Supplemental Digital Content is available in the text.
Keywords: acute stroke care, intracranial hemorrhages, stroke, telemedicine, time-to-treatment, tissue-type plasminogen activator
Abstract
Background and Purpose—
Faster treatment with intravenous alteplase in acute ischemic stroke is associated with better outcomes. Starting in 2015, Kaiser Permanente Northern California redesigned its acute stroke workflow across all 21 Kaiser Permanente Northern California stroke centers to (1) follow a single standardized version of a modified Helsinki model and (2) have all emergency stroke cases managed by a dedicated telestroke neurologist. We examined the effect of Kaiser Permanente Northern California’s Stroke EXpediting the PRrocess of Evaluating and Stopping Stroke program on door-to-needle (DTN) time, alteplase use, and symptomatic intracranial hemorrhage rates.
Methods—
The program was introduced in a staggered fashion from September 2015 to January 2016. We compared DTN times for a seasonally adjusted 9-month period at each center before implementation to the corresponding 9-month calendar period from the start of implementation. The primary outcome was the DTN time for alteplase administration. Secondary outcomes included rate of alteplase administrations per month, symptomatic intracranial hemorrhage, and disposition at time of discharge.
Results—
This study included 310 patients treated with alteplase in the pre–EXpediting the PRrocess of Evaluating and Stopping Stroke period and 557 patients treated with alteplase in the EXpediting the PRrocess of Evaluating and Stopping Stroke period. After implementation, alteplase administrations increased to 62/mo from 34/mo at baseline (P<0.001). Median DTN time decreased to 34 minutes after implementation from 53.5 minutes prior (P<0.001), and DTN time of <60 minutes was achieved in 87.1% versus 61.0% (P<0.001) of patients. DTN times <30 minutes were much more common in the Stroke EXpediting the PRrocess of Evaluating and Stopping Stroke period (40.8% versus 4.2% before implementation). There was no significant difference in symptomatic intracranial hemorrhage rates in the 2 periods (3.8% versus 2.2% before implementation; P=0.29).
Conclusions—
Introduction of a standardized modified Helsinki protocol across 21 hospitals using telestroke management was associated with increased alteplase administrations, significantly shorter DTN times, and no increase in adverse outcomes.
More rapid treatment of acute ischemic stroke patients with intravenous alteplase is associated with improved outcomes.1,2 Because of the importance of earlier treatment with intravenous alteplase, the highest recognition in the American Heart Association/American Stroke Association’s Target: Stroke initiative is currently awarded to primary stroke centers (PSC) who achieve a door-to-needle (DTN) time for alteplase administration within 60 minutes in ≥75% of patients with acute ischemic stroke and within 45 minutes in ≥50% of these patients.3 Several approaches to reducing DTN times for alteplase administration have been studied, including in-hospital system-level bundles, such as the Helsinki model4,5 and use of centralized decision making via remote digital-network neurological specialty consultation (telestroke care).6–12 Although the Helsinki model has been found to significantly reduce DTN times,4,5 it remains unclear whether telestroke initiatives can have an additional impact on treatment times.10,11,13
The Helsinki stroke thrombolysis model includes 12 measures designed to streamline the process of alteplase administration in ischemic stroke, including hospital prenotification by emergency services, direct transfer of patients for computed tomography (CT) immediately after rapid neurological evaluation in the emergency department (ED), premixing of alteplase, rapid interpretation of CT results, and administration of alteplase in the CT suite.4,5 The model has been shown to improve DTN time for alteplase in single-center studies,4,5 but an implementation of these model components has not yet been reported for a large-scale system of care. Telestroke interventions allow centralization of expertise to a small number of stroke-trained neurologists by connecting the patient and stroke expert virtually using a secure video link and remote evaluation of medical imaging.11,12,14–17 The Helsinki model and telestroke have conceptually independent advantages, but, to date, the results of combining these 2 methods in a large number of hospitals have not been reported.
Here, we present the results of the Kaiser Permanente Northern California (KPNC) Stroke EXpediting the PRocess of Evaluating and Stopping Stroke (EXPRESS) program. Stroke EXPRESS combines a modified Helsinki protocol with a centralized telestroke service across 21 community hospitals in an integrated care delivery system that serves >4 million members.
Methods
Study Setting
KPNC provides care in 21 hospitals and 60 clinics, with ≈17 million outpatient and ED visits and 250 000 hospitalizations each year (including ≈3500 new stroke discharges per year). Patients who are demographically similar to the overall population of Northern California are cared for by a single provider group, The Permanente Medical Group, with 8500 physicians (including 112 neurologists). All 21 KPNC hospitals are Joint Commission–certified PSC and 2 are certified comprehensive stroke centers providing endovascular stroke treatment.
Intervention
Before Stroke EXPRESS, the process of patient evaluation and alteplase decision making occurred in a serial fashion with the initial evaluation performed by the ED physician and imaging studies completed typically before obtaining the local on-call neurologist consultation (Figure I in the online-only Data Supplement). No telestroke was used before the Stroke EXPRESS.
