Abstract
HIV infection and antiretroviral therapy (ART) are associated with bone loss and poor vitamin D status in Caucasian populations, though their relative roles are not known. No previous studies have examined longitudinal changes in areal bone mineral density (aBMD), measured by DXA, or in vitamin D status in HIV-positive African women. Of 247 premenopausal, urban, black African women from Soweto, South Africa, initially recruited, 187 underwent anthropometry, DXA scanning and blood and urine collections at both baseline and 12 months. Of these, 67 were HIV-negative throughout (Nref), 60 were HIV-positive with preserved CD4 counts at baseline (Ppres) and 60 were HIV-positive with low CD4 counts at baseline, eligible for ART by South African standards of care at the time (Plow). No participant had been exposed to ART at baseline. By 12 months, 51 Plow women had initiated ART, >85% of whom took combined tenofovir disoproxil fumarate (TDF), lamivudine and efavirenz. By 12 months, Plow and Nref, but not Ppres, increased in body weight and fat mass (group-by-timepoint p ≤0.001, p=0.002 respectively). Plow had significant decreases in aBMD of 2-3%, before and after size adjustment, at the femoral neck (p ≤0.002) and lumbar spine (p ≤0.001), despite significant weight gain. These decreases were associated with increased bone turnover but there were no significant differences or changes over time in vitamin D status, serum phosphate concentrations or renal phosphate handling. Excluding data from 9 Plow women unexposed to ART and 11 Ppres women who had initiated ART accentuated these findings, suggesting the bone loss in Plow was related to ART exposure. This is the first study describing DXA-defined bone loss in HIV-positive Sub-Saharan African women in association with ART. Further work is required to establish if bone loss continues with on-going ART and, if so, whether this results in increased fracture rates.
Keywords: Africa, Antiretroviral Therapy, Bone Health, HIV, Premenopausal Women, Vitamin D
Introduction
HIV-infection is associated with weight loss and with increased morbidity and mortality related to infectious complications of immune suppression. Since the advent of effective combination antiretroviral therapy (ART) survival has increased dramatically,(1) and in more recent years there has been a shift in focus from HIV-associated communicable diseases to non-communicable diseases including osteoporosis and associated fragility fractures.(2)
HIV and ART have both been associated with bone loss and increased risk of fracture.(3–6) Evidence is mixed but the general consensus is that ART-exposure, particularly to tenofovir disoproxil fumarate (TDF), is associated with bone loss.(7,8) Even though the mechanism(s) is not entirely understood, it is hypothesised that the bone loss is related to TDF-induced renal phosphate wasting with subsequent skeletal demineralisation.(9) In a meta-analysis(3) HIV-positive individuals were three times more likely to have lower areal bone mineral density (aBMD) than controls and the prevalence of dual-energy X-ray absorptiometry (DXA) defined osteoporosis was as high as 15%.
A potential contributor to bone loss in HIV-infected individuals is poor vitamin D status, which has been described in several HIV-positive populations, both in ART-naïve patients(10) and in association with ART-exposure.(11,12) The precise mechanism underlying these observations is unknown but it has been suggested that it may be a consequence of decreased dermal synthesis and/or as a result of ART-induced up-regulation of the metabolism of 25-hydroxyvitamin D (25(OH)D). Adequate vitamin D status, as measured by serum 25(OH)D, has a well-established role in skeletal health.
To date, available data about the possible effects of HIV-infection on bone health and vitamin D status are heavily biased towards Caucasian males in resource-rich societies who have a much lower lifetime risk of osteoporotic fracture than women. Some of these studies are also limited by retrospective design and lack of an HIV-negative control group. The effects of HIV and ART on bone health and vitamin D status in Sub-Saharan Africa, where the burden of HIV-infection lies, and where osteoporotic fracture rates are predicted to rise,(13) are largely unstudied(14) and, although data are emerging,(15–17) there are important gaps and an urgent need for targeted research.(14)
We have shown, in a cross-sectional study of urban South African premenopausal women which compared those with HIV-infection but ART-naïve with those who were HIV-negative, that there were no significant differences in aBMD or vitamin D status related to HIV-status, despite HIV-positive women with low CD4 counts having less body fat than both HIV-positive women with preserved CD4 counts and HIV-negative women.(18) HIV-positive women in the low CD4 group were eligible to start ART under the South African medical guidelines current at the time of the study (2010). We hypothesised, based on studies from Western countries, that HIV-positive African women would lose bone mineral over time, accompanied by changes in weight and body composition, in vitamin D status and in calcium and phosphate metabolism, and that the bone loss would be accentuated in those exposed to ART. The aim of the study presented in this paper was to follow these three groups of women for 12 months, in order to investigate the effects of HIV-infection and ART over time on bone mass, body composition, vitamin D status and markers of calcium and phosphorus metabolism.
Participants and Methods
Study design
The study was designed as a 12-month longitudinal investigation of urban South African premenopausal women with and without HIV-infection. Participants attended for study visits at the SAMRC/Wits Developmental Pathways for Health Research Unit (DPHRU) in Soweto, Johannesburg, SA, at baseline and 12 months. At each visit anthropometry and DXA scans were performed, and blood and urine samples were collected for laboratory analysis, as described below. The participants with HIV-infection continued to attend their usual primary health care facilities for monitoring and management of their disease.
Study details, inclusion and exclusion criteria at enrollment, baseline characteristics and dietary intakes have been described in full elsewhere.(18,19) In brief, 247 urban, black South African women were recruited into three groups of approximately equal size from clinics in Soweto, Johannesburg, between February and July 2010. All women were premenopausal and not pregnant or lactating at the time of enrollment. The three groups were: HIV-negative women to act as the reference group (Negative-reference: Nref, n = 98); HIV-positive women with preserved CD4 counts (≥ 350 x 106 cells/l) anticipated not to require ART-initiation for at least 12 months (Positive-preserved: Ppres, n = 74) and HIV-positive women with low CD4 counts (≤200 x 106 cells/l) who were eligible to commence ART soon after the baseline visit (Positive-low: Plow n = 75). At the 12-month visit, Nref participants were offered repeat HIV-antibody testing using the Alere Determine™ rapid HIV-antibody test (Alere San Diego, Inc. San Diego, CA, USA). Those who had a reactive HIV test were referred to a local clinic for confirmatory testing and CD4 count.
The University of the Witwatersrand Human Research Ethics Committee (HREC number: M101525) and the Gauteng Department of Health, South Africa, approved the study. All participants provided informed written consent prior to enrollment.
Anthropometry
Height was measured to the nearest 0.1 cm using a permanent wall-mounted stadiometer (Holtain, Crosswell, UK). Weight was measured to the nearest 0.1kg using an electronic digital scale (Tanita, TBF-410 MA Body Composition Analyzer, Tanita Corporation of America, Inc., Illinois, USA) with participants wearing light clothing. Body Mass Index (BMI) was calculated as weight in kilograms divided by the square of height in metres (kg/m2). Waist and hip circumferences were measured to the nearest 0.1cm using a non-stretchable plasticised tape measure.
