Abstract
Given recent progress in regenerative medicine, we need a means to expand chondrocytes in quantity without losing their regenerative capability. Although many reports have shown that growth factor supplementation can have beneficial effects, the use of growth factor–supplemented basal media has widespread effect on the characteristics of chondrocytes. Chondrocytes were in vitro cultured in the 2 most widely used chondrocyte growth media, conventional chondrocyte culture medium and mesenchymal stem cell (MSC) culture medium, both with and without fibroblast growth factor-2 (FGF2) supplementation. Their expansion rates, expressions of extracellular matrix–related factors, senescence, and differentiation potentials were examined in vitro and in vivo. Our results revealed that chondrocytes quickly dedifferentiated during expansion in all tested media, as assessed by the loss of type II collagen expression. The 2 basal media (chondrocyte culture medium vs. MSC culture medium) were associated with distinct differences in cell senescence. Consistent with the literature, FGF2 was associated with accelerated dedifferentiation during expansion culture and superior redifferentiation upon induction. However, chondrocytes expanded in FGF2-containing conventional chondrocyte culture medium showed MSC-like features, as indicated by their ability to direct ectopic bone formation and cartilage formation. In contrast, chondrocytes cultured in FGF2-supplemented MSC culture medium showed potent chondrogenesis and almost no bone formation. The present findings show that the chosen basal medium can exert profound effects on the characteristics and activity of in vitro–expanded chondrocytes and indicate that right growth factor/medium combination can help chondrocytes retain a high-level chondrogenic potential without undergoing hypertrophic transition.
Keywords: hyaline cartilage, chondrocytes, dedifferentiation, differentiation, in vitro expansion
Introduction
Cartilage tissues show low cellularity and are among the few tissues that lack blood vessels. When damaged, therefore, cartilage undergoes limited repair due to the lack of support from blood vessels or nearby cells. As cartilage can accordingly benefit from tissue-engineering approaches, numerous studies have sought to maximize cartilage tissue regeneration through the application of biocompatible scaffolds with stem cells or chondrocytes. The use of mesenchymal stem cells (MSCs) for cartilage regeneration, however, has been limited by their tendency to undergo hypertrophic transition and/or calcification.1 Other approaches have employed cell- or tissue-engineering-based procedures, including first- or second-generation autologous chondrocyte implantation, in which the patient’s own chondrocytes are used to repair acutely damaged articular cartilage in young patients.2–4
The abovementioned cell-based therapies and tissue-engineering applications require large quantities of chondrocytes. Unfortunately, however, there are significant limits on the size of donor tissues available from biopsy and the number of chondrocytes that may be isolated from such tissues. We previously introduced costal cartilage as a new source of chondrocytes for autologous hyaline cartilage regeneration and confirmed that hyaline cartilage cells engineered from this source were comparable to chondrocytes obtained from articular cartilage in terms of their cell yield, in vitro expansion, and differentiation characteristics.5 Numerous other reports support the idea of using chondrocytes from costal cartilage as an ideal cell source for articular cartilage regeneration.6–12 However, the initial cell yield, extent of in vitro expansion, and chondrogenic capacity of human chondrocytes have been reported to decrease with donor age.5,13–15 Therefore, despite the recent advancements in cartilage tissue engineering, it remains difficult to secure a sufficient supply of chondrocytes.
The in vitro expansion of chondrocytes is also intrinsically associated with their dedifferentiation, wherein the differentiation potential is progressively lost as the number of cell passages increases.16 Several previous reports showed that chondrocytes lose their chondrocytic phenotype during in vitro expansion, instead exhibiting a fibroblast-like morphology characterized by decreased type II collagen expression and increased type I collagen expression. Such chondrocytes tend to lose the functionality needed for tissue reconstruction and/or regeneration. Thus, as the passage number increases, in-vitro-expanded human articular chondrocytes become dedifferentiated and lose their ability to produce well-differentiated cartilage tissue.17–19 The chondrogenic differentiation potential of in-vitro-expanded human articular chondrocytes can also be predicted based on population doublings (PDs), as they reportedly lose their intrinsic capacity for chondrogenesis at around 3.57 to 4.19 PDs.20
The addition of growth factors has been shown to increase the extent of in vitro expansion and support the retention of differentiation among cultured chondrocytes. For example, the addition of fibroblast growth factor-2 (FGF2) to the culture medium was shown to induce a rapid but reversible dedifferentiation during the in vitro expansion period, followed by cartilage-specific gene expression and matrix accumulation upon the induction of differentiation.18,21–23 However, although growth factors are known to help chondrocytes retain their differentiation potential during in vitro expansion, few previous studies have investigated how the chosen basal medium affects this process.
Here, we applied the 2 most widely used chondrocyte growth media, with and without FGF2, and compared the characteristics, quality, and differentiation potential of the in vitro–expanded chondrocytes. Indeed, we found that the basal medium exerts profound effects on the tested parameters of in vitro–expanded chondrocytes. These results demonstrate that the influence of the basal medium cannot be ignored if we hope to produce highly active and functional chondrocytes for cartilage regeneration.
