Abstract
Background/Aims
Esophageal baseline impedance, which is decreased in gastroesophageal reflux disease (GERD) patients, is related to the severity of acid reflux and the integrity of the esophageal mucosa. The study aims to compare the baseline impedance and the dilated intercellular spaces (DIS) within patients with typical reflux symptoms and to evaluate the correlation of baseline impedance with DIS, esophageal acid exposure, as well as the efficacy of proton pump inhibitor (PPI) treatment.
Methods
Ninety-two patients and 10 healthy controls were included in the study. Erosive esophagitis (EE) was defined by esophageal mucosal erosion under upper endoscopy. Patients without mucosa erosion were divided into groups with pathologic acid reflux (non-erosive reflux disease [NERD]) or with hypersensitive esophagus. The biopsies of esophageal mucosa were taken 2–4 cm above the gastroesophageal junction Z-line during upper endoscopy for DIS measurement. All the patients received esomeprazole 20 mg twice-daily treatment for 8 weeks. The efficacy of esomeprazole was evaluated among all patients.
Results
The intercellular spaces were dilated in both EE and NERD patients (P < 0.05). The value 0.73 μm could be used as the cut-off DIS value to distinguish patients from controls (area under the curve [AUC] = 0.849, P < 0.01). One thousand seven hundred sixty-four ohms could be used as the cut-off impedance values to distinguish patients from controls (AUC = 0.794, P < 0.01). The baseline impedance was decreased in both EE patients and NERD patients, and negatively correlated to the acid exposure time (r = −0.527, P < 0.05). There was a weak correlation between DIS and baseline impedance (r = −0.230, P < 0.05). “Baseline impedance > 1764 Ω” was an independent predictor for PPI failure (OR, 11.9; 95% CI, 2.4–58.9; P < 0.01).
Conclusions
The DIS and decreased baseline impedance was observed in patients with mucosa erosion or pathological acid reflux. The baseline impedance reflected the mucosal integrity, it was more sensitive to esophageal acid exposure. Patients with high impedance might not benefit from the PPI treatment.
Keywords: Impedance, Intercellular spaces, Proton pump inhibitor
Introduction
Esophageal pH monitoring could help in detecting pathological acid reflux, but it has limitations in identifying non-acid reflux. Mutlichannel intraluminal impedance (MII) monitoring is a tool designed to detect the bolus movement by measuring the changes of electrical resistance caused by fluid or gas. The liquid bolus with more ions will have lower impedance than the baseline, while the gas with fewer ions will have a higher value.1 Combined MII and pH monitoring can define both acid and non-acid reflux and help to stratify patients without mucosal erosion to the group with pathological acid reflux or with hypersensitive esophagus. During the esophageal MII-pH monitoring, the esophageal wall contacts the catheter at rest, making it possible to use the impedance level to indicate the integrity of the esophageal mucosa.2 The baseline impedance was decreased in patients with erosive esophagitis (EE),3,4 but the impedance pattern of non-erosive reflux disease (NERD) patients has not been clarified well.
Dilated intercellular space (DIS) has been believed to be histological lesion for gastroesophageal reflux disease (GERD) patients, and the DIS was more prominent in EE patients than that in NERD patients.5 So DIS can be a potential structural marker for mucosa injury, especially for those without macroscopic erosions.5,6 It has been shown that the increased mucosal permeability for ions may be present before or even without the development of DIS.7–9 Thus the change of baseline impedance may be prior to DIS to reflect the mucosal integrity.
Currently about 20% to 30% GERD patients have inadequate response to the proton pump inhibitor (PPI) treatment.10 Esophageal baseline impedance may become a candidate as a predictor for treatment efficacy due to the potential association with the severity of acid exposure, but there is still lack of sufficient evidence to support this hypothesis.
Thus, the aims of the current study are (1) to define the baseline impedance pattern of NERD patients, (2) to assess whether the baseline impedance correlated to the severity of esophageal acid exposure and DIS, and (3) to find out whether DIS and the baseline impedance can predict on the efficacy of PPI treatment.