Bone mineral density and body composition by DXA
DXA was performed using an Hologic QDR 4500A DXA (Model: Discovery W (S/N 71201) software version 12.5:7 Hologic, Inc., Waltham, MA, USA). Scans of the whole body, lumbar spine L1-L4, total hip and femoral neck were conducted with participants wearing light clothing, and performed using the automatic scan mode. Whole body was analysed as ‘whole body less head’ (WBLH). For each individual, their follow-up scans were compared to baseline to ensure consistent placement of regions of interest. DXA bone measures were bone mineral content (BMC, g), bone area (BA, cm2) and aBMD (g/cm2) at each site and body composition measures were whole body lean mass (g) and fat mass (g). Daily calibration and long-term DXA scanner stability monitoring were conducted using manufacturer phantoms. Short-term repeat scan precision on 30 participants with repositioning was 0.65% for lumbar spine and 0.97% for proximal femur; the coefficient of variation on daily quality control scans during the period was <0.5%. The extent to which individuals experienced aBMD loss in excess of the least significant change was determined using the conventional DXA 0.03g/cm2 threshold, which is based on a notional instrument precision of 1%.(20)
Laboratory measures
Full details of the blood and urine collection, processing and analytical procedures are given in the supplemental information. In brief, blood was collected in the morning after an overnight fast by venepuncture and processed as EDTA plasma for parathyroid hormone (PTH) analysis and as serum for other analytes relating to calcium, phosphorus and vitamin D metabolism (calcium, phosphate, magnesium, albumin, 25(OH)D) and bone turnover (total alkaline phosphatase (TALP); bone alkaline phosphatase (BALP); serum type 1 procollagen N-terminal (P1NP); and serum collagen type 1 cross-linked β-C-telopeptide (β-CTX). All plasma and serum samples were stored frozen, initially at -20°C and subsequently at -80°C. Urine was collected into a sterile container at the second void of the day after an overnight fast acidified with concentrated hydrochloric acid and stored at -20°C. Serum was analysed for 25(OH)D in the laboratory at DPHRU in duplicate using a chemiluminescent immunoassay (Liaison, DiaSorin Inc., Stillwater, MN, USA). The DPHRU laboratory participates in the international Vitamin D External Quality Assessment Scheme (DEQAS, www.deqas.org) and holds the certificate of proficiency. All other analyses were conducted at MRC Human Nutrition Research (HNR), Cambridge, UK; the methods and assay performance are given in the supplementary material. The number of samples successfully collected, transported and analysed varied depending on the analyte and timepoint. The footnote to Table 5 details the numbers of biochemical datapoints for each analyte by group and timepoint.
Table 5.
Biochemistry at baseline and 12 months by initial HIV status
| Nref |
Ppres |
Plow |
Group-by-timepoint |
||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | p | |
| Serum | |||||||
| 25(OH)D nmol/l | 58.0±15.7 | 63.3±16.6 | 60.5±17.0 | 67.1±18.6h | 60.6±22.6 | 61.3±20.3e | 0.24 |
| Phosphate mmol/l | 1.10±0.19 | 1.16±0.20h | 1.10±0.20 | 1.20±0.19h | 1.15±0.16b | 1.22±0.21 | 0.26 |
| Calciumcorr mmol/l | 2.49±0.10 | 2.48±0.10 | 2.51±0.10 | 2.48±0.10 | 2.52±0.13 | 2.49±0.11 | 0.44 |
| Magnesium mmol/l | 0.80±0.06 | 0.81±0.06 | 0.80±0.06 | 0.80±0.07 | 0.78±0.07 | 0.80±0.06 | 0.60 |
| Albumin g/l | 41.8±3.9 | 40.7±3.3 | 39.6±3.0c | 38.7±4.5b | 36.0±6.0a,d | 38.7±4.8b,g | ≤0.001 |
| Creatinine μmol/l | 84.0±7.7 | 84.5±8.3 | 80.7±7.8 | 81.9±8.9 | 80.5±8.5 | 80.8±9.0b | 0.70 |
| eGFR ml/min/1.73m2 | 80.4±11.9 | 79.7±11.4 | 82.8±10.4 | 81.1±11.9 | 84.2±12.3 | 83.0±11.2 | 0.40 |
| TALP¶ U/l | 43.4[37.7,55.6] | 51.0[40.7,61.1]i | 45.5[36.5,53.8] | 50.0[40.7,59.9]h | 48.7[36.4,60.9]e | 79.1[56.7,92.8]a, d, g | ≤0.001 |
| BALP¶ U/l | - | 18.3[15.4,21.1] | - | 16.9[14.4,19.5] | - | 23.3[17.5,30.8]a, d | ≤0.001# |
| P1NP¶ μg/l | - | 47.5[37.0,65.5] | - | 51.8[39.5,73.9] | - | 72.8[55.4,100.3]a, e | ≤0.001# |
| β-CTX¶ ng/l | - | 117[73,179] | - | 153[98,231]c | - | 171[117,254]a | ≤0.001# |
| PTH¶ ng/l | - | 23.6[19.1,31.6] | - | 20.2[12.7,27.9]c | - | 22.9[17.3,37.5] | 0.04# |
| Urine | |||||||
| Phosphate:Cre¶ | 0.97[0.76,1.34] | 0.92[0.68,1.34] | 1.23[0.94,1.49] | 1.32[0.89,1.84]b | 0.76[0.61,1.14] | 1.29[0.87,1.63] | 0.31 |
| Calcium:Cre¶ | 0.08[0.03,0.17] | 0.07[0.03,0.15] | 0.08[0.04,0.15] | 0.08[0.03,0.12] | 0.07[0.03,0.14] | 0.07[0.03,0.18] | 0.63 |
| Magnesium:Cre | 0.16±0.07 | 0.17±0.08 | 0.15±0.09 | 0.15±0.07 | 0.16±0.07 | 0.24±0.11d | 0.002 |
| TmP/GFR mmol/l | 1.19±0.29 | 1.27±0.29 | 1.14±0.30 | 1.22±0.26 | 1.33±0.32 | 1.30±0.30 | 0.30 |
Data are means ± SDs for normal distributions, for those with positive skew (¶) are median [25,75 percentile]. # indicates p-value is for group effect at 12 months. Abbreviations are 25(OH)D, 25-hydroxyvitamin D; eGFR-MDRD, estimated glomerular filtration rate using the MDRD formula; TALP, total alkaline phosphatase; BALP, bone alkaline phosphatase; P1NP, serum type 1 procollagen N-terminal; β-CTX, serum collagen type 1 cross-linked β-C-telopeptide; and serum type 1 procollagen N-terminal; PTH, parathyroid hormone; Cre, urine creatinine used to develop urine mineral ratios in mmol/mmol; TmP, tubular maximum reabsorption rate of phosphate. Values for BALP, P1NP, CTX and PTH are available at 12 months only. Significance of differences from Scheffé post-hoc tests from hierarchical linear models of the variable in natural logarithms with timepoint (0/12 months), group (Nref/Ppres/Plow), ID (nested within group) and a group-by-timepoint interaction, as follows: between Ppres or Plow and Nref at each timepoint a ≤0.001, b≤0.01, c ≤0.05; between Ppres and Plow at each timepoint: d ≤0.001, e≤0.01, f ≤0.05; between baseline and 12 months in each group: g ≤0.001, h≤0.01, i ≤0.05. Numbers of biochemical datapoints are: 25(OH)D - baseline: Nref = 67; Ppres = 60; Plow = 60; 12 months: Nref = 64; Ppres = 60; Plow = 59; Other blood analytes - baseline: Nref = 41; Ppres = 37; Plow = 51; 12 months: Nref = 65; Ppres = 60; Plow = 60; Urine mineral ratios - baseline: Nref = 50; Ppres = 42; Plow = 38; 12 months: Nref = 60; Ppres = 55; Plow = 42; TmP/GFR - baseline: Nref = 28; Ppres = 27; Plow = 33; 12 months: Nref = 58; Ppres = 55; Plow = 42.
