Supplemental Digital Content is available in the text.
Keywords: amputation-free survival, critical limb ischemia, endovascular therapy, propensity score, surgical reconstruction
Abstract
Background—
The aim of this study was to compare clinical outcomes between surgical reconstruction and endovascular therapy (EVT) for critical limb ischemia (CLI) in today’s real-world settings.
Methods and Results—
This multicenter, prospective, observational study registered and followed 548 Japanese CLI patients. The registration was in advance of revascularization; 197 patients were scheduled to receive surgical reconstruction, and the remaining 351 were scheduled to receive EVT. The primary end point was 3-year amputation-free survival, compared between the 2 treatments in an intention-to-treat manner, using propensity score matching. Interaction analysis was additionally performed to explore which subgroups had better outcomes with surgical reconstruction or EVT. After propensity score matching, the 3-year amputation-free survival was not significantly different between the 2 groups (52% [95% confidence interval, 43%–60%] and 52% [95% confidence interval, 44–60%]; P=0.26). Subsequent interaction analysis identified (1) Wound, Ischemia, and foot Infection (WIfI) classification W-3, (2) fI-2/3, (3) history of ipsilateral minor amputation, (4) history of revascularization after CLI onset, and (5) bilateral CLI as the factors more favorable for surgical reconstruction, whereas (1) diabetes mellitus, (2) renal failure, (3) anemia, (4) history of nonadherence to cardiovascular risk management, and (5) contralateral major amputation were as those less favorable for surgical reconstruction.
Conclusions—
The 3-year amputation-free survival was not different between surgical reconstruction and EVT in the overall CLI population. The subsequent interaction analysis suggested that there would be a subgroup more suited for surgical reconstruction and another benefiting more from EVT.
Clinical Trial Registration—
URL: http://www.umin.ac.jp/ctr/. Unique identifier: UMIN000007050.
WHAT IS KNOWN
Previous studies demonstrated that endovascular therapy and surgical reconstruction were similarly effective revascularization strategies for critical limb ischemia.
However, for the past decade, techniques for revascularization have improved, whereas population ageing, diabetes mellitus pandemic, and the spread of chronic kidney disease have become global burdens.
It remains unknown whether the 2 treatments are still comparable to each other in today’s real-world settings.
WHAT THE STUDY ADDS
The SPINACH study (Surgical Reconstruction Versus Peripheral Intervention in Patients With Critical Limb Ischemia) demonstrated that the 3-year amputation-free survival was not different between surgical reconstruction and endovascular therapy in the overall critical limb ischemia population.
The subsequent risk stratification analysis suggested that critical limb ischemia patients with severe limb status would be more suited for surgical reconstruction, whereas patients with a poor general condition would benefit more from endovascular therapy.
Critical limb ischemia (CLI) is the most advanced form of peripheral arterial disease characterized by ischemic rest pain and ulcer/gangrene.1–3 Revascularization is positioned as the first-line treatment.4 Clinical guidelines recommend both surgical reconstruction and endovascular therapy (EVT), largely based on the findings of the BASIL trial (British Angioplasty versus Surgery in Ischemic Legs).5 Although this classical trial was no doubt an important and informative study, clinical settings have changed during the past decade. For example, technical development has advanced swiftly in the field of EVT. It remains uncertain whether the evidence presented by the trial would be still valid and true in current clinical practice. The aim of this study was to compare clinical outcomes between surgical reconstruction and EVT for CLI in today’s real-world settings.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. This SPINACH registry (Surgical Reconstruction Versus Peripheral Intervention in Patients With Critical Limb Ischemia) was a prospective, multicenter, observational study that enrolled patients who had CLI caused by atherosclerotic arterial disease, either with or without suprainguinal disease, in 23 centers (12 vascular surgery departments and 11 interventional cardiology departments) in Japan.6 Hemodynamically significant stenosis and occlusion were defined as arterial disease. Patients undergoing primary major amputation were excluded. Registration was in advance of revascularization, and the intended revascularization strategy was recorded at registration. Patients had either surgical reconstruction planned (Surg group) or EVT alone planned (EVT group). The treatment strategy was determined by a team of vascular specialists including vascular surgeons and interventional cardiologists in each local manner in clinical practice. Note that each hospital could select both surgical and endovascular treatment. During EVT, a stent was implanted in aortoiliac or superficial femoral lesion as commonly as in clinical practice. During surgical reconstruction, an autogenous vein graft was preferably used for infrainguinal bypass surgery, and hybrid therapy with EVT was allowed. There are no podiatrists in Japan and, therefore, vascular surgeons, interventional cardiologists, plastic surgeons, and dermatologists were cooperatively involved in wound care for respective cases. After revascularization, the follow-up assessments were scheduled at 1, 3, 6, 12, 24, and 36 months, with a tolerance of ±1 month. Attending doctors examined clinical symptoms, hemodynamic status (mainly with ankle–brachial index, ankle pressure, and skin perfusion pressure [SPP]7), and vessel patency (mainly with duplex ultrasound) in clinical settings. The study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of each center registering patients. Written informed consent was obtained.