Materials and Methods
Isolation and Expansion of Costal Chondrocytes
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of the Biosolution Research Institute (MCTTIACUC ASP 09-002). Costal cartilage chondrocytes (CCs) were isolated as previously described5 from 5 New Zealand white rabbits (4 to 5 months old; Cheonan Yonam College, Korea). Briefly, the soft adhering tissues (including the perichondrium) were removed, the costal cartilage was minced into 1 to 2 mm3 pieces, and the cartilage tissue fragments were digested overnight in an enzyme cocktail solution containing collagenase D (2 mg/mL), hyaluronidase (1 mg/mL), and DNase (0.75 mg/mL) at 37 °C with 5% CO2. The enzymes were purchased from Roche Diagnostics (Mannheim, Germany). The viable cells were detected by trypan blue exclusion and counted under a hemocytometer, and equal amounts of cells were separated into 4 groups. Cells of the D group (CCs-D) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Hyclone, Waltham, MA, USA). Cells of the M group (CCs-M) were cultured in MSC growth medium (MSCGM; Lonza, Walkersville, MD, USA). Cells of the DF group (CCs-DF) were cultured in DMEM with 10% FBS and FGF2 (1 ng/mL; R&D Systems, Minneapolis, MN, USA). Cells of the MF group (CCs-MF) were cultured in MSCGM with FGF2 (1 ng/mL). Cells were plated at 1 × 104 cells/cm2 to culture dishes containing the appropriate medium. The medium was changed twice a week, and confluent primary cells were subcultured up to passage 8.
Isolation and Expansion of Bone Marrow–Derived Mesenchymal Stem Cells (BM-MSCs)
Rabbit BM-MSCs were obtained by femoral puncture followed by bone marrow aspiration. Red blood cells were removed by Ficoll gradient centrifugation (GE Healthcare Life Sciences, IL, USA), and the mononuclear cell fraction was directly plated to a culture dish containing MSCGM and cultured at 37 °C with 5% CO2. On day 2 of culture, the nonadherent cells were removed by repeated washes with phosphate-buffered saline (PBS). The adherent cells were cultured until obvious fibroblastic colonies became visible. When the cells were approximately 80% confluent, the adherent cells were trypsinized and subcultured. BM-MSCs between passages 2 and 6 were used for our in vitro and in vivo experiments.
Cell Senescence Assay
Cells were fixed and senescence-associated β-galactosidase (SA-β-gal) expression was assessed using a Senescence Assay Kit (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. Briefly, the cells were fixed, treated with the SA-β-gal substrate solution, and incubated overnight at 37 °C. The development of a blue color was observed under phase-contrast microscopy. The fraction of cells showing β-gal activity (positive for the blue color) was normalized to the total number of cells.
Western Blot Analysis
CCs-D or CCs-M were collected at passages 1 to 4, lysed with lysis buffer containing 2% sodium dodecyl sulfate (SDS), resolved by SDS-PAGE (poly-acrylamide gel electrophoresis), and transferred to a nitrocellulose membrane overnight at 250 mV. The membrane was blocked with 5% skim milk, incubated for 90 min with antibodies against type I collagen (Southern Biotech Associates, Birmingham, AL, USA; cat. # 1310-08, polyclonal) or α-tubulin (Sigma-Aldrich, St. Louis, MO, USA; cat. # T5168, monoclonal, clone # B-5-1-2), and developed with a Chemiluminescence Detection Kit (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The expression level of type I collagen was normalized with respect to that of α-tubulin.
Immunological Staining
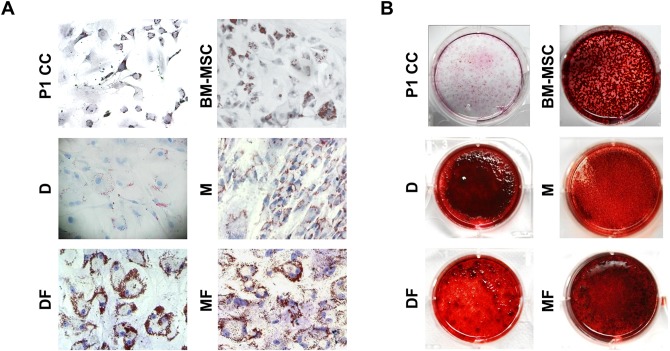
Chondrocytes cultured in D, M, DF, or MF (CCs-D, CCs-M, CCs-DF, or CCs-MF) were plated on cover slips, cultured for 2 d, fixed with 3.7% formalin in PBS, and permeabilized with 0.2% Triton X-100. For immunohistochemical (IHC) staining, anti-type I collagen antibody (Southern Biotech Associates) was applied to the cover slips at 4 °C overnight, and the results were developed with 0.1% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Vector Laboratories, Burlingame, CA, USA) in PBS for 5 min. Nuclei were counterstained with hematoxylin. For immunofluorescence staining, cover slips were blocked with 20% normal goat serum and then reacted with anti-type II collagen (Millipore; cat. # MAB8887, monoclonal, clone # 6B3), anti-α-SMA (smooth muscle actin) (DAKO, Glostrup, Denmark; cat. # M0851, monoclonal, clone # 1A4), anti-CD41 (Abcam, Cambridge, MA, USA; cat. # ab63983, polyclonal), or anti-CD29 (DAKO; cat. #M0889, monoclonal, clone # K20). Fluorescein isothiocyanate–labeled goat anti-mouse IgG (Vector Laboratories) was used as a secondary antibody, and nuclei were counterstained for 5 min with 4′,6-diamidino-2-phenylindole.