Materials and Methods
Subjects
Consecutive outpatients with at least moderate typical symptoms (heartburn and/or regurgitation) were enrolled in the study. All patients were PPI naive. Their symptoms should have lasted for at least 3 months and the frequency was at least 2 days per week. The severity of symptoms was evaluated using a validated scale: mild, symptoms could be tolerated and had little impact on patients’ sleep; moderate, daily life and sleep were affected substantially; and severe, symptom made patients unable to carry out normal activities. Patients would be excluded if they had the following conditions: functional heartburn (with normal acid exposure, negative symptom association, and a negative response to acid suppression treatment), Barrett’s esophagus, previous esophageal or gastrointestinal surgery, peptic ulcer, gastrointestinal tumor, primary or secondary severe esophageal motility disorders, and severe cardiac and renal or pulmonary disease. Another 10 volunteers without gastrointestinal symptoms, systemic disorders or major abdominal surgery and with normal 24-hour MII-pH monitoring results were recruited as healthy controls. Anti-acid and prokinetic drugs must have been stopped at least 1 week prior to the study.
The grades for severity of esophagitis under endoscopy were based on the Los Angeles (LA) classification.11 All patients received esomeprazole 20 mg twice-daily treatment for 8 weeks.12 Esomeprazole therapy was defined as effective if the patients without esophagitis were symptom-free or with only one mild episode during the final week of the therapy, otherwise the therapy was considered a failure. For EE patients, if the mucosal erosion did not heal in the final week of esomeparozle therapy, or the symptom relief could not meet above “effective criteria,” the treatment was also considered failure.
The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (IRB No. [2010] 31). Written informed consent was obtained from all individuals before each procedure.
Intercellular Space Measurement Under Transmission Electron Microscopy
The biopsies were taken from the site no more than 3 cm above the esophagogastric junction during endoscopy. The tissues were fixed by 2% glutaraldehyde in neutral saline phosphate buffer and stored at 4°C for several days, then treated using the standard protocol and osmicated by 1% osmium tetroxide followed by infiltrated in Epon’sresin.4 Ultra-thin sections were cut and collected on copper grids. The basal cells in squamous epithelium were examined and photographed by the transmission electron microscope (Tecnai G2 spirit Twin; FEI, Hillsboro, OR, USA). For each biopsy, 10 photomicrographs were taken from the esophageal mucosa basal layers (×5800). One cell that had clear demarcation with surrounding 5–6 cells was chosen in each image and 10 randomly selected perpendicular trans-sections to adjacent cell membranes were measured (Fig. 1). The intercellular space diameter (ISD) was analyzed by the Image-pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA). The assessment of intercellular space was made by 2 investigators blinded to the endoscopic performance and the reflux pattern of patients.
Figure 1.
The measurement of dilated intercellular spaces. Ten randomly selected perpendicular trans-sections to adjacent cell membranes were measured on the chosen cell which had clear demarcation with surrounding 5–6 cells. BL, basal length.
Twenty-four-hour Multichannel Intraluminal Impedance-pH Monitoring
All subjects underwent 24-hour monitoring by using an ambulatory MII-pH monitoring system (Sleuth; Sandhill Scientific, Inc, Highland Ranch, CO, USA) 3–5 days after upper endoscopy. The pH electrode was placed at 5 cm above the upper margin of the lower esophageal sphincter (LES), and 6 impedance values were recorded at 6 sites (3, 5, 7, 9, 15, and 17 cm above the LES, respectively). The mealtime was excluded from the analysis. The patients without esophagitis were divided into group with pathologic acid reflux (acid exposure time [AET]% ≥ 4.00%, NERD group), with hypersensitive esophagus (without pathological acid reflux, but with symptom association probability ≥ 95.00%).
