Abstract
Context:
With the increasing use of unregulated dietary supplements, athletes are at continued risk from adverse medical events and inadvertent doping.
Evidence Acquisition:
A review of Clinical Key, MEDLINE, and PubMed databases from 2012 to 2017 was performed using search terms, including dietary supplement, contamination, doping in athletes, inadvertent doping, and prohibited substances. The references of pertinent articles were reviewed for other relevant sources.
Study Design:
Clinical review.
Level of Evidence:
Level 3.
Results:
Poor manufacturing processes and intentional contamination with many banned substances continue to occur in dietary supplements sold in the United States. Certain sectors, such as weight loss and muscle-building supplements, pose a greater threat because they are more likely to be contaminated.
Conclusion:
Athletes will continue to be at risk for adverse events and failed doping tests due to contaminated dietary supplements until legislation changes how they are regulated. In the interim, there are several steps that can be taken to mitigate this risk, including improved education of medical staff and athletes and use of third party–certified products.
Keywords: dietary supplements, prohibited contaminants, inadvertent doping
By all accounts, swimmer Jessica Hardy did everything she was supposed to leading up to her competing in the 2008 Beijing Olympics. She was projected to be a gold medal contender in her events. In an effort to gain strength and muscle mass, she took a dietary supplement, Advocare Arginine Extreme. First, she had researched the product, consulted with a nutritionist and team psychologist, and contacted the company about its safety and purity. Before the Games, Ms Hardy failed a drug test because her sample contained clenbuterol, a beta agonist with anabolic properties that is on the United States Anti-Doping Agency (USADA)/World Anti-Doping Agency (WADA) prohibited lists. Despite her conscientiousness, she was banned from the Olympic Games and served a 1-year suspension. This suspension was a reduced penalty because the Court of Arbitration for Sport found that she had exercised sufficient diligence in her investigation of the supplement.82
Annually, the USADA and WADA publish a list of prohibited substances and methods in an effort to keep sports clean and protect athletes (Table 1).87 The National Collegiate Athletic Association (NCAA) and most professional leagues have similar categories of banned substances, including illicit or street drugs.47 A substance is prohibited if it meets 2 of 3 criteria: it has potential to enhance or enhances sports performance, it represents an actual or potential health risk to the athlete, or it violates the spirit of sports.73 The WADA code also states that athletes have a personal duty to ensure that no prohibited substance enters their body and that they are responsible for any prohibited substance or its metabolite or markers found to be present in their samples.85 The biggest cause of inadvertent doping is the careless and misinformed use of supplements.64 Although it is difficult to quantify the extent of the problem, it is estimated that 6.4% to 8.8% of doping cases are caused by tainted dietary supplements.53
Table 1.
World Anti-Doping Agency prohibited list (January 2017)
| ID No. | Prohibited Substance/Method | Type | When Prohibited |
|---|---|---|---|
| S0 | Non-approved substances | Substance | At all times |
| S1 | Anabolic agents | Substance | At all times |
| S2 | Peptide hormones, growth factors, related substances, and mimetics | Substance | At all times |
| S3 | Beta 2 agonists | Substance | At all times |
| S4 | Hormone and metabolic modulators | Substance | At all times |
| S5 | Diuretics and masking agents | Substance | At all times |
| S6 | Stimulants | Substance | In competition |
| S7 | Narcotics | Substance | In competition |
| S8 | Cannabinoids | Substance | In competition |
| S9 | Glucocorticoids | Substance | In competition |
| M1 | Manipulation of blood and blood components | Method | At all times |
| M2 | Chemical and physical manipulation | Method | At all times |
| M3 | Gene doping | Method | At all times |
| P1 | Alcohol | Substance | Particular sports |
| P2 | Beta blockers | Substance | Particular sports |
Background
Athletes have been trying to fulfill the Olympic motto of “faster, higher, stronger” by adding food and other substances to their diets since 776 bc, when the Greeks used dried figs, mushrooms, and strychnine to improve athletic performance. A dietary supplement is defined as a product intended to augment the diet that bears or contains 1 or more of the following ingredients: a vitamin; a mineral; a herb or other botanical; an amino acid; a dietary substance for use by a human to supplement the diet by increasing the total dietary intake; or a concentrate, metabolite, constituent, extract, or combination of any ingredient described above.63 Dietary supplements are used by as many as 53% of American adults based on data from a National Health and Nutrition Examination Survey (NHANES; 2007-2010).8 This shows a substantial increase in usage from 1971-1974 NHANES data in which the usage rate was 33%. As of 2015, dietary supplements are a $38.8 billion industry in the United States.51 There are more than 85,000 dietary supplements currently marketed in the United States.17
In general, dietary supplement use is greater in athletes than in the general population, with elite athletes consuming more than nonelite athletes.35,42,53 Several studies cite rates of dietary supplement use among elite athletes ranging from 69% to 94%.10,13,24,42,71 Multivitamins remain the most commonly taken in all studies, with 39% to 73% of athletes taking something other than multivitamins. A survey of 77 elite Australian swimmers revealed that they took 207 different products, including an herbal preparation in 61%.10 Many athletes do not consider sports drinks and meal replacements to be dietary supplements.24
Athletes take dietary supplements for many reasons, including to aid in recovery, maintain health, improve performance, prevent or treat illness, support their immune system, manipulate body composition, and compensate for poor diet.24,43,72 This is despite the lack of evidence that all but a few substances are effective.43,71 In general, vitamin and mineral supplementation is unnecessary for athletes who consume a diet consisting of nutrient-dense foods.12 Dietary supplements can be considered if the athlete is on an energy-restricted diet or is unwilling or unable to consume sufficient dietary variety. Vegetarian/vegan athletes are at risk for deficiencies in energy, fat, protein, iron, zinc, vitamin B12, omega 3 fatty acids, creatine, and carnosine.70
Along with risks for inadvertent doping, dietary supplement use is strongly associated with intentional doping in elite and amateur sports. Adolescents who used dietary supplements had significantly stronger doping intentions and more positive attitudes and favorable behaviors toward doping.50 Dietary supplement use can be seen as a “gateway to doping,” with doping being 3.5 times higher in a dietary supplement user group than a nonuser group.7