Multipotent Differentiation Capacities of Dedifferentiated CCs In Vitro
To test the multipotent differentiation capacities of the dedifferentiated CCs in vitro, we used cells of passage 8 from each medium group. To assess the adipogenic differentiation capacity, 2 × 104 cells/cm2 were cultured for 21 d in DMEM supplemented with 500 µM 3-isobutyl-1-methylxanthin, 0.2 mM indomethacin, 100 nM dexamethasone, 10 μg/mL insulin, and 10% FBS (all supplements from Sigma-Aldrich) and then stained with oil red O for detection of fat droplets. To assess the osteogenic differentiation capacity, 2 × 104 cells/cm2 were cultured for 28 d in high-glucose DMEM supplemented with 10 mM β-glycerol phosphate (Sigma), 100 nM dexamethasone, 50 µg/mL ascorbic acid (Sigma), and 10% FBS and then stained with 40 mM alizarin red for detection of calcium deposits. To assess the chondrogenic differentiation capacity, 1 × 106 cells were cultured in 15 mL polystyrene tubes containing DMEM supplemented with 1% ITS+3 Liquid media supplement (Sigma), 100 nM dexamethasone, 50 µg/mL ascorbic acid, 40 µg/mL proline (Sigma), and 10 ng/mL transforming growth factor (TGF)-β (ProSpec TechnoGene, East Brunswick, NJ, USA). After 14 d, the cells were pelleted, the pellets were sectioned (5 µm thickness), and the sections were stained with safranin O and fast green for morphological examination and to detect glycosaminoglycan (GAG) expression. CCs of passage 1 cultured in D, and BM-MSCs were used as negative and positive controls, respectively, for multipotency. Apoptotic cells contained in the chondrogenic pellets were visualized using a TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) Apoptosis Detection Kit (Millipore) according to the manufacturer’s instructions.
Ectopic Bone Formation In Vivo
CCs-D, CCs-M, CCs-DF, or CCs-MF of passage 8 (2 × 106 cells) were loaded to 40 mg aliquots of hydroxyapatite/tricalcium phosphate particles (HA-TCPs; Biomatlante, France) and incubated for 1 h at 37 °C with gentle mixing. BALB/c nude mice (7 to 8 wk; Orient Bio, Korea) were anesthetized, and 2 small transverse incisions were made along the dorsum of each mouse’s back. Left and right blunt dissections under the skin were performed to form 4 pockets for implants, and the cell-loaded HA-TCPs were inserted subcutaneously. At 8-, 12-, and 16-wk postimplantation, the implants were harvested (n = 3), fixed with formaldehyde, and decalcified with Calci-Clear Rapid (National Diagnostics, Atlanta, GA, USA). Thin paraffin sections (6 μm) were obtained and stained with hematoxylin and eosin. Five micrographs were taken from each section using a digital camera connected to a light microscope (magnification, 100×). Histological features were quantified using the Image J software Version 1.48 (National Institutes of Health, Bethesda, USA), and the percentage of cartilaginous or osseous matrix versus the total interstitial tissue was calculated. For IHC staining of rabbit type I collagen, sections were permeabilized with 0.2% Triton X-100 in PBS, incubated with 0.2% hyaluronidase at 37 °C for 1 h, exposed to the primary antibody, and developed with 0.1% DAB in PBS for 5 min. Fast red dye was used for counterstaining. BM-MSCs were used as a positive control for ectopic bone formation.
Ability of MF-Cultured Dedifferentiated CCs to Repair Rabbit Articular Cartilage Defects
To assess the ability of fully dedifferentiated CCs-MF to repair a cartilage defect, we used a rabbit full-thickness cartilage defect model. Autologous CCs from 12 male rabbits aged 4 to 5 months old (2.5 kg in weight) were isolated and expanded in MF as described above. Each rabbit was anesthetized, and a 5-mm diameter defect was created on the patellar groove of the femur using a low-speed drill (1.5 mm in depth). CCs-MF of passage 8 (1 × 106 cells/site) were mixed with 20 μL of fibrin glue (FG; Green Cross, Korea) and transplanted into the defect (CCs-MF group). A drop of FG was applied as a control (defect control group). No cast was applied, and the rabbits were allowed to move freely after recovering from anesthesia. At 6- and 12-wk posttransplantation, the rabbits (7- to 8 month old; 3.5 to 4 kg in weight) were sacrificed (n = 6), and the gross morphology of each knee was examined for color, integrity, contour, and smoothness. Specimens obtained from the transplanted areas were photographed, dissected, fixed with 10% buffered formalin, and decalcified using Calci-Clear Rapid. Paraffin sections (6 µm thick) were deparaffinized and stained with safranin O and fast green for examination of histological features and GAG expression. For IHC staining, sections were treated as described above and then reacted with antibodies against type I collagen (Southern Biotech Associates), type II collagen (Millipore), or aggrecan (R&D Systems; cat. # MAB1220, monoclonal, clone # 179509). For histological grading, sections corresponding to the center of each defect were selected by comparison of several adjacent sections. Images of whole sections were subjected to blind evaluation by 2 investigators using the histological grading scale described by Wakitani et al.,24 which comprises 5 categories, each of which is scored from 0 (normal cartilage) to 14 (no repair tissue; Table 1).
Table 1.
Histological Grading Scale for Cartilage Defects.
| Category | Points | |
|---|---|---|
| Cell morphology | Hyaline cartilage | 0 |
| Mostly hyaline cartilage | 1 | |
| Mostly fibrocartilage | 2 | |
| Mostly noncartilage | 3 | |
| Noncartilaginous tissue | 4 | |
| Matrix staining (metachromasia) | Normal (compared with host adjacent cartilage) | 0 |
| Slightly reduced | 1 | |
| Significantly reduced | 2 | |
| No metachromatic stain | 3 | |
| Surface regularity | Smooth (>3/4a) | 0 |
| Moderate (1/2 < 3/4a) | 1 | |
| Irregular (1/4 < 1/2a) | 2 | |
| Severely irregular (<1/4a) | 3 | |
| Thickness of the cartilage | 0 > 2/3b | 0 |
| 1/3 < 2/3b | 1 | |
| <1/3b | 2 | |
| Integration of donor with host adjacent cartilage | Both edges integrated | 0 |
| One edge integrated | 1 | |
| No integration | 2 | |
| Total maximum | 14 | |
aTotal smooth area of reparative cartilage compared with the whole area of the cartilage defect.
bAverage thickness of reparative cartilage compared with that of surrounding cartilage.