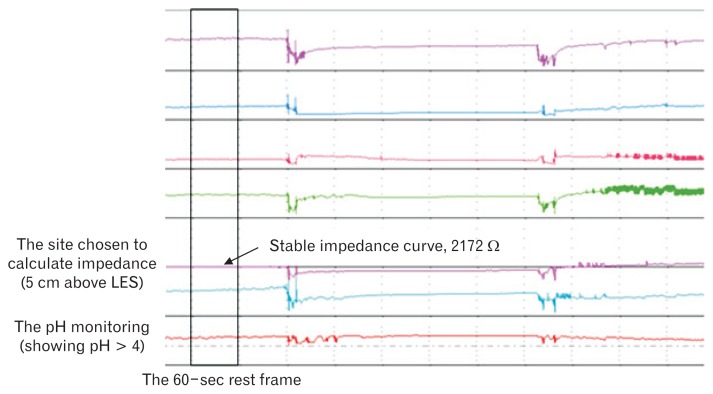
The baseline impedance was commonly calculated by averaging the values assessed at regular time or regular intervals.13–15 In this study, the mean nocturnal baseline impedance (MNBI) values were assessed on the same site as pH monitoring (above LES 5 cm) and in the first stable 60-second times in every hour during the night when the individuals take supine position (Fig. 2). The mean values were taken as the baseline impedance. This procedure was done by one of the investigators blinded to the endoscopic performance and the reflux pattern of patients.
Figure 2.
The calculation of baseline impedance. The impedance values were assessed on the site above lower esophageal sphincter (LES) 5 cm and in the first stable 60-second times in every hour during the night when the individuals take supine position. Then the average values were taken as the mean nocturnal baseline impedance.
Statistical Methods
Data were presented with either mean ± SD or median (inter-quartile range). One-way ANOVA was used to compare the differences on DIS and baseline impedance between the patients and controls. Receiver operating characteristic curve was used to confirm the cut-off values of ISD and impedance used to distinguish the patients from the control group. Pearson rank correlation was used to investigate the correlation between ISD and the baseline impedance, and Spearman rank correlation was used to explore the association between AET% and the impedance. Logistic regression analysis was used to investigate the association of baseline impedance with the efficacy of PPI treatment. The P-value less than 0.05 was considered statistically significant. Statistical analysis was completed by using SPSS 20.0 (SPSS Inc, Chicago, IL, USA).
Results
Demographic Characteristics of the Patients
A total of 108 patients with heartburn and/or regurgitation as their main complaint were screened. Six patients with Barrett’s esophagus or peptic ulcer during upper endoscopy and 10 patients with functional heartburn were excluded from the study. Finally, 35 EE patients (9 patients with LA-A grade, 25 patients with LA-B grade, and 1 patient with LA-D grade), 57 patients without mucosal erosion, and 10 healthy controls were included in the analysis (Table 1). The subgroups without mucosal erosion included 29 NERD patients and 28 patients with hypersensitive esophagus.
Table 1.
Demographic Data of Patients and Controls
| EE (n = 35) | Pathological acid reflux (n = 29) | Hypersensitive esophagus (n = 28) | Control (n = 10) | P-value | |
|---|---|---|---|---|---|
| Age (yr) | 51.00 (37.00, 64.00) | 47.00 (39.00, 55.00) | 48.00 (35.00, 55.00) | 26.00 (24.00, 29.00) | < 0.05 |
| Male (%) | 68.57 | 51.72 | 50.00 | 50.00 | 0.392 |
| BMI (kg/m2) | 24.32 ± 2.82 | 23.28 ± 2.71 | 23.33 ± 3.64 | 21.37 ± 2.64 | 0.057 |
EE, erosive esophagitis; BMI, body mass index.
The age was expressed as medians (interquartile range), the BMI was expressed as mean ± SD.