Definitions
CLI was diagnosed when patients had (1) chronic ischemic foot rest pain with ankle pressure <50 mm Hg, toe pressure <30 mm Hg, or SPP ≤30 mm Hg, or ischemic foot ulcer/gangrene with ankle pressure <70 mm Hg, toe pressure <40 mm Hg, or SPP ≤40 mm Hg (ankle pressure/toe pressure/SPP-proved critical ischemia); or (2) ischemic foot rest pain or ulcer/gangrene with critical ischemia indicated by other modalities.6
Severity of arterial lesions was assessed using the Trans-Atlantic Inter-Society Consensus II classification for aortoiliac and femoropopliteal segments2 and the Trans-Atlantic Inter-Society Consensus classification for infrapopliteal lesions.1 Wound severity was primarily assessed in a prespecified manner by the combination of the Rutherford classification, the University of Texas classification, and the presence of infection (primary analysis). Furthermore, in line with the groundbreaking development of the Wound, Ischemia and foot Infection (WIfI) classification8 after the start of our study, we alternatively used this more sophisticated classification (secondary analysis). The WIfI classes were retrospectively determined using the photographs of pedal wounds and medical records including laboratory examinations at registration. The judgment was first made at each participating center and was thereafter reviewed by an independent plastic surgeon. Disagreements were discussed and resolved in a subsequent committee attended by the plastic surgeon, a vascular surgeon, and an interventional cardiologist. SPPs of 31 to 40 mm Hg and ≤30 mm Hg were treated as WIfI I-2 and I-3, respectively. Subjects analyzed in the secondary analysis were limited to those presenting WIfI I-3 with rest pain and I-2/3 with ulcer/gangrene.
Quality of life (QOL) at registration was assessed using the Japanese version of the Short Form 36 questionnaire9 (generic QOL) and that of the questionnaire10 (disease-specific QOL). Nonadherence to cardiovascular risk management was defined as default on clinical appointment for cardiovascular risk management, for example, antidiabetic, antihypertensive, or antihyperlipidemic treatment.
End Points
The primary end point was the 3-year amputation-free survival (AFS), that is, freedom from the composite of major amputation and all-cause mortality. The secondary end points included limb salvage (ie, freedom from major amputation), overall survival (ie, freedom from all-cause mortality), freedom from major adverse limb event (ie, major amputation and major reintervention11), freedom from major amputation and any reintervention, wound-free limb salvage (freedom from major amputation and unhealed wounds), wound-free survival (freedom from all-cause mortality, major amputation, and unhealed wounds), initial technical success, and 30-day perioperative adverse events. Initial technical success was evaluated from both anatomic (angiographic) and hemodynamic aspects.6 Anatomic success was defined as at least one straight line reaching the foot for EVT and as a patent bypass graft perfusing blood directly to the foot for surgical reconstruction. Hemodynamic success referred to an increase in ankle–brachial index of >0.1 or an increase in SPP of >10 mm Hg.6
Statistical Analysis
Data are presented as the mean±SD for continuous variables and the number (percentage) for discrete variables, if not otherwise mentioned. P<0.05 was considered significant. The intergroup differences in baseline characteristics were tested by unpaired t tests for continuous variables and χ2 tests for discrete variables.
Clinical outcomes were compared after propensity score matching. The propensity score was derived from a logistic regression model including sex, age, residence status, ambulatory status, comorbidities (including dialysis-dependent renal failure), QOL, history of nonadherence to cardiovascular risk management, history of lower extremity treatment, plan for infrapopliteal revascularization, severity of wound and arterial lesions, and contralateral limb status. Wound severity was assessed by the Rutherford classification, the University of Texas classification, and the presence of infection in the primary analysis and by the WIfI classification in the secondary analysis. Matching was reperformed for the secondary analysis. Matching was based on the logit of the propensity score, within a caliper of 0.2 SD of the value. The matching was performed in an intention-to-treat manner; patients with EVT intended at registration were matched to those with surgical reconstruction intended at registration. To maximize the statistical power to detect intergroup prognostic differences, we extracted as many matched samples in the EVT group to one in the Surg group as possible. A sample size of 145 subjects in the Surg group and twice as many paired subjects in the EVT group was calculated to have ≥80% power to detect a difference of 10% in the 3-year AFS rate with a dropout rate of 25%, on the hypothesis that 14% were saved by selecting surgical reconstruction instead of EVT, and 4% were saved by selecting EVT instead of surgical reconstruction, or vice versa. The comparison after matching was performed using stratification by the pairs, and weighted descriptive statistics are reported. Time-to-event outcomes were analyzed by the Kaplan–Meier method and the log-rank test, except for nonfatal limb-related outcomes, which were investigated by competing risk analysis. The survival figures for these nonfatal outcomes represent the complement of the cumulative incidence function used in competing risks analysis. The 95% confidence intervals (CI) were obtained from bootstrapping with 10 000 resamples.
We additionally explored which subgroups had better outcomes with surgical reconstruction or EVT. We first screened candidates for interaction using a crude stratified Cox model. A candidate was selected when it yielded a ≥1.5-fold (or its reciprocal ≤0.67-fold) interaction effect on the association of surgical reconstruction versus EVT with AFS. We subsequently classified the study population according to the accumulation of these interacting factors and assessed the intertreatment prognostic difference in subgroups.
All statistical analyses were performed with R version 3.1.0 (R Development Core Team, Vienna, Austria), adopting MatchIt, lawstat, survival, and cmprsk packages.
Results
Between January 2012 and March 2013, 550 CLI patients in whom revascularization was planned were registered. One patient who was later diagnosed with vasculitis and another patient who later voluntarily withdrew from the study were excluded. The remaining 548 were followed, and 437 patients (80%) completed the 3-year follow-up. During the follow-up period, 47 patients underwent major amputation, 24 (51%) of which were because of uncontrollable infection, and 237 patients died. The leading causes of death was cardiovascular disease (48%), followed by pneumonia (16%), sepsis (14%), others (13%), and unknown causes (8%).