Statistical Analysis
All experiments were repeated at least 3 times unless otherwise indicated in the Methods and Materials section or the figure legends. The differences across all medium groups were analyzed by analysis of variance; this analysis yielded P < 0.0001, indicating that the difference was highly significant. Between-group differences were evaluated using the paired t-test, with P < 0.05 taken as reflecting a significant difference.
Results
Proliferation Potential of CCs Cultured as Monolayers in D, M, DF, and MF
To examine the effect of different culture conditions on the proliferation of primary-cultured chondrocytes, we compared accumulative cell growth among CCs maintained in conventional chondrocyte growth medium (D) or commercialized MSCGM (M), each with or without FGF2 supplementation. As shown in Fig. 1 and Table 2, we observed large differences in the growth of CCs cultured in the various media: the D and M groups showed almost no proliferation after passages 4 and 6, respectively, whereas CCs grown in the presence of FGF2 exhibited proliferation until passage 8 in both media. At passage 8, the cell numbers of the D, M, DF, and MF groups had cumulatively increased by 103-, 5 × 104-, 105-, and 107-folds, respectively, compared with the initial cell numbers (Fig. 1A). The growth rate ratios between cells of the MF and D groups were approximately 115- and 14,495-fold at passages 4 and 8, respectively. Comparison of the total PDs at passage 8 confirmed that the chondrocytes of the various groups varied in their tendency for proliferation: Chondrocytes persisted beyond 8 PD only in the presence of FGF2, and M group induced more PDs than D group regardless of the presence of FGF2 (Fig. 1B).
Fig. 1.
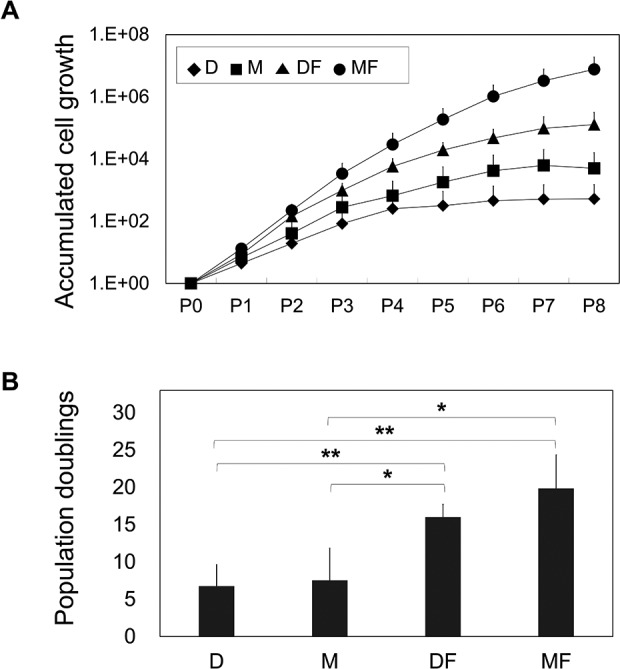
Comparison of cell expansion. (A) Chondrocytes cultured in various media were compared up to passage 8 (n = 5). Accumulated cell growth is shown as the fold difference relative to the number of cartilage chondrocytes obtained by primary culture. The growth rate was calculated by dividing the number of cells recovered at each passage by the number of cells seeded, and the results are plotted in log scale. (B) Total population doublings (PDs) at passage 8 (n = 5) were calculated as follows: PD = log2 (growth rate). *P < 0.05; **P < 0.005.
Table 2.
Population Doubling Time (PDT) at Each Passage.
| Media types p0 | p1 | p2 | p3 | p4 | p5 | p6 | p7 | |
|---|---|---|---|---|---|---|---|---|
| D | 1.96 ± 0.51 | 2.86 ± 0.50 | 2.76 ± 0.58 | 3.14 ± 1.04 | 48.04 ± 46.29 | – | – | – |
| M | 1.82 ± 0.70 | 2.80 ± 2.49 | 2.51 ± 8.55 | 6.96 ± 9.30 | 9.79 ± 24.36 | 11.92 ± 29.81 | 68.25 ± 11.97 | – |
| DF | 1.68 ± 0.24 | 1.10 ± 0.29a** | 1.30 ± 0.44a** | 1.37 ± 0.46a*, b* | 2.19 ± 0.53 | 3.87 ± 1.71 | 6.18 ± 3.04b** | 12.66 ± 4.88 |
| MF | 1.45 ± 0.31 | 1.21 ± 0.52a** | 1.02 ± 0.99a* | 1.30 ± 1.20a* | 1.64 ± 0.82c* | 1.88 ± 0.82 | 2.96 ± 1.43b** | 5.95 ± 1.16c* |
Note: PDT was calculated from the mean culture time and the mean cell expansion fold at each cell passage (n = 5).
aSignificance compared with group D.
bSignificance compared with group M.
cSignificance compared with group DF.
*P < 0.05.
**P < 0.005.
Cellular Characteristics of CCs Cultured in D, M, DF, or MF
To monitor dedifferentiation, we examined the expression levels of extracellular matrix (type I collagen and type II collagen) and markers for stem cell status (CD29 and SMA) among chondrocytes cultured in the various media.
Comparison of cells grown in D and M without FGF2 showed that both media tended to induce the rapid dedifferentiation of chondrocytes, as shown by gradual increases in type I collagen expression and decreases in type II collagen expression with increasing passages (Fig. 2A and B). Culture in D and M was also associated with senescence, as measured by SA-β-gal staining (Fig. 2C): CCs-D entered senescence as early as passage 3, while CCs-M tended to enter senescence at passage 5. Dedifferentiation (increased type I collagen expression) increased more drastically at passages 3 and 4 in CCs-M compared with CCs-D (Fig. 2A). In contrast, senescence (SA-β-gal positivity) was more evident in CCs-D, indicating that chondrocytes cultured in D media failed to retain their functionality during in vitro expansion.