Serum calcium was corrected for albumin (calciumcorr) by normalising to an albumin concentration of 40 g/l:(21) Calciumcorr (mmol/l) = SCa +[0.02 x (40 – Salbumin)], where SCa and Salbumin are the serum concentrations of calcium (mmol/l) and albumin (g/l) respectively.
The ratio of tubular maximum reabsorption rate of phosphate to glomerular filtration rate (TmP/GFR) was derived using the following equations after calculation of the tubular resorption of phosphate (TRP):(22)
-
(a)
if TRP ≤0.86 then TmP/GFR mmol/l = SP x TRP or
-
(b)
if TRP >0.86 then TmP/GFR mmol/l = 0.3 x SP x [TRP/(1-(0.8 x TRP))]
where TRP = [(UP/SP) x (SCr/UCr)] and SP and SCr are the serum phosphate (mmol/l) and creatinine (mmol/l) concentrations and UP (mmol/l) and UCr (mmol/l) are their respective fasting urine concentrations.
Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula for females(23) but without the factor for African-American ethnicity,(24) as per the South African guidelines: eGFR (ml/min/1.73m2) = 141 × min (SCr/0.7, 1)-0.329 × max(SCr/0.7, 1)-1.209 × 0.993age × 1.018, where SCr is serum creatinine concentration in mg/dl (i.e SCr in μmol/l x 0.0113) and age is in years.
Statistical methods
Data were analysed using DataDesk 6.3.1 (Data Description Inc, Ithaca, NY). Summary statistics are presented as mean ± standard deviation (SD) for normally distributed data or median [25th percentile, 75th percentile (IQR)] for skewed distributions. Based on findings from the baseline study, fat mass-to-lean mass2 (fat:lean2) was used to compare body composition between the groups.(18) All continuous variables were transformed to natural logarithms prior to data manipulation and analysis. This enabled the differences between groups and between timepoints to be expressed as a sympercent ([difference/mean] x 100) (25) and, for positively skewed data, normalised the distribution. Summary sympercent data are presented as percentage mean difference ± SE.
Two approaches were used to evaluate and compare the changes in each variable over time within the 3 groups, utilising Linear Model software in DataDesk, with Scheffé post hoc tests.
Firstly, using repeat-measures ANOVA and ANCOVA in hierarchical models constructed for each variable of interest, with individual identifier (nested by group), timepoint, group and a group-by-timepoint interaction term. Weight and bone area were included to adjust bone mineral data for the possible effects of bone and body size(26); height was not included because it was not anticipated to change in these women over 12 months. These models evaluated the size and significance of between-group differences at baseline and 12 months and of within-group differences between values at baseline and 12 months. They also tested whether the change from baseline differed significantly between groups.
Secondly, ANCOVA models were constructed with the value at 12 months of each variable of interest as the dependent variable and baseline value and group as independent variables. Adjustment for the possible influence of differences and change in bone and body size was achieved by including mean height, mean and change in weight, and mean and change in bone area between baseline and 12 months. These models quantified the size effect of the difference between each pair of groups in the change from baseline to 12 months.
The summary data and models presented are only for those women included in the dataset at 12 months, as detailed in the results section. Including all women who had participated at baseline, but not at 12 months, made no material difference to the results described. We also present models with no additional adjustment for covariates such as lifestyle factors, because they did not differ significantly between the groups. A number of women in Ppres had initiated ART during the 12 months since baseline (n = 11 of 60), while some Plow women who had been expected to initiate ART had not done so (n = 9 of 60). To more closely consider the possible effects of ART on the measured outcomes the models were repeated excluding these 18 women. The two groups of HIV-positive women in the dataset restricted by ART status are designated as PpresN (Ppres not ART exposed) and PlowY (Plow exposed to ART).
Results
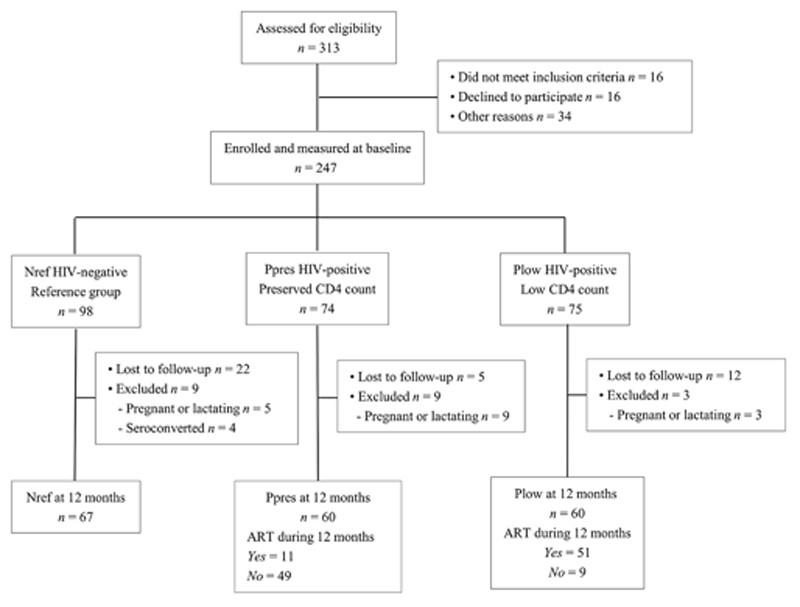
The flow of participants through the study and the reasons for loss-to-follow up are detailed in Figure 1. Of the 247 women measured at baseline, 39 were not available at 12 months for, generally because they could not be contacted. In addition, data from 21 women were excluded because of pregnancy/lactation in the interim period or, in the Nref group, because they had become HIV-positive. There were no significant differences in baseline CD4 count, bone and biochemical variables and most of the anthropometry between women in the same group who were included at 12 months and those who were not. The exceptions were that women included at 12 months in the Nref and Ppres groups were significantly older, and in both the Nref and Plow groups were heavier with greater BMI, fat mass, waist circumference and hip circumference at baseline than those in the same group who were not included at 12 months (data not shown).