The standardized protocol used in Stroke EXPRESS included ambulance prenotification, regional electronic medical record medical history review, rapid neurological evaluation, prenotification of ambulance transport for possible transfer for endovascular stroke treatment, rapid transport to the CT suite, and administration of alteplase in the CT suite (Table 1). The telestroke system connects all 21 hospitals to a single teleneurologist from the Stroke EXPRESS team who takes dedicated acute stroke call for all hospitals. For patient arrivals via ambulance, an ED staff contacts the on-call teleneurologist by calling a centralized number after being notified by prehospital providers of possible incoming acute stroke. For patient arrivals via walk-in, the triage nurse would activate the stroke alert and call teleneurology. The teleneurologist then performs remote video neurological examination together with the on-site ED physician and supervises all aspects of acute stroke care using a single standardized protocol for all hospitals. The teleneurologist would order alteplase to be mixed and hold if the patient meets all criteria except for completion of the noncontrast head CT scan. The telemedicine platform for all of the EDs is mobile and allows the stroke expert to virtually move with the patient from the ED to the CT suite and back. The Stroke EXPRESS program was rolled out in a staggered fashion to each of the 21 KPNC medical centers and was available during the hours of 7 am to midnight at each center during the study period. The program was not operated overnight because of a combination of resource considerations and few stroke arrivals eligible for intravenous alteplase in the midnight to 7 am time period. Between midnight and 7 am, acute stroke evaluations were made by the ED physician and the local on-call neurologist in an identical fashion to the pre-EXPRESS workflow.
Table 1.
Elements of the Stroke EXPRESS Program
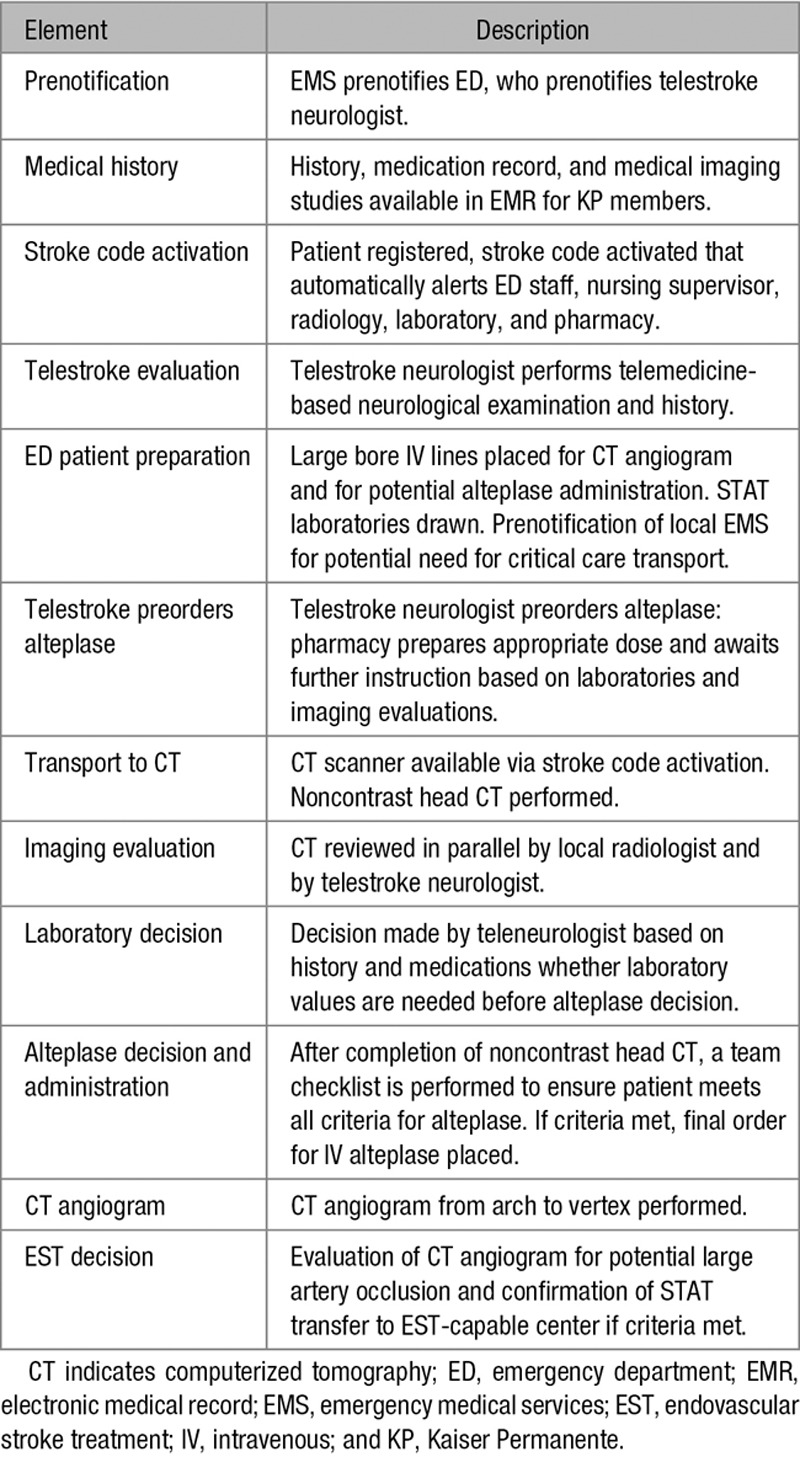
Each stroke center underwent an implementation training day with a regional simulation team. Role cards for each member of an acute stroke treatment team (including ED physician, teleneurologist, primary nurse, ED manager, radiology technician, radiologist, etc) were developed and used during all training and actual stroke alerts. Each teleneurologist underwent the same protocol training and conducted observed mock stroke alerts before taking part in the Stroke EXPRESS program. Monitoring of the program during the study period was performed at weekly telestroke meetings and monthly local stroke committee meetings, as well as at the regional healthcare system level.
