increased the risk of these infections in a clinical setting.38,39
Inflammation of the brain and BBB dysfunction are acknowledged as key contributors to the expansion of perihematomal edema after ICH onset.4,28,40 In that context, we postulate that MCC950 may mitigate the brain’s inflammatory milieu to confer protection from the effects of ICH. In support of this view, we found that the benefit of MCC950 treatment was abolished in mice depleted of microglia via a CSF1R inhibitor. That outcome, together with the finding that NLRP3 inflammasome is expressed primarily in microglia after ICH, suggests that the benefit of MCC950 involves its action on microglia. Other than microglia, Gr-1+ myeloid cells (neutrophils and monocytes) also express NLRP3 inflammasome. Of interest, depletion of Gr-1+ myeloid cells using an anti–Gr-1 MAb also diminished the benefit of MCC950. Given the ability of microglia, neutrophils, and monocytes to disrupt the BBB and cause brain edema in ICH, these combined results reinforce the likelihood that the effect of MCC950 on microglia and Gr-1+ myeloid cells contributes to the benefit of MCC950 treatment after ICH. Nevertheless, the precise operating mechanisms by which MCC950 restricts brain inflammation after ICH remains uncertain and requires future investigation.
Conclusions
We have demonstrated that the NLRP3 inflammasome inhibitor, MCC950, attenuates brain injury and inflammation after ICH. Therefore, MCC950 may serve as a promising candidate for further investigation in advanced preclinical ICH studies.
Disclosures
None.
Sources of Funding
This study was supported in part by American Heart Association grant 16SDG27250236; National Science Foundation of China grant 81471535; and Ministry of Human Resources and Social Security of China grant.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.117.018904/-/DC1.
References
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, et al. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol. 2014;115:64–91. doi: 10.1016/j.pneurobio.2013.09.002. doi: 10.1016/j.pneurobio.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–731. doi: 10.1016/S1474-4422(12)70104-7. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Arima H, Yang J, Zhang S, Wu G, Woodward M, et al. INTERACT2 Investigators. Mannitol and outcome in intracerebral hemorrhage: propensity score and multivariable intensive blood pressure reduction in acute cerebral hemorrhage trial 2 results. Stroke. 2015;46:2762–2767. doi: 10.1161/STROKEAHA.115.009357. doi: 10.1161/STROKEAHA.115.009357. [DOI] [PubMed] [Google Scholar]
- 6.Kuramatsu JB, Huttner HB, Schwab S. Advances in the management of intracerebral hemorrhage. J Neural Transm (Vienna) 2013;120(suppl 1):S35–S41. doi: 10.1007/s00702-013-1040-y. doi: 10.1007/s00702-013-1040-y. [DOI] [PubMed] [Google Scholar]
- 7.Sheth KN, Rosand J. Targeting the immune system in intracerebral hemorrhage. JAMA Neurol. 2014;71:1083–1084. doi: 10.1001/jamaneurol.2014.1653. doi: 10.1001/jamaneurol.2014.1653. [DOI] [PubMed] [Google Scholar]
- 8.Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9:971–988. doi: 10.1038/nrd3295. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- 9.Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol. 2014;75:209–219. doi: 10.1002/ana.24070. doi: 10.1002/ana.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan B, Shen H, Lin L, Su T, Zhong S, Yang Z. Recombinant adenovirus encoding NLRP3 RNAi attenuate inflammation and brain injury after intracerebral hemorrhage. J Neuroimmunol. 2015;287:71–75. doi: 10.1016/j.jneuroim.2015.08.002. doi: 10.1016/j.jneuroim.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Yao ST, Cao F, Chen JL, Chen W, Fan RM, Li G, et al. NLRP3 is required for complement-mediated caspase-1 and IL-1beta activation in ICH. J Mol Neurosci. 2017;61:385–395. doi: 10.1007/s12031-016-0874-9. doi: 10.1007/s12031-016-0874-9. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Li Z, Ren H, Jin WN, Wood K, Liu Q, et al. Colony stimulating factor 1 receptor inhibition eliminates microglia and attenuates brain injury after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2017;37:2383–2395. doi: 10.1177/0271678X16666551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun N, Shen Y, Han W, Shi K, Wood K, Fu Y, et al. Selective sphingosine-1-phosphate receptor 1 modulation attenuates experimental intracerebral hemorrhage. Stroke. 2016;47:1899–1906. doi: 10.1161/STROKEAHA.115.012236. doi: 10.1161/STROKEAHA.115.012236. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Rogove AD, Tsirka AE, Tsirka SE. Protective role of tuftsin fragment 1-3 in an animal model of intracerebral hemorrhage. Ann Neurol. 2003;54:655–664. doi: 10.1002/ana.10750. doi: 10.1002/ana.10750. [DOI] [PubMed] [Google Scholar]
