Supplemental Digital Content is available in the text
Keywords: children, fecal incontinence, functional constipation, long-term use, PEG3350, PEG4000
ABSTRACT
Objective:
The long-term efficacy and safety of polyethylene glycol (PEG) in constipated children are unknown, and a head-to-head comparison of the different PEG formulations is lacking. We aimed to investigate noninferiority of PEG3350 with electrolytes (PEG3350 + E) compared to PEG4000 without electrolytes (PEG4000).
Methods:
In this double-blind trial, children aged 0.5 to 16 years with constipation, defined as a defecation frequency of <3 times per week, were randomized to receive either PEG3350 + E or PEG4000. Primary outcomes were change in total sum score (TSS) at week 52 compared to baseline, and dose range determination. TSS was the sum of the severity of 5 constipation symptoms rated on a 4-point scale (0–3). Noninferiority margin was a difference in TSS of ≤1.5 based on a 95%-confidence interval [CI]. Treatment success was defined as a defecation frequency of ≥3 per week with <1 episode of fecal incontinence.
Results:
Ninety-seven subjects were included, of whom 82 completed the study. Mean reduction in TSS was −3.81 (95% CI: −4.96 to −2.65) and −3.74 (95%CI: −5.08 to −2.40), for PEG3350 + E and PEG4000, respectively. Noninferiority criteria were not met (maximum difference between groups: −1.81 to 1.68). Daily sachet use was: 0 to 2 years: 0.4 to 2.3 and 0.9 to 2.1; 2 to 4 years: 0.1 to 3.5 and 1.2 to 3.2; 4 to 8 years: 1.1 to 2.8 and 0.7 to 3.8; 8 to 16 years 0.6 to 3.7 and 1.0 to 3.7, in PEG3350 + E and PEG4000, respectively. Treatment success after 52 weeks was achieved in 50% and 45% of children, respectively (P = 0.69). Rates of adverse events were similar between groups, and no drug-related serious adverse events occurred.
Conclusions:
Noninferiority regarding long-term constipation-related symptoms of PEG3350 + E compared to PEG4000 was not demonstrated. However, analysis of secondary outcomes suggests similar efficacy and safety of these agents.
What Is Known
Polyethylene glycol is recommended as a first-line laxative for both fecal disimpaction and maintenance treatment in pediatric functional constipation.
Different formulations of polyethylene glycol have been developed, using polyethylene glycol 3350 and polyethylene glycol 4000 (with a molecular weight of 3350 and 4000 g/mol, respectively), with or without the addition of electrolytes.
What Is New
Although noninferiority criteria were not met, polyethylene glycol 3350 with electrolytes and polyethylene glycol 4000 without electrolytes appear to be similarly efficacious and safe during long-term use.
After 1 year of follow-up, treatment success was achieved in approximately half of all subjects.
Constipation is a common problem in childhood with a reported prevalence ranging from 0.7% to 29.6% (median 12%) (1). In most cases, there is no organic cause, and it is thus referred to as functional constipation (2,3). Functional constipation is usually treated with behavioral interventions and laxatives. Polyethylene glycol (PEG) is recommended as a first-line laxative for both fecal disimpaction and maintenance treatment (3,4). PEG (or macrogol) is a polymer which is not metabolized, and minimally absorbed in the gastrointestinal tract (5). Consequently, it creates an osmotic gradient in the lumen of the colon, leading to retention of fluid in the intestine, resulting in softening and loosening of stools. Various formulations of PEG have been developed, using PEG 3350 and PEG 4000 (with a molecular weight of 3350 and 4000 g/mol, respectively), with or without the addition of electrolytes (6). Both PEG 3350 and PEG 4000 are effective in childhood constipation (6,7).
It has been demonstrated that PEG is safe to use for up to 6 months in adults with chronic constipation (8). Similarly, analyses of small groups of patients suggest that PEG is safe for long-term use in children (9,10). Recently, however, concerns have been raised that compounds such as ethylene glycol or diethylene glycol may be ingested as impurities of PEG or may be formed when PEG is degraded after ingestion, leading to adverse effects (11). Consequently, the Food and Drug Administration has decided to sponsor a study on the safety of PEG in children (12).