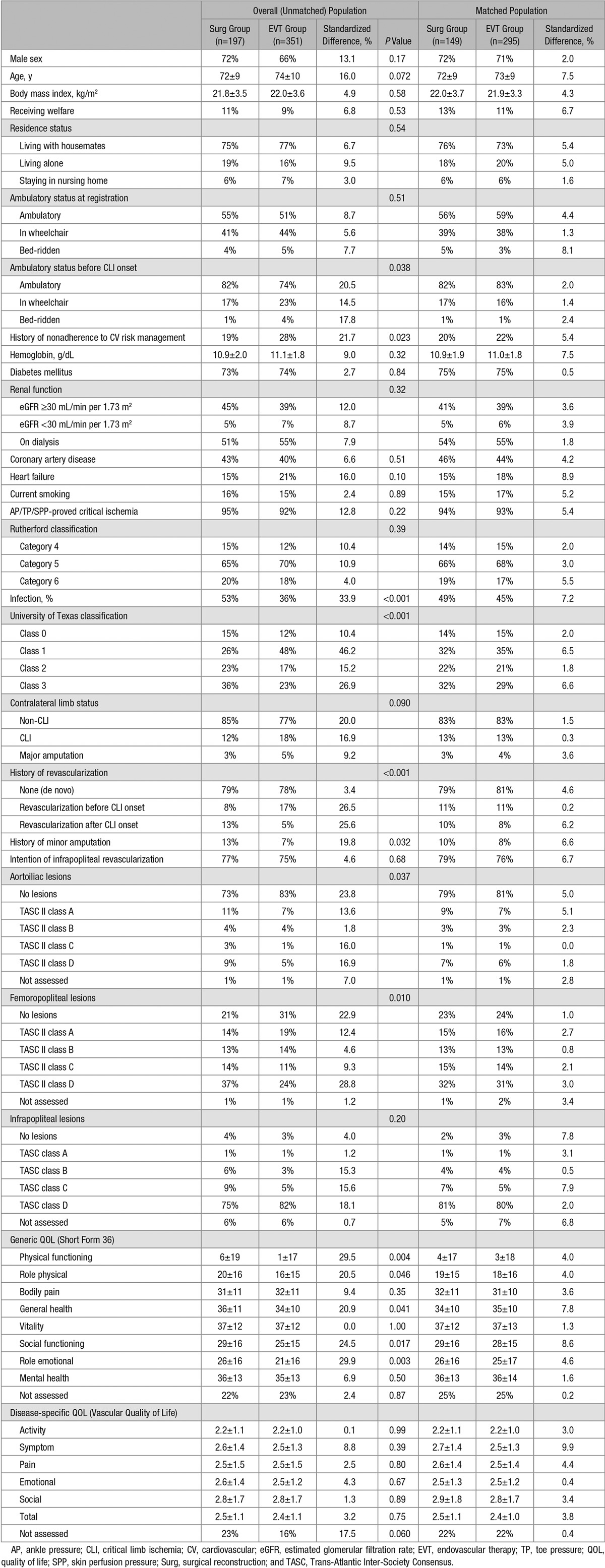
Of the 548 patients, 197 had surgical reconstruction planned (Surg group), whereas 351 had EVT alone planned (EVT group). The prevalence of tissue loss, diabetes mellitus, and regular dialysis was 84%, 73%, and 53%, respectively. Infrapopliteal revascularization was planned at registration in 76%. As summarized in Table 1, background characteristics were basically similar between the 2 groups, except for previous ambulatory status (less ambulatory in the EVT group), history of nonadherence to cardiovascular risk management (more prevalent in the EVT group), severity of wounds and arterial lesions (more severe in the Surg group), history of revascularization (less frequent before and more frequent after CLI onset in the Surg group), minor amputation (more frequent in the Surg group), and generic QOL (lower in the EVT group). The sensitivity analysis of baseline continuous variables by nonparametrical assessments are shown in Table I in the Data Supplement.
Table 1.
Baseline Characteristics in Overall (Unmatched) and Matched Population
In the EVT group, 1 patient (0.3%) died before revascularization and the others (99.7%) received EVT alone, with 45% undergoing stent implantation (7% drug-eluting stent implantation) and 72% undergoing infrapopliteal revascularization. None were crossed over to surgical reconstruction. In the Surg group, 4 patients (2%) crossed over to EVT alone, whereas the remaining 193 (98%) received surgical reconstruction, with 39 (20%) undergoing hybrid therapy (21 iliac EVT plus infrainguinal bypass±endarterectomy, 7 femoropopliteal EVT plus distal bypass, and 11 others). Infrainguinal bypass surgery was performed in 95% of patients, of whom 8% had a prosthetic graft used, and the remaining 92% had an autogenous vein graft alone used. The distal anastomoses were performed to crural or pedal arteries in 78% of cases undergoing infrainguinal bypass surgery.
