Abstract
One of the serious obstacles of the aortopathies research is a considerable shortage of human aortic smooth muscle cells (SMCs), which can be used to model the disease. SMC in most cases come from the whole aorta of transplant donors, which are rather difficult to access. In the course of coronary artery bypass graft (CABG) surgery, a fragment of aortic tissue is excised to make a bypass root. In this study, we show a possibility to use CABG leftover fragments of thoracic aorta as a source of human SMC for in vitro research. We isolated SMC from the fragments of aortic tissues obtained during CABG procedure and compared these cells to the cells that were isolated from aortic tissue of transplant donors. The content of key SMC contractile markers (SMA, SM22α, and vimentin) as well as proliferation and migration rates, metalloproteases MMP-2 and MMP-9 activities were similar in CABG-derived SMC and in transplant donor–derived SMC. In conclusion, leftovers of ascending thoracic aorta obtained during CABG can be used as a source of human aortic SMCs for in vitro research.
Keywords: coronary artery bypass graft, aorta, smooth muscle cells, human
Introduction
Smooth muscle cells (SMCs) are the main functional units of the aortic wall and are responsible for the aortic wall integrity because of its contractile properties and their ability to produce extracellular matrix.1 Human aortic SMCs derived from transplant donors are mostly used in the research of the cellular mechanisms of aortic pathology. However, aortic tissues derived from healthy donors are quite difficult to obtain in a research laboratory. Besides, the donors usually have younger age (30 to 50) compared to the patients with aortic pathologies (50 and more). Coronary artery bypass graft (CABG) surgery is conducted in order to decrease the risk of mortality from coronary artery disease and heart failure.2 The CABG technique consists of harvesting arteries or veins of the patient and grafting them into aortic wall in order to bypass atherosclerotic narrowing and to improve the supply of blood to the myocardium.3 During this procedure, a small piece of aortic tissue is excised to make a bypass. We show here that it is possible to obtain SMC culture in vitro from these aortic leftovers. Comparison of functional properties of CABG-derived SMC with SMC derived from transplant donor showed a high level of similarities. Thus, we propose a new source of SMC for functional studies of aorta, which could be used in particular in thoracic aortic aneurysm research and in the research of cellular mechanisms of other aortic pathologies.
Materials and Methods
The clinical research protocol was approved by the local ethical committee of the Almazov Federal Medical Research Center and was in accordance with the principle of the Declaration of Helsinki. All patients gave informed consent. Samples of the thoracic aorta were harvested during coronary artery bypass graft at the Almazov Federal Medical Research Center.
Specimens were sampled from the patients undergoing CABG (n = 10). Tissue samples were obtained from CABG surgical cases from male patients with stenosis of a. descendance and a. circumflexus. The average patient age was 64 ± 7 (sochinila). All patients had no prior myocardial infarcation and had a scheduled surgery with no signs of acute coronary syndrome. Control aortic specimens were obtained from transplant donors aged 39 ± 7. All tissues were sampled from the outer curvature of the thoracic aorta.