Comparison of Intercellular Space Diameter Between Patients and Healthy Controls
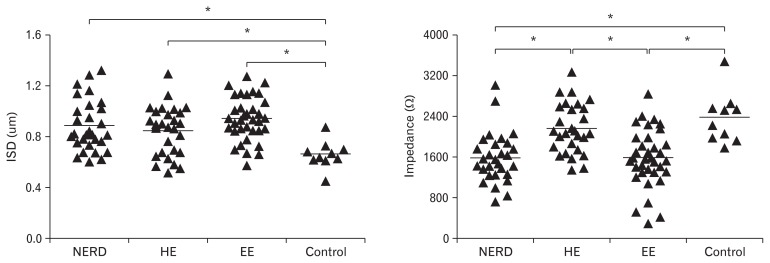
The ISD value was 0.94 ± 0.17 μm for EE patients. Within groups without mucosal erosion, the ISD value was 0.89 ± 0.20 μm for NERD patients, and 0.85 ± 0.19 μm for patients with hypersensitive esophagus (HE). The ISD value was 0.66 ± 0.11 μm for healthy controls. The ISD of patients were larger than that of healthy controls, whether with or without esophagitis (P < 0.05). However, the differences within 3 subgroups were not significant (P > 0.05) (Fig. 3).
Figure 3.
The comparison of intercellular space diameter (ISD) and mean nocturnal baseline impedance among 3 subgroups and controls. The ISD of patients were larger than that of healthy controls, whether with or without esophagitis (P < 0.05). The baseline impedance in erosive esophagitis (EE) and non-erosive reflux disease (NERD) patients was lower than that in patients with hypersensitive esophagus (HE) and healthy controls (P < 0.05).
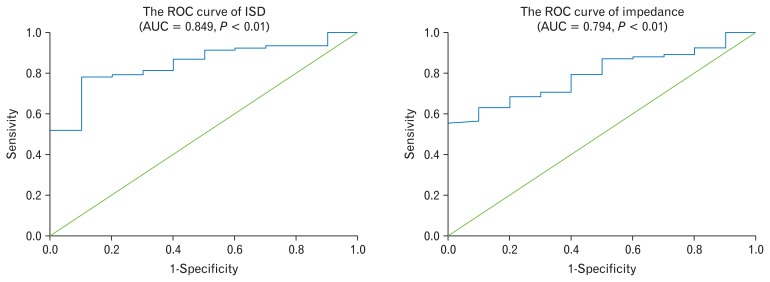
The cut-off value of ISD to distinguish patients from controls was 0.73 μm (AUC = 0.849, P < 0.01) (Fig. 4). The sensitivity was 78.30% and the specificity was 90.00%.
Figure 4.
The receiver operating characteristic (ROC) curve of intercellular space diameter (ISD) and Impedance. AUC, area under the curve.
Comparison of Baseline Impedance Between Patients and Healthy Controls
The value of baseline impedance was 1571.09±567.54Ω for EE patients. Within groups without esophagitis, the value was 1581.07±494.61Ω for NERD patients and 2156.01±495.55Ω for patients with hypersensitive esophagus (HE). The impedance value was 2364.67 ± 500.70 Ω for controls.
The baseline impedance in EE patients was similar to that of NERD patients (P > 0.05), while lower than that in HE patients and healthy controls (P < 0.05). However, the difference was not significant between HE patients and healthy controls (P > 0.05) (Fig. 3).
The cut-off value of impedance to distinguish the patients from controls was 1764 Ω (AUC = 0.794, P < 0.01) (Fig. 4). The sensitivity was 55.40% and the specificity was 100.00%.
Baseline Impedance Correlated With Dilated Intercellular Space and Esophageal Acid Exposure in Patients
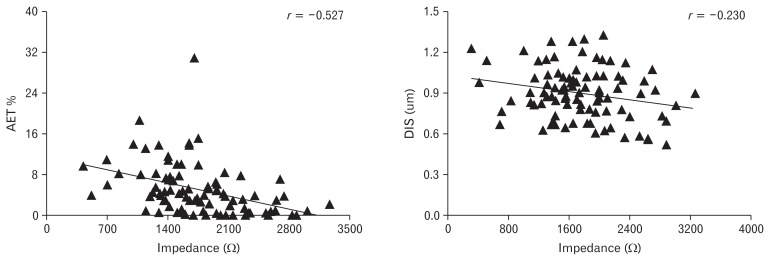
There was a negative weak linear correlation between baseline impedance and DIS (r = −0.230, P < 0.05). The impedance correlated to the acid exposure time (AET%) negatively (r = −0.527, P < 0.01). But there was no correlation between DIS and AET% (P > 0.05) (Fig. 5).