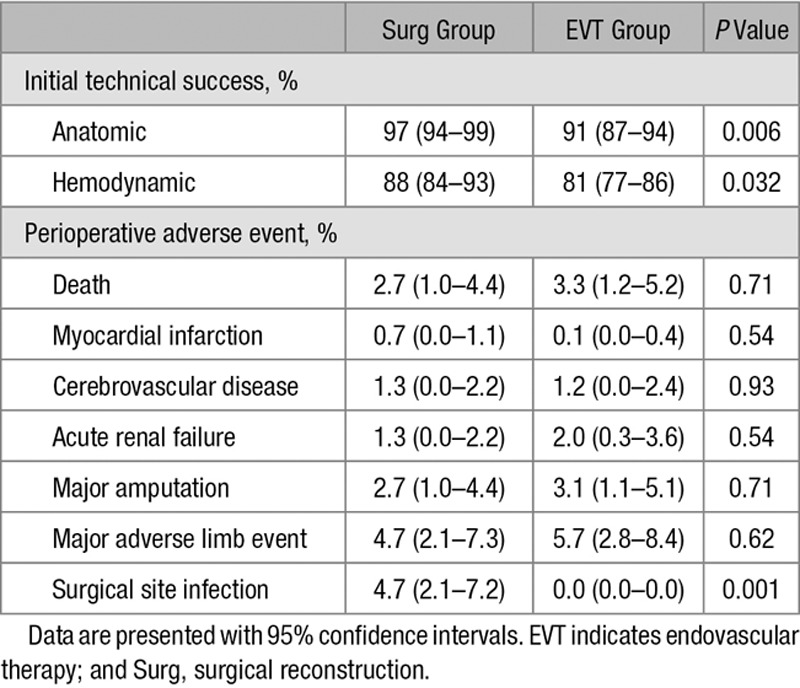
The propensity score matching extracted 149 patients in the Surg group and 295 patients in the EVT group (25, 38, and 86 pairs were ≥3:1, 2:1, and 1:1 matching, respectively). There was no remarkable intergroup difference in baseline characteristics (Table 1; Table I in the Data Supplement). As Figure 1A shows, the 3-year AFS rate was not different between the groups (52%; [95% CI, 43%–60%] in the Surg group versus 52% [95% CI, 44%–60%] in the EVT group; P=0.26). Neither was the 3-year limb salvage rate (90% [95% CI, 85%–95%] versus 92% [95% CI, 87%–96%]; P=0.82) or the 3-year overall survival rate (57% [95% CI, 49%–66%] versus 53% [95% CI, 45%–61%]; P=0.24). A significant intergroup difference was not observed in the 3-year freedom rate from major adverse limb event (78% [95% CI, 71%–85%] versus 85% [95% CI, 80%–90%]; P=0.37; Figure 1B) but was in the 3-year freedom rate from major amputation and any reintervention (64% [95% CI, 56%–72%] versus 51% [95% CI, 43%–58%]; P=0.001; Figure 1C). The proportion of wound-free limb salvage and that of wound-free survival were not significantly different between the groups at 3 years, whereas those proportions were significantly higher in the Surg group at 1 month (Figure 1D and 1E). Compared with the EVT group, the Surg group was more likely to achieve initial technical success, whereas they had a higher proportion of surgical site infection (Table 2).
Figure 1.
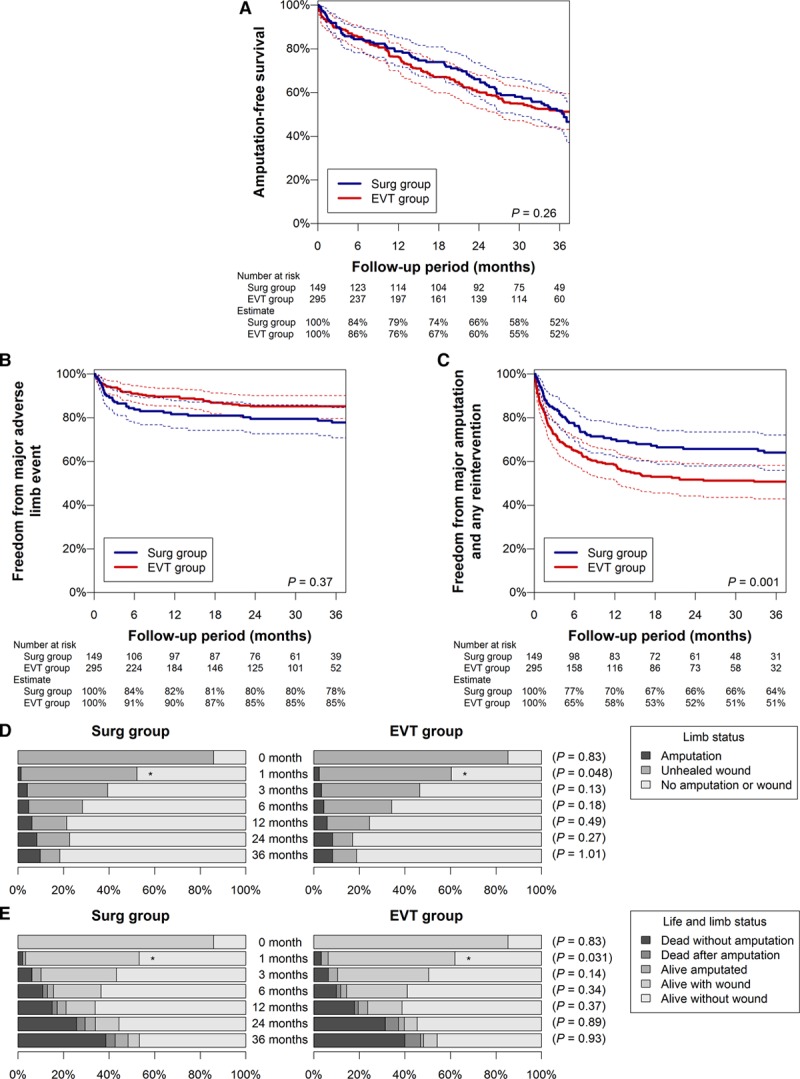
Prognosis in matched population (primary analysis). A–C, Amputation-free survival (A), freedom from major adverse limb event (B), and freedom from major amputation and any reintervention (C). Dotted lines indicate 95% confidence intervals. D and E, Limb status (D) and life and limb status (E). P values are for the intergroup difference in the proportion of wound-free limb salvage (D) and wound-free survival (E). *P<0.05. EVT indicates endovascular therapy; and Surg, surgical reconstruction.
