Abstract
Background:
Biomechanical predictors of a second anterior cruciate ligament (ACL) injury after ACL reconstruction (ACLR) and return to sport (RTS) have been identified; however, these measures may not be feasible in a standard clinical environment.
Purpose/Hypothesis:
The purpose of this study was to evaluate whether standard clinical measures predicted the risk of second ACL injuries. The hypothesis tested was that a combination of strength, function, and patient-reported measures at the time of RTS would predict the risk of second ACL injuries with high sensitivity and specificity.
Study Design:
Case-control study; Level of evidence, 3 and Cohort study (prognosis); Level of evidence, 1.
Methods:
A total of 163 participants (mean age, 16.7 ± 3.0 years) who underwent primary ACLR and were able to RTS were evaluated. All participants completed an assessment of isokinetic strength, hop testing, balance, and the Knee Injury and Osteoarthritis Outcome Score (KOOS). Participants were tracked for a minimum of 24 months to identify occurrences of a second ACL injury. The initial 120 participants enrolled were used to develop a clinical prediction model that utilized classification and regression tree (CART) analysis, and the remaining 43 participants enrolled were used as a validation dataset. Additional analyses were performed in all 163 participants using Kaplan-Meier analysis and Cox proportional hazards modeling.
Results:
Approximately 20% (23/114) of the initial subset of the cohort suffered a second ACL injury. CART analysis identified age, sex, knee-related confidence, and performance on the triple hop for distance at the time of RTS as the primary predictors of a second ACL injury. Using these variables, a model was generated from which high-risk (n = 53) and low-risk groups (n = 61) were identified. A total of 22 participants in the high-risk group and 1 participant in the low-risk group suffered a second ACL injury. High-risk participants fit 1 of 2 profiles: (1) age <19 years, triple hop for distance between 1.34 and 1.90 times body height, and triple hop for distance limb symmetry index (LSI) <98.5% (n = 43) or (2) age <19 years, triple hop for distance >1.34 times body height, triple hop for distance LSI >98.5%, female sex, and high knee-related confidence (n = 10). The validation step identified the high-risk group as being 5 times (odds ratio, 5.14 [95% CI, 1.00-26.46]) more likely to suffer a second ACL injury, with a sensitivity of 66.7% and specificity of 72.0%.
Conclusion:
These findings recognize measures that accurately identify young patients at high risk of sustaining a second ACL injury within 24 months after RTS. The development of a clinical decision algorithm to identify high-risk patients, inclusive of clinically feasible variables such as age, sex, confidence, and performance on the triple hop for distance, can serve as a foundation to re-evaluate appropriate discharge criteria for RTS.
Keywords: ACL, second injury, clinical prediction rule
Outcomes after anterior cruciate ligament reconstruction (ACLR) and return to sport (RTS) are less than optimal in a young, athletic population. A second ACL injury (ipsilateral graft rupture or contralateral ACL injury) after ACLR and RTS in young athletes has been reported to be as high as one-third.27,31,34 The ability to return to competitive preinjury levels of activity in the first year after ACLR may be as low as one-half.1 The incidence of osteoarthritis after ACL injury may be as high as 50% to 90%20 and may begin to present as early as 1 year after injury.6 In consideration of these outcomes, some authors suggest a delay of return to activity for up to 2 years after ACLR.26 Collectively, these data highlight the need to improve care provided to patients with ACL injury to ensure their short-term ability to engage in activity and long-term joint health.
One factor in the ACLR rehabilitation process currently fraught with wide variation is the discharge criteria utilized to determine readiness to safely RTS. In a 2004 systematic review, Kvist18 noted that of 34 articles published between 1998 and 2003, roughly one-third of authors reported the use of isokinetic testing or measures of clinical impairment, such as range of motion, in their RTS decision-making after ACLR. More recently, in a systematic review of articles published between 2001 and 2011, Barber-Westin and Noyes3,4 reported that few objective functional criteria were used to determine a patient’s readiness to RTS after ACLR. Collectively, these authors have highlighted the lack of standardized measures used to determine readiness to safely RTS at the conclusion of rehabilitation after ACLR. Further, measures that currently may be used to determine readiness to discharge from physical therapy, such as time from surgery,25 isokinetic strength,30 and functional performance on hop testing,2 have failed to identify readiness to safely participate in sport with a minimal risk of second injury.