Regulation of Dietary Supplements
It is important to understand the past and present federal regulation of dietary supplements. The single most influential piece of legislation was the Dietary Supplement Health and Education Act (DSHEA) of 1994.63 DSHEA gives authority to the US Food and Drug Administration (FDA) to regulate dietary supplements but as food instead of a type of pharmaceutical drug. Dietary supplement manufacturers are responsible for substantiating the safety of ingredients and ensuring product labeling, but they are not required to obtain FDA approval before producing or selling dietary supplements, nor are they required to demonstrate clinical efficacy. It is the burden of the FDA to prove adulteration or misbranding or risk of illness/injury before a dietary supplement can be removed from the market. A dietary supplement may not claim to diagnose, treat, cure, or prevent any disease. However, a study of 443 websites of 8 bestselling herbal products showed 81% made 1 or more health claims; 55% claimed the product treated, prevented, diagnosed, or cured specific diseases; 52% of those making claim(s) omitted the federal disclaimer; and only 12% provided references without a link to a distributor or vendor.45 In another study, 75% of most popular herbal supplement labels contained none of the key safety messages about warnings for medical conditions, drug interactions, and side effects.61
The DSHEA did give the FDA authority to establish good manufacturing practices (GMPs) to ensure quality. GMPs include testing products to ensure quality, confirming absence of contaminants, verifying accuracy of labeling, maintaining minimal standards for marketing and packaging, monitoring and reporting adverse events, and making all records available for FDA inspection.76,78 An FDA report published in 2013 revealed that 70% of manufacturers were in violation of GMPs, especially with regard to finished product verification.39 The FDA also has scant resources for dietary supplement oversight and enforcement, with only 4% of the FDA Center for Food Safety and Nutrition budget dedicated to dietary supplements.5
The DSHEA passed with support on 4 main points: providing access to safe dietary supplements, improving the health of Americans, empowering consumers to make choices about preventative health, and stimulating growth in the dietary supplements industry. Of these points, some argue that only the last point was achieved.5
The Anabolic Steroid Control Act of 1990 made it a criminal offense to purchase, traffic, or use an anabolic-androgenic steroid (AAS). This was followed by the Designer Anabolic Steroid Control Act of 2014 (DASCA), which added more known AASs to the controlled substances list and provided an easier process to make additions to the list. The DASCA enabled the Drug Enforcement Administration (DEA) to act faster and have better enforcement tools to prosecute offenders.88
The Dietary Supplement and Nonprescription Drug Consumer Protection Act of 2006 requires dietary supplement manufacturers, packers, or distributors to record and report adverse events.62 However, 28% of facilities failed to register with the FDA, and 72% of registrants failed to provide complete and accurate information.37
Athletes’ Knowledge of Dietary Supplements
Athletes consume dietary supplements at a high rate but have limited knowledge about important aspects of their use. In a study on college-aged athletes, 86% were unaware that dietary supplements can have adverse effects.71 Elite Australian athletes did not know the active ingredient (62%), side effects (57%), mechanism of action (54%), or recommended dose (52%) of their dietary supplements.19 In elite, young German athletes, only 36% were aware of issues with contamination and, surprisingly, only 34% of the unaware desired more information.13
In general, the sources of information for athletes with regard to dietary supplements is also of poor quality, with the majority of information coming from family members, fellow athletes, friends, coaches, and trainers.24,71 In a military survey of more than 16,000 personnel, less than 30% discussed supplement use with their health care provider, and the leading source of information was magazines.34 In elite Italian cyclists, most received their information from the Internet, TV, and radio.40 There is also a lack of concordance between dietary supplement use and the reasons for use by athletes, which demonstrates an urgent need to provide athletes with information to make informed decisions.57
Lack of knowledge also affects medical providers, including physicians. In a study of sports medicine physicians, only 51% had a reliable source of information on dietary supplements; 58% routinely asked about dietary supplements; and although 71% had encountered an adverse event, only 10% confirmed reporting it. Of respondents, 68% stated a lack of knowledge of where to report adverse events.55 In a survey of internal medicine residents, 37% were not aware that FDA did not regulate the safety or efficacy of dietary supplements, and 65% were unaware that serious adverse events should be reported to FDA via MedWatch.6
Contaminants
Failure of a doping test can result from ingestion of a banned substance (intentional, inadvertent), passive exposure (secondhand smoke), food (tainted meat, poppy seeds), or abnormally high physiologic levels.3 Contamination can occur accidentally, due to poor manufacturing practices, or intentionally by unscrupulous manufacturers. In general, dietary supplements such as multivitamins/multiminerals are safe and not often contaminated. There was a case of cross-contamination of vitamin tablets with steroids using the same production line.26 The amount of contamination does not have to be great to cause an abnormal doping test. One capsule of a tainted dietary supplement resulted in all having urinary levels of steroid metabolites above the WADA threshold.81 Ingestion of as little as 2.5 µg of 19-norandrostendione, a steroid prohormone, in a dietary supplement (0.00005% contamination) can result in transient elevations of metabolite in urine to fail a doping test.83
The FDA Tainted Supplements List,79 maintained since 2007, contains 781 dietary supplements with the following category breakdown: sexual enhancement (46%), weight loss (39%), muscle building (12%), and other (2%). Of the 465 Class 1 FDA drug recalls between 2004 and 2012, 51% were classified as dietary supplements, with the same most common categories of sexual enhancement (40%), body building (31%), and weight loss (27%). Unapproved drug ingredients accounted for all the recalls.31 Most of the high-risk dietary supplements listed at the USADA are AASs and stimulants.72 These categories are at high risk for adulteration due to the need for rapid and noticeable results by the consumer to promote continued use of the product. To achieve these results, pharmaceutical medications are often added by the manufacturers.