Table 2.
Perioperative Outcomes in Matched Population
Adaptation of WIfI Classification (Secondary Matching Analysis)
Twenty-eight patients (5%) did not meet WIfI classification I-3 with rest pain or I-2/3 with ulcer/gangrene and were, therefore, excluded from the secondary matching analysis. This secondary analysis extracted 129 patients in the Surg group and 289 patients in the EVT group (29, 27, and 73 pairs were ≥3:1, 2:1, and 1:1 matching, respectively; Tables II and III in the Data Supplement). As Figure I and Table IV in the Data Supplement show, the findings were almost the same as the primary matching analysis. The 3-year AFS and freedom from major adverse limb event, as well as the 3-year wound-free limb salvage and wound-free survival, were not different between the groups, whereas the Surg group had a higher rate of freedom from major amputation and any reintervention, the short-term wound-free limb salvage and wound-free survival, and initial technical success and had a higher proportion of surgical site infection.
Interaction Analysis
As Figure 2 shows, the screening analysis identified (1) WIfI classification W-3, (2) fI-2/3, (3) history of ipsilateral minor amputation, (4) history of revascularization after CLI onset, and (5) bilateral CLI as the factors more favorable for surgical reconstruction, whereas (1) history of nonadherence to cardiovascular risk management, (2) lower hemoglobin levels (<10 g/dL), (3) diabetes mellitus, (4) renal failure (including regular dialysis), and (5) contralateral major amputation were extracted as those less favorable for surgical reconstruction. We, therefore, provisionally developed a favorability score for surgical reconstruction, with one point added for each of the former 5 factors and 1 point subtracted for each of the latter 5 (Figure 3A). The score was positively associated with the favorability for surgical reconstruction over EVT in AFS (P<0.001), as well as overall survival (P<0.001) and major amputation (P=0.009). As Figure 3B shows, the fourth quartile of the score benefited more from surgical reconstruction (P<0.001), whereas the first quartile benefited more from EVT (P=0.018). The score had no significant interaction effect on the association of surgical reconstruction versus EVT with other clinical outcomes (all P>0.05), except for the 3-year wound-free survival (P=0.012), the failure of which was mainly attributed to mortality and major amputation (ie, failure of AFS) rather than the presence of unhealed wounds.
Figure 2.
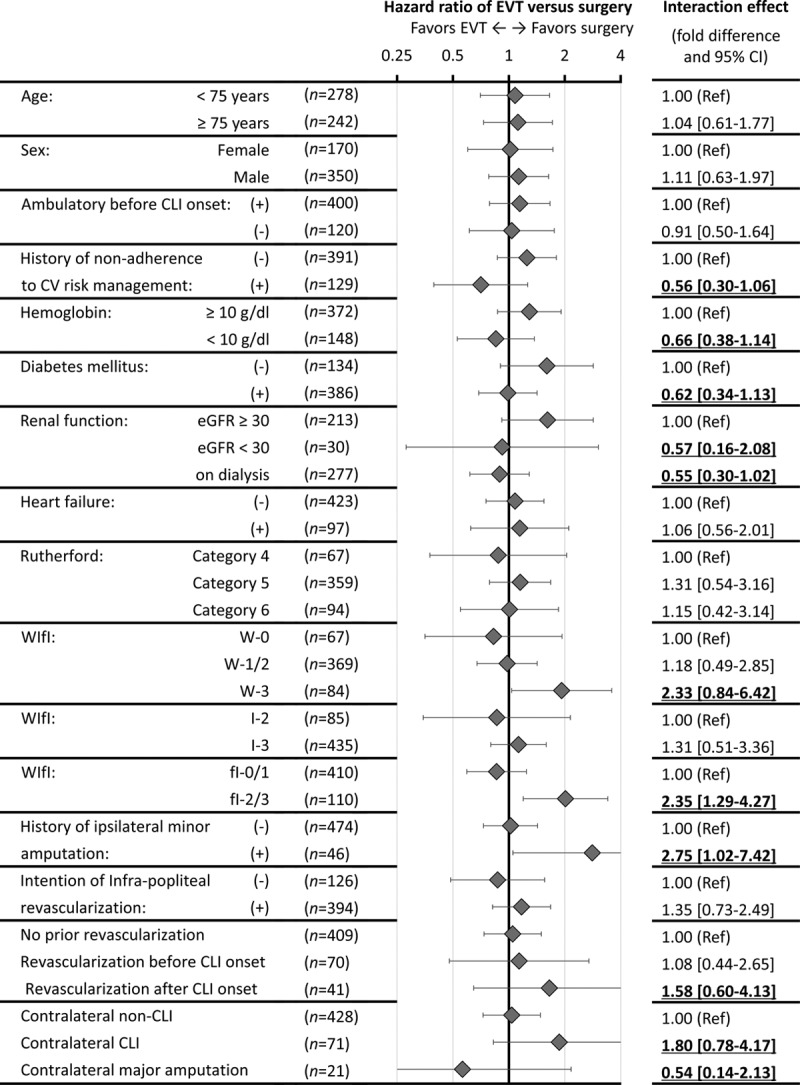
Prognostic impact of endovascular therapy (EVT) vs surgical reconstruction in subgroups. Plots and error bars are hazard ratios of EVT vs surgical reconstruction for the failure of AFS and their 95% confidence intervals (CIs), calculated from the Cox proportional hazards model with stratification on the propensity score. Interaction effects yielding a ≥1.5-fold or ≤0.67-fold difference are underlined. CLI indicates critical limb ischemia; CV, cardiovascular; eGFR, estimated glomerular filtration rate; and WIfI, Wound, Ischemia, and foot Infection.
