Abstract
Reversing pathologic alterations in vascular microRNA (miRNA) expression represents a potential therapeutic strategy for pulmonary hypertension. While polyethylenimine (PEI) has previously been shown to be an effective vehicle for vascular lung-directed delivery of plasmid DNA, it remains unclear whether this utility is generalizable to miRNAs. Here we show that despite elevated lung levels, the intravenous infusion of PEI–miRNA mimic complexes fails to provide lung-selective delivery in rats.
Keywords: microRNA, pulmonary hypertension, lung delivery, polyethylenimine, mimic
Introduction
MicroRNAs (miRNAs) are short non-protein-coding RNAs and key regulators of gene expression. Alterations in miRNA expression have been implicated in the pathobiology of many vascular diseases, including pulmonary hypertension (PH).1 Supplementation of pathologically decreased miRNAs with synthetic mimics has proven useful for probing disease mechanisms and potential therapeutic targets in preclinical rodent models of PH.2,3 Although lung-selective delivery of miRNA mimics is possible through direct intratracheal or intranasal instillation, these routes of administration may preferentially favor uptake within pulmonary airway epithelial cells.4 Thus, these routes may be suboptimal for lung-targeted delivery of mimics to vascular and, in particular, endothelial cells. This is an important consideration given that therapeutic efficacy may depend not only on the level of mimic achieved in the target tissue, but also on the specificity of delivery, which may be critical to limiting potential off-target effects and toxicity.5 Intravenous administration routes offer the most direct access to the vascular endothelium, but are not inherently lung-specific. This limitation could potentially be overcome with polyethylenimine (PEI) carriers, which have previously been reported to be an effective vehicle for vascular lung-targeted delivery of plasmid DNA.6–8 However, no study has investigated the tissue-specificity associated with systemic administration of PEI–miRNA mimic complexes. Therefore, we sought to confirm whether the intravenous delivery of miRNA mimics with PEI can selectively enrich target miRNA levels in rat lungs.
Methods
All animal procedures were approved by the University of Ottawa’s Animal Care Ethics Committee and complied with the principles and guidelines of the Canadian Council on Animal Care. A synthetic miRNA mimic with no mammalian homologue (cel-miR-39, ThermoFisher) was specifically chosen to facilitate the quantification of mimic levels, by circumventing the endogenous basal levels associated with mammalian miRNAs in rats. In addition to linear PEI (in vivo-jetPEI, cat# 201-10G, Polyplus Transfection), we evaluated a second commonly used liposome-based transfection reagent/vehicle (invivofectamine, cat# IVF3001, ThermoFisher) to serve as a control. Male Sprague-Dawley rats (∼ 250 g) were anesthetized under 5% isoflurane and received an intravenous infusion (via jugular vein) of 0.5 nmol of cel-miR-39 mimic suspended in either invivofectamine 3.0 (5% v/v in 1 × PBS) or in vivo-jetPEI (N/P ratio = 7, in 5% glucose solution) in a total volume of 50 µL/rat, prepared according to manufacturer recommendations. Rats were euthanized 2 h and 24 h after mimic delivery (n = 3 rats/time point/vehicle). miRNA quantification was performed as previously described.9 Briefly, total RNA was extracted from ∼ 25 mg of non-perfused tissue using the miRNeasy mini kit (Qiagen). Two micrograms of total RNA was used for reverse transcription in the miScript II RT kit (Qiagen). The quality of RNA was reflected by absorbance ratios of ∼ 2, and Agilent Bioanalyzer RIN numbers > 7.0. MiRNA levels were subsequently quantified using miScript polymerase chain reaction (PCR) reagents, primers, and PCR cycling conditions according to manufacturer instructions (Qiagen) using a CFX384 PCR machine (Biorad). Normalized relative miRNA expression levels were calculated using the formula 2 −ΔCq, where ΔCq =Cq(target miRNA) − Cq(reference control, RNU6−2). A quantification cycle (Cq) threshold of 35 was predefined as the PCR detection limit based on manufacturer recommendations. Statistical tests were performed with Graphpad Prism V7.0, on log transformed data to facilitate a normal distribution. Between-group differences were evaluated by one-way ANOVA and Dunnett’s multiple comparison test vs. the lung group.