Consumers are at risk of ingesting these unknown added medications, and therefore being affected by their side effects and drug-drug interactions. Risks go beyond normal side effects of the medication due to megadosing, polypharmacy practices, the fact that these drugs are often untested in humans, and the effects of combining with exercise.32,66 Ten-year data from US emergency rooms between 2004 and 2013 estimated 23,000 visits due to adverse effects from dietary supplements, resulting in 2156 hospitalizations.25 This number may underestimate the true incidence of adverse events because many events are not accurately classified as related to dietary supplements. The most commonly implicated dietary supplements were for weight loss and energy.25 This is supported by the increased reports of dietary supplement–associated hepatotoxicity that saw liver injury due to dietary supplements, reported to the Drug Induced Liver Injury Network, rise from 7% to 20% between 2004 and 2013.48 Dietary supplements for bodybuilding were the most commonly implicated.48
Dietary Supplements for Muscle Building
In the WADA 2015 Antidoping Rules Violation Report, the most common sports implicated for infractions were athletics, bodybuilding, cycling, weightlifting, and power lifting.86 The most frequent class of contaminants in doping testing is AAS, accounting for 50% of the 4500 adverse analytical findings at WADA laboratories, likely due to the growing problem with unapproved and/or designer substances.28 Starting in 2000, the first solid evidence for steroid contamination of dietary supplements showed that of 634 nonhormonal dietary supplements from 13 countries and 215 suppliers, 14.8% were contaminated with hormones or prohormones.27 The majority of hidden ingredients in tainted supplements in the muscle-building category were steroids (89%) followed by aromatase inhibitors (11%).76 Since 2002, 20% of legally sold sports nutrition products contained potent, synthetic oral AAS, with many bona fide AASs listed openly on product labels.26,54
Starting in 2003 with the Bay Area Laboratory Co-Operative scandal involving several professional and Olympic athletes, the creation of “the Clear” and “the Cream” represented the onset of designer steroids developed to evade tests for doping. Both were meant to improve performance but not raise flags on usual doping tests. Designer steroids are also known as prohormones, natural steroids, or testosterone boosters.59 They are AASs synthesized from a known parent steroid molecule and chemically modified with the intent to circumvent controlled substance laws and achieve AAS results from legal products.26,59 Most of these designer AASs are analogs of 1960s compounds that were abandoned for better alternatives.59 Designer steroids, in many cases, were developed as “pro-drugs,” which means that they are converted into active AASs in the body after ingestion. An example is prostanozolol, which is converted to stanozolol (Winstrol).9,26 These AASs share a common mechanism to testosterone acting on androgen receptors, but many are chemically novel and have never been studied in human subjects for toxicological or pharmacological effect. An example is the compound methasteron (Superdrol), a 17-α-alkylated steroid. This modification allows better oral bioavailability but was shown to be hepatotoxic, resulting in several cases of hepatitis and liver failure.1,59
The health consequences of unknown ingestion of these AASs are profound, including cholestasis, renal failure, gynecomastia, hypertension, acne, polycythemia, decreased high-density lipoprotein cholesterol, and psychological dependence.44 It is also possibly the most common cause of hypogonadism in men of reproductive age.59 Ingestion by women can lead to absence of menses, virilization, alopecia, and clitoromegaly.32,38 Young athletes can see accelerated puberty and stunted growth due to premature closure of growth plates.32,38
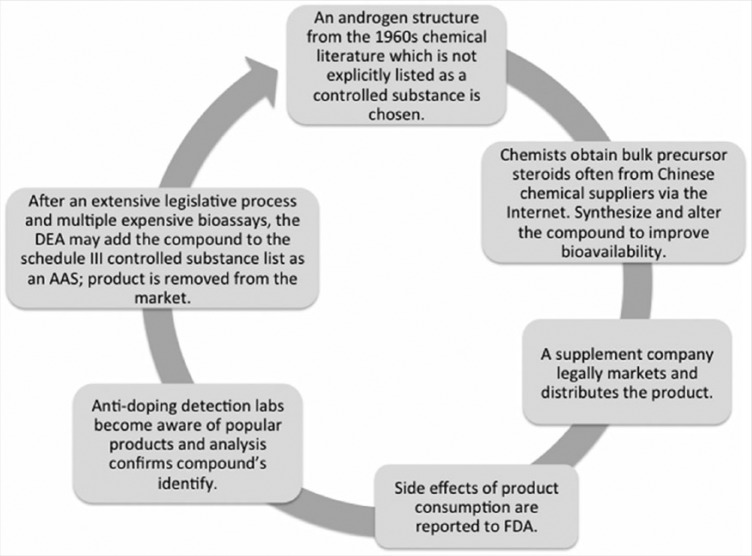
Determining whether a specific product contains AAS is difficult without chemical analysis.26 Ingestion of designer steroids has produced positive doping tests in many unsuspecting athletes.54 Once the new AAS is identified and reported to the FDA, the new compound is banned by DEA/FDA, and often, a new compound emerges in the marketplace to replace it (Figure 1).
Figure 1.
Designer steroid cycle. AAS, anabolic-androgenic steroid; DEA, Drug Enforcement Administration; FDA, Food and Drug Administration.
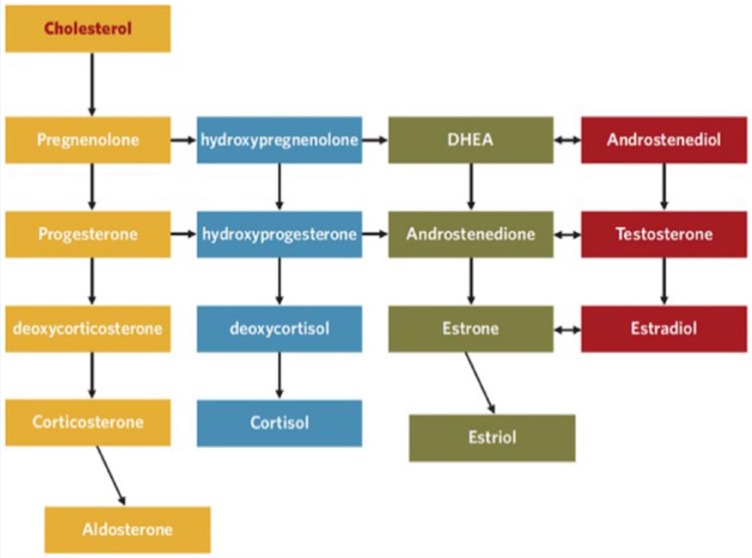
Prohormones, such as androstenedione and dehydroepiandrosterone (DHEA), became popular in 1996 due to baseball star Mark McGwire’s open endorsement. There is no evidence that DHEA increases circulating testosterone levels, muscle mass, strength, or performance despite the theory that they are converted to testosterone in vivo (Figure 2). The ergogenic potential for DHEA remains largely a myth.30 Many are now listed on banned substances lists. Despite the known dangers of AAS, there are hundreds of thousands of Internet sites that sell AAS and prohormones directly to consumers.14
Figure 2.
Steroid hormone cascade (used with permission from Brett Osborn). DHEA, dehydroepiandrosterone.
More recently, other substances have been developed, such as selective androgen receptor modulators, which are nonsteroidal alternatives to activate the androgen receptors on muscle and bone tissue.69 New analytical methods for their detection have been developed since 2006. As with designer steroids, newer substances are constantly entering the supplement market and are a substantial concern for misuse.28
Dietary Supplements for Weight Loss
As many as 34% of patients attempting to lose weight will use a dietary supplement. Fifty-three percent of these users believe that the FDA has evaluated the dietary supplement before marketing for safety and efficacy.58 Weight-loss dietary supplements represent 39% of the FDA’s tainted dietary supplements list and have been contaminated with many different agents with various mechanisms of action, including appetite suppressants, stimulants, antidepressants, diuretics, and laxatives. Specific contaminants include sibutramine (Meridia) and its analogs, phenolphthalein, locaserin (Belviq), fluoxetine (Prozac), dimetylamylamine (DMAA), orlistat (Alli), furosemide (Lasix), and fenproporex (Perphoxene).79
By far the most common adulterant in this class of dietary supplement is sibutramine (Meridia), which was approved in 1997 for management of obesity by Abbott but was voluntarily withdrawn in November 2010 due to increased risks of cardiovascular events, including myocardial infarction and stroke. Sibutramine is a serotonin norepinephrine reuptake inhibitor structurally related to amphetamine.56
Supplements containing ephedra or Ma Huang were very popular in the 1990s and 2000s. This stimulant was banned in 2004 due to cardiovascular toxicity. Since then, manufacturers have been marketing several “ephedra-free” products. In a cycle similar to that of designer steroids, designer stimulants have been created, basing the compounds on 1930s/1940s pharmaceutically developed sympathomimetics. Marketed as nasal decongestants and stimulants originally, these substances were abandoned long ago due to safety and abuse concerns.60
The re-emergence of these compounds started with dimethylamylamine (DMAA), also known as “geranium oil extract,” a potent vasopressor in a class known as phenylethylamines. As reports of cardiac arrests, cerebral hemorrhages, and other adverse events started to appear, DMAA was banned in 2012 after it was determined it was a new dietary ingredient and was not found in geraniums. What followed was a succession of chemically related compounds with structures and effects similar to DMAA and methamphetamines, with initials like DMBA, BMPEA, NADEP, and NN-DMPAA, each claiming to be occurring naturally from sources but later disproven as being created synthetically (Table 2).60 Some of these compounds are very closely related to one another; DMAA differs from DMBA by 1 carbon chain. This cycle of new stimulants closely resembles the succession of designer steroids seen in Figure 1.