To date, several head-to-head studies in adults with constipation have been performed that compare different PEG formulations, showing comparable efficacy and safety (13–16). A recent meta-analysis concluded that the addition of electrolytes to PEG does not appear to offer any clinical benefits in the management of adults with constipation (17).
Presently, head-to-head data comparing different PEG formulations for treatment of childhood constipation are lacking. Furthermore, it is unknown whether PEG is safe and efficacious for long-term use in children.
Therefore, we aimed to investigate the noninferiority of PEG 3350 with electrolytes (PEG3350 + E) compared to PEG 4000 without electrolytes (PEG4000) with respect to constipation-related symptoms in children. Secondary aims were to compare efficacy, rates of adverse events, and average dose between the 2 agents during 1 year of treatment.
METHODS
Study Design
We performed a double-blind, randomized, multicenter, parallel-group controlled trial in 1 academic hospital and 3 teaching hospitals in the Netherlands. The respective Medical Ethics Committees of the participating hospitals reviewed and approved the research protocol. This study is registered at ClinicalTrials.gov (NCT01810653).
Study Population
Between January 2006 and December 2007, children were recruited from the outpatient clinics of the participating hospitals. Eligible children were aged 6 months to 16 years, with <3 spontaneous bowel movements per week. Patients with drug-induced constipation or an organic cause for constipation (ie, neurological disorders, Hirschsprung disease, or anal anomalies) were excluded, as well as patients with previous gastrointestinal surgery (except appendectomy), known metabolic or endocrine disorders, intellectual disability, or cerebral palsy. Furthermore, use of PEG within 2 months before inclusion or current use of other drugs influencing gastrointestinal function (eg, lactulose, loperamide, cisapride) was not allowed. Before inclusion, written informed consent was obtained from subjects’ parents or legal representatives. Additionally, subjects ages 12 years or older provided written informed consent themselves.
Randomization, Treatment Allocation, and Blinding
Subjects were randomly assigned to either PEG3350 + E or PEG4000 at a ratio of 1:1. Subject randomization numbers were generated by a licensed Clinical Research Organization and incorporated into double blind labeling. A set of subject numbers and associated treatments were sent to the investigators. Randomization numbers were allocated sequentially in the order in which the subjects were enrolled. Subjects were stratified into 4 different age groups: 6 months to 2 years (0–2 years), 2 years to 4 years (2–4 years), 4 years to 8 years (4–8 years), and 8 years to 16 years (8–16 years). Randomization number allocation was performed by an independent employee of the clinical research organization via telephone. Study medication was provided in blank, single-dose sachets. Identical packaging and labeling ensured treatment blinding. Neither the patient nor the study personnel was aware of the treatment allocation.
Protocol
Eligible subjects recorded a bowel diary 1 week before the start of the study medication to assess gastrointestinal symptoms at baseline. In this week, the use of laxatives was not allowed. When baseline assessment confirmed a defecation frequency <3 times per week, subjects were randomized.
During the first 3 days of treatment, all subjects received a rectal enema once daily for 3 consecutive days to remove any rectal fecal impaction. Subjects ages 6 months to 3 years received enemas of 5 mL containing 45 mg of sodium laurylsulfoacetate, 450 mg of sodium citrate, and 3.125 g of sorbitol (Microlax, Johnson & Johnson Consumer b.v., Amersfoort, the Netherlands). Subjects ages 3 to 6 years received enemas of 60 mL containing 60 mg sodium docusate and 15 g sorbitol. Subjects older than 6 years received enemas of 120 mL containing 120 mg of sodium docusate and 30 g of sorbitol.
On the fourth day of treatment, subjects started with either PEG3350 + E (Transipeg, Bayer Consumer Care AG, Basel, Switzerland) or PEG4000 (Forlax, Zambon, Amersfoort, the Netherlands). PEG4000 sachets contained 4 g of PEG with a molecular weight of 4000 g/mol. PEG3350 + E sachets contained 2.95 g of PEG with a molecular weight of 3350 g/mol and electrolytes: 37.5 mg potassium chloride, 73 mg sodium chloride, 284 mg sodium sulfate, and 84 mg sodium hydrogen carbonate.