It should be noted that our telemedicine model differs from the traditional hub-and-spoke model. In Stroke EXPRESS, there is no single hospital serving as the hub. The program is based on the centralization of expertise of stroke neurologists recruited from across the network serving as a virtual hub, not a physical hub. The number of teleneurologists ranged from 9 to 12 during the study period. In addition, the teleneurologists use a single standardized protocol to handle all stroke alerts from all centers.
Data Source and Subjects
The data that support the findings of this study are available from the corresponding author on reasonable request. Data for the present study were extracted from the electronic medical record and associated relational databases as part of a quality initiative to evaluate Stroke EXPRESS. Nine months of EXPRESS data were extracted from the electronic medical record, starting with the staggered go-live date for each center, and were compared with data extracted for the 9-month period before implementation, matched for calendar dates to account for any potential seasonal variations. Cases were included for analysis only if the ED triage time was between 7 am and midnight for both periods because this was the availability time for Stroke EXPRESS for the study period.
To ensure accurate data on medical history, comorbidities, and outcomes, only enrolled KPNC members were included in the present analysis.
Permission to publish fully anonymized, aggregate data from this quality initiative, with waiver of the need for institution review board evaluation and waiver of individual patient consent, was granted by the KPNC Research Determination officer. This research was conducted according to the principles of the Declaration of Helsinki.
Measurements
For each patient, we collected basic demographic information (age, sex, self-reported race/ethnicity), last known well time (in the post-EXPRESS cohort), telestroke activation time, ED arrival time, alteplase bolus time, medical comorbidities (hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, congestive heart failure), Charlson comorbidity index,18 modified National Institutes of Health Stroke Scale (mNIHSS) score on arrival and mNIHSS score19 recorded during hospitalization, mode of ED arrival, history prior stroke or transient ischemic attack, length of inpatient stay, and hospital discharge status. Symptomatic intracerebral hemorrhage was determined based on European Cooperative Acute Stroke Study criteria: any hemorrhage on repeat CT scan performed up to 48 hours from presentation associated with at least a 4-point increase in the mNIHSS.20 The mNIHSS differs in only minor ways from the original scale and has been extensively validated against the parent scale21; we have previously reported its use in the context of our KPNC data source.22