Figure 1. Flow through of study participants in the three groups from recruitment to 12 months.
Nref = HIV-negative women; Ppres = HIV-positive women with preserved CD4 counts at baseline; Plow = HIV negative women with low CD4 counts at baseline; ART = antiretroviral therapy.
Table 1 gives the ages and other characteristics at baseline and 12 months of the participants included in the follow-up study by their original group at baseline. On average, Ppres women were older and had more pregnancies that Nref but there were no significant differences in other characteristics. Table 1 also gives, for HIV-positive women, data on CD4 counts and ART initiation and duration. Over 85% of participants requiring ART were treated with a combination of TDF, lamivudine and efavirenz.
Table 1.
Subject characteristics at baseline and 12 months by initial HIV status
| Timepoint months |
Nref n = 67 |
Ppres n = 60 |
Plow n = 60 |
|
|---|---|---|---|---|
| Age (y) | 0 | 31.4±8.5 | 34.5±6.0a | 33.2±6.2 |
| 12 | 32.2±8.5 | 35.5±6.0a | 34.1±6.2 | |
| Gravidity | 0 | 1 [0,2] | 2 [2,3]b | 2 [1,3] |
| Regular menses (%) | 0,12 | 69, 81 | 83, 82 | 75, 77 |
| Hormonal contraception (%) | 0,12 | 39, 36 | 33, 27 | 35, 32 |
| Any fracture to date (%) | 0,12 | 28, 28 | 23, 23 | 13, 13 |
| Current smoker (%) | 0,12 | 7, 3 | 15, 15 | 8, 8 |
| Alcohol consumer (%) | 0 | 15 | 30 | 22 |
| Calcium intake (mg/d) | 0 | 670±372 | 656±306 | 727±576 |
| ART initiated (n,%) | 12 | - | 11 (18) | 51 (85)c |
| ART duration (d)¶ | 12 | - | 198 [118,279] | 330 [298,347]c |
| CD4 counts (x106/l) | ||||
| All HIV-positive women | 0 | - | 416 [343,466] | 176 [102,224]c |
| 12 | - | 402 [338,474] | 233 [179,333]c | |
| Restricted set# | 0 | - | 428 [352, 478] | 151 [93,212]c,d |
| 12 | - | 419 [350,482] | 230 [178,348]c,d |
Data are mean ± SD, median [IQR] or percentage of subjects reporting ‘Yes’.
Nref = HIV-negative throughout; Ppres = HIV-positive with preserved CD4 counts at baseline; Plow = HIV-positive with low CD4 counts at baseline.
In women who had initiated ART by 12 months (PPres n=11, Plow, n=51)
Restricted set by ART-exposure status at 12 months: PpresN = Ppres not exposed to ART (n=59), PlowY = Plow exposed to ART (n=51).
Significance of group differences at same timepoint, or within-individual timepoint differences in the same group using ANOVA (continuous variables) or Chi-square test (frequencies):
p ≤0.05 different to Nref
p ≤0.01 different to Nref
p ≤0.001 different to Ppres
p ≤0.001 different to baseline
There were no other significant differences.
Changes in anthropometry and bone measures by HIV status at baseline
Table 2 presents the anthropometric, body composition and aBMD data at baseline and 12 months for participants in the follow-up study. Table 2 also details the statistical significance in the hierarchical linear models (Method 1) of within-individual change over time in each variable by group and of group-by timepoint interactions which indicate whether the time effect differed significantly between groups. Table 3 gives the percentage changes over time within each group from the models presented in Table 2. Figure 2 illustrates these changes over time by group for all women and for women defined by their ART status at 12 months. Table 4 gives the percentage differences in change over time between groups from the ANCOVA models (Method 2).
Table 2.
Anthropometry and bone mineral densities at baseline and 12 months by initial HIV status
| Nref |
Ppres |
Plow |
Group-by-timepoint |
||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | p | |
| Anthropometry | |||||||
| Height (m) | 1.58±0.06 | 1.58±0.06 | 1.59±0.06 | 1.60±0.06 | 1.59±0.06 | 1.59±0.06 | 0.66 |
| Weight (kg) | 72.2±17.4 | 74.1±17.5h | 72.1±17.4 | 71.6±17.3a | 64.5±15.7a,d | 66.8±15.4a,d,g | ≤0.001 |
| BMI (kg/m2) | 29.1±7.1 | 29.9±7.2h | 28.3±6.5c | 28.1±6.6a | 25.5±6.0a,d | 26.4±5.9a,d,g | ≤0.001 |
| Fat mass, WBLH (kg) | 27.8±12.1 | 29.4±12.2h | 26.0±9.7c | 26.0±10.3a | 21.3±9.3a,d | 23.7±10.7a,d,g | 0.002 |
| Lean mass, WBLH (kg) | 39.0±5.8 | 39.2±5.8 | 39.5±5.9 | 39.0±5.8 | 36.8±5.0a,d | 37.4±5.6a,d | 0.05 |
| Fat:lean2 (1000*kg/kg2) | 17.8±4.9 | 18.7±4.9i | 16.4±4.6a | 16.8±5.2a | 15.4±5.2a,d | 16.7±5.3a,g | 0.07 |
| Waist (cm) | 88.5±14.7 | 91.4±14.7h | 89.8±15.2 | 90.9±16.6 | 85.1±14.3a,d | 87.5±13.7a,e,h | 0.20 |
| Hip (cm) | 109.7±14.1 | 111.7±14.7i | 107.5±14.2c | 108.4±13.5a | 100.7±13.7a,d | 104.7±12.2a,d,g | 0.01 |
| Waist:hip (cm/cm) | 0.81±0.07 | 0.82±0.07 | 0.84±0.09a | 0.84±0.08 | 0.85±0.06a | 0.83±0.06 | 0.11 |
| Bone mineral density (aBMD g/cm2) | |||||||
| Total hip | 1.018±0.151 | 1.042±0.153g | 0.989±0.134a | 1.008±0.135a,h | 0.985±0.128a | 0.993±0.128a | 0.04 |
| Femoral neck | 0.941±0.120 | 0.939±0.130 | 0.918±0.133a | 0.916±0.134a | 0.923±0.135b | 0.901±0.134a,f,h | 0.02 |
| Lumbar spine | 1.019±0.132 | 1.032±0.127i | 1.023±0.116 | 1.020±0.112 | 1.011±0.135f | 0.990±0.140a,d,g | ≤0.001 |
| WBLH | 0.967±0.088 | 0.966±0.087 | 0.948±0.077a | 0.952±0.075a | 0.949±0.083a | 0.942±0.082a,e,i | 0.02 |
Nref, n = 67, HIV-negative women; Ppres, n = 60, HIV-positive with preserved CD4 counts at baseline; Plow, n = 60, HIV-positive with low CD4 counts at baseline, WBLH = whole body less head. Data are means ± SDs, aBMD data are unadjusted. Significance of differences from Scheffé post hoc tests from hierarchical linear models of the variable in natural logarithms with timepoint (0/12 months), group (Nref/Ppres/Plow), ID (nested within group) and a group-by-timepoint interaction, as follows: between Ppres or Plow and Nref at each timepoint a ≤0.001, b≤0.01, c ≤0.05; between Ppres and Plow at each timepoint: d ≤0.001, e ≤0.01, f ≤0.05; between baseline and 12 months in each group: g ≤0.001, h≤0.01, i ≤0.05.
