Abstract
V-domain Ig suppressor of T-cell activation (VISTA) is a critical negative checkpoint molecule involved in regulating the immune response. Targeting the pathway with an antagonist anti-VISTA antibody designated 13F3 has been shown to enhance disease severity in experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis. To determine if VISTA plays a role in murine lupus, New Zealand Black × New Zealand White (BWF1) mice were treated with 13F3 or control hamster Ig and disease monitored. Onset of proteinuria was earlier and renal damage more profound in mice treated with 13F3. Cell subset analysis showed an increase of activated splenic T cells and inflammatory splenic myeloid cells, but no effect on B cells, in mice receiving 13F3. Examination of the kidney showed an increase in inflammatory myeloid cell infiltration with 13F3 treatment. This study along with previous EAE data, suggests that interventions that enhance VISTA regulatory activity may be effective for the treatment of autoimmune disease.
Keywords: systemic lupus erythematosus, VISTA, myeloid cells
Introduction
Targeting pathways of negative checkpoint regulators (NCRs) such as cytotoxic T-lymphocyte associated-antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1) has revolutionized cancer therapy. The FDA approval of Ipilimumab (anti-CTLA-4 Ab), Pembrolizumab, and Nivolumab (anti-PD-1 Abs) has substantially improved anti-tumor responses in certain cancer patients.1 In February 2016, an antibody targeting the V-domain Ig suppressor of T cell activation (VISTA), an NCR that suppresses T-cell activation with an anti-VISTA antibody, entered Phase 1 clinical trials in patients with advanced solid tumors (NCT02671955).
Perhaps not unexpectedly, as clinical experience grows with the FDA-approved NCR-targeting agents in cancer, autoimmune disease has emerged as a relatively common side effect, suggesting that the downside of NCR inhibition is loss of immune regulation and systemic autoimmunity. For example, it has been reported that Ipilimumab has significant immune-related toxicities including the exacerbation of both rheumatoid arthritis and dermatologic complications.2–4 These clinical observations support the notion that enhancing, rather than inhibiting, NCR activity may be a rational approach to the treatment of primary autoimmune disease.
VISTA (PD-1H,5,6 DD1α,7 Dies1,8 Gi249) is hematopoietically expressed on T cells, monocytes, and neutrophils, with the highest expression in the myeloid compartment. Assessment of the VISTA pathway showed signaling alters both myeloid and T cell function.6,10,11 A previous study showed that blocking the VISTA pathway with an anti-VISTA antibody, 13F3, exacerbates experimental autoimmune encephalomyelitis (EAE), a murine model for human multiple sclerosis. An increase in disease incidence and severity (limb paralysis) was observed in mice treated with 13F3.10 A subsequent study showed that VISTA-deficient (VISTA−/−) mice are predisposed to developing autoimmunity.12 Further examination of this phenotype revealed VISTA−/− mice bred onto 2D2 TCR transgenic mice specific for the autoantigen myelin oligodendrocyte glycoprotein (MOG35–55) had enhanced EAE disease incidence and mortality.12
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease with protean clinical manifestations, characterized immunologically by production of autoantibodies, immune complex formation and deposition, and in some cases immune complex glomerulonephritis.13 Numerous murine models of lupus are available, including New Zealand Black × New Zealand White F1 hybrid (BWF1) mice, in which only female F1 mice develop high levels of nephritogenic anti-dsDNA antibodies and eventually succumb to florid diffuse proliferative glomerulonephritis.14
We recently demonstrated the critical role of VISTA in SLE by breeding Sle1,3 lupus-prone mice onto VISTA−/− mice. Sle1,3 mice genetically deficient in VISTA expression had greatly accelerated disease characterized by severe, rapidly progressive glomerulonephritis associated with enhanced T and myeloid cell dysfunction.15 These findings prompted an investigation of targeting the VISTA pathway with antibodies to further dissect the role of VISTA in female BWF1 mice.
In this study, we show for the first time that targeting VISTA with 13F3 worsened disease in female BWF1 mice. This is demonstrated by the earlier onset of proteinuria and renal damage in anti-VISTA-treated mice compared with control Ig-treated mice. Flow cytometric analysis showed an increase in splenic activated T cells and myeloid cells in 13F3-treated mice. Further studies showed an increase in renal inflammatory myeloid cells in 13F3-treated mice. These findings further establish a critical role of VISTA in the regulation of lupus nephritis in BWF1 mice and suggest that antibodies that augment VISTA activity in vivo may be therapeutic in lupus and other autoimmune diseases.