Statistical Analysis
Univariable analyses comparing subjects in the pre-EXPRESS and EXPRESS periods were performed with the Fisher exact test for categorical variables and the nonparametric Kruskal–Wallis equality-of-populations rank test for continuous data. The numbers of alteplase administrations in the 2 study periods were compared with simple Poisson regression.
Multivariable analyses were conducted with mixed-effects linear and logistic models, allowing random intercepts for medical center. Models included adjustment for age, sex, race, baseline NIHSS score, Charlson comorbidity index, mode of ED arrival (ambulance/other), history of hypertension, atrial fibrillation, coronary heart disease, diabetes mellitus, heart failure, and prior stroke.
Statistical analyses were performed with SAS version 9.3 (SAS, Cary, NC) and Stata, version 14.1 (Stata, College Station, TX).
Results
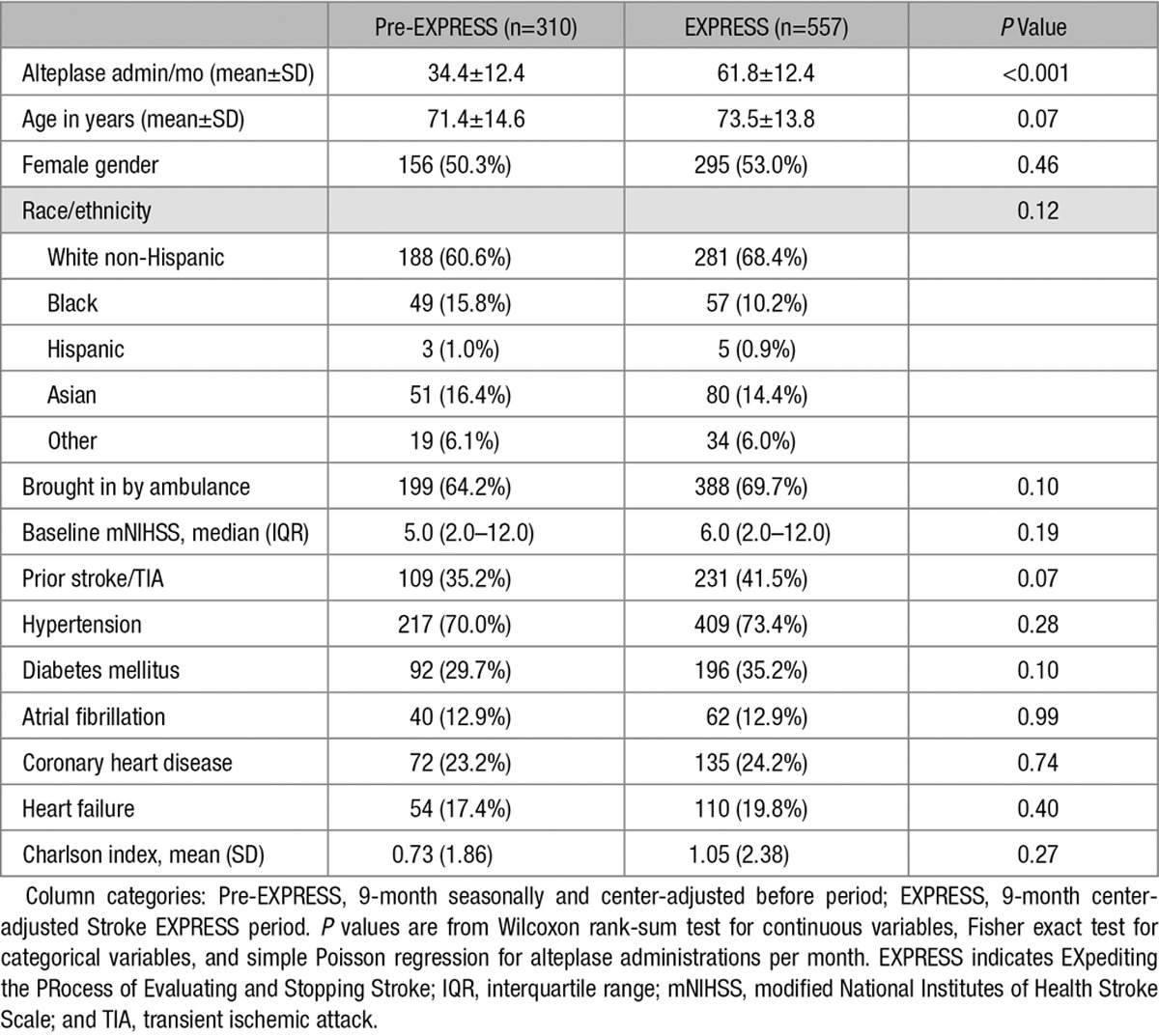
There were 310 patients who received alteplase during the pre-EXPRESS period and 557 patients who received alteplase during the corresponding EXPRESS period (difference in alteplase administrations per month, P<0.001; Table 2; Figure I in the online-only Data Supplement). On average, there were 8.8 stroke alerts daily in the pre-EXPRESS period compared with 11.7 stroke alerts daily in the EXPRESS period across 21 centers. The rates of alteplase administration were 13.1% (310 cases/2375 stroke alerts) and 17.6% (557 cases/3168 stroke alerts; P<0.001), respectively. There were no significant differences in patient characteristics between these 2 alteplase cohorts for demographics, mode of ED arrival, baseline mNIHSS, and comorbidities (Table 2). Among those who received alteplase, there were 12 (3.87%) in the pre-EXPRESS period who did not have a discharge diagnosis of stroke (ie, stroke mimics) compared with 38 (6.82%) in the EXPRESS period (P=0.04).
Table 2.
Patient Characteristics
Both continuous and categorical DTN times decreased significantly in the Stroke EXPRESS period (Tables 3 and 4; Figure). Mean DTN time decreased to 41.8 minutes from 63.2 minutes, and median DTN time decreased to 34.0 minutes from 53.5 minutes (P<0.001; Table 3). The percentage of cases treated within 30 minutes increased to 40.8% from 4.2% (P<0.001; Table 4). In addition, median DTN times decreased consistently across nearly all medical centers regardless of volumes of alteplase cases (Figure II in the online-only Data Supplement).
Table 3.
Time Between ED Arrival and Alteplase Administration (Continuous Door-to-Needle Time in Minutes)
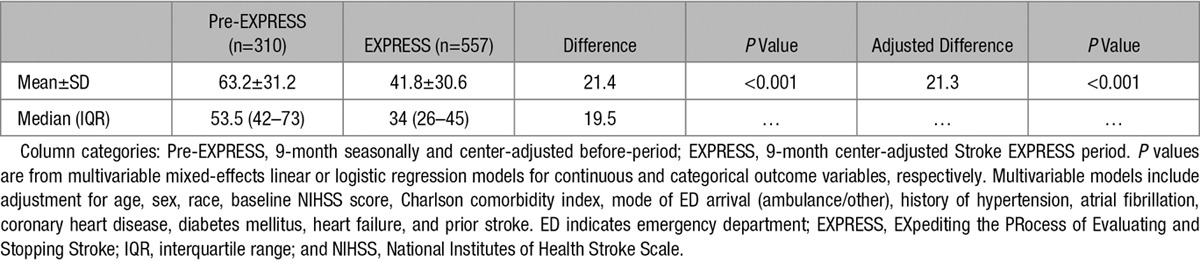
Table 4.
Time Between ED Arrival and Alteplase Administration (Categorical Door-to-Needle Time in Minutes)
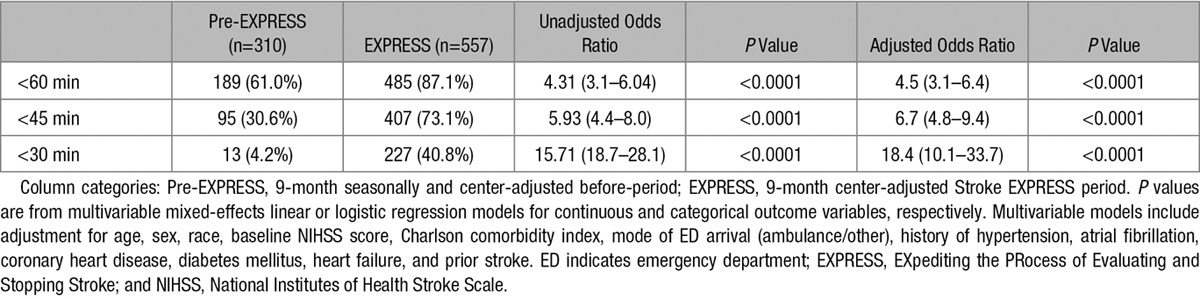
Figure.