Participants younger than 2 years initially received 1 sachet per day. Participants older than 2 years initially received 2 sachets per day. The dose could be adjusted based on individual titration during the follow-up visits, with a maximum of 4 sachets per day. When defecation did not occur within 3 consecutive days, rescue medication was allowed. In children younger than 6 years, rescue medication was an enema (as described above). In older children, rescue medication was either an enema or an oral dose of 5-mg bisacodyl. In total, 8 visits were scheduled (weeks 0, 1, 2, 4, 8, 12, 26, and 52 after enrollment).
Outcome Assessment
During the first 12 weeks of the study, and the weeks before the visits at week 26 and 52, parents or legal representatives recorded the following in daily diaries: defecation frequency, stool consistency, fecal incontinence frequency, and adverse events. Furthermore, for subjects younger than 2 years, diaries were used to score the following 5 constipation-related symptoms on a 4-point scale (0 = none, 1 = mild/sometimes, 2 = moderate/regular, and 3 = severe/often): crying attributed to abdominal pain/cramps; diarrhea; flatulence; crying during defecation; redness attributed to straining during defecation. For subjects older than 2 years, diaries were used to score the following symptoms on the same scale: abdominal pain/cramps; diarrhea; flatulence; painful defecation; straining at defecation. The Total Sum Score (TSS) was calculated as the sum of the scores of the respective 5 constipation-related symptoms, ranging from 0 to 15 points, with higher scores reflecting more (severe) symptoms.
There were 2 primary outcomes: the change in TSS at week 52 compared to baseline, and dose range determination, calculated as the number of sachets used per patient per day per age group. Secondary outcomes included the proportion of subjects with treatment success (defined as a defecation frequency >3 times per week and <1 episodes of fecal incontinence per week), defecation frequency, stool consistency (hard, normal, soft, or watery) and fecal incontinence frequency. Duration of treatment was calculated as the number of days from inclusion to the date of last intake of study medication. If the date of last intake of study medication was not available (eg, if the subject was lost to follow-up), the last visit date was used instead. Safety was assessed by evaluating the number of (serious) adverse events. Whether an adverse event was considered to be drug-related was determined by the reporting local investigator based on the temporal sequence from drug administration, the recovery on discontinuation and the recurrence on reintroduction of study medication, underlying, concomitant or intercurrent diseases, concomitant medication or treatment, and the pharmacology and pharmacokinetics of the study medication.
Statistical analysis
All randomized children were included in the intention-to-treat (ITT) population. With the exception of the primary analysis, all analyses were performed in the ITT population. The primary analysis was performed in the per-protocol (PP) population, which consisted of all subjects who completed the study according to the protocol. Subjects were excluded from the PP population in case of any of the following: >1 of the 7 study visits missing; missing data at baseline and/or at week 52; data missing for several weeks; nonresponse as evidenced by use of rescue medication for >2 consecutive weeks; use of laxatives in the week before inclusion up to the day of randomization; withdrawal from the study. The primary analysis was performed to evaluate noninferiority of PEG3350 + E to PEG4000 with respect to change in TSS at week 52 compared to baseline. A 2-sided 95% confidence interval [CI] was used in the noninferiority analysis, with a noninferiority margin of an absolute difference in TSS of 1.5. Thus, noninferiority of PEG3350 + E to PEG4000 could be concluded if the 2-sided 95% CI of the absolute difference in mean change in TSS between treatment groups was entirely below the value 1.5 (which is equivalent to 1-sided testing with a CI of 97.5%).
For continuous data with a normal distribution, means and standard deviations (SD) were reported, t tests were used to evaluate differences between 2 groups and 1-way analysis of variances (with post-test for linear trend when appropriate) were used to evaluate differences between >2 groups. For continuous data with a non-normal distribution, medians and interquartile ranges (IQR) were reported, and Mann–Whitney U tests were used to evaluate differences between groups. For categorical data, Fisher exact tests were used. Significance was set at P < 0.05. Statistical analysis was performed using SAS software, Version 9 of the SAS System for Windows (SAS Institute Inc. Cary, NC).
Sample Size
Assuming equality, with for each group an estimated mean TSS of 7.5 with a SD of 2.4, a sample size of 41 evaluable subjects per treatment group was required to demonstrate noninferiority of 2 treatment groups with a power of 80% (β = 0.2), a significance level of 0.05 (α = 0.05) and a noninferiority margin of an absolute difference in TSS score of 1.5.