Primary Cultures
To obtain SMC cultures, the cells were isolated as previously described4 with modifications.5 The fragment of aortic tissue was excised during CABG procedure and put into a sterile tube containing 5 mL of phosphate-buffered saline (PBS) with penicillin/streptomycin (Thermo scientific, Waltham, MA). The specimen was kept at +4° C up to 24 h before further proceeding. Under sterile conditions in cell culture hood, the postoperative tissue fragments were put into 10 cm Petri dish, washed several times with PBS, and then dissected away from the adventitia and chopped with glass scissors until the size of the fragments was not more than 1 mm3. After washing in PBS, the tissue fragments were first incubated for 90 min at 37° C in DMEM containing 0.1% collagenase (Collagenase, Type 3, Worthington Biochemical Corporation, Lakewood, NJ, USA). Then tissue samples were put in culture medium (Dulbecco’s modified eagle’s medium [DMEM] 20% fetal bovine serum, 1% glutamine, 1% penicillin/streptomycin, all reagents were from Invitrogen, Waltham, MA, USA). The surface of culture flask was scratched by forceps. The tissue fragments were placed onto a scratched culture 25 cm2 or 12.5 cm2 flask (Corning, Munich, Germany) in a minimal volume of culture medium covering the surface. After 5 to 6 d, bigger fragments were detached from the flask and washed out, whereas small fragments tended to stay attached and gave the population of migrating cells. The flasks with tissue fragments were left for 4 to 5 wks. DMEM culture medium was supplemented with 20% fetal bovine serum, 1% glutamine, and 1% penicillin/streptomycin (Invitrogen, USA). The medium was very gently changed every second day with a 1000P pipette tip, allowing the fragments staying attached and the cells migrate along the scratches on the plastic. SMC firstly emerged from tissue explants at 5th to 10th d of in vitro culture. After 4 to 5 wks, SMC formed confluent monolayer and were reseeded using 0.125 g/L trypsin solution on Petri dishes and routinely cultured up to 5th to 6th passages. In the experiments, the cells of 2 to 5 passages were used.
Human umbilical vein endothelial cells (HUVECs), skin fibroblasts, and adipose tissue–derived mesenchymal stem cells (MSCs) were used for comparison in immunohistochemical staining experiments. All types of the cells were obtained by standard methods described previously from umbilical cords, skin biopsy, and adipose tissue biopsies correspondingly in the Biobank of Almazov Federal Medical research Centre6,7
Cell Migration Assay
Cell migration was determined using a “scratch” wound assay as described previously.5 SMCs were seeded at a density of 105 cells in a 6-well plate grown to confluence on 6-well plates; after the cells formed a monolayer, the medium was exchanged for serum-free medium containing 10 mM hydroxyurea (Sigma, Munich, Germany) and10 ng/mL platelete-derived growth factor (PDGF-BB) (Peprotech, Rocky Hill, NJ) growth factor to inhibit proliferation and to stimulate migration; the cell monolayer was scraped with a 200P pipette tip to create a cell-free zone. The number of cells, which migrated into the wounded area, was counted using microscopic images after 6 and 24 h. Three independent images were counted per 1 time point per cell line. Experiments were performed in triplicates.
Cell Proliferation Assay
Cell proliferation rate was determined as follows. The cells were seeded at 24-well plates at a density of 10,000 cells per well and then counted every second day of culture in duplicates in Neubauer chamber for 4 independent cultures. Experiments were performed in triplicates.
Immunocytochemistry
For immunocytochemical staining, the cells were grown on round cover slides with diameter of 15 mm (Carl Roth, Germany) in the Petri dishes until 70% to 80% of confluency. Then the cells were washed 2 times with PBS and fixed with 1% Paraformaldehyde (Sigma, Munich, Germany) in PBS for 10 min at room temperature. Then the slides were washed 3 times with PBS, blocked for 30 min with PBS containing 1% bovine serum albumin (Applichem, Germany), and then a primary antibody diluted in PBS containing 1% bovine serum albumin (Applichem, Germany) was added for 1 h at room temperature. The slides were washed 3 times with PBS and a secondary antibody diluted in PBS containing 1% bovine serum albumin (Applichem, Germany) was added for 1 h at room temperature. After washing 3 times in PBS, the slides were quickly washed in 4’,6-diamidino-2-phenylindole (DAPI; Sigma, Munich, Germany) diluted 1:10000 in PBS to visualize nuclei. Then the following primary antibodies were used: alpha smooth muscle actin, or SMA(sc-32251, Santa Cruz Biotechnology, Dallas, TX, USA, dilution 1:300); SM22alpha(ab14106, Abcam, USA, dilution 1:100), and vimentin (sc-6260, Santa Cruz, USA, dilution 1:200). Secondary antibodies conjugated with Alexa488 or Alexa546 (Invitrogen, USA) were used in 1: 1000 dilution. DAPI was used to visualize nuclei. Microphotographs were taken using AxioObserver Microscope (Zeiss) at 20× and 40× magnification with AxioVision software (version 3.0).