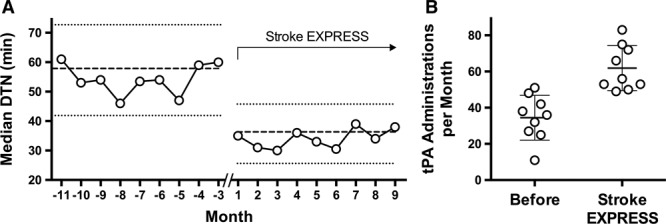
Impact of Stroke EXpediting the PRocess of Evaluating and Stopping Stroke (EXPRESS) program on door-to-needle (DTN) times and alteplase use. A, Median DTN time in minutes for each month across the 2 periods. The scale of the x axis is centered on 1 for the first month of the Stroke EXPRESS program introduction at each center, with a 3-month gap between the before-period and the Stroke EXPRESS period (interrupted x axis line). Dashed horizontal lines correspond to the overall median DTN time for each period, flanked by horizontal dotted lines indicating the interquartile range. B, Alteplase administrations per month in the before period and Stroke EXPRESS period. Circles correspond to the number of alteplase administrations in each month, overlaid on the mean (solid lines)±SD (thin lines) for each period. tPA indicates tissue-type plasminogen activator.
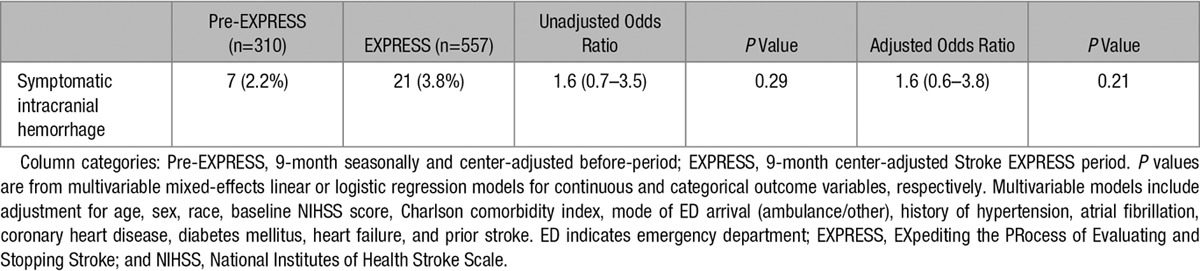
Rates of symptomatic intracerebral hemorrhage during the 2 study periods were not significantly different (3.8% versus 2.2% before implementation; P=0.29; Table 5). We performed a sensitivity analysis on DTN times for more appropriately treated patients by removing the stroke mimics and found the median DTN times similar to those seen with the primary analysis, decreased from 53 to 34 minutes (P<0.001).
Table 5.
Rates of Symptomatic Intracranial Hemorrhage
Although we required Kaiser Permanente membership for the primary analyses reported here (to ensure complete capture of comorbidities and other information), secondary analysis comparing Kaiser Permanente members and nonmembers treated in the same Stroke EXPRESS period at each hospital showed similar mean DTN times (41.8±30.6 minutes for Kaiser Permanente members [n=557] versus 40.0±33.1 minutes for nonmembers [n=141]; P=0.54). In addition, we also observed a significant decrease in the mean DTN time for nonmembers in the EXPRESS period compared with pre-EXPRESS period (40±33.1 versus 61.2±36 minutes; P<0.001).
During the study period, teleneurology was only available from 7 am to midnight. Sensitivity analysis for the hours from midnight to 7 am showed 20 treated cases in the pre-EXPRESS period and 43 cases in the post-EXPRESS period and a smaller and nonsignificant difference in mean DTN times in pre-EXPRESS compared with EXPRESS period (69.2±34.7 versus 56±35.7 minutes; P=0.09).
There was no significant difference in the median length of hospital stay (3.1 versus 3.5 days before implementation; P=0.14) or discharge disposition to home (54.8% versus 55.4% before implementation; P=0.83) between the periods.
Discussion
We found that a standardized system combining a modified Helsinki model with a multicenter telestroke program led to increased alteplase use and substantially shorter DTN times among patients with acute ischemic stroke. This success was observed across nearly all medical centers regardless of volumes of treated stroke. Despite a 72% relative increase in the symptomatic intracerebral hemorrhage rate, this was not a statistically significant increase in the setting of 967 total patients treated. There was a small increase in the number of possible stroke mimics, which may have resulted from the increased speed of the new workflow, but this would not be sufficient to explain the larger increase in alteplase usage.