Materials and methods
Mice
NZBWF-1 (BWF1) female mice were purchased from Jackson Laboratories and C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD). All mice were housed in the pathogen-free facility at The Geisel School of Medicine at Dartmouth.
Treatment
For lupus studies, BWF1 mice were treated with 300ug hamster Ig (BioXCell) or 13F320 three times a week by i.p. injection.
Proteinuria
Proteinuria levels (mg/dl) were recorded weekly using Chemstrip test trips (Roche Diagnostics).
Antibodies
All directly conjugated antibodies for flow cytometry were purchased from Biolegend. These include: B220, CD45, CD3, CD4, CD8, CD69, CD44, F4/80, Gr1, CD11b, CD11c, MHCII and CCR2. CD4+Foxp3+ regulatory T cells (Tregs) were identified using the commercially available Treg staining kit (eBioscience). To detect VISTA expression, 13F3 Ab from our laboratory was used.10
Cytokine/chemokine analysis
Quantification of chemokine and cytokine levels was determined with the 32 Milliplex Mouse Cytokine/Chemokine Magnetic Bead Panel Luminex assay (Milipore) and analyzed on the Bio-plex 200 Systems (Life Science Research, Bio Rad). All data were analyzed using the Bio Plex Manager 6.0 software.
Flow cytometry
Flow cytometric analysis of splenocytes and renal cells was performed as previously described.15
Confocal imaging
Confocal imaging was performed to detect C3/IgG immune complexes as previously described.15
Cell sorting
Splenocytes were stained to identify cell population of interest and sorted on the BD FACSAria III (BD Bioscience).
Migration assays
Migration assays were established using a transwell system. Splenocytes were isolated from Ham-Ig or 13F3 treated NZBWF-1 mice and placed in upper chamber (5.0 µm, HTS Transwell®-96, Corning Life Sciences). The lower chamber was coated with monocyte chemoattractant protein-1 (MCP-1) and CXCL13 (B-cell chemoattractant) recombinant proteins (PeproTech). After 6 hours, cells were harvested from both chambers and stained to identify inflammatory monocytes (Gr1+ Ly6C+ F4/80+ CD11b+ cells) expressing CCR2. Flow cytometric analysis was performed and cell migration calculated as percentage of cells migrated into lower chamber compared with upper chamber.
Heat map
The heat map was generated as described previously.15
Graphs and statistical analysis
GraphPad Prism 6 software (Graph Pad, San Diego, CA) was used to present data and statistical analysis determined by the Student t-test (two-tailed); ***p < 0.005; **p < 0.025; *p < 0.05.
Results
VISTA blockade enhances murine arthritis and lupus disease progression
Proteinuria-negative lupus-prone female BWF1 mice (21 weeks old) were treated three times a week by i.p. injection with PBS (control), 300 µg hamster-Ig (Ham-Ig) or the VISTA pathway blocking antibody 13F3. Disease progression was monitored weekly by proteinuria and weight loss.
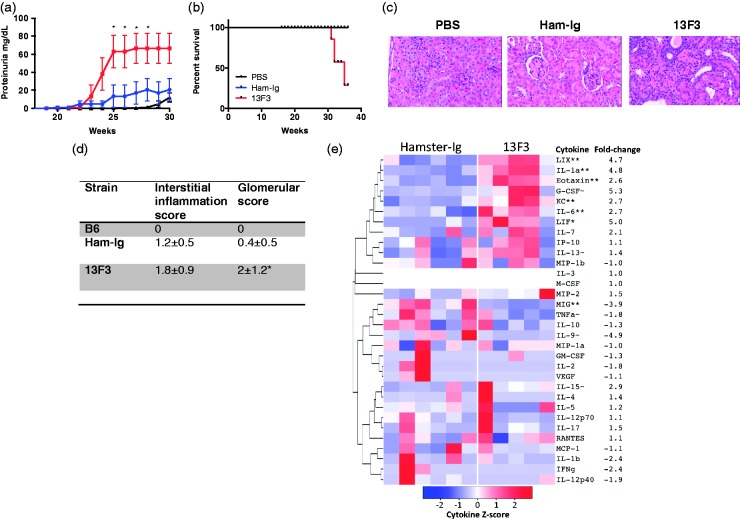
Treatment with 13F3 accelerated the development of proteinuria compared with untreated mice (Figure 1(a)) and reduced survival (Figure 1B), independent of weight loss (data not shown). Pathologic examination of kidney sections from sacrificed mice showed greater cellular infiltration in 13F3-treated mice (Figure 1(c), representative H&E staining). A semi-quantitative grading of interstitial and glomerular disease in kidney sections demonstrated significantly greater glomerular scores in 13F3 compared with Ham-Ig-treated mice (Figure 1(d)). Next, the in vivo systemic effect of 13F3 was determined by profiling serum from Ham-Ig and 13F3-treated mice by Luminex assay for a pathogenic disease signature. Analysis showed statistically significant increases in LIX/CXCL5, KC/CXCL1 and IL-6 (Figure 1(e)), which have been associated with SLE pathogenesis.16–18
Figure 1.