Results
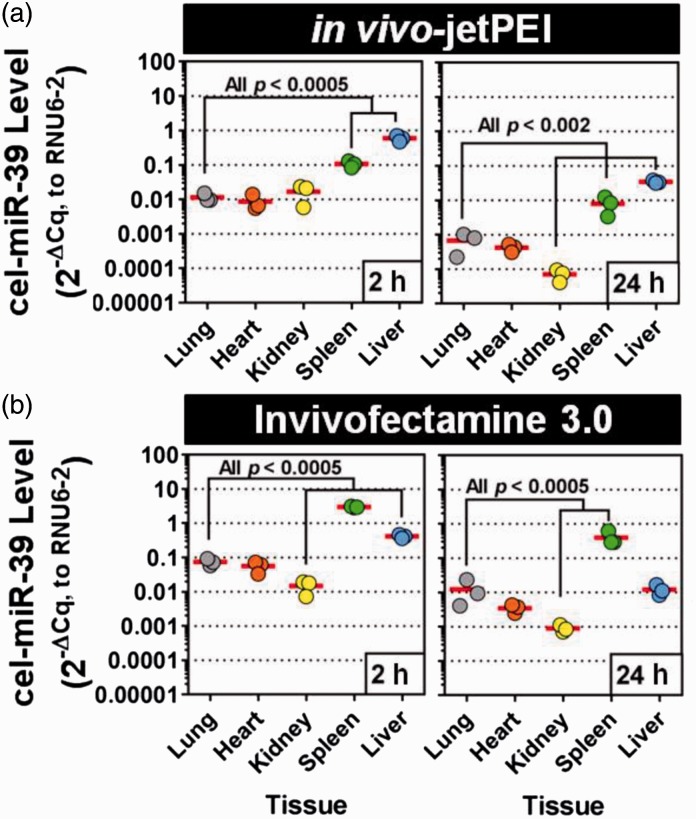
Intravenous delivery of cel-miR-39 with jetPEI leads to a marked increase in pulmonary tissue levels at 2 h post delivery (mean ± SD = 0.01 ± 0.003, 2 -ΔCq relative units, Fig. 1a, representing ∼ 11 Cq or > 1000-fold above the PCR detection limit; Supplementary Fig. 1). However, jetPEI-mediated delivery showed no evidence of lung selectivity, as cel-miR-39 levels were comparable in the heart (0.009 ± 0.005, P = 0.76) and kidney (0.02 ± 0.009, P = 0.91), and significantly higher in the spleen (0.1 ± 0.02, P = 0.0004) and liver (0.6 ± 0.1, P = 0.0001) (Fig. 1a). The lung selectivity of jetPEI-mediated mimic delivery was also assessed at a later time point 24 h post infusion. Pulmonary levels of cel-miR-39 were 14-fold lower at this later time point (0.0007 ± 0.0004, 2 -ΔCq units), but still ∼ 7 Cq above the PCR detection limit. Mimic levels in other tissues exhibited a similar level of decline, such that the relative pattern of tissue selectivity was largely preserved between 2 h and 24 h post delivery. With exception of the kidney, in which cel-miR-39 levels were significantly lower (0.00007 ±0.00003, P = 0.002) than levels observed in the lung at 24 h, the other tissues showed comparable (heart: 0.0004 ±0.0001, P = 0.87) or higher levels (spleen: 0.008 ± 0.004, P = 0.0005; liver: 0.03 ± 0.003, P = 0.0001).
Fig. 1.
Tissue biodistribution of cel-miR-39 mimic after jugular vein infusion with jetPEI or invivofectamine vehicle. Relative normalized levels of cel-miR-39 were quantified by RT-qPCR at 2 h and 24 h after intravenous delivery using in vivo-jetPEI (a) or invivofectamine 3.0 (b) transfection reagents as vehicles. Individual biological replicates are shown (n = 3 rats/time point/vehicle). Horizontal lines denote mean levels.