Overall rates of alteplase administration for ischemic stroke are increasing in the United States,23,24 but remain low, from 4% to 6% as of 2010.24 Even among patients presenting to the ED within 2 hours of ischemic stroke onset, ≈25% are not treated with alteplase.25 The main reason reported for nontreatment is mild or rapidly improving stroke.25
Earlier treatment of acute ischemic stroke patients with intravenous thrombolysis leads to improved outcomes,1,2 particularly when alteplase is administered in the first hour after stroke onset.26 Despite these goals, rapid treatment with alteplase shortly after hospital arrival remains elusive in many academic and community settings, with a large number of parallel factors influencing DTN, including presence or lack or ambulance prenotification systems,27 time to imaging performance,28 laboratory protocols, and presence or absence of alteplase premixing for high-probability alteplase candidates. Paradoxically, patients presenting earlier after stroke onset have been reported to have longer DTN times.29
Several system-level interventions have been explored in attempts to reduce time delays in thrombolytic administration.4,5,30–32 The Helsinki model has been the most successful of these interventions.4,5 The initial experience in a Helsinki hospital reduced DTN times significantly,4 and external replication in a Melbourne hospital achieved similar results.5 To date, the experience with the Helsinki model has been limited to single-center efforts.
The Target: Stroke initiative by the American Heart Association/American Stroke Association has been introduced in many certified PSC in the United States This was a national quality improvement initiative focused on reducing DTN times for eligible patients being treated with intravenous alteplase. Despite involving only broad guidance and recommendations across >1000 centers, and an award recognition program, Target: Stroke has also been found to reduce DTN times.30 Participating Target: Stroke hospitals reduced average DTN time from 74 to 59 minutes and increased alteplase administrations within 60 minutes from 29.6% to 53.3%.30
Telestroke care has also been used successfully for management of acute stroke treatment in a variety of settings, with a focus on bringing stroke expertise to more remote or underserved areas.6,7,11 A horizontal networking approach of a hubless system of stroke specialists across multiple hospitals has been previously reported from the British National Health Service.33 Overall, telemedicine may be associated with improved alteplase use, but it remains unclear whether telemedicine can also substantially reduce DTN times.10,11,13 In 1 randomized trial of telemedicine consultation versus telephone consultation, there was no difference in alteplase use despite a higher percentage of correct treatment decisions made in the telemedicine group.13
The Stroke EXPRESS program reported here takes a modified Helsinki protocol for acute stroke workflows and scales it to 21 hospitals in an integrated care delivery system by using telestroke to provide standardized, efficient care across the network. Because the whole program was rolled out as a bundle of multiple interventions, we could not identify one or more specific elements as being responsible for the dramatic improvement in DTN time and increased alteplase usage. However, the biggest changes made in this program on acute stroke alert management included a reduction in the time between ED arrival and stroke neurologist’s involvement in the case and reduction in ED arrival to order to mix alteplase. In the pre-EXPRESS period, a stroke alert activation and a call to the neurologist were not made until after the ED physician had evaluated the potential stroke patient. The new protocol called for immediate activation of stroke alert and call to telestroke for all potential acute stroke cases. Furthermore, no alteplase mix and hold was ever ordered before the completion of a head CT scan with the old protocol because such mechanism was not available pre-EXPRESS. Having access to early electronic medical record review in KPNC members was an added benefit, but this was not a required part of reducing DTN time as shown by the similar DTN time results for non-KPNC members. The successful rollout of this program demonstrates that telemedicine may have roles beyond the traditional hub-and-spoke model: we find that a large group of hospitals in an integrated system can collectively create a stroke telemedicine network that is virtually, but not physically, centralized.
Our study has limitations. This is a retrospective analysis of data collected prospectively from a multicenter quality initiative, and randomization did not occur at the patient, physician, or hospital level. The results can only be generalizable to acute stroke care provided during the hours of 7 am to midnight because telestroke was not available from midnight to 7 am during the study period. However, there was a trend for decreased DTN time overnight post-EXPRESS suggesting a possible influence of the new workflow even in the absence of teleneurologist. This was a before-and-after single-cohort study and therefore subject to confounding by secular trends (although the sharp change in alteplase DTN times associated temporally with the staggered rollout should serve to minimize the impact of such confounding). Because this is a before-and-after study, it did not include an additional long-term follow-up phase to assess for sustainability although the overall trend in administration times seems to be stable. Although the KPNC population is generally representative of the population of Northern California,34 KPNC members by definition have medical insurance, and thus more indigent populations are under-represented. However, with the new telestroke program, similar mean DTN times were achieved for members and nonmembers. As noted, all 21 centers were certified as PSC or comprehensive stroke centers before the intervention. Therefore, it remains unclear whether this intervention could be translated to non-PSC.
In summary, implementation of a modified Helsinki model with multicenter telestroke management across 21 hospital centers was associated with an increase in alteplase administration rates and significantly shorter DTN times with a symptomatic intracerebral hemorrhage rate that was not statistically different from the rate in preimplementation. Future studies are needed to better evaluate the long-term sustainability of the intervention and its effect on clinical outcomes, including mortality and long-term disability.
Acknowledgments
We thank the Kaiser Permanente Northern California Stroke Force Operating Remote Cerebrovascular Experts (FORCE) team for its dedication to providing the best care possible for our patients with acute stroke, including Jonathan Artz, MD, Nandini Bakshi, MD, Molly Burnett, MD, Elizabeth A. Cahill, MD, Sheila Chan, MD, Jai H. Cho, MD, Tara Dutta, MD, Nancy J. Edwards, MD, Kenneth Fox, MD, Lindsey K. Frischmann, MD, John J. Geraghty, MD, Jet K. Ho, MD, Jina L. Janavs, MD, Kevin S.L.J. Sawchuk, MD, and Deepinder Singh, MD.