Fig. 2.
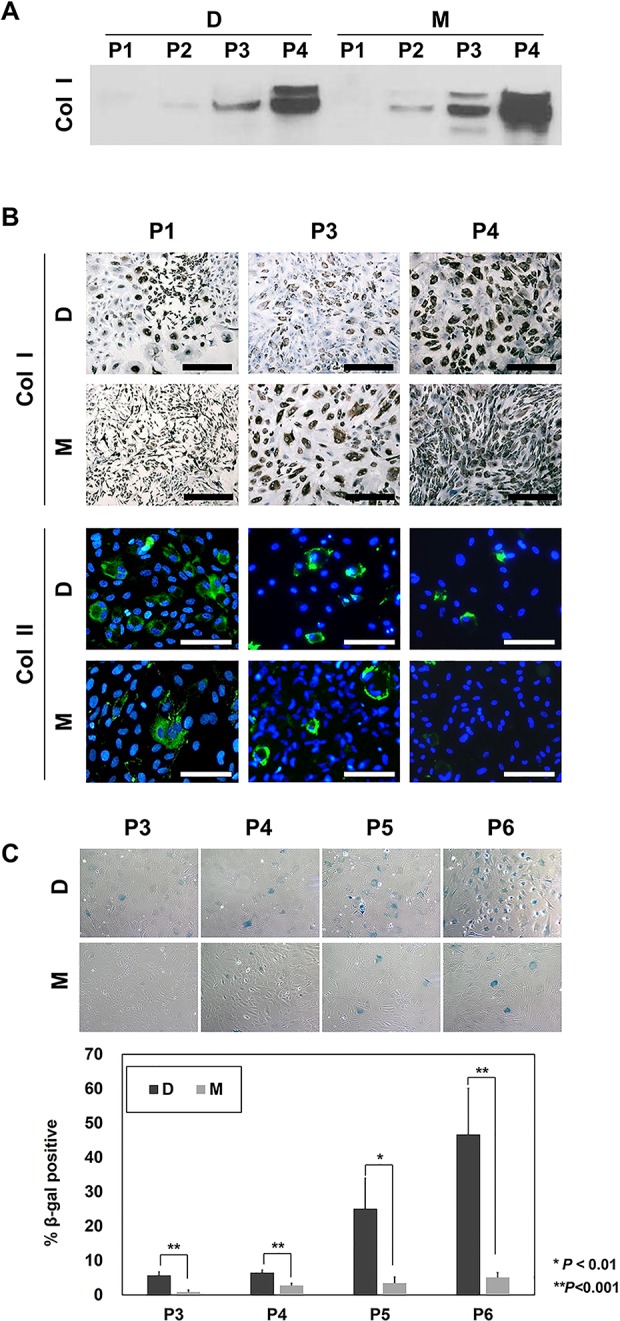
Dedifferentiation and senescence of costal chondrocytes (CCs) during in vitro expansion. (A) Western blot assay for type I collagen expression in CCs-D or CCs-M. (B) Immunohistochemical (IHC) staining for type I collagen and immunofluorescence (IF) staining for type II collagen was performed in CCs-D or CCs-M. For visualization of total cells, nuclei were counterstained with hematoxylin and 4′,6-diamidino-2-phenylindole for IHC and IF, respectively. Scale bar, 100 µm. (C) Senescence-associated β-galactosidase staining of CCs-D or CCs-M (n = 5). The fraction of cells positive for β-gal activity (%) was normalized by the total number of cells. *P < 0.01; **P < 0.001.
To examine the combined effect of the medium plus a growth factor, we cultured CCs in D and M with and without FGF2 and examined the expressions of type I collagen, type II collagen, CD29 (which is reportedly associated with the chondrogenic potential of stromal cells),25 and α-SMA in early and late passage cells. Based on the loss of type II collagen expression, DF and MF induced dedifferentiation as early as passage 2 and thus earlier than seen with D or M (Fig. 3A and B). DF and MF were also associated with decreased expression of type I collagen, indicating that FGF2 was associated with a somewhat different kind of dedifferentiation. Regarding CD29 (Fig. 3A and B), chondrocytes cultured in D group showed relatively high expression of CD29 at passage 2, while only a few cells expanded in M expressed CD29 at this time point. At passage 8, the majority of cells in the D and M groups showed CD29 expression. Cells of the DF and MF groups showed even stronger expression of CD29 at passage 8 (Fig. 3A), indicating that FGF2 supported the development of stromal cell characteristics. The expression of α-SMA was significantly higher in cells of the D and M groups at passage 8, whereas this enhancement was decreased in cells of the DF and MF groups (Fig. 3A).
Fig. 3.