Recent evidence has identified biomechanical and neuromuscular factors present at the time of RTS, such as hip internal rotation moment, knee valgus, asymmetric sagittal-plane knee moment during the landing of a drop vertical jump, and altered postural stability, that predict second ACL injuries after ACLR and RTS.28 These factors may provide a highly sensitive and specific predictive model of second ACL injuries; however, these measures are not feasible to be included in a discharge algorithm that could be universally implemented in all facilities, as they require equipment not readily available to all clinicians. More recently, attempts at injury prediction with clinically feasible tools have included the identification of female athletes at risk of having high knee abduction moments, a risk factor for a primary ACL injury in previously uninjured athletes,24 and patellofemoral pain.23 These studies have introduced the feasibility of using clinical measures to screen for risk factors; however, a critical gap exists, as a clinician-friendly algorithm composed of simple screening assessments that could identify patients at high risk for future injuries after ACLR and RTS has yet to be recognized and implemented.
The purpose of this study was to evaluate whether standard clinical measures could predict the risk of second ACL injuries in a young healthy population of athletes after ACLR. The tested hypothesis was that a combination of strength, function, and patient-reported measures at the time of RTS would predict the risk of second ACL injury with high sensitivity and specificity. The identification of such measures represents an important foundational step in the development of new discharge criteria for patients after ACLR who seek to return to participation in sport.
Methods
Participants
A prospective case-cohort design was used to identify the clinical predictors of a second ACL injury after a primary ACL injury, ACLR, and RTS. One hundred sixty-three athletes (105 female, 58 male) who sustained an ACL injury, underwent ACLR, completed rehabilitation, and were released to prior levels of activity by their surgeon and rehabilitation professional were recruited to participate in this study (Table 1). Inclusion criteria required the patients to be between the ages of 10 and 27 years (mean age, 16.7 ± 3.0 years) with no history of contralateral ACL injuries as well as no bilateral lower extremity or lower back injuries during the prior 12 months. Participants were recruited from the tristate region and were medically managed by a diverse group of surgeons and physical therapists. Graft type and rehabilitation were not controlled in this study. All participants stated that they planned to return to their preinjury level of a pivoting or cutting sport (level 1 or 2)7 at a minimum of 50 hours per year. Participants were excluded if they elected not to return to pivoting or cutting sports or were yet to be released to preinjury levels of function. The patients primarily participated in level 1/2 sports such as basketball, soccer, volleyball, and football.
TABLE 1.
Patient Demographicsa
| Overall (N = 163) | Initial Cohort (n = 120) | Validation Cohort (n = 43) | P Value | |
|---|---|---|---|---|
| Age, mean ± SD, y | 16.7 ± 3.0 | 17.0 ± 2.9 | 16.0 ± 3.3 | >.05 |
| Weight, mean ± SD, kg | 67.1 ± 16.4 | 67.6 ± 16.1 | 65.8 ± 17.6 | >.05 |
| Height, mean ± SD, cm | 167.6 ± 11.1 | 168.5 ± 10.3 | 165.0 ± 12.9 | >.05 |
| Sex, female/male, n | 105/58 | 78 /42 | 27 /16 | >.05 |
| Graft type, HS/BPTB/ALLO, n | 95/53/15 | 60/49/11 | 35/4/4 | <.001 |
| Time from ACLR to RTS testing, mean ± SD, mo | 8.3 ± 2.5 | 8.1 ± 2.1 | 8.9 ± 3.3 | >.05 |
aACLR, anterior cruciate ligament reconstruction; ALLO, allograft; BPTB, bone–patellar tendon–bone graft; HS, hamstring graft; RTS, return to sport.