Table 2.
Recent designer stimulants in dietary supplements
| Chemical Name | Alias Names | Popular Brands (Company) | Reported Natural Origin | FDA Ban Date |
|---|---|---|---|---|
| 1.3-Dimethylamylamine | DMAA Geranamine |
Napalm (Muscle Warfare) OxyElite Pro (USP Labs) Jack3d (USP Labs) |
Geranium oil | Apr 2013 |
| Aegeline | OxyElite Pro (USP Labs) | Bael trees | Nov 2013 | |
| N-α-diethyl phenethylamine | NADEP | Craze (Driven Sports) Detonate (Gaspari Nutrition) |
Dendrobium orchid | Apr 2014 |
| β-Methylphenethyl-amine | BMPEA | Black Widow (Hi-Tech Pharmaceuticals) Lipodrene (Hi-Tech Pharmaceuticals) |
Acacia rigidula | Apr 2015 |
| 1.3-Dimethylbutyl-amine | DMBA AMP, AMP Citrate |
Contraband (Iron Forged Nutrition) MD2 Meltdown (VPX) |
Pouchang tea | Sep 2015 |
| Methylsynephrine | Oxilofrine p-Hydroxyephedrine octopamine |
Hyperdrive 3.0 (ALR) Miami Lean (Skyline Nutrition) |
Orange peel Bitter orange |
Mar 2016 |
| N,N-dimethyl-2-phenylpropan-1-amine | NN-DMPAA | NOXPUMP (DY Nutrition) | Acacia rigidula |
Weight loss can also be achieved by excretion of stool and urine. Diuretics are often seen in dietary supplements marketed for weight loss and body image and represented 12% of the adverse analytical findings at WADA laboratories in 2015.86 Diuretics not only excrete water for rapid weight loss but also mask the presence of banned substances by diluting any doping agent or metabolite in the urine. At-risk sports include weight class–divided sports (wrestling, boxing, judo, weight lifting) and sports where athletes maintain low body weight (gymnastics and ballet).
Dietary Supplements for Energy
Caffeine is found naturally in food, such as chocolate, and many beverages, including coffee, tea, and energy drinks. Caffeine and other stimulants increase alertness, improve focus, decrease reaction time, and delay fatigue. In a survey of college student athletes, 86% took a preworkout supplement, and 73% consumed energy drinks.10,24 Most athletes do not consider sports drinks and meal replacements as dietary supplements.24 Caffeine is also found naturally in other less-known sources such as guarana (Paullinica cupana), green tea extract (Camellia sinensis), and yerba mate (Ilex paraguariensis), often found in combination with caffeine in energy drinks and dietary supplements.84 Excessive intake is associated with risk of heart palpitations and psychiatric, autonomic, and gastrointestinal adverse events.38 There was a 10-fold increase in emergency room visits between 2005 and 2009 due to energy drinks, likely because “proprietary blends” and the combination of several ingredients led to larger-than-recommended amounts of caffeine being consumed.23 The FDA recently issued a warning letter to manufacturers of pure caffeine powder, which is available for purchase through the Internet, due to the narrow dose window between safe and toxic.77 One teaspoon of caffeine powder is equivalent to 28 cups of regular coffee. Caffeine is not banned by the WADA/USADA but is on the monitored list. The NCAA limits caffeine levels to <15 µg/mL in urine samples. This level can be achieved with intakes of 500 mg or 6 to 8 cups of brewed coffee 2 to 3 hours prior to competition.46
Other
Two percent of the FDA CDER list79 for tainted supplements were in the category of “other,” with most of these being various nonsteroidal anti-inflammatory drugs; however, they also included benzodiazepines and corticosteroids, which are prohibited. Narcotics are not often found in dietary supplements, but a dietary supplements ingredient found in energy drinks that is increasing in use is mitragynine (Kratom), a herbal preparation with stimulating and opioid effects.29 Kratom is on WADA monitored list as a narcotic in competition and has been found in some brands to be adulterated with tramadol (Ultram).4 The first failed drug tests noted due to kratom ingestion were in 2015.29
Herbal Preparations
Herbal preparations are a particularly difficult class to regulate and study due to the multiple, complex compounds found in herbs. Any part of the plant, including leaves, stems, seeds, roots, and flowers, can be used as an herbal medication. Botanicals represent 18% of the US dietary supplements market, and Internet sales increased by an astonishing 19% in the past year.51 In a study using DNA barcoding to assess authentic contents of herbal preparations, 68% of samples had product substitution, and 59% contained plant species not listed on the label.49 A military survey noted more adverse events with combinations found in herbal dietary supplements than in single-ingredient dietary supplements.36
Third-Party Testing
The FDA has established quality standards for prescription drugs but not for dietary supplements. There are several third-party companies that will assess dietary supplements for quality, purity, potency, and composition. If a supplement passes, it will receive a “seal of approval” (Figure 3) to display on its package label. The programs are fee-based, and participation is voluntary. Cost may be an issue for smaller companies, and larger companies may not see any market advantage to certification and forego the added scrutiny.2,16 In a study of military commissaries, only 12% of the dietary supplements on shelves were independently certified.16 However, there seems to be an increase in the number of dietary supplement manufacturers seeking certification. The number of United States Pharmacopeia (USP)–certified products rose from 6 to more than 100 in 2015.66,80 Even when a product goes through testing to obtain certification, it is impossible for manufacturers to claim that a product is “free from all banned substances” simply because it is not possible to test for all of them. In short, supplement certification cannot completely eliminate the risk that a dietary supplement is contaminated, but it has strong evidence of reduced risk.15
Figure 3.