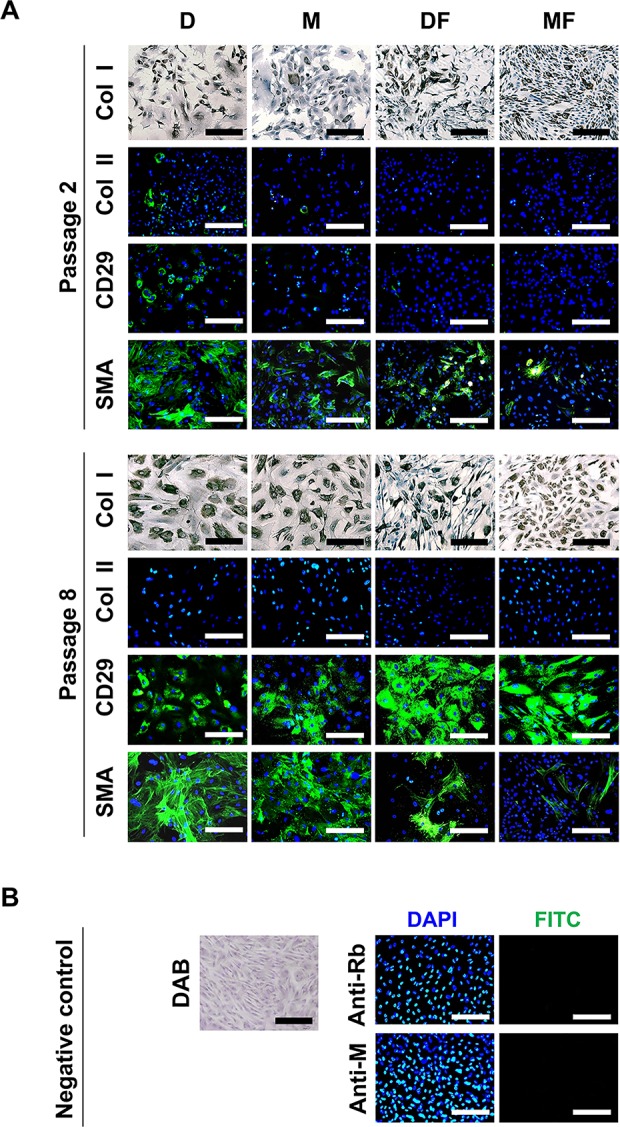
Characteristics of CCs during in vitro expansion. (A) IHC staining for type I collagen and IF staining for type II collagen, CD29, and α-SMA were performed in CCs cultured in D, M, DF, or MF at passages 2 and 8 (n = 5). To visualize total cells, nuclei were counterstained with hematoxylin and 4′,6-diamidino-2-phenylindole for IHC and IF, respectively. (B) Negative control experiments confirmed the specificities of the IHC and IF signals. Scale bar, 100 µm. DAB, 3,3′-diaminobenzidine; anti-Rb, antirabbit immunoglobulin; anti-M, antimouse immunoglobulin; CCs, costal chondrocytes; IHC, immunohistochemical; IF, immunofluorescence.
Multipotency of Dedifferentiated CCs
We next investigated the effects of the various media on the in vitro differentiation potential of chondrocytes. Newly isolated, nonexpanded CCs of passage 1 showed moderately positive adipogenic potential and extremely low osteogenic potential compared with BM-MSCs, which were used as the positive control (Fig. 4). After 8 passages of in vitro expansion in the various media, all cell groups accumulated more lipid droplets and calcified matrix when cultured in adipogenic and osteogenic media, respectively. However, there were between-group differences in their in vitro differentiation potentials. Cells cultured in DF and MF groups showed superior adipogenic differentiation, while cells of the M group outperformed those of the D group in this regard (Fig. 4A). Osteogenic differentiation was clearly increased following in vitro expansion (Fig. 4B), and the extent of calcification varied across the tested groups. However, as it is currently agreed that the osteogenic differentiation potential can truly be evaluated only in an in vivo model,26,27 we did not compare the intensity of alizarin red staining among the groups.
Fig. 4.
Multipotent differentiation capacities of fully dedifferentiated costal chondrocytes (CCs) in vitro. Representative micrographs of the adipogenesis (A) and osteogenesis (B) of passage 8 CCs, passage 1 CCs, and bone marrow–derived mesenchymal stem cells (BM-MSCs; n = 3). Lipid droplets and calcium-rich matrix accumulation were visualized with oil red O and alizarin red S, respectively.
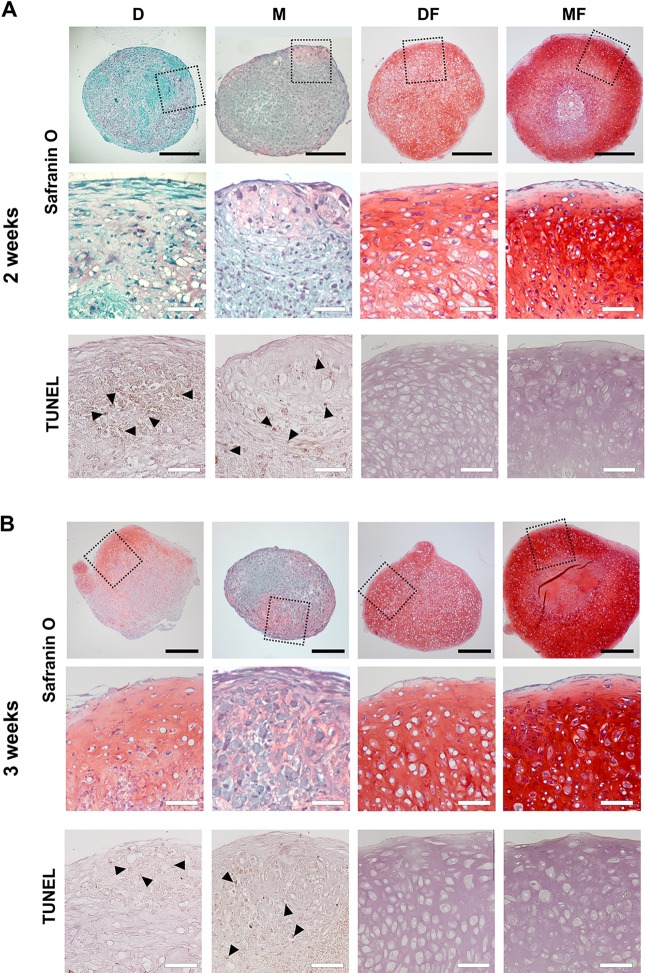
We tested the chondrogenic differentiation potentials of chondrocytes cultured in each medium and found that the different treatment groups produced cartilage pellets of varied quality upon in vitro chondrogenic induction. After 2 wk of chondrogenic induction, CCs-D produced mostly fibrous tissues and CCs-M showed little GAG-containing matrix, whereas CCs-DF and CCs-MF produced GAG-containing cartilaginous matrix (Fig. 5A). After 3 wk of chondrogenic induction, CCs-D produced a GAG-positive cartilaginous pellet with no fibrous morphology, whereas the pellet formed by CCs-M differed little from that observed at 2 wk in terms of GAG-rich cartilaginous matrix formation (Fig. 5B). The CCs-DF pellets comprised chondrocytes that were consistently larger than those of the CCs-MF pellets at 2 and 3 wk, and CCs-MF produced more GAG-rich matrix than CCs-DF (Fig. 5A and B). At all tested time points, the presence of FGF2 had beneficial effects, based on the fact that apoptotic cells were not present and increasing the deposition of GAG-rich cartilaginous matrix (Fig. 5A and B). Among the FGF2-containing media, MF yielded better chondrogenesis than DF, with cells of the MF group forming more GAG-rich matrix and showing fewer hypertrophic changes than DF (Fig. 5A and B).