Histochemistry
Aortic tissue fragments were embedded into cryopreservation medium TissueTec and frozen in liquid nitrogen; the cryosections have been made using cryostate (Thermo Scientific, USA) and stained with hematoxylin/eosin kit (Leica) by a standard technique. Microphotographs were taken using AxioObserver Microscope (Zeiss) at 20× magnification with AxioVision software.
Zymography
MMP activity was assayed by a modified gelatin zymography method.8 Activity and content of MMP-2 and MMP-9 were expressed in QuantiScan arbitrary units.
Statistical Analysis
Groups were compared using the Mann–Whitney nonparametric test. A P value of ≤0.05 was considered significant.
Results and Discussion
We examined histologically aortic tissue fragments that derived from CABG surgery (Fig. 1). Thoracic aortic wall of CABG patients had normal structure with no dissection, necrosis, or atherosclerotic plaques.
Fig. 1.
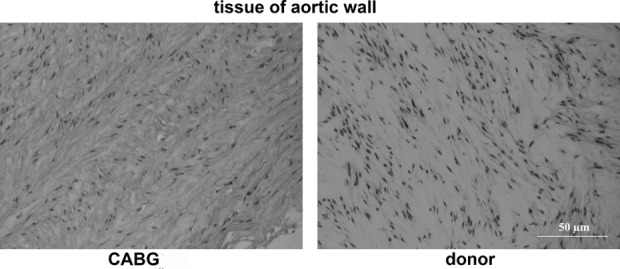
Thoracic aortic wall of a coronary artery bypass graft (CABG) patient in comparison to thoracic aortic wall of a donor (donor). Hematoxylin/eosin staining; magnification 20×. No visible structural anomalies are seen in the aortic wall of CABG patient.
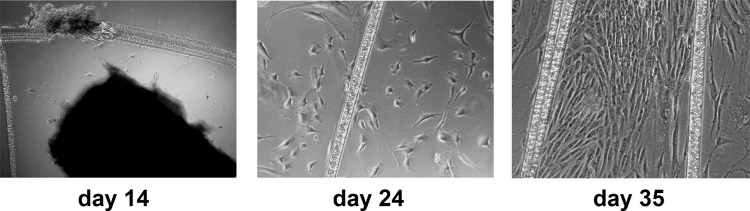
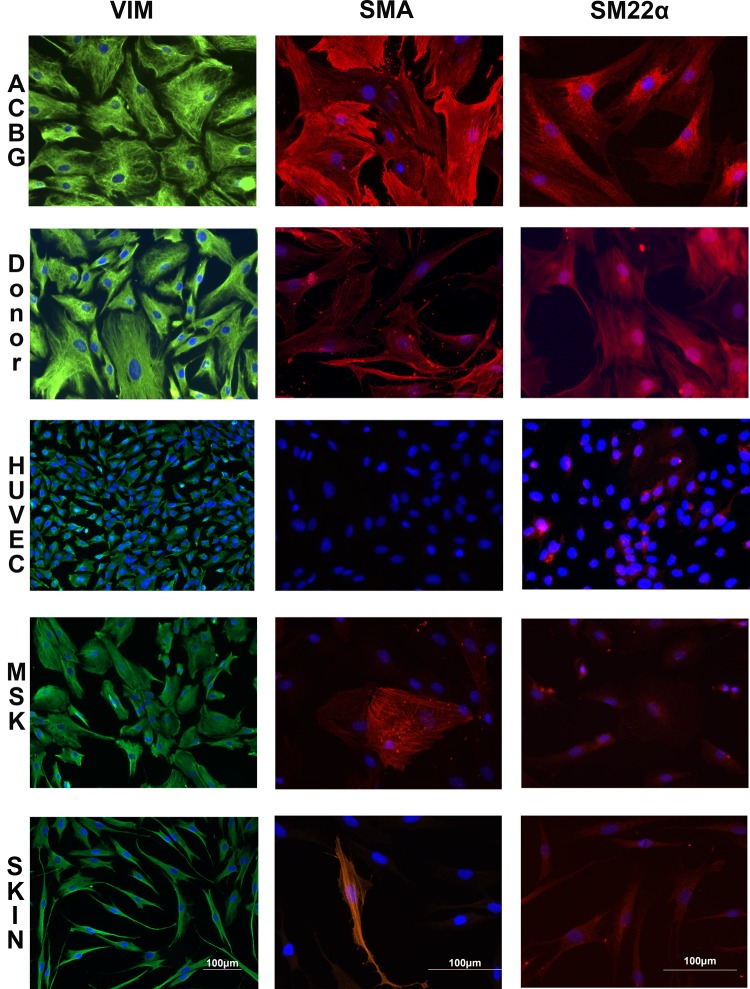
Figure 2 demonstrates the stages of SMC migration from a fragment of aortic tissue derived from a CABG patient. After 4 to 5 wks, the cells reached confluence and it was possible to passage them for 4 to 6 passages. To verify that the cells were SMCs, we stained them for contractile markers of SMC: vimentin, SM22α, and αSMA (Fig. 3). For comparison, we used several primary cell lines that do not contain SMCs: HUVEC, skin fibroblasts, and adipose tissue–derived