13F3 induces disease progression in lupus-prone BWF1 mice.
21 week old BWF1 female mice were treated with PBS (n = 8), 300µg Ham-Ig (n = 8) or 300µg 13F3 (n = 8) and (a) proteinuria monitored weekly and (b) survival recorded. To determine clinical effect of 13F3, paraffin-embedded kidneys were (c) H&E stained and (d) clinically scored for interstitial inflammation and glomerular score. (e) To examine effect of systemic impact of 13F3, serum was collected from Ham-Ig or 13F3 treated mice (week 30) and chemokine/cytokines quantified by luminex assay and shown as heat map (see material and methods). Data representative of 3 experiments and shown as mean +/- SEM. Statistical analysis performed as previously described (see materials and methods).
VISTA blockade increases splenic T-cell activation and inflammatory myeloid cell function
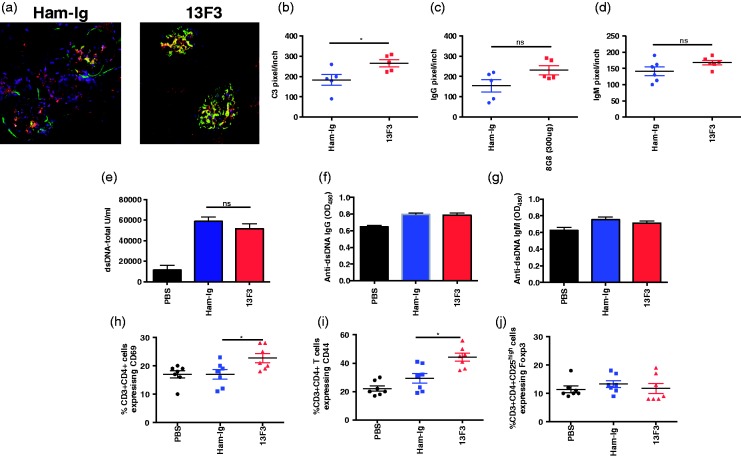
Autoantibody production with resultant immune complex deposition is a hallmark of lupus nephritis.19 To determine if blocking the VISTA pathway with 13F3 exerted its accelerated effects on nephritis via enhanced renal immune complexes (IC) deposition, immunofluorescence staining was performed on frozen kidney sections from Ham-Ig or 13F3 with IgG and C3. No difference in IgG/C3 IC deposition were detected between treatment groups (samples processed at week 30) (Figure 2A). Further quantification revealed upon 13F3 treatment, a significant increase in C3 levels (Figure 2B) independent of IgG deposition (Figure 2C). In parallel, IgM deposition was examined and no difference observed (Figure 2D). Furthermore, serum analysis from Ham-Ig- and 13F3-treated mice showed no difference in total anti-dsDNA antibody (Figure 2E), anti-dsDNA-IgG antibody (Figure 2F) and dsDNA-IgM antibody (Figure 2G) titers.
Figure 2.
13F3 increases splenic activated T cells and myeloid cells independent of B-cell function in BWF1 mice.
(a) To determine role of VISTA pathway in the B cell compartment, immunofluorescence staining was performed on frozen kidney specimens from Ham-Ig or 13F3 treated mice (age 30 weeks) to detect immune complex (C3/IgG) deposits using confocal microscopy. Quantification of (b) C3, (c) IgG and (d) IgM levels in the kidney were determined by pixels per inch. Analysis of (e) anti-dsDNA (total), (f) anti-dsDNA-IgG and (g) anti-dsDNA IgM antibody titers in serum from control versus 13F3-treated mice were determined by ELISA. To determine effect of 13F3 on cellular subsets, splenocytes were stained for activated CD4+ T cells expressing (h) CD69 (i) CD44, (J) CD4+ Tregs and analysed by flow cytometry. Data is representative of 3 experiments and displayed as the mean +/- SEM. Statistical analysis performed as previously described (see materials and methods).