Missing Data
If ≤2 of 5 TSS components were missing, it was assumed that the respective symptoms did not occur. If >2 of 5 constipation-related symptoms were missing, the TSS of that week was considered missing. Subjects with insufficient data to calculate the TSS at baseline were excluded from the PP population. In analyses of treatment success, subjects with missing data were considered treatment failures.
RESULTS
Subjects
Between April 2006 and December 2007, a total of 97 children (ITT population) were included and randomized, of whom 82 completed the study (Fig. 1). Follow-up was completed in December 2008. Reasons for withdrawal were lost to follow-up (n = 5), insufficient response (n = 2), consent withdrawal (n = 2), noncompliance (n = 1), and other (n = 5). Another 20 subjects were excluded from the PP population because of missing baseline data (n = 16), because of the use of laxatives up until inclusion (n = 2), or because of the use of rescue medication for >2 consecutive weeks, indicating nonresponse (n = 2). The PP population consisted of 62 subjects. Baseline characteristics in both the ITT population and the PP population were similar between groups (Table 1 and Supplemental Table 1, Supplemental Digital Content 1). Mean age at the onset of constipation was 23.9 (SD 30.5) months and 21.4 (SD 20.5) months in the PEG3350 + E and PEG4000 groups, respectively (P = 0.64; ITT population). Children younger than 2 years had a mean number of 1.2 (SD 1.1) and 1.6 (SD 0.5) bowel movements per week, in the PEG3350 + E and PEG4000 groups, respectively (ITT population). In children older than 2 years, the mean defecation frequency on the toilet was 1.6 (SD 0.8) and 1.2 (SD 0.9) in the PEG3350 + E and PEG4000 groups, respectively (ITT population). Almost all (99%) subjects fulfilled Rome III criteria for functional constipation (Supplemental Table 2, Supplemental Digital Content 2).
FIGURE 1.
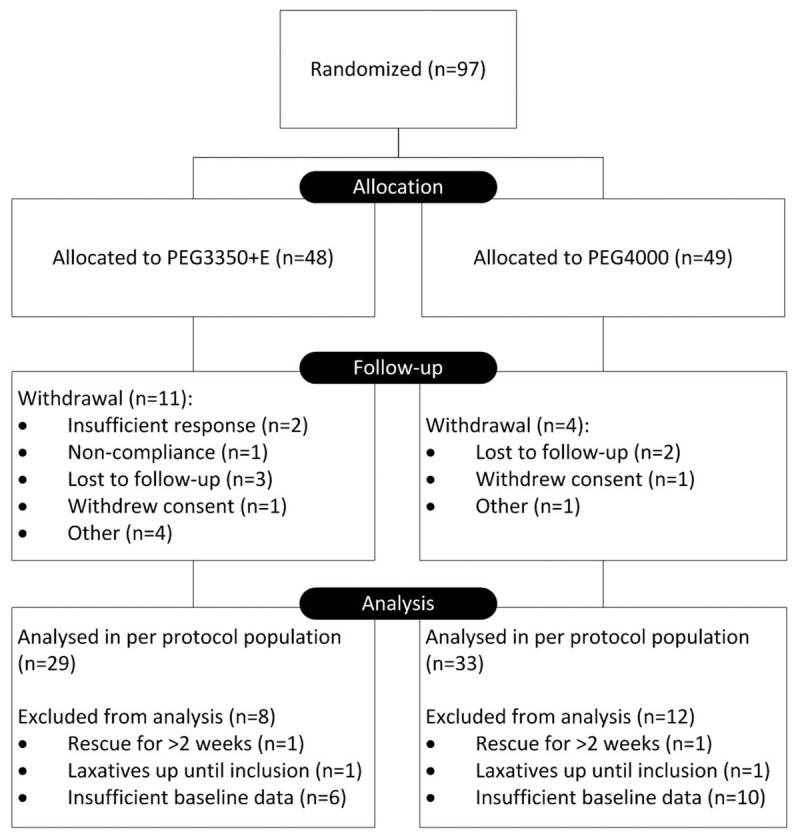
Flow diagram of the progress through trial phases.
TABLE 1.