Seals for third-party certification companies.
The USADA provides guidance on choosing a third-party testing company. Third-party testing companies should be free from conflict of interest, have external accreditation, conduct audits for GMPs, evaluate the dietary supplements for overall safety and quality, and have validated and accredited methods to test for prohibited substances.74
USP80 is the standard-setting organization for quality, purity, and safety for prescription medications as well as dietary supplements. Compliance with USP standards is mandatory for prescription and over-the-counter medications sold in the United States but voluntary for dietary supplements. USP certification consists of annual facility audit, compliance with GMPs, and random marketplace sampling for final product analysis. Consumer Labs (consumerlab.com), National Sanitation Foundation (NSF.org), Banned Substances Control Group (bscg.org), and Informed-Choice/Informed-Sport (Informed-Sport.com) offer a variety of services including raw material and facility certification, GMP compliance, label verification, and final product testing. For sports certification, the final products are typically analyzed for over 200 banned substances, and approved lot/batch numbers are listed on the company’s websites. Aegis Shield (aegisshield.com) offers a list of dietary supplements that have had their label contents cross-referenced with 7 different banned substance lists, but they do not analyze products for contaminants. Several of these companies offer mobile applications and are endorsed by professional organizations such as Major League Baseball, National Football League, National Hockey League, and WADA.
Future Considerations
Until regulatory action corrects the problems associated with the DSHEA, it appears that athletes and patients will be at significant health and professional risks due to possible exposure to unapproved prohibited contaminants, especially designer steroids and stimulants.28,68 Appropriate corrective actions would involve 4 recommendations: only allow claims supported by research evidence, require manufacturers to list known adverse effects on labels, require the FDA to analyze the contents of dietary supplements, and restrict definition of dietary ingredients to vitamins and minerals meant to supplement the diet. A separate regulatory framework would be established for nonvitamin, nonmineral ingredients such as botanicals, herbs, and other medicinal products to be regulated like pharmaceuticals.21,41 Senators Richard Durbin (D-Illinois) and Richard Blumenthal (D-Connecticut) introduced the Dietary Supplement Labeling Act of 2013, which was designed to give consumers a clearer understanding of what they are taking.22 However, this bill was not enacted into law.
New and improved testing procedures in doping laboratories have resulted in more cases of adverse findings but may be able to distinguish between inadvertent and intentional doping.28 Between 2002 and 2008, 22 new steroidal compounds were identified by WADA-accredited labs.28 Newer analytic techniques are helping to detect, identify, and characterize new compounds. Testing of dietary supplements for performance-enhancing drugs is a challenge, as it is difficult to develop reliable methods to extract contaminants from different matrices like tablets, capsules, powders, gels, bars, beverages, and so on. Prohibited substances can be missed because sophisticated analytical testing techniques look for certain performance-enhancing drugs and not for others.
Legal action against dietary supplement companies has recently been used with some good results. The US Department of Justice fined GNC for selling a tainted supplement, OxyElite Pro, which contained aegeline and DMAA, causing the company to change its procedures to improve the quality and purity of dietary supplements.75 Some have proposed state and local governments help regulate the dietary supplement industry as they have some experience regulating products that affect public health, such as tobacco.65 The legal route can also be used by individual athletes, as was shown with a German soccer player who had court rule in his favor after testing positive for banned substance (nonlisted AAS) from his dietary supplement and was awarded damages, legal fees, and lost earnings.67
The Department of Defense has implemented a new program called Operation Safe Supplement, which consists of a high-risk dietary supplements list, education for health care professionals, and a rapid adverse event reporting system.20 A novel idea of a “supplement response team” consisting of toxicologists, clinicians, pharmacologists, and chemists has been proposed to react to FDA alerts.17 Currently, it is still possible to purchase FDA-banned dietary supplements months after a recall.18 Once a dietary supplement is deemed unsafe, it should be mandated that it is removed from all retail and Internet stores.
Team physicians and athletic trainers need to engage athletes on the topic of dietary supplements because they are in a position to mitigate the risks. First, the athlete needs to be evaluated for the need of supplementation, as simple diet adjustments may suffice and referral to a sports nutritionist may be appropriate. Questioning may be more fruitful if it is socioculturally sensitive. Positive response rates to dietary supplement use increased from 47% to 53% when adding terms such as “infusions, teas, herbs from garden, and natural remedies.”11 If a dietary supplement is needed or desired, the medical staff needs to consider 5 question areas: safety, effectiveness (plausible action), specific product information, risks/benefits, and potential for failed test.12 Since there may be a disconnect between an athlete’s reasons for taking a dietary supplement and its actual use,57 medical staff can help match the specific dietary supplements to the individual athlete. It is imperative that the staff have knowledge of prohibited substances for the athlete’s sport, doping testing procedures, adverse event reporting mechanisms, and adequate resources to refer to for up-to-date, accurate information (Table 3). The Australia Institute of Sport (www.ausport.gov.au/ais) ranks sports supplements as low risk, unknown risk, restricted, or banned based on scientific evidence whether they are safe, legal, and effective. Use of such an unbiased, up-to-date, evidence-based resource should help lessen the risk of inadvertent doping.
Table 3.
Resources for health professionals and athletes
| Organization | Website |
|---|---|
| Nutrition | |
| American Dietetic Association | eatright.org |
| Australian Institute of Sport | ausport.gov.au/ais |
| Gatorade Sports Science Institute | gssiweb.org |
| My Sports Dietician | mysportsdconnect.com |
| Sports, Cardiovascular, and Wellness Nutritionists | scandpg.org |
| United States Olympic Committee | teamusa.org |
| Dietary supplements information | |
| American Botanical Council | herbalgram.com |
| Australian Institute of Sport | ausport.gov.au |
| Center for Food Safety and Applied Nutrition | cfsan.fda.gov |
| Dietary Supplement Database (part of National Library of Medicine) | dsld.nim.nih.gov |
| United States Food and Drug Administration | fda.gov |
| Human Performance Research Center (Department of Defense initiative) | hprc-online.org |
| National Center for Alternative and Complimentary Medicine | nccam.nih.gov |
| National Center for Drug Free Sport | drugfreesport.com |
| Natural Medicines (formerly Natural Standard and Natural Medicines Comprehensive Database) | naturalmedicines.com |
| Office of Dietary Supplements | ods.od.nih.org |
| United States Anti-Doping Agency–Supplement 411 | USADA.org |
| Third-party testing | |
| Aegis Shield | Aegisshield.com |
| Banned Substances Control Group | BSCG.org |
| Consumer Labs | consumerlab.com |
| Informed-Choice/Informed Sport | Informed-Sport.org |
| National Sanitation Foundation | NSF.org |
| US Pharmacopeia | USP.org |
| Prohibited substances | |
| World Anti-Doping Agency | wada.org |
| United States Anti-Doping Agency | GlobalDRO.org |
| National Center for Drug Free Sport | drugfreesport.com |
| National Collegiate Athletic Association | NCAA.org |
| Adverse event reporting | |
| Med Watch | safetyreporting.hhs.gov |
Dr William Osler once said, “One of the first duties of the physician is to educate the masses not to take medicine.”52 This sage advice coupled with the adage “first, do no harm” seems appropriate when discussing the use of dietary supplements with our patients and athletes due to their limited efficacy and known safety risks. There is no 100% guarantee that tested products are entirely free of every prohibited substance,33 but the risk is significantly reduced with a quality assurance system in place, supported by GMPs, and an appropriate and accredited prohibited substance testing program. The only way to get a 100% guarantee that a dietary supplement will not cause a positive test or adverse effect is to avoid using any supplements at all.32
Acknowledgments
The author would like to thank Susan Hansen and Jacqueline Grove for their insights and editorial assistance in the preparation of this article.