Table 3.
Percentage change over 12 months within each group by initial HIV status
| Nref |
Ppres |
Plow |
||||
|---|---|---|---|---|---|---|
| %Δ±SE | P | %Δ±SE | P | %Δ±SE | p | |
| Anthropometry | ||||||
| Weight | +2.7±0.8 | 0.004 | -0.7±0.9 | 0.70 | +3.8±0.9 | ≤0.001 |
| BMI | +2.6±0.8 | 0.007 | -1.0±0.9 | 0.50 | +3.8±0.9 | ≤0.001 |
| Fat mass | +6.2±2.0 | 0.008 | -0.8±2.1 | 0.93 | +9.4±2.1 | ≤0.001 |
| Lean mass | +0.5±0.6 | 0.63 | -1.2±0.6 | 0.17 | +0.7±0.6 | 0.50 |
| Fat:lean2 | +5.1±1.9 | 0.03 | +1.5±2.0 | 0.75 | +8.0±2.0 | ≤0.001 |
| Waist | +3.3±0.9 | 0.002 | +1.0±1.0 | 0.56 | +2.8±1.0 | 0.01 |
| Hip | +1.9±0.7 | 0.04 | +1.0±0.8 | 0.47 | +4.1±0.8 | ≤0.001 |
| Waist:hip | +1.4±0.9 | 0.28 | +0.1±1.0 | 0.99 | -1.3±1.0 | 0.37 |
| Bone mineral density, unadjusted | ||||||
| Total hip | +2.5±0.6 | ≤0.001 | +1.9±0.6 | 0.007 | +0.4±0.6 | 0.78 |
| Femoral neck | -0.1±0.6 | 0.99 | -0.3±0.7 | 0.92 | -2.4±0.7 | 0.002 |
| Lumbar spine | +1.4±0.5 | 0.03 | -0.2±0.5 | 0.94 | -2.0±0.6 | ≤0.001 |
| WBLH | -0.2±0.3 | 0.86 | +0.4±0.3 | 0.51 | -0.9±0.3 | 0.03 |
| Bone mineral density, size-adjusted | ||||||
| Total hip | +0.7±0.6 | 0.49 | -0.3±0.6 | 0.84 | -1.7±0.6 | 0.02 |
| Femoral neck | -0.4±0.7 | 0.84 | -0.2±0.7 | 0.94 | -2.7±0.7 | ≤0.001 |
| Lumbar spine | +1.2±0.5 | 0.07 | -0.2±0.6 | 0.92 | -2.3±0.6 | ≤0.001 |
| WBLH | -0.0±0.3 | 0.99 | +0.3±0.3 | 0.62 | -0.7±0.4 | 0.14 |
Nref, n = 67, HIV-negative women; Ppres, n = 60, HIV-positive with preserved CD4 counts at baseline; Plow, n = 60, HIV-positive with low CD4 counts at baseline; WBLH, whole body less head. Data are mean percentage changes (%Δ) ± SEs expressed as sympercents from hierarchical linear models. All continuous variables were transformed to natural logarithms. Predictor variables were time (0/12 months), group (Nref/Ppres/Plow) and ID (nested within group) and a group-by-timepoint interaction. Adjustment of aBMD for bone and body size was achieved by including bone area and weight in the models. The p-values for the interaction are in Table 1.
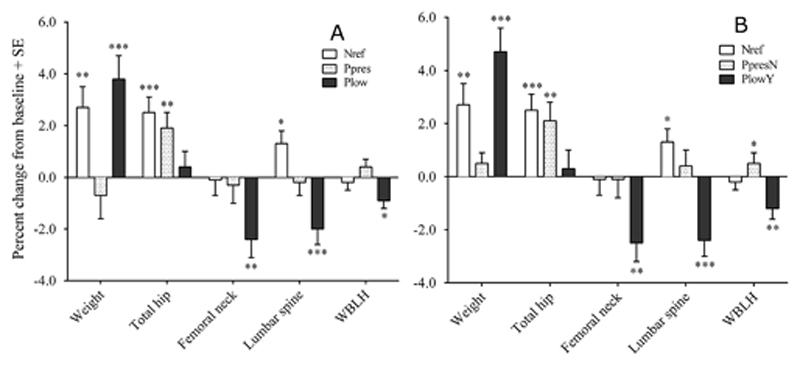
Figure 2. Percentage changes in weight and unadjusted aBMD from baseline to 12 months in HIV-negative and HIV-positive women (A) according to group designation at baseline and (B) ART status at 12 months.
ART = antiretroviral therapy; Nref = HIV-negative at baseline and 12 months, n = 67; Ppres = HIV-positive women with preserved CD4 counts at baseline, n = 60; Plow = HIV negative women with low CD4 counts at baseline n = 60; PpresN = Ppres women not exposed to ART by 12 months, n = 49; PlowY = Plow women exposed to ART by12 months, n = 51;. Data are mean percentage changes from baseline to 12 months obtained from Scheffé post-hoc tests from hierarchical linear models of the variable in natural logarithms with timepoint (0/12 months), group (Nref/Ppres/Plow), ID (nested within group) and a group-by-timepoint interaction; error bars are SEs. Significance of change at 12 months from baseline: *p ≤0.05; **p ≤0.01; ***p ≤0.001.
Table 4.