Baseline characteristics (ITT population)
| PEG3350 + E (n = 48) | PEG4000 (n = 49) | |
| Age, y, mean (SD) | 5.5 (3.9) | 5.0 (3.3) |
| Males, n (%) | 22 (46%) | 18 (37%) |
| Weight, kg, mean (SD) | 22.9 (15.6) | 19.3 (7.9) |
| Height, cm, mean (SD) | 112.2 (27.7) | 109.3 (21.9) |
| Race | ||
| White, n (%) | 43 (90%) | 46 (94%) |
| Black, n (%) | 0 (0%) | 1 (2%) |
| Other, n (%) | 5 (10%) | 2 (4%) |
| <2 y of age: bowel movements per week, mean (SD) | 1.2 (1.1) | 1.6 (0.5) |
| ≥2 y of age: bowel movements on toilet per week, mean (SD) | 1.6 (0.8) | 1.2 (0.9) |
| ≥2 y of age: proportion of subjects with fecal incontinence (%) | 76% | 82% |
| ≥2 y of age: urinary incontinence (%) | 18% | 16% |
| Mean age at first complaints of constipation, mo, mean (SD) | 23.9 (30.5) | 21.4 (20.5) |
| Previous laxative treatment, n (%) | 34 (71%) | 38 (78%) |
ITT = intention-to-treat; PEG = polyethylene glycol; SD = standard deviation.
Primary Outcomes
Total Sum Scores
Average TSSs in the PP population during the study are shown in Figure 2. Mean reduction in TSS at week 52 compared to baseline was −3.81 (95% CI: −4.96 to −2.65; n = 26) in the PEG3350 + E group and −3.74 (95% CI: −5.08 to −2.40; n = 29) in the PEG4000 group. Mean difference between groups in change between baseline and week 52 was −0.07 with a 95% CI of −1.81 to 1.68. Consequently, noninferiority criteria were not met. In both groups, TSS scores decreased as the study progressed.
FIGURE 2.
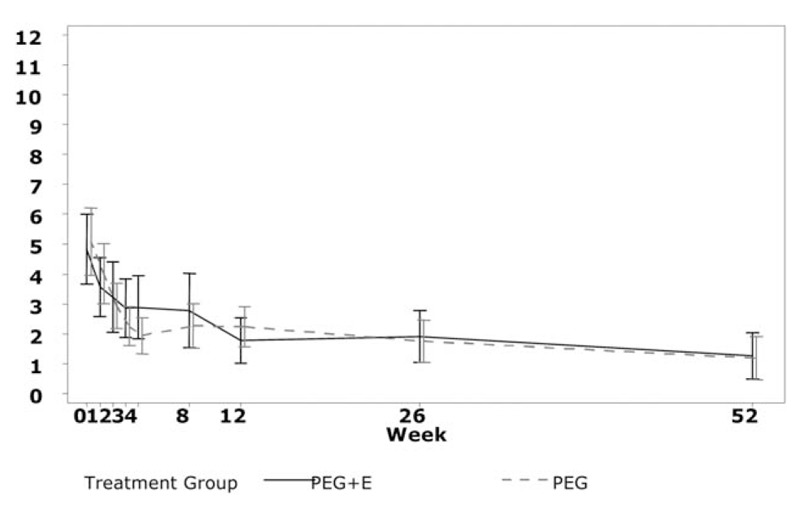
Average total sum score by week and treatment group. Whiskers indicate 95% confidence intervals (per-protocol population). PEG = polyethylene glycol.
Dose Range Determination
The dose range based on the number of sachets used per day was 0.4 to 2.3 and 0.9 to 2.1 in subjects 0 to 2 years; 0.1 to 3.5 and 1.2 to 3.2 in subjects 2 to 4 years; 1.1 to 2.8 and 0.7 to 3.8 in subjects 4 to 8 years; 0.6 to 3.7 and 1.0 to 3.7 in subjects 8 to 16 years, in PEG3350 + E and PEG4000, respectively.