Figure 3.
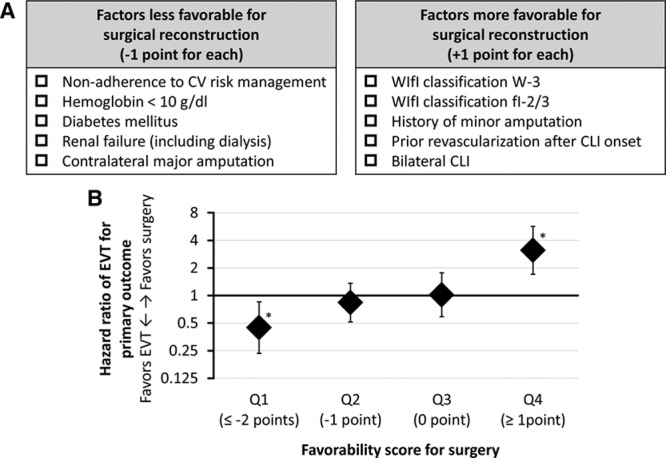
Classification by favorability score for surgical reconstruction. A, Development of the favorability score for surgical reconstruction vs endovascular therapy (EVT). B: Hazard ratios of EVT vs surgical reconstruction for the failure of amputation-free survival and their 95% CIs, calculated from the Cox proportional hazards model with stratification on the propensity score. The population was classified according to the quartiles of the developed favorability score. *P<0.05. CLI indicates critical limb ischemia; CV, cardiovascular; and WIfI, Wound, Ischemia, and foot Infection.
Discussion
The SPINACH study compared the 3-year AFS between surgical reconstruction and EVT for CLI patients in current real-world settings. The AFS rate was not significantly different between the 2 treatments. The subsequent interaction analysis suggested that there would be a subgroup more suited for surgical reconstruction, and another benefiting more from EVT.
Peripheral arterial disease has become more prevalent, presumably because of global trends in population ageing, diabetes mellitus pandemic, and the spread of chronic kidney disease.12–16 Importantly, these risk factors often predispose a patient to more distal vessel involvement.17,18 Infrainguinal, and especially infrapopliteal, lesions are now common in clinical practice. On the other hand, revascularization techniques have improved in the past decade. Femoral stent implantation has become common in EVT,19 whereas the efficacy of autogenous vein grafts has been reevaluated, and they are preferentially used in bypass surgery.4 Furthermore, hybrid therapy, which could minimize surgical invasiveness and reduce operative risk, has been increasingly used.20
In the current study, reflecting today’s clinical practice, almost half the patients in the EVT group underwent stent implantation, one fifth in the Surg group underwent hybrid therapy, and most infrainguinal bypass surgeries used autogenous vein grafts. In addition, most patients received infrapopliteal revascularization, and patients on dialysis were not excluded. Our primary finding confirmed that AFS was not different between surgical reconstruction and EVT, which was validated by the secondary analysis adopting the WIfI classification. Our study would provide reliable evidence for today’s clinical management of CLI.
Given that AFS and freedom from major adverse limb event had no significant intergroup difference, EVT seemed as effective as bypass surgery against major adverse events. However, initial success rate and freedom rate from any reintervention and major amputation were lower in the EVT group. Furthermore, the proportion of wound-free limb salvage and that of wound-free survival were lower in the short term, although they were not in the long term. These findings would reflect the inferiority of EVT to surgical reconstruction in terms of a prompt and abundant supply of blood flow,21–23 which would be a key factor for freedom from reintervention and for prompt wound healing. On the other hand, surgical site infection was more prevalent in the Surg group, indicating that the management of surgical sites would be easier in EVT than in surgical reconstruction.
The subsequent interaction analysis suggested that some patients would receive more benefits from either surgical reconstruction or EVT in terms of AFS. First, history of ipsilateral minor amputation, history of revascularization after CLI onset (ie, requirement of redo revascularization), and bilateral CLI were likely associated with the favorability for surgical reconstruction. These clinical phenotypes might reflect long and extensive exposure to end-stage arteriosclerosis, which might benefit more from surgical reconstruction supplying sufficient blood flow. Furthermore, WIfI W-3 and fI-2/3 also suggested the favorability for surgical reconstruction. Major tissue loss and severe infection, both of which are well-known risk factors for delayed wound healing and major amputation,24–26 often require abundant blood flow for limb salvage. In this respect, surgical reconstruction, better at sufficient blood flow supply, might be more effective. The current study did not identify the Rutherford classification as an interacting factor like the WIfI classification, presumably because it was not so accurate in grading wound severity. At least in selecting revascularization strategies, the WIfI classification would be more informative than the Rutherford classification.