Testing Protocol
The study was approved by an institutional review board, and informed consent was obtained from all participants and guardians (if applicable) before enrollment and testing. All demographic data, including height, weight, and body mass index, and appropriate surgical information were collected from all participants. Patient-reported outcomes were completed by all participants, inclusive of the International Knee Documentation Committee (IKDC) subjective form16 and the Knee Injury and Osteoarthritis Outcome Score (KOOS).29 Knee confidence was determined based on the participant’s response to question 3 of the KOOS quality of life (QOL) subscale. Specifically, the question asks, “How much are you troubled with lack of confidence in your knee?” Each participant then completed a dynamic clinical assessment, inclusive of standard measures to determine readiness to RTS,3,18 consisting of strength, postural stability, functional performance, mobility, and knee laxity measures executed within 4 weeks of their medical clearance to RTS.
Quadriceps strength was assessed isometrically at 60° of knee flexion, and quadriceps and hamstring strength was tested isokinetically at 180 deg/s and 300 deg/s with a dynamometer (Biodex Medical Systems) using previously described methods.30 Isokinetic hip abduction strength was assessed using a Biodex dynamometer in a standing position as previously described.5 Postural stability was assessed in a single-leg standing position on a Biodex stabilometer, as described in prior work.28
Functional performance was assessed using 3 single-leg hop for distance measures (single hop, triple hop, and triple crossover hop) and 1 single-leg timed hop test30 (Figure 1). The single hop for distance required 1 maximal effort jump, taking off and landing on the same limb. The triple hop required 3 consecutive maximal effort jumps in a straight line. The triple crossover hop required 3 consecutive hops, crossing over a midline each time, with the goal of maximizing this horizontal distance from the takeoff point to the final landing point. For each of these 3 hop tests, the distance from the toe of the takeoff point to the toe of the final landing point was measured. The timed hop required the patient to jump as fast as possible for 6 m, with the time to execute the task recorded. For each of the strength, postural stability, and functional hop measures, raw performance data were recorded for both limbs, and limb symmetry indices (LSIs) were calculated, with 100% representing perfect symmetry between limbs on the measure and less than 100% representing a deficit in the involved limb. In addition, raw values of the distance hopped on the 3 distance-based hop tests were normalized to body height. Anterior-posterior knee laxity was assessed using the CompuKT arthrometer (MEDmetric) with the knee at 20° of flexion.35 Participants were tracked for 24 months after RTS to identify any second ACL injury that occurred. In the 24 months after RTS, 32 participants sustained a second ACL injury to either the ipsilateral or contralateral knee.
Figure 1.
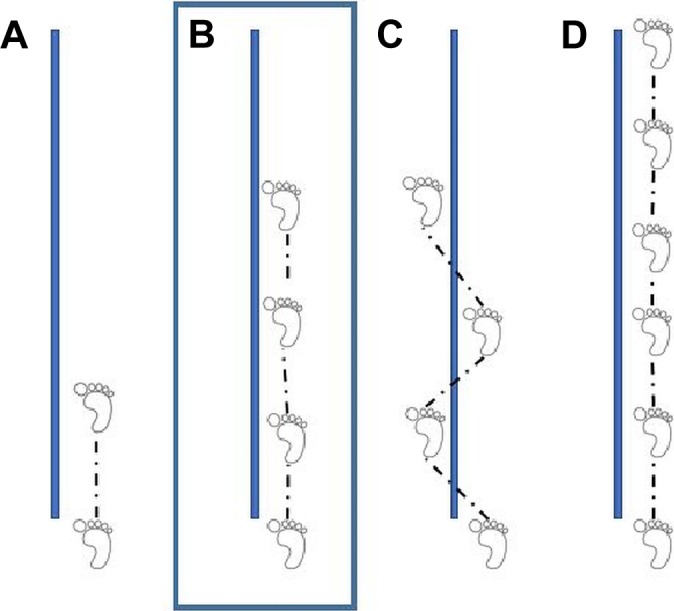
Single-leg hop tests, including (A) the single hop for distance in centimeters, (B) the triple hop for distance in centimeters, (C) the triple crossover hop in centimeters, and (D) the 6-m timed hop in seconds.