Figure 5.
The baseline impedance correlated to acid exposure time (AET)% and dilated intercellular spaces (DIS) in the patients. The impedance correlated to the AET% negatively (r = −0.527, P < 0.01), and there was also a negative weak linear correlation between baseline impedance and DIS (r = −0.230, P < 0.05).
Association of Baseline Impedance and the Efficacy of Esomeprazole Treatment
Seventy-three patients finished the follow-up studies. The esomeprazole therapy was effective in 60 patients, while ineffective in 13 patients. In the PPI responders, there were 26 (43.33%) patients with esophagitis, 17 (28.33%) patients with NERD, and 17 (28.33%) patients with hypersensitive esophagus. In the PPI no-response group, there were 3 (23.08%) patients with esophagitis, 2 (15.38%) patients with NERD, and 8 (61.54%) patients with hypersensitive esophagus.
It seemed that the PPI response group included fewer patients with hypersensitive esophagus, and the baseline impedance was lower (P < 0.05). However, the AET% was higher in this group (P < 0.05) (Table 2). The cut-off value of baseline impedance was used to divide all patients into 2 groups and the PPI effective group included more patients with lower impedance (P < 0.05).
Table 2.
The Comparison of Metrics Between Patients With and Without Response to Proton Pump Inhibitor Treatment
| PPI effective (n = 60) | PPI failure (n = 13) | P-value | |
|---|---|---|---|
| Age (yr) | 49.47 ± 13.47 | 45.31 ± 11.98 | 0.308 |
| BMI (kg/m2) | 24.30 ± 2.98 | 23.27 ± 2.49 | 0.249 |
| Male (n [%]) | 35.00 (58.33) | 6.00 (46.15) | 0.422 |
| Erosive esophagitis (n [%]) | 26.00 (43.33) | 3.00 (23.08) | 0.176 |
| Hypersensitive esophagus (n [%]) | 17.00 (28.33) | 8.00 (61.54) | 0.049 |
| Hiatal hernia (n [%]) | 9.00 (15.00) | 0.00 (0.00) | 0.305 |
| DIS (μm) | 0.91 ± 0.18 | 0.82 ± 0.17 | 0.114 |
| Baseline impedance (Ω) | 1621.26 ± 561.17 | 2117.48 ± 428.68 | 0.004 |
| AET% | 14.95 (4.53, 28.83) | 8.00 (1.70, 13.45) | 0.008 |
| Total reflux episodes | 64.50 (51.00, 78.75) | 62.00 (33.00, 93.00) | 0.779 |
| Liquid reflux episodes | 52.27 ± 21.53 | 57.15 ± 39.66 | 0.673 |
| Acid reflux episodes | 30.98 ± 16.43 | 24.38 ± 18.38 | 0.203 |
| Non-acid reflux episodes | 19.00 (10.25, 30.25) | 21.00 (13.50, 45.50) | 0.177 |
| Gas reflux episodes | 10.00 (7.00, 14.00) | 9.00 (6.50, 17.50) | 0.897 |
| Proximal reflux episodes | 26.00 (20.00, 38.00) | 21.00 (12.00, 32.50) | 0.133 |
| Symptom associated with acid (n) | 27 | 7 | 0.562 |
| Symptom associated with non-acid reflux (n) | 13 | 6 | 0.140 |
PPI, proton pump inhibitor; BMI, body mass index; DIS, dilated intercellular spaces; AET, acid exposure time.
The age, BMI, DIS, impedance, liquid reflux, and acid reflux episodes were expressed as mean ± SD, and the AET%, total reflux, non-acid reflux, gas reflux, and proximal reflux episodes were expressed as median (interquartile range).
Logistic regression was done to find the predictor for PPI failure within these patients. The factors included in the logistic regression were AET%, with HE and baseline impedance. The result showed that the baseline impedance > 1764 Ω was the only independent predictor for the PPI failure (OR, 11.9; 95% CI, 2.4–58.9; P < 0.01).