Secondary Outcomes
Dosing and Duration of Treatment
No difference was found in mean daily number of sachets used during the whole study period (PEG3350 + E: 1.74 [SD 0.78]; PEG4000: 1.80 [SD 0.60]; P = 0.67). Mean daily dose of PEG relative to body weight was 0.29 g/kg (SD: 0.17) and 0.43 g/kg (SD: 0.17), for PEG3350 + E and PEG4000, respectively (P < 0.001). In both treatment groups, older subjects used lower doses of PEG relative to body weight (0 to 2 years: 0.45 and 0.65 g/kg; 2 to 4 years: 0.32 and 0.48 g/kg; 4 to 8 years: 0.25 and 0.35 g/kg; 8 to 16 years: 0.15 and 0.26 g/kg; P < 0.001 and P < 0.0001; linear trend: P < 0.0001 and P < 0.0001; in PEG3350 + E and PEG4000, respectively). Mean duration of treatment was 261 days (SD 147) in the PEG3350 + E group and 327 days (SD 88) in the PEG4000 group (P = 0.009).
Treatment Success and Use of Rescue Medication
The rate of treatment success ranged from 33% and 25% at week 1 to 50% and 45% at week 52, for subjects treated with PEG3350 + E and PEG4000, respectively (Fig. 3). At all visits, no significant differences were found in rates of treatment success between treatment groups. Rescue medication was used at least once in 35% (17/48) of the subjects in the PEG3350 + E group and 18% (9/49) in the PEG4000 group (P = 0.07).
FIGURE 3.
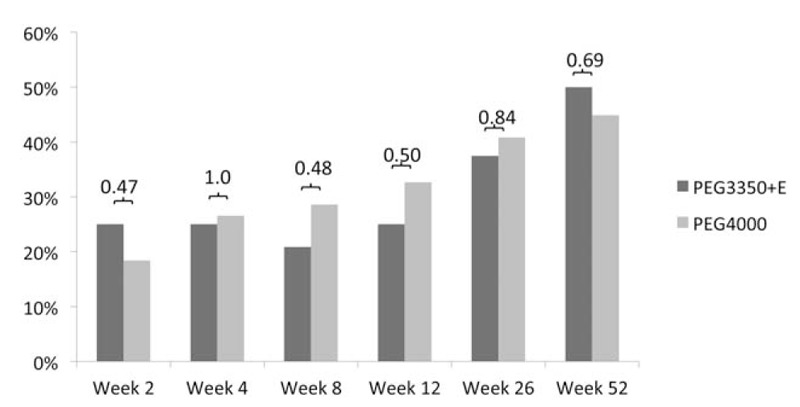
Success rates of PEG3350 + E vs PEG4000 at all visits after start of treatment (intention-to-treat population). No difference was found between treatment arms. PEG = polyethylene glycol.
Safety
A total of 143 adverse events were reported by 56 subjects (58%; Supplemental Table 3, Supplemental Digital Content 3). Five serious adverse events were reported in 2 subjects (2%): dehydration (n = 2), upper respiratory tract infection (n = 1), metabolic acidosis (n = 1), constipation (n = 1). Both subjects were randomized to PEG3350 + E. None of the serious adverse events were considered to be drug-related (Supplemental Table 4, Supplemental Digital Content 4). Six subjects (6%) reported a total of 8 adverse events that were suspected to be related to the study drug (Supplemental Table 4, Supplemental Digital Content 4). No differences in rates of adverse events, serious adverse events, or drug-related adverse events were found between groups.
DISCUSSION
The primary aim of this study was to evaluate whether PEG3350 + E was noninferior to PEG4000 with respect to long-term constipation-related symptoms in children with constipation. The primary endpoint was not met and thus noninferiority of PEG3350 + E versus PEG4000 was not demonstrated. Consequently, we cannot rule out that PEG3350 + E is inferior to PEG4000. However, there are 2 potential other reasons why noninferiority criteria were not met. First, standard deviations of the change in the primary outcome were larger than expected, leading to a lower statistical power than anticipated. Second, the PP population, in which the primary outcome was assessed, was smaller than anticipated, owing to a large number of subjects with insufficient baseline data. Indeed, noninferiority criteria were met if the number of evaluable subjects per group was the anticipated 41 (assuming no change in means and standard deviations; data not shown). No difference between the treatment arms was found with respect to constipation symptoms, treatment success, and adverse events, suggesting that both treatments are likely to have similar long-term efficacy and safety.