Statistical Analysis
To develop the clinical predictors of a second ACL injury after ACLR and RTS, the data obtained from the first consecutive 120 participants recruited and enrolled in the study were used as the training sample, and the remaining 43 participants were used as the holdout validation sample. Among the first 120 participants, classification and regression tree (CART) analysis was used to identify important predictors and their potential interactions. CART analysis or recursive binary tree modeling took interactive steps for the identification of the strongest predictor of second ACL injury events. Binary splitting was performed repeatedly with the goal of allocating participants into groups such that they would have a similar risk of second ACL injuries. We used 10-fold cross-validation for the development of clinical decision rules in the first 120 participants of the training sample. We refined the model by choosing clinically meaningful cut points, based on current best evidence and clinical expertise, and the order of entering the model, which was determined by the CART model. Subsequently, several alternative models were generated. We chose the final model by maximizing the model fit according to 10-fold cross-validation and the best model diagnostic characteristics (receiver operating characteristic, sensitivity, specificity, positive predictive value, negative predictive value) in the holdout validation data. This was to avoid overfitting and to ensure model internal and external validity. Based on the final CART model, we classified participants into the high-risk group if the estimated probability of a second ACL injury was greater than 30% and into the low-risk group otherwise. At last, we compared the survival rate between the high-risk and low-risk groups using Kaplan-Meier analysis in all 163 participants.
Results
A predictive model that identified young athletes at high risk for a second ACL injury in the first 24 months after RTS, ACLR, and completion of rehabilitation was developed from the dataset. Among the initial 120 participants enrolled in the study, 114 had complete datasets and were included in CART analysis that was designed to identify the most important clinical variables that predicted a second ACL injury. Twenty percent of the initial subset of the cohort (23/114) suffered a second ACL injury, inclusive of 8 ipsilateral graft failures and 15 contralateral ACL injuries. CART analysis identified younger age (<19 years), higher knee-related confidence from question 3 of the QOL subscale of the KOOS, female sex, and normalization of as well as LSI performance on the triple hop for distance as the most important predictors of a second ACL injury. Further, CART analysis dichotomized triple hop for distance limb symmetry (LSI <98.5%) and trichotomized triple hop for distance normalized to height in the involved limb at <1.34 times body height, between 1.34 and 1.90 times body height, and >1.90 times body height. Using these variables, a model with 7 leaf nodes was generated, from which a high-risk group (n = 53; 43 females, 10 males) and low-risk group (n = 61; 32 females, 29 males) were identified. No differences in the distribution of patients with different graft types in the high-risk and low-risk groups were observed (P = .78).
Twenty-two (41.5%) participants in the high-risk group and 1 (1.6%) participant in the low-risk group suffered a second ACL injury in the 24 months after RTS. High-risk participants fit into 1 of 2 profiles. One profile included 43 patients and was categorized by younger age (<19 years), triple hop for distance between 1.34 and 1.90 times body height, and triple hop for distance LSI <98.5% (Table 2). Nineteen (44%) of these patients suffered a second ACL injury. The second profile within the high-risk group included 10 patients, and they were younger (<19 years), had triple hop for distance >1.34 times body height, had triple hop for distance LSI >98.5%, were female, and reported high knee-related confidence on the KOOS QOL subscale (Table 2). Three (30%) of these patients suffered a second ACL injury.
TABLE 2.
Summary of High-Risk Profilesa
| High-Risk Profile 1 | High-Risk Profile 2 |
|---|---|
|
|
aLSI, limb symmetry index.
The remaining 43 participants in the study were used for further validation of the predictive model. No significant differences in age, height, weight, proportion of female:male participants, and time between ACLR and testing were seen between groups (see Table 1). A difference in the distribution of graft types was seen between groups, as the validation cohort had a greater percentage of patients with hamstring grafts than the initial cohort. Of the final 43 participants, 34 had complete datasets and were included in the validation. In this subset, 9 of 34 participants (26.5%) suffered a second ACL injury. Eighteen patients were classified into the low-risk group. Three of the patients in the low-risk group (16.7%) suffered a second ACL injury. Thirteen participants were classified as high risk. Six of the participants (46.2%) in the high-risk group suffered a second ACL injury in the 24 months after RTS. The participants included in the validation of the predictive model identified within the high-risk group were 5 times (odds ratio, 5.14 [95% CI, 1.00-26.46]) more likely to suffer a second ACL injury than the low-risk group, with a sensitivity of 66.7%, specificity of 72.0%, positive predictive value of 46.15%, and negative predictive value of 85.71%. A Kaplan-Meier survival curve that compares second ACL injuries in the high-risk group with those in the low-risk group is provided in Figure 2.