Footnotes
The author reports no potential conflicts of interest in the development and publication of this article.
References
- 1. Abbate V, Kicman AT, Evans-Brown M, et al. Anabolic steroids detected in bodybuilding dietary supplements - a significant risk to public health. Drug Test Anal. 2015;7:609-618. [DOI] [PubMed] [Google Scholar]
- 2. Akabas SR, Vannice G, Atwater JB, Cooperman T, Cotter R, Thomas L. Quality certification programs for dietary supplements. J Acad Nutr Diet. 2016;116:1370-1379. [DOI] [PubMed] [Google Scholar]
- 3. Anderson JM. Evaluating the athlete’s claim of an unintentional positive urine drug test. Curr Sports Med Rep. 2011;10:191-196. [DOI] [PubMed] [Google Scholar]
- 4. Arndt T, Claussen U, Güssregen B, et al. Kratom alkaloids and O-desmethyltramadol in urine of a “Krypton” herbal mixture consumer. Forensic Sci Int. 2011;208:47-52. [DOI] [PubMed] [Google Scholar]
- 5. Ashar BH. The dietary supplement health and education act: time for a reassessment: comment on “acute selenium toxicity associated with a dietary supplement.” Arch Intern Med. 2010;170:261-263. [DOI] [PubMed] [Google Scholar]
- 6. Ashar BH, Rice TN, Sisson SD. Physicians’ understanding of the regulation of dietary supplements. Arch Intern Med. 2007;167:966-969. [DOI] [PubMed] [Google Scholar]
- 7. Backhouse SH, Whitaker L, Petroczi A. Gateway to doping? Supplement use in the context of preferred competitive situations, doping attitude, beliefs, and norms. Scand J Med Sci Sports. 2013;23:244-252. [DOI] [PubMed] [Google Scholar]
- 8. Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173:355-361. [DOI] [PubMed] [Google Scholar]
- 9. Baume N, Mahler N, Kamber M, Mangin P, Saugy M. Research of stimulants and anabolic steroids in dietary supplements. Scand J Med Sci Sports. 2006;16:41-48. [DOI] [PubMed] [Google Scholar]
- 10. Baylis A, Cameron-Smith D, Burke LM. Inadvertent doping through supplement use by athletes: assessment and management of the risk in Australia. Int J Sport Nutr Exerc Metab. 2001;11:365-383. [DOI] [PubMed] [Google Scholar]
- 11. Ben-Arye E, Halabi I, Attias S, Goldstein L, Schiff E. Asking patients the right questions about herbal and dietary supplements: cross cultural perspectives. Complement Ther Med. 2014;22:304-310. [DOI] [PubMed] [Google Scholar]
- 12. Bonci L. Supplements: help, harm, or hype? How to approach athletes. Curr Sports Med Rep. 2009;8:200-205. [DOI] [PubMed] [Google Scholar]
- 13. Braun H, Koehler K, Geyer H, Kleiner J, Mester J, Schanzer W. Dietary supplement use among elite young German athletes. Int J Sport Nutr Exerc Metab. 2009;19:97-109. [DOI] [PubMed] [Google Scholar]
- 14. Brennan BP, Kanayama G, Pope HG., Jr. Performance-enhancing drugs on the web: a growing public-health issue. Am J Addict. 2013;22:158-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cadwallader AB, Murray B. Performance-enhancing drugs I: understanding the basics of testing for banned substances. Int J Sport Nutr Exerc Metab. 2015;25:396-404. [DOI] [PubMed] [Google Scholar]
- 16. Cancio A, Eliason MJ, Mercer J, Tran T, Deuster PA, Stephens MB. Third-party certification of dietary supplements: prevalence and concerns. Mil Med. 2012;177:1460-1463. [DOI] [PubMed] [Google Scholar]
- 17. Cohen PA. Hazards of hindsight—monitoring the safety of nutritional supplements. N Engl J Med. 2014;370:1277-1280. [DOI] [PubMed] [Google Scholar]
- 18. Cohen PA, Maller G, DeSouza R, Neal-Kababick J. Presence of banned drugs in dietary supplements following FDA recalls. JAMA. 2014;312:1691-1693. [DOI] [PubMed] [Google Scholar]
- 19. Dascombe BJ, Karunaratna M, Cartoon J, Fergie B, Goodman C. Nutritional supplementation habits and perceptions of elite athletes within a state-based sporting institute. J Sci Med Sport. 2010;13:274-280. [DOI] [PubMed] [Google Scholar]
- 20. Deuster PA, Lieberman HR. Protecting military personnel from high risk dietary supplements. Drug Test Anal. 2016;8:431-433. [DOI] [PubMed] [Google Scholar]
- 21. Dodge T. Consumers’ perceptions of the dietary supplement health and education act: implications and recommendations. Drug Test Anal. 2016;8:407-409. [DOI] [PubMed] [Google Scholar]
- 22. Durbin D. With adverse event reporting on the rise, legislation needed to protect consumers of dietary supplements [press release]. Newsroom, Senator Durbin website, August 1, 2013. http://www.durbin.senate.gov/newsroom/press-releases/durbin-blumenthal-with-adverse-event-reporting-on-the-rise-legislation-needed-to-protect-consumers-of-dietary-supplements. Accessed January 25, 2017.