Discussion
In this study, we evaluated the changes of impedance and inter-cellular spaces in different phenotypes of patients with typical reflux symptoms and to find whether the baseline impedance correlated with mucosal integrity, as well as the efficacy of PPI treatment. It turned out that the baseline impedance was decreased in GERD patients and correlated to AET% and DIS negatively. Though it seemed that intercellular space became dilated as exposure time increased, there was no correlation between DIS and AET%. The PPI treatment would be more effective in patients with lower impedance.
According to the traditional notion, the inflammation would follow the chemical injury caused by the contact of gastric juice to the esophageal epithelial cells. Thus, the basal cells and papillary hyperplasia and DIS would be more severe in patients with mucosa erosion and excessive acid reflux.16,17 However, in our study, we found that the dilation of intercellular spaces was similar in the 3 subgroups. This was partially due to the fact that most patients included in the EE group were LA-A and LA-B grade since severe esophagitis is less prevalent in China.18 Recent studies suggested that the cause for esophagitis may be cytokine mediated. The esophageal epithelial cells could be stimulated by the refluxate to release cytokines, then the inflammatory cells are recruited and infiltrate from the submucosa upward to the surface, and the histological microscopic injury would appear before mucosa erosion.17,19 Kanazawa et al20 found that contact with refluxate could cause the elevation of IL-8 in the mucosa of NERD patients. IL-8 promoted the aggregation of neutrophils and the active substance from neutrophils could cause mucosa injury. Even the immune reaction induced by the psychological stress could cause DIS in patients without mucosal erosion.21 This was an important cause for the symptoms of patients with hypersensitive esophagus. So it was not surprising to find no significant difference in DIS between patients with or without pathological acid reflux in our study, and no correlation between DIS and AET%. We defined the cut-off value of 0.73 μm for DIS to distinguish patients with typical reflux symptoms from healthy controls, which was consistent with a previous report.22
Patel et al23 found that the distal MNBI was different between patients with and without elevated AET%. In our study, the lower baseline impedance was only found in patients with macroscopic lesions or with pathological acid reflux. Though they calculated the MNBI at stable nocturnal periods as Martinucci et al13 reported, their values were similar to ours. This result supported that there was a good correlation between short-time and long-time measurement.13 Frazzoni et al24 found that MNBI could be used to segregate different phenotypes of patients with PPI responsive heartburn from healthy controls. This was in line with our study, but the cutoff value was a little higher than ours. This may result from more pH-negative patients with higher impedance included in their study.
We found the baseline impedance negatively correlated to the DIS and AET%, which supported that the baseline impedance could indeed reflect the paracellular permeability and the severity of acid reflux. The change of DIS was due to the inflammation, even the biopsy failed to find DIS, it could not rule out the possibility that the barrier function had changed.25 The DIS is located in the basal cell layer, it seemed that a proton does not need such large alteration to penetrate through the epithelium. Therefore, the DIS may not be a sensitive marker to reflect the functional change of mucosal integrity. Because DIS was observed by an electron microscope, its use in clinical practice would be limited. Since the impedance is the equivalent of the resistance for an alternating current, reduced baseline impedance could be explained by the increase permeability of the esophageal epithelium to ions. Therefore, the change of baseline impedance may be prior to DIS in the early stage of mucosal injury. This is an explanation as to why the correlation between impedance and DIS was weak.
It was interesting to find that the baseline impedance was an independent predictor for PPI failure. Previous studies have reported similar conclusions.23,26 This was supported by the result that the PPI effective group had more severe acid exposure. Because pathological acid reflux was not the only cause for DIS, it was not surprising to find that DIS had no impact on the efficacy of PPI treatment.