Figure 2.
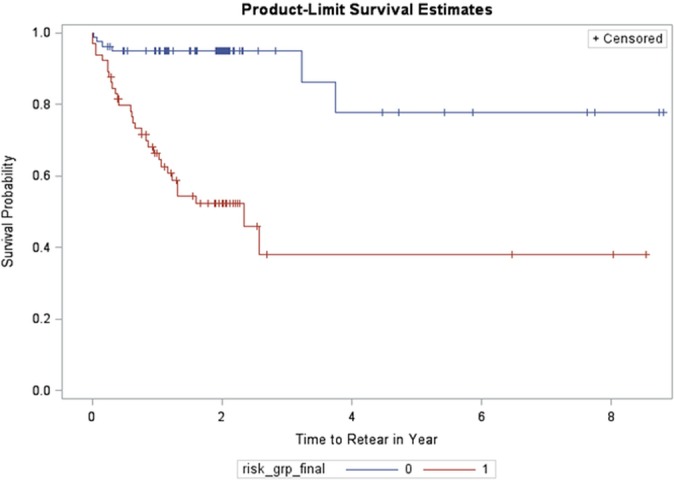
Kaplan-Meier survival curve demonstrating second anterior cruciate ligament injury in the high-risk group (red) and the low-risk group (blue) in years after return to sport.
Discussion
The findings of this study support the tested hypothesis that standard clinical measures can identify patients at high risk for second ACL injury after ACLR and RTS. Specifically, younger patients who present with moderate normalized triple hop for distance performance (1.34-1.90 times body height) and greater limb asymmetry (<98.5%) on the triple hop for distance test at the time of RTS and those patients who are female, have high self-reported confidence, normalized performance on the triple hop for distance >1.34 times body height, and greater limb symmetry (>98.5%) are at greatest risk for a second ACL injury after ACLR and RTS. To our knowledge, this represents the first report using a subset of standardized clinical assessments typically conducted at the conclusion of rehabilitation in preparation for RTS, interpreted in such a way to successfully predict the risk of second ACL injury in a population of young active athletes returning to pivoting and cutting sports.
Two prior studies have attempted to examine differences in outcomes in populations of patients after ACLR who have achieved all recommended criteria of a discharge algorithm including strength, hop testing, and patient-reported outcomes. Grindem et al10 reported that patients who failed to pass all assessments included in their discharge algorithm, including patient-reported outcomes (>90/100) as well as quadriceps strength and hop testing, with symmetry scores of greater than 90% were at greater risk of injuries after RTS. These injuries included, but were not limited to, second ACL injuries. Kyritsis et al19 studied a cohort of male professional soccer players after ACLR and measured a battery of discharge criteria before RTS, including isokinetic strength testing, dynamic running tests, and functional hop testing. These authors reported that patients who failed to meet all clinical discharge criteria were at 4 times greater risk for graft ruptures. Each of these studies evaluated the efficacy of meeting all measures in current standard discharge algorithms to identify the risk of future injuries.
The unique contribution of these current data is the identified high-risk profiles of patients based on a subset of current discharge criteria that include individual limb performance as well as limb symmetry with functional hop testing. Two profiles of patients were included in the high-risk category. The single common variable in both profiles is age. Both profiles indicate that a younger patient is at higher risk. This is consistent with current evidence that identifies younger age as a risk factor for future injury, which is likely because of increased activity by these younger patients.32
In addition to younger age, the first profile represents a population with moderate functional performance on the involved limb in the presence of limb asymmetry. Forty-four percent of participants in the initial training set (n = 120) who fit this profile suffered a second ACL injury within the first 24 months after they returned to sport. Functional hop testing is traditionally used as an assessment tool to determine readiness to RTS after ACLR2,3,18; however, limb symmetry is often reported as the primary metric to determine function. Specifically, the athlete’s ability to hop an equal distance on the involved and uninvolved limbs is then calculated into the limb symmetry score, with results less than 100% indicating a deficit in the involved limb. The primary hop tests reported in the literature include the single-leg hop for distance, the single-leg triple hop for distance, and the single-leg triple crossover hop. Historically, 85% to 90% LSI or greater was deemed sufficient to RTS,30 although recent evidence has suggested that these criteria may not be stringent enough to achieve safe RTS.33 These data indicate that utilization of the triple hop data, with a focus on performance, specifically distance jumped on the involved limb, normalized to body height in addition to more stringent limb symmetry requirements may be a more representative measure of patient function and ability to safely RTS.