To gain insight into whether the above effects were unique to jetPEI, intravenous delivery of cel-miR-39 was repeated using a different transfection reagent based on cationic liposomes (i.e. invivofectamine 3.0). At 2 h post delivery, pulmonary levels of cel-miR-39 were robustly detected (0.07 ± 0.02, 2 -ΔCq relative units) after infusion with invivofectamine, and levels were significantly higher (sevenfold, P = 0.01) compared to rats that had received jetPEI. A lack of lung-enriched targeting was also evident in the tissue biodistribution profile of cel-miR-39. With the exception of the kidney, which showed five- to 14-fold lower mimic levels at 2 h and 24 h compared to the lungs (P ≤ 0.0005), other tissues showed comparable (i.e. heart at 2 h and 24 h, liver at 24 h) or significantly higher levels in the range of 5–40-fold (i.e. spleen at 2 h and 24 h, liver at 2 h, all P ≤ 0.0005). Of note, the underlying quality and quantity of total RNA was largely comparable between groups, as reflected by the relatively stable levels of the internal reference control (Supplementary Fig. 1), which argues against potential false negative interpretations of lung selectivity.
Discussion
In this study, we investigated whether vascular lung-targeted delivery of miRNA mimics could be achieved using the PEI carrier, jetPEI, which has been reported to result in highly selective lung targeting when complexed with plasmid DNA.6–8 Our results showed that in rats, jetPEI-mediated systemic delivery of miRNA mimics lead to a broad uptake in all tissues assessed including the lung, heart, kidney, spleen, and liver. A lack of pulmonary selectivity was also demonstrated using the cationic liposome-based vehicle, invivofectamine.
Previous studies have shown that PEI, in combination with plasmid DNA, can serve as an effective approach for lung-targeted delivery after intravenous injection, as evidenced by enhanced reporter gene activity in the lung up to two orders of magnitude higher than in other tissues.6–8 However, we were not able to reproduce this tissue selectivity using miRNA mimics. Instead, direct quantification of miRNA mimic levels by quantitative reverse transcription PCR (RT-qPCR) showed a preferential transfer to the liver and spleen compared to the lung (by at least tenfold), and this effect was observed consistently at both 2 h and 24 h post delivery. The mechanism by which PEI–DNA complexes selectively target the lung has not been clearly defined, though it has been suggested that alterations in the physicochemical properties of PEI–nucleic acid structures can enhance interactions and retention within the pulmonary circulation.4 Therefore, the difference between our findings and prior studies with plasmid DNA could be related to the smaller size and charge density of the miRNA mimics (∼22 nt), or possibly the chemical modifications used to enhance their stability and resistance to ribonuclease digestion.
A number of studies have reported robust downregulation of pulmonary gene expression after delivering small-interfering RNA (siRNA) intravenously with PEI (as reviewed elsewhere),10 suggesting that PEI may also be able to facilitate lung-targeted delivery of small RNA species. However, there are conflicting data on whether lung selectivity is actually achieved through intravenous delivery of siRNA and PEI. Whereas surrogate measures of siRNA activity assessed through reporter gene assays11 and in vivo bioluminescence-imaging12 may be consistent with lung enrichment, direct quantification of radio-labeled siRNA levels have indicated preferential uptake within the liver and spleen.13,14 To date, there remains very limited information on the ability of PEI to transfer miRNA selectively to the lungs after systemic infusion. While two studies have shown that jetPEI can facilitate intravenous transfer of miRNA mimics to the lungs of mice with bacteria-induced pulmonary inflammation, the specificity of delivery was not directly confirmed (i.e. by quantifying mimic levels).15,16 Interestingly, the biologic effects associated with mimic activity, such as a change in bacterial burden or messenger RNA target levels, were shown to be consistently altered in multiple organs. This result argues against the likelihood of lung-selective delivery and supports our findings.