Fig. 5.
Comparison of the chondrogenic capabilities of fully dedifferentiated costal chondrocytes (CCs) in vitro. Chondrogenic pellets produced by CCs expanded in each medium were subjected to safranin O staining for comparison of cartilaginous matrix production after 2 wk (A) and 3 wk (B) of in vitro chondrogenic differentiation (n = 3). Apoptotic cells in the chondrogenic pellets were visualized by TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) assay and counterstained with hematoxylin. Arrowheads indicate apoptotic cells. Closed scale bar, 500 µm and open scale bar, 100 µm.
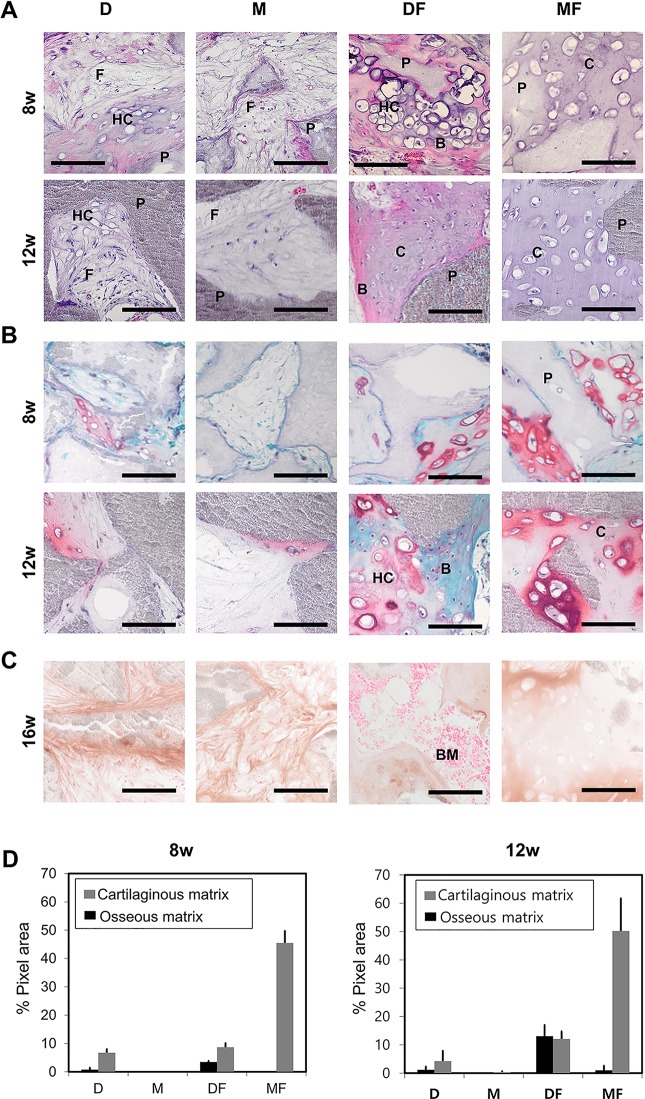
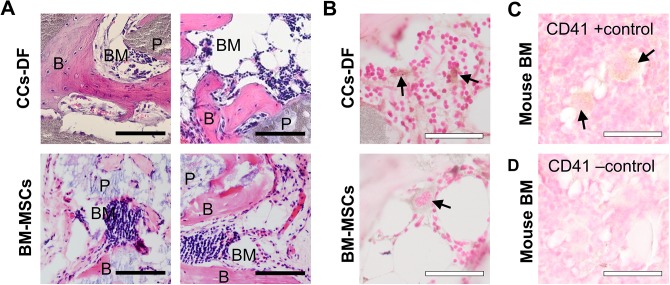
The multidifferentiation potential exhibited by the dedifferentiated chondrocytes indicates that they gained MSC-like characteristics with respect to differentiation. Given this, and the current notion that true osteogenic potential can be accurately assessed only by monitoring in vivo osteogenesis,26,28,29 we examined the osteogenic potential of CCs-D, CCs-M, CCs-DF, and CCs-MF in an in vivo ectopic bone model using HA/TCP bone biomaterials. Interestingly, some chondrocyte groups produced considerable amounts of bone tissues in this ectopic bone model (Fig. 6). Chondrocytes expanded in DF showed abundant cartilaginous matrix formation by 8 and 12 wk of in vivo incubation. At 8 wk, many parts of the cartilaginous tissue formed by the CCs-DF exhibited a hypertrophic morphology, indicating a transition from chondrogenesis to osteogenesis. At 12 wk, there was considerable amount of osseous matrix formation with embedded osteocytes. At this time point, the bone tissues formed by CCs-DF were morphologically similar to those formed by rabbit BM-MSCs in the ectopic bone model (Fig. 7). Moreover, a portion of the newly formed bone matrix provided a niche for hematopoiesis, as indicated by its positive expression of the hematopoietic marker, CD41 (Fig. 7B). Together, the extent of in vivo hypertrophic transition at 8 wk and in vivo osteogenesis at 12 wk suggests that CCs-DF-led bone formation occurs via endochondral ossification.