MSCs. CABG-derived cells demonstrated the pattern of staining for contractile markers similar to that seen in the SMC derived from transplant donor–derived SMC (Fig. 3). None of the cultures used as comparison demonstrated similar staining pattern. These data suggested that the cultures that had been obtained from CABG leftovers were indeed SMCs.
Fig. 2.
Stages of smooth muscle cell (SMC) culture from aortic explants derived from coronary artery bypass graft (CABG) patients. A phase contrast microscope image, magnification 40×. The cells migrating from the aortic patch along the scratches are seen.
Fig. 3.
The cells derived from coronary artery bypass graft (CABG) patient express markers of smooth muscle cell (SMC) similar to the cells derived from the aortic tissue of the donors. Human umbilical cord endothelial cell (HUVEC), skin fibroblasts, and adipose tissue–derived mesenchymal stem cells (MSCs) that do not contain SMCs were used for comparison. VIM, vimentin; α-SMA, alpha smooth muscle actin. Fluorescent microscope images; bars indicate magnification.
Next, we looked at proliferative rate, as it is an important parameter of primary cell cultures. CABG-derived SMC had proliferation rate slightly lower than that of donor-derived SMC (Fig. 4); however, they proliferated until passages 5 to 6 similar to donor-derived SMC.
Fig. 4.
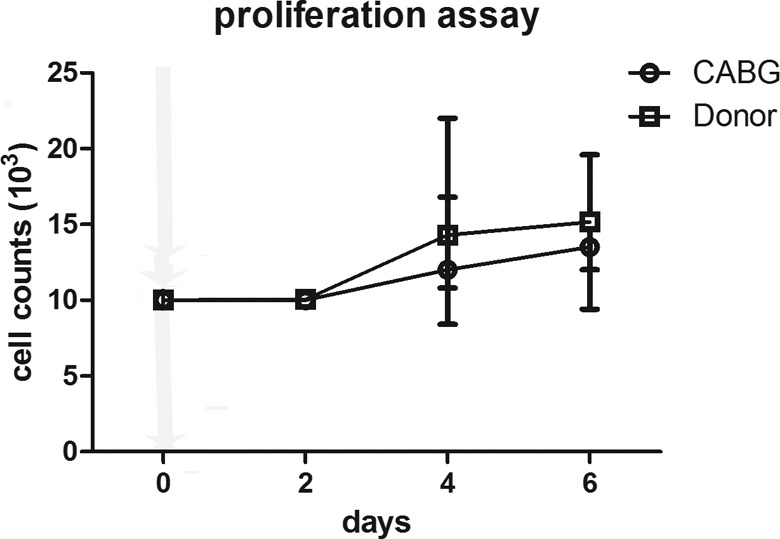
Proliferation assay. The cells derived from coronary artery bypass graft (CABG) patient have similar proliferation rate to the cells derived from the aortic tissue of the donors. The cells were seeded at identical numbers and then were counted every second day. Each point represents a mean for 4 independent cultures. Bars represent standard deviation.