In contrast, the prognostic superiority of surgical reconstruction was likely diminished in patients with systemic comorbidities including diabetes mellitus, renal failure, and anemia, and contralateral major amputation. In general, major amputation, preferably performed in poor-risk patients and considerably impairing activities of daily living by itself, is a marker of a poor general condition. Surgical reconstruction is more invasive than EVT, and a poor general condition indicated by these clinical features might accentuate the risk of perioperative complications and subsequent adverse events.27,28 Furthermore, history of nonadherence to cardiovascular risk management was also likely associated with diminished superiority of surgical reconstruction. In patients with the history, comorbidities might be poorly controlled, which would increase perioperative risks of invasive surgical treatments. In addition, surgical reconstruction often requires more careful postoperative management including regular graft surveillance programs. These patients might likely fail to adhere to the postoperative management, which would lead to poor outcomes after surgery.
The current study had several limitations. First, the SPINACH registry was not a randomized controlled trial. However, propensity score–matching analysis based on prospectively collected data could reduce bias as much as possible. Second, detailed information on wound status, wound management, and lesion morphology was limited. Furthermore, vessel patency, hospital length of stay, and cost were not assessed. Third, some novel endovascular devices including drug-eluting balloons and atherectomy devices were not used. Fourth, this study, representing a CLI population in Japan,24–26 had a higher prevalence of renal failure compared with overseas. However, the prevalence is rapidly increasing worldwide.29 Data are to date scarce on CLI patients with renal failure, and we think that our data would provide clinically relevant information. Fifth, in the interaction analysis, the candidates were nominated through discussion of the study investigators and not on the basis of strict or objective selection criteria. This process would give a bias of the study. In addition, we had no validation data set. Future validation studies are needed. Sixth, although the developed favorability score could successfully classify CLI patients in terms of AFS and the 3-year wound-free survival, the score did not discriminate the intertreatment differences in other clinical outcomes. The subgroups classified by the score would still have room for further classification from the viewpoint of these other clinical outcomes.
In conclusion, the SPINACH study, cooperatively performed by vascular surgeons and interventional cardiologists, compared clinical outcomes between current optimal surgical reconstruction and EVT for CLI patients in real-world clinical settings. The 3-year AFS were not different between the 2 treatment strategies in the overall population. The subsequent interaction analysis suggested that CLI patients with severe wound status might be more suited for surgical reconstruction, whereas those with a poor general condition might benefit more from EVT in terms of AFS.
Sources of Funding
The SPINACH study (Surgical Reconstruction Versus Peripheral Intervention in Patients With Critical Limb Ischemia) was sponsored by Abbott Vascular Japan Co, Ltd, Boston Scientific Japan K.K., Cook Japan Incorporated, Goodman Co, Ltd, Johnson & Johnson K.K., Kaken Pharmaceutical Co, Ltd, Kaneka Medix Corporation, Medicon Inc, Medikit Co, Ltd, Medtronic Japan Co, Ltd, Mitsubishi Tanabe Pharma Corporation, MSD K.K., St. Jude Medical Japan Co, Ltd, Taisho Toyama Pharmaceutical Co, Ltd, Terumo Corp, and W.L. Gore & Associates, Co, Ltd (in alphabetic order).
Disclosures
None.
Supplementary Material
Footnotes
The Data Supplement is available at http://circinterventions.ahajournals.org/lookup/suppl/doi:10.1161/CIRCINTERVENTIONS.117.005531/-/DC1.
References
- 1.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg. 2000;31(1)(pt 2):S1–S296. [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Bell K, Caporusso J, Durand-Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E, 3rd, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(suppl 1):S1–S75. doi: 10.1016/j.ejvs.2006.09.024. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:1425–1443. doi: 10.1161/CIR.0b013e31828b82aa. doi: 10.1161/CIR.0b013e31828b82aa. [DOI] [PubMed] [Google Scholar]
- 4.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471. doi: 10.1161/CIR.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, Storkey H BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 6.Azuma N, Iida O, Takahara M, Soga Y, Kodama A. Surgical reconstruction versus peripheral intervention in patients with critical limb ischemia - a prospective multicenter registry in Japan: the SPINACH study design and rationale. Vascular. 2014;22:411–420. doi: 10.1177/1708538113518204. doi: 10.1177/1708538113518204. [DOI] [PubMed] [Google Scholar]
- 7.Yamada T, Ohta T, Ishibashi H, Sugimoto I, Iwata H, Takahashi M, Kawanishi J. Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs–comparison with other noninvasive diagnostic methods. J Vasc Surg. 2008;47:318–323. doi: 10.1016/j.jvs.2007.10.045. doi: 10.1016/j.jvs.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 8.Mills JL, Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59:220–34.e1. doi: 10.1016/j.jvs.2013.08.003. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 10.Morgan MB, Crayford T, Murrin B, Fraser SC. Developing the Vascular Quality of Life Questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg. 2001;33:679–687. doi: 10.1067/mva.2001.112326. doi: 10.1067/mva.2001.112326. [DOI] [PubMed] [Google Scholar]