Sources of Funding
The research in this article was funded by a grant from The Permanente Medical Group.
Disclosures
The corresponding author, Dr Nguyen-Huynh, received funding from The Permanente Medical Group for the analysis of this study. The other authors report no conflicts.
Supplementary Material
Footnotes
Presented in part at the International Stroke Conference, Houston, TX, February 22–24, 2017.
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.117.018413/-/DC1.
References
- 1.Saver JL. Time is brain–quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 2.Meretoja A, Keshtkaran M, Saver JL, Tatlisumak T, Parsons MW, Kaste M, et al. Stroke thrombolysis: save a minute, save a day. Stroke. 2014;45:1053–1058. doi: 10.1161/STROKEAHA.113.002910. doi: 10.1161/STROKEAHA.113.002910. [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, Smith EE, Saver JL, Reeves MJ, Hernandez AF, Peterson ED, et al. Improving door-to-needle times in acute ischemic stroke. Stroke. 2011;42:2983–2989. doi: 10.1161/STROKEAHA.111.621342. [DOI] [PubMed] [Google Scholar]
- 4.Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79:306–313. doi: 10.1212/WNL.0b013e31825d6011. doi: 10.1212/WNL.0b013e31825d6011. [DOI] [PubMed] [Google Scholar]
- 5.Meretoja A, Weir L, Ugalde M, Yassi N, Yan B, Hand P, et al. Helsinki model cut stroke thrombolysis delays to 25 minutes in Melbourne in only 4 months. Neurology. 2013;81:1071–1076. doi: 10.1212/WNL.0b013e3182a4a4d2. doi: 10.1212/WNL.0b013e3182a4a4d2. [DOI] [PubMed] [Google Scholar]
- 6.Audebert HJ, Schultes K, Tietz V, Heuschmann PU, Bogdahn U, Haberl RL, et al. Telemedical Project for Integrative Stroke Care (TEMPiS) Long-term effects of specialized stroke care with telemedicine support in community hospitals on behalf of the Telemedical Project for Integrative Stroke Care (TEMPiS). Stroke. 2009;40:902–908. doi: 10.1161/STROKEAHA.108.529255. doi: 10.1161/STROKEAHA.108.529255. [DOI] [PubMed] [Google Scholar]
- 7.Amarenco P. “Telethrombolysis”: stroke consultation by telemedicine. Lancet Neurol. 2008;7:763–765. doi: 10.1016/S1474-4422(08)70172-8. doi: 10.1016/S1474-4422(08)70172-8. [DOI] [PubMed] [Google Scholar]
- 8.Handschu R, Scibor M, Willaczek B, Nückel M, Heckmann JG, Asshoff D, et al. Telemedicine in acute stroke. J Neurol. 2008;255:1792–1797. doi: 10.1007/s00415-008-0066-9. [DOI] [PubMed] [Google Scholar]
- 9.Mutgi SA, Zha AM, Behrouz R. Emerging subspecialties in neurology: telestroke and teleneurology. Neurology. 2015;84:e191–e193. doi: 10.1212/WNL.0000000000001634. doi: 10.1212/WNL.0000000000001634. [DOI] [PubMed] [Google Scholar]
- 10.Sanders KA, Patel R, Kiely JM, Gwynn MW, Johnston LH. Improving telestroke treatment times in an expanding network of hospitals. J Stroke Cerebrovasc Dis. 2016;25:288–291. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.030. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Johansson T, Wild C. Telemedicine in acute stroke management: systematic review. Int J Technol Assess Health Care. 2010;26:149–155. doi: 10.1017/S0266462310000139. doi: 10.1017/S0266462310000139. [DOI] [PubMed] [Google Scholar]
- 12.Wechsler LR. Advantages and limitations of teleneurology. JAMA Neurol. 2015;72:349–354. doi: 10.1001/jamaneurol.2014.3844. doi: 10.1001/jamaneurol.2014.3844. [DOI] [PubMed] [Google Scholar]
- 13.Meyer BC, Raman R, Hemmen T, Obler R, Zivin JA, Rao R, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7:787–795. doi: 10.1016/S1474-4422(08)70171-6. doi: 10.1016/S1474-4422(08)70171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wechsler LR, Demaerschalk BM, Schwamm LH, Adeoye OM, Audebert HJ, Fanale CV, et al. American Heart Association Stroke Council; Council on Epidemiology and Prevention; Council on Quality of Care and Outcomes Research. Telemedicine quality and outcomes in stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e3–e25. doi: 10.1161/STR.0000000000000114. doi: 10.1161/STR.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 15.Schwamm LH, Chumbler N, Brown E, Fonarow GC, Berube D, Nystrom K, et al. American Heart Association Advocacy Coordinating Committee. Recommendations for the implementation of telehealth in cardiovascular and stroke care: a policy statement from the American Heart Association. Circulation. 2017;135:e24–e44. doi: 10.1161/CIR.0000000000000475. doi: 10.1161/CIR.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 16.Müller-Barna P, Hubert GJ, Boy S, Bogdahn U, Wiedmann S, Heuschmann PU, et al. TeleStroke units serving as a model of care in rural areas: 10-year experience of the TeleMedical project for integrative stroke care. Stroke. 2014;45:2739–2744. doi: 10.1161/STROKEAHA.114.006141. doi: 10.1161/STROKEAHA.114.006141. [DOI] [PubMed] [Google Scholar]