There are some limitations in our study. Firstly, patients with “functional heartburn” were not included in the current study. The negative results of 24-hour MII-pH monitoring only was not enough to diagnose functional heartburn and these patients were with poor compliance to take PPI in our study. In fact, this cluster of patients would be very good subjects to investigate the role of baseline impedance and DIS in differentiation between “real GERD” and functional heartburn. Secondly, the sample size of patients finished the 8-week follow-up was relatively small, and there was lack of data on the changes of baseline impedance after PPI treatment, which would be useful in supporting baseline impedance as a candidate marker to reflect the efficacy of PPI treatment. Thirdly, the control group was young. However, we have made a comparison between young and old patients, and it turned out that the age was not an influential factor for baseline impedance and DIS (1878.25 ± 452.14 Ω for young patients and 1650.97 ± 657.93 Ω for old patients, P > 0.05). Finally, the association between the symptom severity and the level of both baseline impedance and DIS was not investigated in the current study. Further study is needed to define this relationship.
In summary, our findings suggested that DIS and baseline impedance were useful in distinguishing patients with typical reflux symptoms from controls. The esophageal baseline impedance reflected mucosal integrity and it was decreased in GERD patients including NERD. Patients with high baseline impedance might not benefit from PPI treatment due to relative low acid reflux.
Footnotes
Financial support: The work was supported by the grant from the China National Natural Science Foundation 81400582 (Yingli-an Xiao).
Conflicts of interest: None.
Author contributions: Study concept, acquisition of data, analysis, drafting: Chenxi Xie; acquisition of data and analysis: Daniel Sifrim and Yuwen Li; and study concept, acquisition of data, analysis, drafting, study supervision, and finalizing the manuscript: Minhu Chen and Yinglian Xiao.
References
- 1.Bredenoord AJ, Tutuian R, Smout AJ, Castell DO. Technology review: esophageal impedance monitoring. Am J Gastroenterol. 2007;102:187–194. doi: 10.1111/j.1572-0241.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 2.Farré R, Blondeau K, Clement D, et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut. 2011;60:885–892. doi: 10.1136/gut.2010.233049. [DOI] [PubMed] [Google Scholar]
- 3.Zhong C, Duan L, Wang K, et al. Esophageal intraluminal baseline impedance is associated with severity of acid reflux and epithelial structural abnormalities in patients with gastroesophageal reflux disease. J Gastroenterol. 2013;48:601–610. doi: 10.1007/s00535-012-0689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weijenborg PW, Smout AJ, Verseijden C, et al. Hypersensitivity to acid is associated with impaired esophageal mucosal integrity in patients with gastroesophageal reflux disease with and without esophagitis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G323–G329. doi: 10.1152/ajpgi.00345.2013. [DOI] [PubMed] [Google Scholar]
- 5.van Malenstein H, Farré R, Sifrim D. Esophageal dilated intercellular spaces (DIS) and nonerosive reflux disease. Am J Gastroenterol. 2008;103:1021–1028. doi: 10.1111/j.1572-0241.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- 6.Yerian L, Fiocca R, Mastracci L, et al. Refinement and reproducibility of histologic criteria for the assessment of microscopic lesions in patients with gastroesophageal reflux disease: the Esohisto Project. Dig Dis Sci. 2011;56:2656–2665. doi: 10.1007/s10620-011-1624-z. [DOI] [PubMed] [Google Scholar]
- 7.Farré R, van Malenstein H, De Vos R, et al. Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut. 2008;57:1366–1374. doi: 10.1136/gut.2007.141804. [DOI] [PubMed] [Google Scholar]
- 8.Carney CN, Orlando RC, Powell DW, Dotson MM. Morphologic alterations in early acid-induced epithelial injury of the rabbit esophagus. Lab Invest. 1981;45:198–208. [PubMed] [Google Scholar]