The utility of functional hop testing in relation to the quantification of lower limb strength, power, and function has been debated in the literature. Early reports of performance on the single-leg hop for distance demonstrated only moderate correlations to isokinetic strength.9 More recently, Hamilton et al11 reported specifically on the triple hop for distance and noted moderate to strong correlations between performance on the triple hop and measures of isokinetic quadriceps and hamstring strength and power measured with the vertical jump test. Despite this, a recent meta-analysis that reviewed physical performance tests noted limited and conflicting evidence in the utility of these tests; however, the authors did report that the relationship between hop testing and future injuries has yet to be reported in the literature.12 The current data indicate that performance on the triple hop for distance test, inclusive of both distance hopped normalized to height and limb symmetry, may be good indicators of the risk of future ACL injuries after ACLR and RTS in young athletes.
The second high-risk profile developed from these data represents young female athletes who present with moderate to high performance normalized to leg length on the single-leg triple hop for distance (>1.34 times body height), excellent limb symmetry on this test (>98.5%), and high self-reported confidence on the KOOS QOL subscale. Thirty percent of the patients in the initial training set who fit this profile suffered a second ACL injury. Interestingly, the participants who fit this profile represent high performers on the triple hop for distance, with high distances hopped and nearly perfect symmetry between limbs, in addition to having high confidence in their knee. Confidence was determined by a self-report to the question in the QOL subscale of the KOOS, which asked, “How much are you troubled with lack of confidence in your knee?” Those who answered “not at all” were classified as confident in their knee. One might theorize that an athlete who possesses high physical capacity, as evident by triple hop performance, in addition to having a high level of confidence may represent an athlete willing to RTS sooner, potentially before complete graft maturation, and at a higher intensity of play. Higher intensity play and/or insufficient tissue healing inherently may result in an increased likelihood of ACL injuries. Finally, the patients who fit this profile are all female, who are known to have a higher risk of ACL injuries when compared with male patients in comparable sports.21 Theoretically, this group may simply represent previously identified high-risk female athletes who have regained sufficient strength, function, and confidence after ACLR to return to high-risk activity. In this case, a focus to improve outcomes in this subset may be to address the modifiable preinjury factors that inherently place female athletes at high risk for ACL injuries, such as altered neuromuscular and biomechanical factors.13–15
These data represent an important initial step to redefining discharge criteria after ACLR. Currently, there is wide variation and little consistency in standard discharge criteria used to determine readiness to safely RTS after ACLR.3,4,18 Typically, an assessment of isokinetic strength,22 functional hop testing,8 anterior-posterior knee laxity,17 and patient-reported outcome scales16 may be included in the decision-making process to release a patient to return to pivoting and cutting sports after ACLR. Unfortunately, few of these measures have been shown to relate with success after ACLR17 or predict second ACL injuries after ACLR. Biomechanical assessments have identified abnormal movement and altered postural stability as predictors of a second ACL injury after ACLR and RTS28; however, these measures require equipment not typically available in the clinical setting. The development of these patient profiles that can predict second ACL injuries after ACLR, based on measures able to be assessed in all clinical settings, may represent an important first step in the advancement of more valid and clinically applicable assessments to be utilized in RTS decision-making after ACLR. In addition, the application of this information can spread to second injury prevention planning. Although sex and age would be considered “nonmodifiable” risk factors, an increased focus on targeting the remaining “modifiable” risk factors, inclusive of triple hop performance and impairments, which may limit performance on this measure, may help reduce the risk of second ACL injuries.