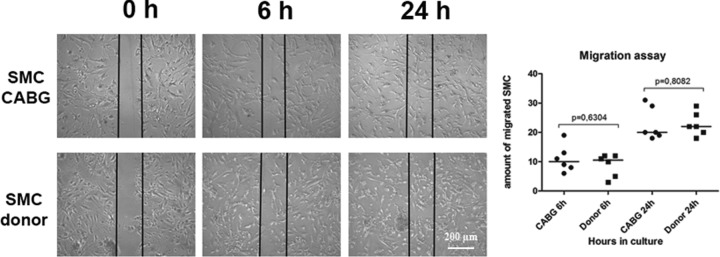
Migration ability is an important characteristic of SMC and is used to estimate functional potential of SMC, for example, in the aortic aneurysm research.5 Therefore, we compared the migration rate of CABG-derived SMC and that of donor-derived SMC. Migration rate of CABG SMC was similar to that of donor SMC (Fig. 5).
Fig. 5.
Migration assay. The cells derived from coronary artery bypass graft patient (CABG, n = 6) have similar migration rate comparing to the cells derived from the aortic tissue of the donors (n = 6). The groups are compared using Mann–Whitney nonparametric test. The lines represent the median.
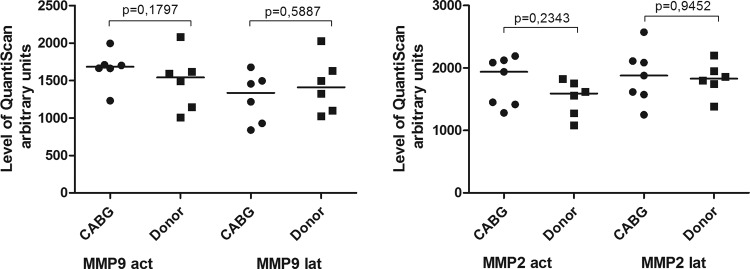
Activity of various metalloproteases is an essential characteristic of smooth muscle cell functionality.9 For example, SMCs derived from the patients with thoracic aortic aneurysm associated with disintegration of aortic wall due to SMC dysfunction have significantly higher activity of MMP9.5 CABG-derived SMC and donor-derived SMC had similar MMP2 and MMP9 content and activity (Fig. 6).
Fig. 6.
MMP-2,9 activity in coronary artery bypass graft (CABG) smooth muscle cell (SMC) and donor SMC. Latent and active form of MMP-2 and MMP-9 activity was measured by zymography in the supernatants from corresponding cell cultures. The cells derived from CABG patient ( n = 7) have similar MMP activity levels to the cells derived from the aortic tissue of the donors (n = 6). The groups are compared using Mann–Whitney nonparametric test. The lines represent the median.
In conclusion, we show here the possibility to obtain functionally normal SMCs from tiny pieces of aortic wall that rest at CABG surgery. To our knowledge, this is the first report of such a source of human SMC. We consider this an important finding in several terms. These SMCs represent population that comes from aged patients, which is most often the case when the diseased state is studied, for example, in the case of aortic aneurysm, where the median age is 60 and more, whereas transplant donors are mostly younger with median age around 40. Second important suggestion is that when aortic SMC come from transplant donor aortas, the whole thoracic aorta is used to obtain the cell culture, especially in commercially available cultures, while it has been shown that SMCs, which come from different regions of aorta, are functionally different.10 CABG-derived SMCs come from ascending part of the aorta, and thus the cells are in particular suitable for the research and modeling the cellular mechanisms of thoracic/ascending aortic aneurysm and other pathologies of ascending aorta.