No difference was found in the mean daily number of sachets used. However, the mean daily dose of PEG relative to body weight was significantly lower in subjects receiving PEG3350 + E compared to subjects receiving PEG4000. Furthermore, older subjects used significantly lower doses of PEG relative to body weight in both treatment groups. These findings suggest that the recommended starting dose of PEG maintenance treatment of 0.4 g · kg−1 · day−1(3) may not be universally applicable, but instead depends on a subjects’ age or body weight, and the specific PEG formulation. To our knowledge, no previous head-to-head comparisons between various PEG formulations have been performed in children.
Duration of treatment was shorter in subjects receiving PEG3350 + E compared to subjects receiving PEG4000, potentially indicating that PEG3350 + E could successfully be tapered and stopped earlier in the course of treatment. It may, however, also reflect that subjects receiving PEG4000 were less eager to taper and stop the treatment, because PEG4000 may be more palatable than PEG3350 + E (15).
One year after inclusion, approximately half of subjects in both groups were successfully treated. This appears to be somewhat lower than reported in most previous studies investigating the efficacy of PEG for childhood constipation, although reported response rates vary widely (9,18–23). A potential explanation for the relatively low response rate may be our long follow-up compared to other studies, considering that a large proportion of children with constipation will eventually relapse after successful treatment (24). However, in the present study, therapeutic effect appeared to gradually increase over time. Furthermore, the response rate may also be an underestimation, resulting from conservative handling of missing data with respect to treatment success.
Recently, concerns have been raised regarding the long-term safety of PEG (11). Therefore, safety was an important secondary outcome of this study. Both PEG3350 + E and PEG4000 were well tolerated. Although the majority of subjects in both groups experienced an adverse event, only a small proportion of subjects experienced drug-related adverse events, and none of these were severe. Although these findings are reassuring, results from an ongoing study will help definitively determine whether long-term use of PEG in children is safe (12).
Strengths of our study are its prospective, randomized, double-blind, head-to-head nature and the long follow-up. To our knowledge, our study has the longest prospective follow-up of the efficacy and safety of laxatives in childhood constipation. A weakness is the high proportion of withdrawals and incomplete baseline data. Furthermore, Rome criteria—often considered a criterion standard for the diagnosis of pediatric functional constipation—were not used for the selection of subjects. However, almost all included subjects fulfilled Rome III criteria for functional constipation. Another limitation is that the TSS was used as a primary outcome measure, which has never been used previously. Indeed, the value of the TSS in determining the outcome of treatment for functional constipation remains to be determined. However, there is no consensus on how the efficacy of treatment for childhood constipation should be assessed, and a wide variety of outcome measures are used (25).
In conclusion, PEG3350 + E and PEG4000 are efficacious and well tolerated in the treatment of pediatric functional constipation. No significant differences with respect to efficacy and safety were found between both treatments. However, noninferiority of PEG3350 + E compared to PEG4000 regarding long-term constipation related symptoms could not be demonstrated, probably related to the small sample size.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
This study was funded by Bayer Consumer Care AG, Basel, Switzerland.
www.clinicaltrials.gov registration number: NCT01810653.
Drs Bekkali and Hoekman contributed equally to this article.
M.V. is employed by Bayer. The other authors report no conflict of interest.
REFERENCES
- 1.Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol 2011; 25:3–18. [DOI] [PubMed] [Google Scholar]
- 2.Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology 2006; 130:1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr 2014; 58:258–274. [DOI] [PubMed] [Google Scholar]
- 4.Lee-Robichaud H, Thomas K, Morgan J, et al. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database Syst Rev 2010; CD007570. [DOI] [PubMed] [Google Scholar]
- 5.Brady CE, DiPalma JA, Morawski SG, et al. Urinary excretion of polyethylene glycol 3350 and sulfate after gut lavage with a polyethylene glycol electrolyte lavage solution. Gastroenterology 1986; 90:1914–1918. [DOI] [PubMed] [Google Scholar]
- 6.Hoekman DR, Benninga MA. Functional constipation in childhood: current pharmacotherapy and future perspectives. Expert Opin Pharmacother 2013; 14:41–51. [DOI] [PubMed] [Google Scholar]
- 7.Dziechciarz P, Horvath A, Szajewska H. Polyethylene glycol 4000 for treatment of functional constipation in children. J Pediatr Gastroenterol Nutr 2015; 60:65–68. [DOI] [PubMed] [Google Scholar]
- 8.Dipalma JA, Cleveland MV, McGowan J, et al. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol 2007; 102:1436–1441. [DOI] [PubMed] [Google Scholar]
- 9.Loening-Baucke V, Pashankar DS. A randomized, prospective, comparison study of polyethylene glycol 3350 without electrolytes and milk of magnesia for children with constipation and fecal incontinence. Pediatrics 2006; 118:528–535. [DOI] [PubMed] [Google Scholar]
- 10.Gordon M, Naidoo K, Akobeng A, et al. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst Rev 2012; CD009118. [DOI] [PubMed] [Google Scholar]
- 11.NASPGHAN Neurogastroenterology and Motility Committee. Polyethylene Glycol 3350 (PEG 3350) Frequently Asked Questions [Internet]. 2015 [cited 2016 Apr 4]. Available at: http://naspghn.informz.net/NASPGHN/data/images/PEG 3350 FAQ.pdf. [Google Scholar]
- 12.Heuckeroth R, Piccoli D. Polyethylene glycol safety in children [Internet]. [cited 2016 Apr 4]; Available at: http://grantome.com/grant/NIH/R01-FD005312-01.