Percentage difference between groups in change over 12 months by initial HIV status and by ART status at 12 months
| HIV status at baseline |
ART status at 12 months |
|||||||
|---|---|---|---|---|---|---|---|---|
| Ppres-Nref %∆±SE |
Plow-Nref %∆±SE |
Plow–Ppres %∆±SE |
Group P |
PpresN-Nref %∆±SE |
PlowY-Nref %∆±SE |
PlowY-PpresN %∆±SE |
Group p |
|
| Anthropometry | ||||||||
| Weight (kg) | -3.5±1.2b | +0.4±1.2 | +3.9±1.2b | 0.002 | -3.9±1.3b | +1.4±1.3 | +5.3±1.4a | ≤0.001 |
| Fat mass (kg) | -7.4±2.8c | +1.3±3.0 | +8.7±3.0c | 0.006 | -8.7±3.0c | +3.3±3.1 | +12.1±3.4b | ≤0.001 |
| Lean mass (kg) | -1.6±0.8 | -0.2±0.8 | +1.4±0.9 | 0.12 | -1.6±0.9 | +0.1±0.9 | +1.7±1.0 | 0.13 |
| Fat:lean2 (1000*kg/kg2) | -4.3±2.7 | +1.4±2.8 | +5.7±2.8 | 0.11 | -5.7±2.9 | +2.2±3.0 | +7.9±3.1c | 0.03 |
| Waist (cm) | -2.1±1.3 | -0.9±1.3 | +1.2±1.3 | 0.25 | -2.0±1.4 | -0.5±1.3 | +1.5±1.4 | 0.32 |
| Hip (cm) | -1.2±1.0 | +0.9±1.0 | +2.1±1.0 | 0.12 | -1.7±1.0 | +1.6±1.1 | +3.3±1.1c | 0.02 |
| Waist:hip (cm/cm) | +0.0±1.2 | -0.9±1.2 | -0.9±1.2 | 0.70 | +0.2±1.2 | -1.4±1.2 | -1.6±1.3 | 0.38 |
| Bone mineral density, unadjusted | ||||||||
| Total hip (g/cm2) | -0.8±0.8 | -2.3±0.8c | -1.5±0.8 | 0.02 | -0.5±0.9 | -2.5±0.9c | -1.9±1.0 | 0.02 |
| Femoral neck (g/cm2) | -0.3±0.9 | -2.4±0.9c | -2.1±0.9 | 0.02 | -0.1±1.0 | -2.5±1.0c | -2.5±1.0 | 0.02 |
| Lumbar spine (g/cm2) | -1.5±0.8 | -3.5±0.8a | -1.9±0.8c | ≤0.001 | -0.9±0.8 | -3.9±0.8a | -3.0±0.9b | ≤0.001 |
| WBLH (g/cm2) | +0.4±0.4 | -0.8±0.4 | -1.3±0.5c | 0.02 | +0.6±0.5 | -1.2±0.5c | -1.8±0.5a | ≤0.001 |
| Bone mineral density, size-adjusted | ||||||||
| Total hip (g/cm2) | -1.1±0.7 | -2.1±0.7c | -1.0±0.7 | 0.02 | -1.0±0.8 | -2.5±0.8b | -1.4±0.9 | 0.01 |
| Femoral neck (g/cm2) | +0.2±0.9 | -1.9±0.9 | -2.1±1.0 | 0.06 | +0.5±1.0 | -2.1±1.0 | -2.7±1.1 | 0.04 |
| Lumbar spine (g/cm2) | -1.4±0.8 | -3.2±0.8a | -1.8±0.8 | ≤0.001 | -0.9±0.8 | -4.1±0.8a | -3.2±0.9b | ≤0.001 |
| WBLH (g/cm2) | +0.1±0.5 | -1.0±0.5 | -1.1±0.5 | 0.04 | +0.2±0.5 | -1.3±0.5c | -1.6±0.5b | 0.005 |
Nref, n = 67, HIV-negative women; Ppres, n = 60, HIV-positive with preserved CD4 counts at baseline; Plow, n = 60, HIV-positive with low CD4 counts at baseline; PpresN, n = 49, HIV-positive with preserved CD4 counts at baseline not started on ART by 12 months; PlowY, n = 51, HIV-positive with low CD4 counts at baseline and started on ART during the 12 months; WBLH, whole body less head. Data are mean percentage differences (%∆) ± SEs expressed as sympercents from Scheffé post-hoc tests from ANCOVA models (y = aBMD at 12 months, x = aBMD at baseline; group (Nref/Ppres/Plow)). Adjustment for bone and body size was achieved by including height, change and mean bone area, change and mean weight in the models. All continuous variables were transformed to natural logarithms prior to data analysis. Significance of difference between the groups in each pair: a ≤0.001, b≤0.01, c ≤0.05.
Plow women were significantly lighter at baseline with lower fat mass, lean mass and fat:lean2 ratio than Ppres or Nref. Nref and Plow gained significant amounts of weight and body fat over the 12 months, but Ppres had no significant anthropometric changes. Despite their larger increases in weight and fat mass, Plow remained significantly lighter with less fat mass than Nref.
The mean baseline aBMD values of Ppres and Plow women at the different skeletal sites were generally slightly lower than Nref. These differences were not significant in cross-sectional models, but were statistically significant at the hip and WBLH in the hierarchical longitudinal models. The aBMD of Nref and Ppres women increased significantly at the total hip and aBMD at the lumbar spine was also increased in Nref. These changes were largely associated with the increase in body weight, and were diminished and not significant after size adjustment. Conversely, despite their increase in body weight, by 12 months the aBMD of Plow women had significantly decreased by 2-3% at the femoral neck and lumbar spine, before and after size adjustment, with smaller decreases at the total hip and WBLH.
With the dataset restricted by ART-exposure status at 12 months that included only Ppres women who had not initiated ART (PpresN) and Plow women who had (PlowY), the size effects and statistical significance of the changes within each group (Method 1, Figure 2 A and B) and the differences in change over time between groups (Method 2, Table 4) were generally similar or slightly greater than in the full dataset, despite the smaller numbers of women. These analyses indicate that both PlowY and Nref had gained weight and fat mass relative to PpresN, whereas PlowY experienced significant decreases in aBMD relative to the other two groups, both before and after size adjustment, the greatest differences occurring at the lumbar spine (difference ± SE: -3.9 ± 0.8% relative to Nref, -3.0 ± 0.9% relative to PpresN). A greater number of women in PlowY lost more than 0.03 g/cm2 of aBMD than the other two groups, for example at the lumbar spine (Nref = 8%, PpresN = 4%, PlowY = 50%, p ≤0.0001).
Changes in markers of calcium and phosphorus metabolism
Table 5 presents the data and results of the hierarchical models for 25(OH)D and markers of calcium and phosphorus metabolism. There was no significant group-by-timepoint interaction for 25(OH)D. There were also no significant differences in 25(OH)D over time or between groups other than that Ppres had significantly higher concentrations at 12 months than at baseline and than Plow. There were also no significant group-by-timepoint interactions for serum phosphate, TmP/GFR or eGFR-MDRD. Similar findings were obtained using the dataset restricted by ART-exposure status.
In contrast, a significant group-by-timepoint interaction was observed for TALP (p ≤0.001, Table 5). TALP increased over time in all 3 groups (change over time ± SE: Nref = +10.8 ± 4.4%, p = 0.053; Ppres = +15.6 ± 4.5%, p = 0.003; Plow = +35.2 ± 3.9%, p ≤0.001) but significantly more so in Plow (Plow - Nref = +28.7 ± 5.5%, p ≤0.001; Plow - Ppres = +26.5 ± 5.4%, p ≤0.001). The magnitude of the differences in change over time between Plow and the other groups was accentuated in the dataset restricted by ART-exposure status, (PlowY - Nref = +34.4 ± 5.3%, p ≤0.001; PlowY - PpresN = +39.5 ± 5.6%, p ≤0.001). BALP measured only at 12 months showed similar group differences to TALP (Table 4 and, in the restricted dataset, PlowY – Nref: BALP = +29.0 ± 5.3%, p ≤0.001). The two measures were closely correlated (R2 = 58.9%; p ≤0.001), indicating that the increase in TALP in Plow at 12 months was predominantly due to an increase in the bone isoenzyme.