- 23. Eudy AE, Gordon LL, Hockaday BC, et al. Efficacy and safety of ingredients found in preworkout supplements. Am J Health Syst Pharm. 2013;70:577-588. [DOI] [PubMed] [Google Scholar]
- 24. Froiland K, Koszewski W, Hingst J, Kopecky L. Nutritional supplement use among college athletes and their sources of information. Int J Sport Nutr Exerc Metab. 2004;14:104-120. [DOI] [PubMed] [Google Scholar]
- 25. Geller AI, Shehab N, Weidle NJ, et al. Emergency department visits for adverse events related to dietary supplements. N Engl J Med. 2015;373:1531-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geyer H, Parr MK, Koehler K, Mareck U, Schanzer W, Thevis M. Nutritional supplements cross-contaminated and faked with doping substances. J Mass Spectrom. 2008;43:892-902. [DOI] [PubMed] [Google Scholar]
- 27. Geyer H, Parr MK, Mareck U, Reinhart U, Schrader Y, Schanzer W. Analysis of non-hormonal nutritional supplements for anabolic-androgenic steroids—results of an international study. Int J Sports Med. 2004;25:124-129. [DOI] [PubMed] [Google Scholar]
- 28. Geyer H, Schanzer W, Thevis M. Anabolic agents: recent strategies for their detection and protection from inadvertent doping. Br J Sports Med. 2014;48:820-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guddat S, Gorgens C, Steinhart V, Schanzer W, Thevis M. Mitragynine (Kratom)—monitoring in sports drug testing. Drug Test Anal. 2016;8:1114-1118. [DOI] [PubMed] [Google Scholar]
- 30. Hahner S, Allolio B. Dehydroepiandrosterone to enhance physical performance: myth and reality. Endocrinol Metab Clin North Am. 2010;39:127-139, x. [DOI] [PubMed] [Google Scholar]
- 31. Harel Z, Harel S, Wald R, Mamdani M, Bell CM. The frequency and characteristics of dietary supplement recalls in the United States. JAMA Intern Med. 2013;173:926-928. [DOI] [PubMed] [Google Scholar]
- 32. Hatton CK, Green GA, Ambrose PJ. Performance-enhancing drugs: understanding the risks. Phys Med Rehabil Clin North Am. 2014;25:897-913. [DOI] [PubMed] [Google Scholar]
- 33. Judkins CM, Teale P, Hall DJ. The role of banned substance residue analysis in the control of dietary supplement contamination. Drug Test Anal. 2010;2:417-420. [DOI] [PubMed] [Google Scholar]
- 34. Kao TC, Deuster PA, Burnett D, Stephens M. Health behaviors associated with use of body building, weight loss, and performance enhancing supplements. Ann Epidemiol. 2012;22:331-339. [DOI] [PubMed] [Google Scholar]
- 35. Knapik JJ, Steelman RA, Hoedebecke SS, Austin KG, Farina EK, Lieberman HR. Prevalence of dietary supplement use by athletes: systematic review and meta-analysis. Sports Med. 2016;46:103-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knapik JJ, Trone DW, Austin KG, Steelman RA, Farina EK, Lieberman HR. Prevalence, adverse events, and factors associated with dietary supplement and nutritional supplement use by US Navy and Marine Corps personnel. J Acad Nutr Diet. 2016;116:1423-1442. [DOI] [PubMed] [Google Scholar]
- 37. Levinson DR. Dietary Supplements: Companies May Be Difficult to Locate in an Emergency. Washington, DC: US Department of Health & Human Services; October 2012. Report No. OEI-01-11-00211. https://oig.hhs.gov/oei/reports/oei-01-11-00211.pdf. Accessed January 25, 2017. [Google Scholar]
- 38. Liddle DG, Connor DJ. Nutritional supplements and ergogenic AIDS. Prim Care. 2013;40:487-505. [DOI] [PubMed] [Google Scholar]
- 39. Long J. FDA GMP inspectors cite 70% of dietary supplement firms. Natural Products Insider. 2013. [Google Scholar]
- 40. Loraschi A, Galli N, Cosentino M. Dietary supplement and drug use and doping knowledge and attitudes in Italian young elite cyclists. Clin J Sport Med. 2014;24:238-244. [DOI] [PubMed] [Google Scholar]
- 41. Marcus DM. Dietary supplements: what’s in a name? What’s in the bottle? Drug Test Anal. 2016;8(3-4):410-412. [DOI] [PubMed] [Google Scholar]
- 42. Maughan RJ, Depiesse F, Geyer H. International Association of Athletics Federations. The use of dietary supplements by athletes. J Sports Sci. 2007;25(suppl 1):S103-S113. [DOI] [PubMed] [Google Scholar]
- 43. Maughan RJ, Greenhaff PL, Hespel P. Dietary supplements for athletes: emerging trends and recurring themes. J Sports Sci. 2011;29(suppl 1):S57-S66. [DOI] [PubMed] [Google Scholar]
- 44. Momaya A, Fawal M, Estes R. Performance-enhancing substances in sports: a review of the literature. Sports Med. 2015;45:517-531. [DOI] [PubMed] [Google Scholar]
- 45. Morris CA, Avorn J. Internet marketing of herbal products. JAMA. 2003;290:1505-1509. [DOI] [PubMed] [Google Scholar]
- 46. National Collegiate Athletic Association. Caffeine and athletic performance. 2014. https://www.ncaa.org/sites/default/files/Caffeine%20and%20Athletic%20Performance.pdf. Accessed February 6, 2017.
- 47. National Collegiate Athletic Association. 2016-17 NCAA Banned Drugs. Indianapolis, IN: NCAA; 2016. http://www.ncaa.org/2016-17-ncaa-banned-drugs-list. Accessed February 6, 2017. [Google Scholar]
- 48. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60:1399-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Newmaster SG, Grguric M, Shanmughanandhan D, Ramalingam S, Ragupathy S. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013;11:222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50. Ntoumanis N, Ng JY, Barkoukis V, Backhouse S. Personal and psychosocial predictors of doping use in physical activity settings: a meta-analysis. Sports Med. 2014;44:1603-1624. [DOI] [PubMed] [Google Scholar]
- 51. Nutrition Business Journal. Supplement business report 2016. 2016. https://www.newhope.com/sites/newhope360.com/files/2016%20NBJ%20Supplement%20Business%20report_lowres_TOC.pdf. Accessed February 6, 2017.