Flow cytometric analysis was then performed to study the impact of 13F3 on splenic cell subsets. 13F3-treated mice had a significant increase in the frequency of activated CD4+ T cells as defined by the expression of both CD69 (Figure 2(h)) and CD44 (Figure 2(d)). No differences in frequency of CD4+Tregs (Figure 2(j)) were found.
13F3 increases splenic inflammatory myeloid cells and infiltration into the kidneys
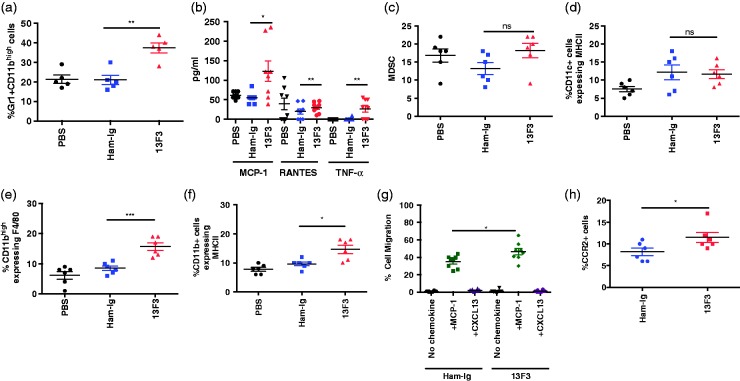
VISTA is highly expressed on myeloid cells20 warranting assessment of 13F3 on the myeloid compartment was investigated A significant increase in splenic inflammatory myeloid cells (Figure 3A) was found in 13F3-treated mice. Furthermore, after in vitro stimulation with IFN-γ, purified splenic inflammatory myeloid cells from 13F3-treated mice exhibited a heightened pro-inflammatory profile with increased elaboration of MCP-1 (monocyte chemoattractant protein-1), RANTES (regulated on activation, normal T cell expressed and secreted), and TNF-α compared to control PBS- and Ham-Ig-treated mice (Figure 3B). No difference in myeloid derived suppressor cells (MDSCs) (Figure 3C) or dendritic cells (CD45+ cells expressing CD11c+MHCII) (Figure 3D) was detected. As lupus is a type 1 driven disease, plasmacyoid dendritic cells (pDC) activation and IFN-γ production was also examined and no difference detected between groups (data not shown). Assessment of infiltrating renal cell populations by flow cytometry showed no difference in T cells (CD44 expression) between untreated and treated mice by flow cytometric analysis and immunofluorescence staining (data not shown). In contrast, significant increases in the percentage of inflammatory myeloid cell populations previously reported in nephritic BWF1 mice.21,22 Renal cell analysis of myeloid cells defined as CD11bhigh cells expressing F4/80 (Figure 3E) and CD11b+ cells expressing MHCII (Figure 3F) were significantly increased in mice treated with 13F3 compared to controls. To determine if this was in part due to 13F3 affecting cell migration, in vitro migration assays were performed with splenocytes from Ham-Ig and 13F3 treated mice in response to MCP-1 and CXCL13 (B lymphocyte chemoattractant). Compared to inflammatory monocytes from Ham-Ig treated mice, a specific and significant increase in migration in response to MCP-1 was found in 13F3-treated mice (Figure 3G). A significant increase in expression of CCR2, the MCP-1 ligand, was also found in 13F3-treated mice (Figure 3H).
Figure 3.
13F3 increases myeloid cell infiltration into the kidneys in BWF1 mice.
To determine the impact of 13F3 on the myeloid compartment, splenoctyes were harvested from PBS, Ham-Ig and 13F3 treated mice and stained with directly conjugated antibodies to identify (a) Gr1+CD11bhigh (c) MDSCs and (d) CD11c+ cells expressing MHCII and flow cytometic analysis was performed. To assess functional significance of 13F3, (b) splenic inflammatory monocytes were isolated and stimulated with IFN-γ for 6 hours. Supernatants were harvested and investigated for MCP-1, RANTES and TNF-α by luminex assay. For renal analysis, flow cytometry was performed in renal cells stained with antibodies to detect (e) CD11bhigh cells expressing F4/80 and (f) CD11bhigh cells expressing MHC class II (gated on CD45+ cells) . For migration assays, splenocytes were placed in the upper chamber of a 0.5µm transwell, and migration to recombinant proteins MCP-1 and CXCL13 coated on the lower chamber was examined. To assess cell migration, transwell assays were established (see materials and methods) and (g) inflammatory monocyte cell migration (h) expressing CCR2 was examined by flow cytometry. Flow cytometry performed using a MACSQuant Analyzer and data analyzed using FlowJo software. All data is representative of 3 experiments and displayed as mean +/- SEM. Statistical analysis performed as previously described (see materials and methods).