- 15.Grossetete M, Rosenberg GA. Matrix metalloproteinase inhibition facilitates cell death in intracerebral hemorrhage in mouse. J Cereb Blood Flow Metab. 2008;28:752–763. doi: 10.1038/sj.jcbfm.9600572. doi: 10.1038/sj.jcbfm.9600572. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Ren H, Sheth KN, Shi FD, Liu Q. A TSPO ligand attenuates brain injury after intracerebral hemorrhage. FASEB J. 2017;31:3278–3287. doi: 10.1096/fj.201601377RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. doi: 10.1038/nm.3806. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stafford JH, Hirai T, Deng L, Chernikova SB, Urata K, West BL, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016;18:797–806. doi: 10.1093/neuonc/nov272. doi: 10.1093/neuonc/nov272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Hong LJ, Huang JY, Jiang Q, Tao RR, Tan C, et al. P2RX7 sensitizes Mac-1/ICAM-1-dependent leukocyte-endothelial adhesion and promotes neurovascular injury during septic encephalopathy. Cell Res. 2015;25:674–690. doi: 10.1038/cr.2015.61. doi: 10.1038/cr.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Condamine T, Kumar V, Ramachandran IR, Youn JI, Celis E, Finnberg N, et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J Clin Invest. 2014;124:2626–2639. doi: 10.1172/JCI74056. doi: 10.1172/JCI74056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Jin WN, Liu Y, Shi K, Sun H, Zhang F, et al. Brain ischemia suppresses immunity in the periphery and brain via different neurogenic innervations. Immunity. 2017;46:474–487. doi: 10.1016/j.immuni.2017.02.015. doi: 10.1016/j.immuni.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Jin WN, Shi SX, Li Z, Li M, Wood K, Gonzales RJ, et al. Depletion of microglia exacerbates postischemic inflammation and brain injury. J Cereb Blood Flow Metab. 2017;37:2224–2236. doi: 10.1177/0271678X17694185. doi: 10.1177/0271678X17694185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou K, Zhong Q, Wang YC, Xiong XY, Meng ZY, Zhao T, et al. Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3beta/PTEN axis. J Cereb Blood Flow Metab. 2017;37:967–979. doi: 10.1177/0271678X16648712. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Liu Q, Sanai N, Jin WN, La Cava A, Van Kaer L, Shi FD. Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation. Nat Neurosci. 2016;19:243–252. doi: 10.1038/nn.4211. doi: 10.1038/nn.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Han B, He Y, Li D, Ma X, Liu Q, et al. MicroRNA-132 attenuates neurobehavioral and neuropathological changes associated with intracerebral hemorrhage in mice. Neurochem Int. 2017;107:182–190. doi: 10.1016/j.neuint.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Urday S, Kimberly WT, Beslow LA, Vortmeyer AO, Selim MH, Rosand J, et al. Targeting secondary injury in intracerebral haemorrhage–perihaematomal oedema. Nat Rev Neurol. 2015;11:111–122. doi: 10.1038/nrneurol.2014.264. doi: 10.1038/nrneurol.2014.264. [DOI] [PubMed] [Google Scholar]
- 29.Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond MD, Taylor RA, Mullen MT, Ai Y, Aguila HL, Mack M, et al. CCR2+ Ly6C(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci. 2014;34:3901–3909. doi: 10.1523/JNEUROSCI.4070-13.2014. doi: 10.1523/JNEUROSCI.4070-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sansing LH, Harris TH, Kasner SE, Hunter CA, Kariko K. Neutrophil depletion diminishes monocyte infiltration and improves functional outcome after experimental intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:173–178. doi: 10.1007/978-3-7091-0693-8_29. doi: 10.1007/978-3-7091-0693-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 33.Sheth KN, Kimberly WT, Elm JJ, Kent TA, Yoo AJ, Thomalla G, et al. Exploratory analysis of glyburide as a novel therapy for preventing brain swelling. Neurocrit Care. 2014;21:43–51. doi: 10.1007/s12028-014-9970-2. doi: 10.1007/s12028-014-9970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng L, Chen Y, Ding R, Fu Z, Yang S, Deng X, et al. P2X7R blockade prevents NLRP3 inflammasome activation and brain injury in a rat model of intracerebral hemorrhage: involvement of peroxynitrite. J Neuroinflammation. 2015;12:190. doi: 10.1186/s12974-015-0409-2. doi: 10.1186/s12974-015-0409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu Y, Liu Q, Anrather J, Shi FD. Immune interventions in stroke. Nat Rev Neurol. 2015;11:524–535. doi: 10.1038/nrneurol.2015.144. doi: 10.1038/nrneurol.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinarello CA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. 2013;25:469–484. doi: 10.1016/j.smim.2013.10.008. doi: 10.1016/j.smim.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.López-Castejón G, Pelegrín P. Current status of inflammasome blockers as anti-inflammatory drugs. Expert Opin Investig Drugs. 2012;21:995–1007. doi: 10.1517/13543784.2012.690032. doi: 10.1517/13543784.2012.690032. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. doi: 10.1016/j.pneurobio.2013.11.003. [DOI] [PubMed] [Google Scholar]