- 52. Osler W. Sir William Osler: Aphorisms From His Bedside Teachings and Writings. 2nd ed. Springfield, IL: Charles C. Thomas; 1961. [Google Scholar]
- 53. Outram S, Stewart B. Doping through supplement use: a review of the available empirical data. Int J Sport Nutr Exerc Metab. 2015;25:54-59. [DOI] [PubMed] [Google Scholar]
- 54. Parr M, Pokrywka A, Kwiatkowska D, Schänzer W. Ingestion of designer supplements produced positive doping cases unexpected by the athletes. Biol Sport. 2011;28:153-157. [Google Scholar]
- 55. Pascale B, Steele C, Attipoe S, O’Connor FG, Deuster PA. Dietary supplements: knowledge and adverse event reporting among American Medical Society for Sports Medicine physicians. Clin J Sport Med. 2016;26:139-144. [DOI] [PubMed] [Google Scholar]
- 56. Pawar RS, Grundel E. Overview of regulation of dietary supplements in the USA and issues of adulteration with phenethylamines (PEAs). Drug Test Anal. 2017;9:500-517. [DOI] [PubMed] [Google Scholar]
- 57. Petróczi A, Naughton DP, Mazanov J, Holloway A, Bingham J. Performance enhancement with supplements: incongruence between rationale and practice. J Int Soc Sports Nutr. 2007;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pillitteri JL, Shiffman S, Rohay JM, Harkins AM, Burton SL, Wadden TA. Use of dietary supplements for weight loss in the United States: results of a national survey. Obesity. 2008;16:790-796. [DOI] [PubMed] [Google Scholar]
- 59. Rahnema CD, Crosnoe LE, Kim ED. Designer steroids—over-the-counter supplements and their androgenic component: review of an increasing problem. Andrology. 2015;3:150-155. [DOI] [PubMed] [Google Scholar]
- 60. Rasmussen N, Keizers PH. History full circle: ‘Novel’ sympathomimetics in supplements. Drug Test Anal. 2016;8:283-286. [DOI] [PubMed] [Google Scholar]
- 61. Raynor DK, Dickinson R, Knapp P, Long AF, Nicolson DJ. Buyer beware? Does the information provided with herbal products available over the counter enable safe use? BMC Med. 2011;9:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. S. 3546–109th Congress: Dietary Supplement and Nonprescription Drug Consumer Protection. 2006. https://www.govtrack.us/congress/bills/109/s3546, Accessed January 25, 2017.
- 63. S. 4325–103rd Congress: Dietary Supplement Health and Education Act of 1994. Public law 103-417, October 25, 1994. [Google Scholar]
- 64. Sargent A, Backhouse S. 2015 world anti-doping code. Sport Exerc Scientist. 2014;42:11. [Google Scholar]
- 65. Starr R. Should states and local governments regulate dietary supplements? Drug Test Anal. 2016;8:402-406. [DOI] [PubMed] [Google Scholar]
- 66. Starr RR. Too little, too late: ineffective regulation of dietary supplements in the United States. Am J Public Health. 2015;105:478-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Striegel H, Vollkommer G, Horstmann T, Niess AM. Contaminated nutritional supplements—legal protection for elite athletes who tested positive: a case report from Germany. J Sports Sci. 2005;23:723-726. [DOI] [PubMed] [Google Scholar]
- 68. Thevis M, Kuuranne T, Walpurgis K, Geyer H, Schanzer W. Annual banned-substance review: analytical approaches in human sports drug testing. Drug Test Anal. 2016;8:7-29. [DOI] [PubMed] [Google Scholar]
- 69. Thevis M, Schanzer W. Synthetic anabolic agents: steroids and nonsteroidal selective androgen receptor modulators. Handb Exp Pharamacol. 2010;195:99-126. [DOI] [PubMed] [Google Scholar]
- 70. Thomas DT, Erdman KA, Burke LM. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Acad Nutr Diet. 2016;116:501-528. [DOI] [PubMed] [Google Scholar]
- 71. Tian HH, Ong WS, Tan CL. Nutritional supplement use among university athletes in Singapore. Singapore Med J. 2009;50:165-172. [PubMed] [Google Scholar]
- 72. US Anti-Doping Agency. High-risk dietary supplement list. 2016. http://www.supplement411.org/hrl/. Accessed January 26, 2017.
- 73. US Anti-Doping Agency. How does a substance get considered for the WADA Prohibited List? 2017. http://www.usada.org/substances/prohibited-list/. Accessed February 6, 2017
- 74. US Anti-Doping Agency. Third-party testing guidance. 2014. http://www.usada.org/substances/supplement-411/reduce-risk-testing-positive-experiencing-adverse-health-effects/third-party-testing-guidance/. Accessed January 26, 2017.
- 75. US Department of Justice. GNC enters into agreement with Department of Justice to improve its practices and keep potentially illegal dietary supplements out of the marketplace [press release]. Washington, DC: Office of Public Affairs, December 7, 2016. [Google Scholar]
- 76. US Food and Drug Administration. Current good manufacturing practices (CGMPs) for dietary supplements. 2015. http://www.fda.gov/Food/GuidanceRegulation/CGMP/ucm079496.htm. Accessed January 26, 2017.
- 77. US Food and Drug Administration. FDA consumer advice on pure powdered caffeine [Alerts & Advisories]. 2015. http://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm405787.htm. Accessed February 6, 2017.
- 78. US Food and Drug Administration. Guidance for industry: questions and answers regarding the labeling of dietary supplements as required by the Dietary Supplement and Nonprescription Drug Consumer Protection Act. 2007; http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/DietarySupplements/ucm179018.htm. Accessed January 25, 2017.
- 79. US Food and Drug Administration. Tainted products marketed as dietary supplements_CDER. 2016. http://www.accessdata.fda.gov/scripts/sda/sdNavigation.cfm?filter&sortColumn=3a&sd=tainted_supplements_cder&displayAll=true. Accessed January 25, 2017.
- 80. US Pharmacopeial Convention website. http://www.usp.org/. Accessed February 6, 2017.
- 81. van der Merwe PJ, Grobbelaar E. Unintentional doping through the use of contaminated nutritional supplements. S Afr Med J. 2005;95:510-511. [PubMed] [Google Scholar]
- 82. WADA v Hardy & USADA (Court of Arbitration for Sport Decisions 2009) Docket CAS 2008/A/1490. Decided May 21, 2010. http://www.usada.org/wp-content/uploads/hardy-cas.pdf. Accessed January 25, 2017.
- 83. Watson P, Judkins C, Houghton E, Russell C, Maughan RJ. Urinary nandrolone metabolite detection after ingestion of a nandrolone precursor. Med Sci Sports Exerc. 2009;41:766-772. [DOI] [PubMed] [Google Scholar]
- 84. Woods DJ. Guarana: Paullinia cupana, P. sorbilis; also known as Brazilian cocoa and ‘zoom’. J Prim Health Care. 2012;4:163-164. [PubMed] [Google Scholar]
- 85. World Anti-Doping Agency. The code. https://www.wada-ama.org/en/what-we-do/the-code. Accessed February 6, 2017.
- 86. World Anti-Doping Agency. 2015 Anti-doping testing figures. 2015. https://www.wada-ama.org/sites/default/files/resources/files/2015_wada_anti-doping_testing_figures_report_0.pdf. Accessed February 6, 2017.
- 87. World Anti-Doping Agency. 2017 List of prohibited substances and methods. 2017. https://www.wada-ama.org/en/prohibited-list. Accessed January 26, 2017. [DOI] [PubMed]
- 88. Young A. Bill seeks to close loophole for anabolic steroids. USA Today. February 11, 2014. [Google Scholar]