- 9.Orlando RC, Powell DW, Carney CN. Pathophysiology of acute acid injury in rabbit esophageal epithelium. J Clin Invest. 1981;68:286–293. doi: 10.1172/JCI110246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weijenborg PW, Cremonini F, Smout AJ, Bredenoord AJ. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: a meta-analysis. Neurogastroenterol Motil. 2012;24:747–757. e350. doi: 10.1111/j.1365-2982.2012.01888.x. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 12.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 13.Martinucci I, de Bortoli N, Savarino E, et al. Esophageal baseline impedance levels in patients with pathophysiological characteristics of functional heartburn. Neurogastroenterol Motil. 2014;26:546–555. doi: 10.1111/nmo.12299. [DOI] [PubMed] [Google Scholar]
- 14.Kessing BF, Bredenoord AJ, Weijenborg PW, Hemmink GJ, Loots CM, Smout AJ. Esophageal acid exposure decreases intraluminal baseline impedance levels. Am J Gastroenterol. 2011;106:2093–2097. doi: 10.1038/ajg.2011.276. [DOI] [PubMed] [Google Scholar]
- 15.Borrelli O, Salvatore S, Mancini V, et al. Relationship between baseline impedance levels and esophageal mucosal integrity in children with erosive and non-erosive reflux disease. Neurogastroenterol Motil. 2012;24:828–e394. doi: 10.1111/j.1365-2982.2012.01947.x. [DOI] [PubMed] [Google Scholar]
- 16.Frierson HF., Jr Histology in the diagnosis of reflux esophagitis. Gastroenterol Clin North Am. 1990;19:631–644. [PubMed] [Google Scholar]
- 17.Dunbar KB, Agoston AT, Odze RD, et al. Association of acute gastroesophageal reflux disease with esophageal histologic changes. JAMA. 2016;315:2104–2112. doi: 10.1001/jama.2016.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou D, He J, Ma X, et al. Epidemiology of symptom-defined gastroesophageal reflux disease and reflux esophagitis: the systematic investigation of gastrointestinal diseases in china (SILC) Scand J Gastroenterol. 2011;46:133–141. doi: 10.3109/00365521.2010.521888. [DOI] [PubMed] [Google Scholar]
- 19.Souza RF, Huo X, Mittal V, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. 2009;137:1776–1784. doi: 10.1053/j.gastro.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 20.Kanazawa Y, Isomoto H, Wen CY, et al. Impact of endoscopically minimal involvement on IL-8 mRNA expression in esophageal mucosa of patients with non-erosive reflux disease. World J Gastroenterol. 2003;9:2801–2804. doi: 10.3748/wjg.v9.i12.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farré R, De Vos R, Geboes K, et al. Critical role of stress in increased oesophageal mucosa permeability and dilated intercellular spaces. Gut. 2007;56:1191–1197. doi: 10.1136/gut.2006.113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calabrese C, Fabbri A, Bortolotti M, et al. Dilated intercellular spaces as a marker of oesophageal damage: comparative results in gastro-oesophageal reflux disease with or without bile reflux. Aliment Pharmacol Ther. 2003;18:525–532. doi: 10.1046/j.1365-2036.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- 23.Patel A, Wang D, Sainani N, Sayuk GS, Gyawali CP. Distal mean nocturnal baseline impedance on pH-impedance monitoring predicts reflux burden and symptomatic outcome in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2016;44:890–898. doi: 10.1111/apt.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frazzoni M, Savarino E, de Bortoli N, et al. Analyses of the post-reflux swallow-induced peristaltic wave index and nocturnal baseline impedance parameters increase the diagnostic yield of impedance-pH monitoring of patients with reflux disease. Clin Gastroenterol Hepatol. 2016;14:40–46. doi: 10.1016/j.cgh.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Tobey NA, Gambling TM, Vanegas XC, Carson JL, Orlando RC. Physicochemical basis for dilated intercellular spaces in non-erosive acid-damaged rabbit esophageal epithelium. Dis Esophagus. 2008;21:757–764. doi: 10.1111/j.1442-2050.2008.00841.x. [DOI] [PubMed] [Google Scholar]
- 26.de Bortoli N, Martinucci I, Savarino E, et al. Association between baseline impedance values and response proton pump inhibitors in patients with heartburn. Clin Gastroenterol Hepatol. 2015;13:1082–1088. e1. doi: 10.1016/j.cgh.2014.11.035. [DOI] [PubMed] [Google Scholar]