P1NP and β-CTX were also significantly higher in Plow compared to Nref at 12 months (Table 5). There was no significant difference in PTH at 12 months between Plow or PlowY and Nref. However, PTH at 12 months in Ppres was significantly lower than Plow (Table 4). For all three of these analytes, restricting the dataset by ART-exposure status increased the magnitude and significance of these differences (PlowY-Nref; P1NP = +47.6 ± 8.2%, p ≤0.001 and CTX = +48.4 ± 11.9%, p ≤0.001; Ppres-Plow: PTH = -32.4 ± 10.5%, p = 0.009).
Significant group-by-timepoint interactions were seen for serum albumin and fasting UMg/Cr (Table 4). Serum albumin was lower in Plow at baseline than the other 2 groups but had increased significantly by 12 months towards Nref values. Serum albumin in Ppres was also lower than Nref at baseline, but higher than Plow, and was little changed at 12 months. There was a marked increase in UMg/Cr in Plow from baseline to 12 months compared to the other two groups (difference over time ± SE: +40.2 ± 10.4%, p ≤0.001).
Discussion
This is the first prospective study comparing DXA-defined changes in aBMD, vitamin D status and biochemistry in HIV-positive women not on ART at baseline and HIV-negative women in Africa. Women who had a CD4 count low enough to warrant ART initiation, based on South African health department guidelines at the time, lost significant amounts of bone mineral over 12 months when assessed using DXA. Bone loss averaged 2-3% over one year, a rate which exceeds the 1-2% annual decreases in aBMD seen in older women in early menopause. The women in this study were premenopausal and, on average, in their early thirties, when no bone loss would be expected and their average aBMD values at baseline were within -0.5 SD (i.e. z-score close to zero) of the HIV-negative reference group.(18). Indeed, increases or no change in aBMD over 12 months were observed in both the reference women and HIV-positive women with preserved CD4 counts.
The magnitude and the statistical significance of the aBMD decreases in Plow were unchanged or increased after excluding Plow women who remained unexposed to ART at 12 months. In addition, the bone loss observed in Plow women, especially those exposed to ART, was in spite of increased body weight and improved CD4 count and serum albumin concentration. These findings, plus the lack of difference in bone measures at baseline between the two HIV-positive groups when they were not exposed to ART, strongly suggests that the observed bone loss in Plow was a result of ART exposure rather than severity of HIV-infection. Decreases in aBMD due to TDF have been reported in longitudinal studies of HIV-negative African women and men receiving pre-exposure prophylaxis to prevent HIV-infection, although generally of a smaller magnitude to those observed in the HIV-positive women in this study, possibly because of lower treatment adherence.(27,28)
Unlike previous reports e.g.(10–12) there was no indication that either HIV-infection or ART-exposure was associated with compromised vitamin D status in these South African women. The mean 25(OH)D concentration in all 3 groups exceeded 50 nmol/l at both baseline and 12 months with no decrease over time in Ppres or Plow. Similarly, there was no evidence from the serum phosphate, TmP/GFR and eGFR data that either HIV-infection or ART-exposure was associated with renal damage, as has been reported by others in connection with TDF exposure. e.g.(29,30) In contrast, the decreases in aBMD in Plow were associated with higher bone turnover at 12 months as shown by serum concentrations of TALP, BALP, P1NP and β-CTX, effects that were accentuated when Plow women not exposed to ART were excluded. This suggests a direct or indirect effect of ART on bone, resulting in an increased rate of bone remodelling and loss of skeletal mineral despite no difference in PTH between the groups. The observed increase in fasting urinary magnesium excretion (UMg/Cr) in Plow also fits with this possibility, although this may also reflect the magnesium stearate present in many TDF preparations.
There were very high rates of overweight and obesity in this cohort of urban, black South African women.(18) Women who initiated ART gained weight rapidly, largely due to an increase in fat mass rather than in lean mass. Greater fat accumulation in relation to ART-associated weight gain has been reported in other settings and it has been suggested that this is due to mitochondrial dysfunction.(31) In the South African women, the deposition of fat rather than muscle with ART may also be a consequence of their poor diet and low physical activity.(19) Whatever the mechanism, such rapid increases in adiposity are likely to increase the risk of poor cardiometabolic outcomes.
This study is limited by its observational design, the absence of data on HIV-viral load, HIV-clade and duration of HIV-infection, and the relatively small numbers. It was conducted in women from Soweto, a poor township of South Africa at latitude 26°S, and the results may not extrapolate to other populations. In addition, we had no data on markers specific for proximal tubule damage as reported in connection with TDF-exposure,(29,30) such as retinol-binding protein or beta-2 microglobulin. The strengths are the longitudinal design, that no participant was exposed to ART at baseline, that an HIV-negative reference group of similar age and background was included, and that >85% of women exposed to ART received standard South African first-line treatment removing the confounding effects of various ART combinations.
In summary, this study suggests that, in urban, black South African women, HIV-infection per se has no discernible effects on bone mineral status over a 12-month period but that exposure to TDF-based ART is associated with loss of bone mineral and an increase in bone turnover. Newer prodrugs of TDF such as Tenofovir Alafemamide Fumarate (TAF) have been demonstrated in controlled trials to be more bone-sparing than TDF.(32) TAF has been licensed in the US and Europe but is not commonly available in Africa. It is unlikely to replace TDF for some time because of its higher cost, a difference in affordability that will increase greatly when generic TDF becomes available after its patent expires in 2017. Given that TDF is currently part of first-line ART in many African nations and other low- and middle-income countries, there is a need to develop better awareness among clinicians of possible decreases in bone mineral density, and to ascertain whether such bone loss is progressive with longer duration of ART exposure, ultimately resulting in osteoporosis and increased fracture risk.
Supplementary Material
Acknowledgements
We wish to acknowledge all of the study participants, staff at DPHRU, ZAZI/PHRU, Nthabiseng and Lilian Ngoyi Clinics, Johannesburg, SA, for their many contributions to the study and to the staff of the Bone Indices Laboratory, MRC Human Nutrition Research, Cambridge, UK, for biochemical analysis.
This work was supported by the UK Medical Research Council [program number U105960371]; MMH was supported by a MRC PhD Clinical Research Training Fellowship. We acknowledge donation of HIV test kits by Alere San Diego, Inc. San Diego, CA, USA.