- 11.Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL, Nehler MR, Powell RJ, Sidawy AN. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg. 2009;50:1462–73.e1. doi: 10.1016/j.jvs.2009.09.044. doi: 10.1016/j.jvs.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 12.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 13.Selvin E, Köttgen A, Coresh J. Kidney function estimated from serum creatinine and cystatin C and peripheral arterial disease in NHANES 1999-2002. Eur Heart J. 2009;30:1918–1925. doi: 10.1093/eurheartj/ehp195. doi: 10.1093/eurheartj/ehp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 15.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radhakrishnan J, Remuzzi G, Saran R, Williams DE, Rios-Burrows N, Powe N, Brück K, Wanner C, Stel VS, Venuthurupalli SK, Hoy WE, Healy HG, Salisbury A, Fassett RG, O’Donoghue D, Roderick P, Matsuo S, Hishida A, Imai E, Iimuro S CDC-CKD Surveillance Team; European CKD Burden Consortium; CKD.QLD group. Taming the chronic kidney disease epidemic: a global view of surveillance efforts. Kidney Int. 2014;86:246–250. doi: 10.1038/ki.2014.190. doi: 10.1038/ki.2014.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diehm N, Shang A, Silvestro A, Do DD, Dick F, Schmidli J, Mahler F, Baumgartner I. Association of cardiovascular risk factors with pattern of lower limb atherosclerosis in 2659 patients undergoing angioplasty. Eur J Vasc Endovasc Surg. 2006;31:59–63. doi: 10.1016/j.ejvs.2005.09.006. doi: 10.1016/j.ejvs.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Wasmuth S, Baumgartner I, Do DD, Willenberg T, Saguner A, Zwahlen M, Diehm N. Renal insufficiency is independently associated with a distal distribution pattern of symptomatic lower-limb atherosclerosis. Eur J Vasc Endovasc Surg. 2010;39:591–596. doi: 10.1016/j.ejvs.2009.11.034. doi: 10.1016/j.ejvs.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 19.Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clément D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Röther J, Sievert H, van Sambeek M, Zeller T ESC Committee for Practice Guidelines. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2851–906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- 20.Slovut DP, Lipsitz EC. Surgical technique and peripheral artery disease. Circulation. 2012;126:1127–1138. doi: 10.1161/CIRCULATIONAHA.111.059048. doi: 10.1161/CIRCULATIONAHA.111.059048. [DOI] [PubMed] [Google Scholar]
- 21.Iida O, Soga Y, Kawasaki D, Hirano K, Yamaoka T, Suzuki K, Miyashita Y, Yokoi H, Takahara M, Uematsu M. Angiographic restenosis and its clinical impact after infrapopliteal angioplasty. Eur J Vasc Endovasc Surg. 2012;44:425–431. doi: 10.1016/j.ejvs.2012.07.017. doi: 10.1016/j.ejvs.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Baumann F, Fust J, Engelberger RP, Hügel U, Do DD, Willenberg T, Baumgartner I, Diehm N. Early recoil after balloon angioplasty of tibial artery obstructions in patients with critical limb ischemia. J Endovasc Ther. 2014;21:44–51. doi: 10.1583/13-4486MR.1. doi: 10.1583/13-4486MR.1. [DOI] [PubMed] [Google Scholar]
- 23.Romiti M, Albers M, Brochado-Neto FC, Durazzo AE, Pereira CA, De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47:975–981. doi: 10.1016/j.jvs.2008.01.005. doi: 10.1016/j.jvs.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Iida O, Soga Y, Yamauchi Y, Hirano K, Kawasaki D, Yamaoka T, Takahara M, Uematsu M. Clinical efficacy of endovascular therapy for patients with critical limb ischemia attributable to pure isolated infrapopliteal lesions. J Vasc Surg. 2013;57:974–981.e1. doi: 10.1016/j.jvs.2012.10.096. doi: 10.1016/j.jvs.2012.10.096. [DOI] [PubMed] [Google Scholar]
- 25.Iida O, Nakamura M, Yamauchi Y, Kawasaki D, Yokoi Y, Yokoi H, Soga Y, Zen K, Hirano K, Suematsu N, Inoue N, Suzuki K, Shintani Y, Miyashita Y, Urasawa K, Kitano I, Yamaoka T, Murakami T, Uesugi M, Tsuchiya T, Shinke T, Oba Y, Ohura N, Hamasaki T, Nanto S OLIVE Investigators. Endovascular treatment for infrainguinal vessels in patients with critical limb ischemia: OLIVE registry, a prospective, multicenter study in Japan with 12-month follow-up. Circ Cardiovasc Interv. 2013;6:68–76. doi: 10.1161/CIRCINTERVENTIONS.112.975318. doi: 10.1161/CIRCINTERVENTIONS.112.975318. [DOI] [PubMed] [Google Scholar]
- 26.Azuma N, Uchida H, Kokubo T, Koya A, Akasaka N, Sasajima T. Factors influencing wound healing of critical ischaemic foot after bypass surgery: is the angiosome important in selecting bypass target artery? Eur J Vasc Endovasc Surg. 2012;43:322–328. doi: 10.1016/j.ejvs.2011.12.001. doi: 10.1016/j.ejvs.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Keats AS. The ASA classification of physical status–a recapitulation. Anesthesiology. 1978;49:233–236. doi: 10.1097/00000542-197810000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Beattie WS, Karkouti K, Wijeysundera DN, Tait G. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology. 2009;110:574–581. doi: 10.1097/ALN.0b013e31819878d3. doi: 10.1097/ALN.0b013e31819878d3. [DOI] [PubMed] [Google Scholar]
- 29.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]