This study is not without limitations. The sample included in this study represents a young active cohort of athletes who were injured playing a pivoting or cutting sport and who hoped to return to their prior level of function after ACLR. As a result, this discharge criteria model may be applicable to this population but may have less utility in patients not returning to pivoting or cutting sports or older athletes. This model should be validated in these populations before generalizing its implementation to these groups. Second, this model looks specifically at variables typically measured in the clinical setting. While this resulted in a feasible model in the current clinical setting, it was not inclusive of variables that may be predictive of outcomes only feasible to measure in select settings, such as biomechanics laboratories. For example, the high-risk group that was young and female and presented with higher performance on the hop tests and minimal asymmetry may present with other biomechanical movement asymmetries previously described as a high-risk variable in this population.28 A stronger model may be able to be developed with the use of a more comprehensive set of variables; however, it would likely be less clinically generalizable in today’s clinical environment. Third, the second injury cohort included both ipsilateral and contralateral second ACL injuries. If unique predictive variables exist for each group, this study would be unable to identify these because of a lack of statistical power with the number of second injuries to date. Future studies need to replicate this methodology using ipsilateral and contralateral second ACL injuries as unique dependent variables to determine whether unique high-risk groups exist for each outcome. Finally, although the validation set demonstrated that patients in the high-risk profile groups were 5 times more likely to suffer a second ACL injury, this was based on a relatively small validation sample. Future studies should confirm these findings in a larger validation set.
Conclusion
The current criteria used to release patients to return to pivoting and cutting sports after ACLR are widely varied and fail to identify patients at high risk for future injuries. Prior attempts to identify variables predictive of future injury have resulted in highly sensitive and specific models but required technology typically unavailable in a clinical setting and therefore are not clinically feasible. The current findings demonstrate that clinically feasible measures assessed at the time of discharge after ACLR may be used to develop a model of athletes at high risk for future ACL injuries. Specifically, performance on the triple hop for distance test in both distance hopped and limb symmetry, sex, and self-reported knee confidence in young athletes can be used to determine who is at high risk for future ACL injuries after ACLR. With this knowledge, future research can focus on the development of new discharge criteria and improving interventions designed to target these readily modifiable variables.
Footnotes
This work was funded by support from the National Institutes of Health (grant F32-AR055844 to L.C.S.), the National Football League Charities Medical Research Grants in 2007, 2008, 2009, 2011 (to M.V.P., T.E.H., and L.C.S.), and the Cincinnati Children's Place Outcome Research Award (to M.V.P. and L.C.S.).
Ethical approval for this study was obtained from the Cincinnati Children’s Hospital Medical Center (No. 2008-0514).
References
- 1. Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48(21):1543–1552. [DOI] [PubMed] [Google Scholar]
- 2. Barber SD, Noyes FR, Mangine RE, McCloskey JW, Hartman W. Quantitative assessment of functional limitations in normal and anterior cruciate ligament-deficient knees. Clin Orthop. 1990;(255):204–214. [PubMed] [Google Scholar]
- 3. Barber-Westin SD, Noyes FR. Factors used to determine return to unrestricted sports activities after anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(12):1697–1705. [DOI] [PubMed] [Google Scholar]
- 4. Barber-Westin SD, Noyes FR. Objective criteria for return to athletics after anterior cruciate ligament reconstruction and subsequent reinjury rates: a systematic review. Phys Sportsmed. 2011;39(3):100–110. [DOI] [PubMed] [Google Scholar]
- 5. Brent JL, Myer GD, Ford KR, Paterno MV, Hewett TE. The effect of sex and age on isokinetic hip-abduction torques. J Sport Rehabil. 2013;22(1):41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Culvenor AG, Collins NJ, Guermazi A, et al. Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation. Arthritis Rheumatol. 2015;67(4):946–955. [DOI] [PubMed] [Google Scholar]
- 7. Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient: a prospective outcome study. Am J Sports Med. 1994;22(5):632–644. [DOI] [PubMed] [Google Scholar]
- 8. Fitzgerald GK, Lephart SM, Hwang JH, Wainner RS. Hop tests as predictors of dynamic knee stability. J Orthop Sports Phys Ther. 2001;31(10):588–597. [DOI] [PubMed] [Google Scholar]