Footnotes
Authors responsibilities: MMH, AP and JMP designed the study; MMH conducted the study; JMP and SAN provided senior oversight of the study in South Africa; KW provided senior oversight and interpretation of the DXA data; AP provided senior oversight and interpretation of the biochemical data; MMH and AP jointly conducted the statistical analysis and drafted the paper; all authors had full access to the data and contributed to interpretation of the findings. MMH and AP have responsibility for data integrity. All authors approved the manuscript for publication.
The authors state that they have no conflicts of interest.
References
- 1.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–53. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–74. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 4.Hoy J. Bone, fracture and frailty. Curr Opin HIV AIDS. 2011;6:309–14. doi: 10.1097/COH.0b013e3283478741. [DOI] [PubMed] [Google Scholar]
- 5.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Güerri-Fernandez R, Vestergaard P, Carbonell C, Knobel H, Avilés FF, Castro AS, et al. HIV Infection is strongly associated with hip fracture risk, independently of age, gender, and comorbidities: a population-based cohort study. J Bone Miner Res. 2013;28:1259–63. doi: 10.1002/jbmr.1874. [DOI] [PubMed] [Google Scholar]
- 7.Stellbrink H, Orkin C, Arribas J, Compston J, Gerstoft J, Van Wijngaerden E, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51:963–72. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 8.Falutz J. Unmasking the bare bones of HIV preexposure prophylaxis. Clin Infect Dis. 2015;15:581–3. doi: 10.1093/cid/civ329. [DOI] [PubMed] [Google Scholar]
- 9.Casado JL. Renal and bone toxicity with the use of tenofovir: understanding at the end. AIDS Rev. 2016;18:59–68. [PubMed] [Google Scholar]
- 10.Shivakoti R, Christian P, Yang WT, Gupte N, Mwelase N, Kanyama C, et al. Prevalence and risk factors of micronutrient deficiencies pre- and post-antiretroviral therapy (ART) among a diverse multicountry cohort of HIV-infected adults. Clin Nutr. 2016;35:183–9. doi: 10.1016/j.clnu.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Bout-van den Beukel CJ, Fievez L, Michels M, Sweep FC, Hermus AR, Bosch ME, et al. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1375–82. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 12.Kwan CK, Eckhardt B, Baghdadi J, Aberg JA. Hyperparathyroidism and complications associated with vitamin D deficiency in HIV-infected adults in New York City, New York. AIDS Res Hum Retroviruses. 2012;28:1025–32. doi: 10.1089/aid.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oden A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of high fracture probability worldwide: secular increases 2010-2040. Osteoporos Int. 2015;26:2243–8. doi: 10.1007/s00198-015-3154-6. [DOI] [PubMed] [Google Scholar]
- 14.Matovu FK, Wattanachanya L, Bekinska M, Pettifor JM, Ruxrungtham K. Bone health and HIV in resource-limited settings: a scoping review. Curr Opin HIV AIDS. 2016;11:306–25. doi: 10.1097/COH.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cournil A, Eymard-Duvernay S, Diouf A, Moquet C, Coutherut J, Ngom Gueye NF, et al. Reduced quantitative ultrasound bone mineral density in HIV-infected patients on antiretroviral therapy in Senegal. PLoS One. 2012;7:e31726. doi: 10.1371/journal.pone.0031726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dave JA, Cohen K, Micklesfield LK, Maartens G, Levitt NS. Antiretroviral therapy, especially efavirenz, is associated with low bone mineral density in HIV-infected South Africans. PLoS One. 2015;10:e0144286. doi: 10.1371/journal.pone.0144286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudfeld CR, Wang M, Aboud S, Giovannucci EL, Mugusi FM, Fawzi WW. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS One. 2012;7:e40036. doi: 10.1371/journal.pone.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamill MM, Ward KA, Pettifor JM, Norris SA, Prentice A. Bone mass, body composition and vitamin D status of ARV-naïve, urban, black South African women with HIV-infection, stratified by CD4 count. Osteoporos Int. 2013;24:2855–61. doi: 10.1007/s00198-013-2373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrottesley SV, Micklesfield LK, Hamill MM, Goldberg GR, Prentice A, Pettifor JM, et al. Dietary intake and body composition in HIV-positive and -negative South African women. Publ Health Nutr. 2014;17:1603–13. doi: 10.1017/S1368980013001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gluer C-C. Monitoring skeletal changes by radiological techniques. J Bone Miner Res. 1999;14:195201962. doi: 10.1359/jbmr.1999.14.11.1952. [DOI] [PubMed] [Google Scholar]
- 21.Ramrakha P, Moore K, Sam A. Oxford Handbook of Acute Medicine. 3rd ed. Oxford: Oxford University Press; 2010. [Google Scholar]
- 22.Payne RB. Renal tubular reabsorption of phosphate (TmP/GFR): indications and interpretation. Ann Clin Biochem. 1998;35(Part 2):201–6. doi: 10.1177/000456329803500203. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 24.van Deventer HE, George JA, Paiker JE, Becker PJ, Katz IJ. Estimating glomerular filtration rate in black South Africans by use of the modification of diet in renal disease and Cockcroft-Gault equations. Clin Chem. 2008;54:1197–202. doi: 10.1373/clinchem.2007.099085. [DOI] [PubMed] [Google Scholar]
- 25.Cole TJ. Sympercents: symmetric differences on the 100 log(e) scale simplify the presentation of log transformed data. Stats Med. 2000;19:3109–25. doi: 10.1002/1097-0258(20001130)19:22<3109::aid-sim558>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr. 1994;60:837–42. doi: 10.1093/ajcn/60.6.837. [DOI] [PubMed] [Google Scholar]
- 27.Mirembe BG, Kelly CW, Mgodi N, Greenspan S, Dai JY, Mayo A, et al. Bone mineral density changes among young, healthy African women receiving oral Tenofovir for HIV preexposure prophylaxis. J Acquir Immune Defic Syndr. 2016;71:287–94. doi: 10.1097/QAI.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasonde M, Niska RW, Rose C, Henderson FL, Segolodi TM, Turner K, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with Tenofovir-Emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi: 10.1371/journal.pone.0090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casado JL, Bañón S, Santiuste C, Serna J, Guzman P, Tenorio M, et al. Prevalence and significance of proximal renal tubular abnormalities in HIV-infected patients receiving tenofovir. AIDS. 2016;30:231–9. doi: 10.1097/QAD.0000000000000901. [DOI] [PubMed] [Google Scholar]
- 30.Ezinga M, Wetzels JF, Bosch ME, van der Ven AJ, Burger DM, et al. Long-term treatment with tenofovir: prevalence of kidney tubular dysfunction and its association with tenofovir plasma concentration. Antivir Ther. 2014;19:765–71. doi: 10.3851/IMP2761. [DOI] [PubMed] [Google Scholar]
- 31.Johansson DL, Ravussin E. The role of mitochondria in health and disease. Current Opinion in Pharmacology. 2009;9:780–6. doi: 10.1016/j.coph.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant PM, Cotter AG. Tenofovir and bone health. Curr Opin HIV AIDS. 2016;11:326–32. doi: 10.1097/COH.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.