Discussion
The data presented in this study show the pronounced acceleration of disease in BWF1 mice in response to 13F3, an anti-VISTA antibody that blocks its function as an NCR. This effect appears to be mediated predominantly through a pro-inflammatory influence on myeloid cells, perhaps by promoting enhanced migration into nephritic kidneys. Following 13F3 treatment, early onset of proteinuria (Figure 1(a)) and renal damage (Figure 1(c and d)) was detected in BWF1 mice compared with control treated mice. Cellular analysis revealed that 13F3 treatment increased the percentage of splenic CD4+ T-cell activation (Figure 2(h and i)) and inflammatory myeloid cells (Figure 3(a)) independent of B-cell activity. These findings are similar to previous reports that showed little if any direct influence of VISTA on B-cell biology.10,15,22
We found that most of the effects of VISTA in the BWF1 model are confined to the myeloid and, to a lesser extent, T-cell compartments. Previous studies have shown that VISTA is highly expressed on myeloid cells.10,11,22 In our study, ex vivo activation of splenic myeloid cells with IFN-γ showed an increase in MCP-1 and TNF-α production, all of which have been correlated with active lupus,23–25 in the 13F3-treated versus Ham-Ig-treated group (Figure 3(b)).
The accelerated renal damage seen with 13F3 treatment prompted an analysis of the specific cellular infiltration in treated versus control kidneys. Flow cytometric analysis showed an increase in myeloid cell infiltration in 13F3-treated mice (Figure 3(e and f)), which suggests that 13F3 alters myeloid activity and/or chemotaxis. This finding was similar to an increase in infiltrating renal myeloid cells seen in a VISTA knockout lupus-prone mouse model we have derived (Sle1,3VISTA mice15). In the current work, in vitro chemotaxis assays suggest that this may in part be due to the enhanced response of inflammatory myeloid cells to MCP-1 (Figure 3(g)). This is intriguing as prolonged survival has been found in CCR2−/− MRL/lpr lupus-prone mice.26 Indeed, other investigators have demonstrated that blocking CCR2 ameliorates renal vasculitis disease in MRL/lpr mice, associated with a reduction in monocyte homing to bone marrow.27,28
The significant increase in CCR2 expression on inflammatory monocytes that we identified after 13F3 treatment (Figure 3H) may be due to intrinsic defects induced by the VISTA pathway. This is supported by the dysregulation of CCR2 expression on transcription factor IFN regulatory factor (Irf)5−/− monocytes, resulting in less responsiveness to MCP-1/CCL2, reducing their response to developing pristine-induced lupus mice.29 We are currently investigating how 13F3 may mediate these defects.
Concluding remarks
The data presented here highlight the role of VISTA in regulating murine lupus. In the future, enhancement rather than inhibition of the activity of NCRs like VISTA may represent a rational approach to the treatment of human autoimmune diseases such as multiple sclerosis or SLE.
Acknowledgements
The authors thank DartLab Core Facility for technical support with flow cytometry and Luminex assay. We also thank Ken Orndorff with his assistance with using the confocal microscope. We thank the department of Pathology at Dartmouth-Hitchcock Medical Center for processing kidney specimens.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RJN is involved with the commercial development of VISTA and is the CSO of ImmuNext Inc. RJN receives consulting fees, research support and salary. RJN and SC are inventors listed on VISTA patents.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an Alliance for Lupus Research/Lupus Research Institute grant awarded to RJN and CMB. RJN is CSO of ImmuNext Inc and receives research support, salary and/or consulting fees. RJN is involved in business development.