- 9. Greenberger HB, Paterno MV. Relationship of knee extensor strength and hopping test performance in the assessment of lower extremity function. J Orthop Sports Phys Ther. 1995;22(5):202–206. [DOI] [PubMed] [Google Scholar]
- 10. Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50(13):804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamilton RT, Shultz SJ, Schmitz RJ, Perrin DH. Triple-hop distance as a valid predictor of lower limb strength and power. J Athl Train. 2008;43(2):144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hegedus EJ, McDonough S, Bleakley C, Cook CE, Baxter GD. Clinician-friendly lower extremity physical performance measures in athletes: a systematic review of measurement properties and correlation with injury. Part 1: the tests for knee function including the hop tests. Br J Sports Med. 2015;49(10):642–648. [DOI] [PubMed] [Google Scholar]
- 13. Hewett TE, Myer GD, Ford KR. Reducing knee and anterior cruciate ligament injuries among female athletes: a systematic review of neuromuscular training interventions. J Knee Surg. 2005;18(1):82–88. [DOI] [PubMed] [Google Scholar]
- 14. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. [DOI] [PubMed] [Google Scholar]
- 15. Hewett TE, Myer GD, Ford KR, Paterno MV, Quatman CE. The 2012 ABJS Nicolas Andry Award. The sequence of prevention: a systematic approach to prevent anterior cruciate ligament injury. Clin Orthop Relat Res. 2012;470(10):2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600–613. [DOI] [PubMed] [Google Scholar]
- 17. Kocher MS, Steadman JR, Briggs KK, Sterett WI, Hawkins RJ. Relationships between objective assessment of ligament stability and subjective assessment of symptoms and function after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(3):629–634. [DOI] [PubMed] [Google Scholar]
- 18. Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Med. 2004;34(4):269–280. [DOI] [PubMed] [Google Scholar]
- 19. Kyritsis P, Bahr R, Landreau P, Miladi R, Witvrouw E. Likelihood of ACL graft rupture: not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br J Sports Med. 2016;50(15):946–951. [DOI] [PubMed] [Google Scholar]
- 20. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. [DOI] [PubMed] [Google Scholar]
- 21. Marshall SW, Padua D, McGrath M. Incidence of ACL injury In: Hewett TE, Shultz SJ, Griffin LY, eds. Understanding and Preventing Noncontact ACL Injuries. Champaign, Illinois: Human Kinetics; 2007:5–30. [Google Scholar]
- 22. Mattacola CG, Perrin DH, Gansneder BM, Gieck JH, Saliba EN, McCue FC., 3rd Strength, functional outcome, and postural stability after anterior cruciate ligament reconstruction. J Athl Train. 2002;37(3):262–268. [PMC free article] [PubMed] [Google Scholar]
- 23. Myer GD, Ford KR, Foss KD, Rauh MJ, Paterno MV, Hewett TE. A predictive model to estimate knee-abduction moment: implications for development of a clinically applicable patellofemoral pain screening tool in female athletes. J Athl Train. 2014;49(3):389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Development and validation of a clinic-based prediction tool to identify female athletes at high risk for anterior cruciate ligament injury. Am J Sports Med. 2010;38(10):2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Myer GD, Martin L, Jr, Ford KR, et al. No association of time from surgery with functional deficits in athletes after anterior cruciate ligament reconstruction: evidence for objective return-to-sport criteria. Am J Sports Med. 2012;40(10):2256–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagelli CV, Hewett TE. Should return to sport be delayed until 2 years after anterior cruciate ligament reconstruction? Biological and functional considerations. Sports Med. 2017;47(2):221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS): development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. [DOI] [PubMed] [Google Scholar]
- 30. Schmitt LC, Paterno MV, Hewett TE. The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2012;42(9):750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Webster KE, Feller JA. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(11):2827–2832. [DOI] [PubMed] [Google Scholar]
- 32. Webster KE, Feller JA, Leigh WB, Richmond AK. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(3):641–647. [DOI] [PubMed] [Google Scholar]
- 33. Wellsandt E, Failla MJ, Snyder-Mackler L. Limb symmetry indexes can overestimate knee function after anterior cruciate ligament injury. J Orthop Sports Phys Ther. 2017;47(5):334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD. Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Am J Sports Med. 2016;44(7):1861–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wordeman SC, Paterno MV, Quatman CE, Bates NA, Hewett TE. Arthrometric curve-shape variables to assess anterior cruciate ligament deficiency. Clin Biomech (Bristol, Avon). 2012;27(8):830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]