This study has several important limitations. Fragments of CABG-derived aortic tissue are quite small, usually not more, than 5 mm3. This limits the amount of cells that could be derived from each patient. However, it is possible to pull up to 20 tissue fragments from different patients together and then to treat them and let growing to confluency in the flask of 25 cm2. This pulling speeds up the process of SMC migration from aortic tissue. It is also possible to harvest the cells from the tissue fragments in a period of up to 72 h after surgery. Some tissue fragments fail to give the good cultures. This feature mainly depends on the age of a patient, and the minimal possible age of a CABG patient has to be chosen. To our knowledge, the success in cell isolation is restricted by age of 80. In our practice of deriving more than 100 cell cultures from CABG leftovers, we noticed transformation of cell culture only once. This, however, should also be taken into account, and obviously transformed cells that actively proliferate after passage 7 should be discarded.
Aortic wall includes different types of cells, while to our experience, fragments from CABG were suitable only for getting SMC but not endothelial cells.
Footnotes
Ethical Approval: The clinical research protocol was approved by the local ethical committee of the Almazov Federal Medical Research Center and was in accordance with the principle of the Declaration of Helsinki.
Statement of Human and Animal Rights: Specimens were sampled by consent from the patients undergoing CABG.
Statement of Informed Consent: All patients gave informed consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by “ITMO University”, grant agreement No. 715791.
References
- 1. Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet. 2008;9:283–302. [DOI] [PubMed] [Google Scholar]
- 2. Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diodato M, Chedrawy EG. Coronary artery bypass graft surgery: the past, present, and future of myocardial revascularisation. Surgery research and practice 2014;2014:726158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirschenlohr HL, Metcalfe JC, Grainger DJ. Cultures of proliferating vascular smooth muscle cells from adult human aorta. Methods Mol Med. 1996;2:319–334. [DOI] [PubMed] [Google Scholar]
- 5. Malashicheva A, Kostina D, Kostina A, Irtyuga O, Voronkina I, Smagina L, Ignatieva E, Gavriliuk N, Uspensky V, Moiseeva O. Phenotypic and functional changes of endothelial and smooth muscle cells in thoracic aortic aneurysms. Int J Vasc Med. 2016;2016(Article ID 3107879):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kostina AS, Uspensky VE, Irtyuga OB, Ignatieva EV, Freylikhman O, Gavriliuk ND, Moiseeva OM, Zhuk S, Tomilin A, Kostareva AA, et al. Notch-dependent EMT is attenuated in patients with aortic aneurysm and bicuspid aortic valve. Biochim Biophys Acta. 2016;1862(4):733–740. [DOI] [PubMed] [Google Scholar]
- 7. Malashicheva A, Bogdanova M, Zabirnyk A, Smolina N, Ignatieva E, Freilikhman O, Fedorov A, Dmitrieva R, Sjöberg G, Sejersen T. Various lamin A/C mutations alter expression profile of mesenchymal stem cells in mutation specific manner. Mol Gen Metab. 2015;115(2):118–127. [DOI] [PubMed] [Google Scholar]
- 8. Oliver GW, Stetler WG, Kleiner DE. Zymography, casein zymography, and reverse zymography: activity assays for proteases and their inhibitors In: Sterchi EE, Stöcker W, editors. Proteolytic Enzymes. Berlin: Springer Verlag; 1999; p. 63–76. [Google Scholar]
- 9. Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. 2013;2012:928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27(6):1248–1258. [DOI] [PubMed] [Google Scholar]