In this study, we address a knowledge gap concerning the pulmonary selectivity of jetPEI-mediated, systemic delivery of miRNA mimics. We demonstrate that the intravenous delivery of miRNA mimics with jetPEI can elevate pulmonary miRNA levels in rats, but lack pulmonary selectivity. This has important implications for potential off-target effects in preclinical animal models and the interpretation of downstream biologic effects. Novel insight into the relative effectiveness of PEI vs. lipid vectors was also examined and revealed a similar tissue biodistribution pattern. Of note, we did not investigate the mechanism underlying the lack of lung selectivity associated with systemic delivery of PEI–miRNA mimic complexes nor the cellular localization of mimics, which represent limitations of this study. Given the importance of miRNA biology in pulmonary vascular disease, and the potential therapeutic value of lung-targeted vascular intervention strategies, the investigation of other types of transfection reagents with reported lung-selective properties (e.g. neutral lipid emulsions)17 may merit further consideration.
Supplementary Material
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work was supported by the Canadian Institutes of Health Research (FDN-143291 to DJS) and the Entelligence Young Investigator's grant from Actelion Pharmaceuticals US, Inc. (KS).
References
- 1.White K, Loscalzo J, Chan SY. Holding our breath: The emerging and anticipated roles of microRNA in pulmonary hypertension. Pulm Circ 2012; 2: 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courboulin A, Paulin R, Giguere NJ, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 2011; 208: 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothman AM, Arnold ND, Pickworth JA, et al. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest 2016; 126: 2495–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Gioia S, Conese M. Polyethylenimine-mediated gene delivery to the lung and therapeutic applications. Drug Des Devel Ther 2009; 2: 163–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chollet P, Favrot MC, Hurbin A, et al. Side-effects of a systemic injection of linear polyethylenimine-DNA complexes. J Gene Med 2002; 4: 84–91. [DOI] [PubMed] [Google Scholar]
- 6.Bragonzi A, Dina G, Villa A, et al. Biodistribution and transgene expression with nonviral cationic vector/DNA complexes in the lungs. Gene Ther 2000; 7: 1753–1760. [DOI] [PubMed] [Google Scholar]
- 7.Goula D, Benoist C, Mantero S, et al. Polyethylenimine-based intravenous delivery of transgenes to mouse lung. Gene Ther 1998; 5: 1291–1295. [DOI] [PubMed] [Google Scholar]
- 8.Song YK, Liu F, Liu D. Enhanced gene expression in mouse lung by prolonging the retention time of intravenously injected plasmid DNA. Gene Ther 1998; 5: 1531–1537. [DOI] [PubMed] [Google Scholar]
- 9.Schlosser K, White RJ, Stewart DJ. miR-26a linked to pulmonary hypertension by global assessment of circulating extracellular microRNAs. Am J Respir Crit Care Med 2013; 188: 1472–1475. [DOI] [PubMed] [Google Scholar]
- 10.Gunther M, Lipka J, Malek A, et al. Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung. Eur J Pharm Biopharm 2011; 77: 438–449. [DOI] [PubMed] [Google Scholar]
- 11.Ge Q, Filip L, Bai A, et al. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci U S A 2004; 101: 8676–8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnet ME, Gossart JB, Benoit E, et al. Systemic delivery of sticky siRNAs targeting the cell cycle for lung tumor metastasis inhibition. J Control Release 2013; 170: 183–190. [DOI] [PubMed] [Google Scholar]
- 13.Hobel S, Koburger I, John M, et al. Polyethylenimine/small interfering RNA-mediated knockdown of vascular endothelial growth factor in vivo exerts anti-tumor effects synergistically with Bevacizumab. J Gene Med 2010; 12: 287–300. [DOI] [PubMed] [Google Scholar]
- 14.Malek A, Merkel O, Fink L, et al. In vivo pharmacokinetics, tissue distribution and underlying mechanisms of various PEI(-PEG)/siRNA complexes. Toxicol Appl Pharmacol 2009; 236: 97–108. [DOI] [PubMed] [Google Scholar]
- 15.Li X, He S, Li R, et al. Pseudomonas aeruginosa infection augments inflammation through miR-301b repression of c-Myb-mediated immune activation and infiltration. Nat Microbiol 2016; 1: 16132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, Li X, Ye Y, et al. MicroRNA-302b augments host defense to bacteria by regulating inflammatory responses via feedback to TLR/IRAK4 circuits. Nat Commun 2014; 5: 3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trang P, Wiggins JF, Daige CL, et al. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther 2011; 19: 1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.