References
- 1.Ceeraz S, Nowak EC, Burns CM, Noelle RJ. Immune checkpoint receptors in regulating immune reactivity in rheumatic disease. Arthritis Res Ther 2014; 16: 469–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol 2016; 28: 254–263. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2016; 2: 234–240. [DOI] [PubMed] [Google Scholar]
- 4.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer 2016; 54: 139–148. [DOI] [PubMed] [Google Scholar]
- 5.Flies DB, Wang S, Xu H, Chen L. Cutting edge: A monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol 2011; 187: 1537–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flies DB, Han X, Higuchi T, et al. Coinhibitory receptor PD-1H preferentially suppresses CD4(+) T cell-mediated immunity. J Clin Invest 2014; 124: 1966–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon KW, Byun S, Kwon E, et al. Control of signaling-mediated clearance of apoptotic cells by the tumor suppressor p53. Science 2015; 349: 1261669–1261669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aloia L, Parisi S, Fusco L, Pastore L, Russo T. Differentiation of embryonic stem cells 1 (Dies1) is a component of bone morphogenetic protein 4 (BMP4) signaling pathway required for proper differentiation of mouse embryonic stem cells. J Biol Chem 2010; 285: 7776–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakr MA, Takino T, Domoto T, et al. GI24 enhances tumor invasiveness by regulating cell surface membrane-type 1 matrix metalloproteinase. Cancer Sci 2010; 101: 2368–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Rubinstein R, Lines JL, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med 2011; 208: 577–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bharaj P, Chahar HS, Alozie OK, et al. Characterization of Programmed Death-1 Homologue-1 (PD-1H) expression and function in normal and HIV infected individuals. Plos One 2014; 9. [DOI] [PMC free article] [PubMed]
- 12.Wang L, Le Mercier I, Putra J, et al. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc Natl Acad Sci U S A 2014; 111: 14846–14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putterman C, Caricchio R, Davidson A, Perlman H. Systemic lupus erythematosus. Clin Dev Immunol 2012; 2012: 437282–437282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol 2011; 2011: 271694–271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceeraz S, Sergent PA, Plummer SF, et al. VISTA deficiency accelerates the development of fatal murine lupus nephritis. Arthritis Rheumatol 2017; 69: 814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus 2004; 13: 339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lech M, Anders HJ. The pathogenesis of lupus nephritis. J Am Soc Nephrol 2013; 24: 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robak E, SysaJedrzejowska A, Stepien H, Robak T. Circulating interleukin-6 type cytokines in patients with systemic lupus erythematosus. Eur Cytokine Netw 1997; 8: 281–286. [PubMed] [Google Scholar]
- 19.Perry D, Sang A, Yin YM, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol 2011; 2011: 271694. [DOI] [PMC free article] [PubMed]
- 20.Bethunaickan R, Berthier CC, Ramanujam M, et al. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J Immunol 2011; 186: 4994–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiffer L, Bethunaickan R, Ramanujam M, et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol 2008; 180: 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lines JL, Pantazi E, Mak J, et al. VISTA Is an immune checkpoint molecule for human T cells. Cancer Res 2014; 74: 1924–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aringer M, Feierl E, Steiner G, et al. Increased bioactive TNF in human systemic lupus erythematosus: Associations with cell death. Lupus 2002; 11: 102–108. [DOI] [PubMed] [Google Scholar]
- 24.Lu MM, Wang J, Pan HF, et al. Increased serum RANTES in patients with systemic lupus erythematosus. Rheumatol Int 2012; 32: 1231–1233. [DOI] [PubMed] [Google Scholar]
- 25.Singh RG, Usha, Rathore SS, Behura SK, Singh NK. Urinary MCP-1 as diagnostic and prognostic marker in patients with lupus nephritis flare. Lupus 2012; 21: 1214–1218. [DOI] [PubMed] [Google Scholar]
- 26.Perez de Lema G, Maier H, Franz TJ, et al. Chemokine receptor Ccr2 deficiency reduces renal disease and prolongs survival in MRL/lpr lupus-prone mice. J Am Soc Nephrol 2005; 16: 3592–3601. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa H, Kohno M, Sasaki M, et al. Antagonist of monocyte chemoattractant protein 1 ameliorates the initiation and progression of lupus nephritis and renal vasculitis in MRL/lpr mice. Arthritis Rheum 2003; 48: 2555–2566. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni O, Pawar RD, Purschke W, et al. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J Am Soc Nephrol 2007; 18: 2350–2358. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Feng D, Bi X, Stone RC, Barnes BJ. Monocytes from Irf5-/- mice have an intrinsic defect in their response to pristane-induced lupus. J Immunol 2012; 189: 3741–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]