- 17.Hubert GJ, Meretoja A, Audebert HJ, Tatlisumak T, Zeman F, Boy S, et al. Stroke Thrombolysis in a Centralized and a Decentralized System (Helsinki and Telemedical Project for Integrative Stroke Care Network). Stroke. 2016;47:2999–3004. doi: 10.1161/STROKEAHA.116.014258. doi: 10.1161/STROKEAHA.116.014258. [DOI] [PubMed] [Google Scholar]
- 18.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 19.Lyden PD, Lu M, Levine SR, Brott TG, Broderick J NINDS rtPA Stroke Study Group. A modified National Institutes of Health Stroke Scale for use in stroke clinical trials: preliminary reliability and validity. Stroke. 2001;32:1310–1317. doi: 10.1161/01.str.32.6.1310. [DOI] [PubMed] [Google Scholar]
- 20.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 21.Meyer BC, Lyden PD. The modified National Institutes of Health Stroke Scale: its time has come. Int J Stroke. 2009;4:267–273. doi: 10.1111/j.1747-4949.2009.00294.x. doi: 10.1111/j.1747-4949.2009.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flint AC, Conell C, Klingman JG, Rao VA, Chan SL, Kamel H, et al. Impact of increased early statin administration on ischemic stroke outcomes: a multicenter electronic medical record intervention. J Am Heart Assoc. 2016;5:e003413. doi: 10.1161/JAHA.116.003413. doi: 10.1161/JAHA.116.003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang MC, Cutler DM, Rosen AB. Trends in thrombolytic use for ischemic stroke in the United States. J Hosp Med. 2010;5:406–409. doi: 10.1002/jhm.689. doi: 10.1002/jhm.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moradiya Y, Crystal H, Valsamis H, Levine SR. Thrombolytic utilization for ischemic stroke in US hospitals with neurology residency program. Neurology. 2013;81:1986–1995. doi: 10.1212/01.wnl.0000436946.08647.b5. doi: 10.1212/01.wnl.0000436946.08647.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messé SR, Khatri P, Reeves MJ, Smith EE, Saver JL, Bhatt DL, et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology. 2016;87:1565–1574. doi: 10.1212/WNL.0000000000003198. doi: 10.1212/WNL.0000000000003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JT, Fonarow GC, Smith EE, Reeves MJ, Navalkele DD, Grotta JC, et al. Treatment with tissue plasminogen activator in the golden hour and the shape of the 4.5-hour time-benefit curve in the National United States Get With The Guidelines-Stroke population. Circulation. 2017;135:128–139. doi: 10.1161/CIRCULATIONAHA.116.023336. doi: 10.1161/CIRCULATIONAHA.116.023336. [DOI] [PubMed] [Google Scholar]
- 27.Desai JA, Smith EE. Prenotification and other factors involved in rapid tPA administration. Curr Atheroscler Rep. 2013;15:337. doi: 10.1007/s11883-013-0337-5. doi: 10.1007/s11883-013-0337-5. [DOI] [PubMed] [Google Scholar]
- 28.Sadeghi-Hokmabadi E, Taheraghdam A, Hashemilar M, Rikhtegar R, Mehrvar K, Mehrara M, et al. Simple in-hospital interventions to reduce door-to-CT time in acute stroke. Int J Vasc Med. 2016;2016:1656212. doi: 10.1155/2016/1656212. doi: 10.1155/2016/1656212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi KC, Liang JW, Wilson N, Tuhrim S, Dhamoon MS. More time is taken to administer tissue plasminogen activator in ischemic stroke patients with earlier presentations. J Stroke Cerebrovasc Dis. 2017;26:70–73. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.031. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Fonarow GC, Zhao X, Smith EE, Saver JL, Reeves MJ, Bhatt DL, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311:1632–1640. doi: 10.1001/jama.2014.3203. doi: 10.1001/jama.2014.3203. [DOI] [PubMed] [Google Scholar]
- 31.Busby L, Owada K, Dhungana S, Zimmermann S, Coppola V, Ruban R, et al. CODE FAST: a quality improvement initiative to reduce door-to-needle times. J Neurointerv Surg. 2016;8:661–664. doi: 10.1136/neurintsurg-2015-011806. doi: 10.1136/neurintsurg-2015-011806. [DOI] [PubMed] [Google Scholar]
- 32.LaMonte MP, Bahouth MN, Magder LS, Alcorta RL, Bass RR, Browne BJ, et al. Emergency Medicine Network of the Maryland Brain Attack Center. A regional system of stroke care provides thrombolytic outcomes comparable with the NINDS stroke trial. Ann Emerg Med. 2009;54:319–327. doi: 10.1016/j.annemergmed.2008.09.022. doi: 10.1016/j.annemergmed.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal S, Day DJ, Sibson L, Barry PJ, Collas D, Metcalf K, et al. Thrombolysis delivery by a regional telestroke network–experience from the U.K. National Health Service. J Am Heart Assoc. 2014;3:e000408. doi: 10.1161/JAHA.113.000408. doi: 10.1161/JAHA.113.000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]