- 13.Seinelä L, Sairanen U, Laine T, et al. Comparison of polyethylene glycol with and without electrolytes in the treatment of constipation in elderly institutionalized patients: a randomized, double-blind, parallel-group study. Drugs Aging 2009; 26:703–713. [DOI] [PubMed] [Google Scholar]
- 14.Chaussade S, Minić M, Minic M. Comparison of efficacy and safety of two doses of two different polyethylene glycol-based laxatives in the treatment of constipation. Aliment Pharmacol Ther 2003; 17:165–172. [DOI] [PubMed] [Google Scholar]
- 15.Szojda MM, Mulder CJJ, Felt-Bersma RJF. Differences in taste between two polyethylene glycol preparations. J Gastrointestin Liver Dis 2007; 16:379–381. [PubMed] [Google Scholar]
- 16.Couturier D. [Comparative study of Forlax and Transipeg in the treatment of functional constipation in the adult]. Ann Gastroenterol Hepatol (Paris) 1996; 32:135–140. [PubMed] [Google Scholar]
- 17.Katelaris P, Naganathan V, Liu K, et al. Comparison of the effectiveness of polyethylene glycol with and without electrolytes in constipation: a systematic review and network meta-analysis. BMC Gastroenterol 2016; 16:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voskuijl W, de Lorijn F, Verwijs W, et al. PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomised, controlled, multicentre trial. Gut 2004; 53:1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nurko S, Youssef NNN, Sabri M, et al. PEG3350 in the treatment of childhood constipation: a multicenter, double-blinded, placebo-controlled trial. J Pediatr 2008; 153:254–261. [DOI] [PubMed] [Google Scholar]
- 20.Thomson MA, Jenkins HR, Bisset WM, et al. Polyethylene glycol 3350 plus electrolytes for chronic constipation in children: a double blind, placebo controlled, crossover study. Arch Dis Child 2007; 92:996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gremse DA, Hixon J, Crutchfield A. Comparison of polyethylene glycol 3350 and lactulose for treatment of chronic constipation in children. Clin Pediatr (Phila) 2002; 41:225–229. [DOI] [PubMed] [Google Scholar]
- 22.Rafati M, Karami H, Salehifar E, et al. Clinical efficacy and safety of polyethylene glycol 3350 versus liquid paraffin in the treatment of pediatric functional constipation. Daru 2011; 19:154–158. [PMC free article] [PubMed] [Google Scholar]
- 23.Candy DCA, Edwards D, Geraint M. Treatment of faecal impaction with polyethelene glycol plus electrolytes (PGE + E) followed by a double-blind comparison of PEG + E versus lactulose as maintenance therapy. J Pediatr Gastroenterol Nutr 2006; 43:65–70. [DOI] [PubMed] [Google Scholar]
- 24.van Ginkel R, Reitsma JB, Büller HA, et al. Childhood constipation: longitudinal follow-up beyond puberty. Gastroenterology 2003; 125:357–363. [DOI] [PubMed] [Google Scholar]
- 25.Kuizinga-Wessel S, Heckert SL, Tros W, et al. Reporting on outcome measures of functional constipation in children—a systematic review Authors. J Pediatr Gastroenterol Nutr 2016; 62:840–846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