Fig. 6.
In vivo ectopic bone formation test of fully dedifferentiated CCs up to passage 8. (A, B) Representative micrographs of ectopic bone formation by dedifferentiated CCs transplanted to the subcutaneous tissues of nude mice, as assessed at 8- and 12-wk postimplantation (n = 3) using hematoxylin and eosin staining (A) or safranin O staining (B). (C) To identify the origin of bone tissues, immunohistochemical staining of antirabbit type I collagen was performed after 16 wk of in vivo osteogenesis. (D) Percentages of cartilaginous or osseous matrix out of total interstitial tissue among the HA-TCP implants, as calculated using the Image J program. The original areas taken up by the HA-TCPs were excluded before image analysis. CCs, costal chondrocytes; P, HA-TCPs; F, fibrotic tissue; HC, hypertrophic cartilage; B, bone tissue; BM, bone marrow; C, cartilaginous tissue; HA-TCPs, hydroxyapatite/tricalcium phosphate particles. Scale bar, 100 µm.
Fig. 7.
Comparison of in vivo osteogenesis in ectopic bone models implanted with costal chondrocytes (CCs)-DF versus bone marrow–derived mesenchymal stem cells (BM-MSCs). (A) Tissue morphology was compared under hematoxylin and eosin staining. (B) The presence of hematopoietic clusters was confirmed by immunohistochemical against CD41. (C) Positive and negative staining of CD41 was confirmed with mouse bone marrow tissues (BM) incubated with or without the primary antibody, respectively. Arrow indicates CD41(+) cell. Closed scale bar, 100 µm and open scale bar, 50 µm.
Chondrocytes expanded in MF showed the highest degree of cartilaginous matrix formation, as evidenced by the extents of in vivo chondrogenesis and GAG-rich matrix formation. The tissues formed by CCs-MF showed hypertrophy in only a few isolated spots, and very little bone matrix was visible even after 12 wk of in vivo incubation (Fig. 6).
To identify the origin of the tissues formed by the implants, samples were obtained at 16-wk postimplantation, sectioned, and subjected to IHC staining for rabbit-specific type I collagen. As shown in Fig. 6C, the fibrous, cartilaginous, and osseous tissues were of donor origin, while the hematopoietic clusters formed by CCs-DF originated from the host.
To numerically compare the extent of chondrogenesis and osteogenesis exerted by CCs expanded in each medium, histological sections were subjected to image analysis using the Image J software (Fig. 6D). For CCs-D, CCs-M, CCs-DF, and CCs-MF implants, cartilaginous tissue comprised approximately 6.74%, 0.01%, 8.67%, and 45.48% of the total tissue areas at 8-wk postsurgery and 4.26%, 0.35%, 12.07%, and 50.19% at 12 wk, respectively, while bone tissue comprised 0.77%, 0%, 3.45%, and 0% of the total tissue areas at 8 wk and 1.2%, 0%, 13.06%, and 0.96% at 12 wk, respectively. Chondrocytes expanded in D and M groups generated the least osseous and cartilaginous matrix at 8 and 12 wk of in vivo incubation; most of the tissues newly formed from CCs-D and CCs-M were fibrotic.
Together, our results show that CCs-DF exhibited in vivo chondrogenic and osteogenic differentiation potential, suggesting that DF induced the chondrocytes to develop MSC-like differentiation potential. In terms of chondrogenic potential, MF triggered the strongest formation of cartilaginous tissue in vitro and in vivo at all tested time points. The differentiation tendency of each chondrocyte group is summarized in Table 3.
Table 3.
In Vitro and In Vivo Differentiation Tendencies of Chondrocytes Expanded in the Various Media.
| Lineages of differentiation Da | Mb | DFc | MFd | |
|---|---|---|---|---|
| Adipogenesise | + | +/++ | +++ | +++ |
| In vitro osteogenesisf | +++ | ++ | +++ | +++ |
| In vivo osteogenesisg | +/− | − | +++ | +/− |
| Chondrogenesish | −/+ | −/+ | ++/+++ | +++ |
aCultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum.
bCultured in mesenchymal stem cell growth medium (MSCGM).
cCultured in DMEM supplemented with 10% fetal bovine serum and fibroblast growth factor-2 (FGF2).
dCultured in MSCGM supplemented with FGF2.
eThe extent of oil red O–positive cells: +, <50%; ++, <75%; +++, 75%.
fThe extent of alizarin red intensity: +, contains empty spot; ++, entirely red; +++, entirely dark red.
gThe extent of mineralized matrix formation at 12 wk in an ectopic bone model: −, absence of hypertrophy; +, presence of hypertrophy; ++, presence of bone matrix; +++, presence of bone matrix and bone marrow tissue.
hThe quality of cartilaginous tissue after 2 wk of in vitro chondrogenesis: −, presence of fast green-stained fibrous tissue; +, light red color (safranin O) at isolated spot; ++, light red color in the entire area; +++, presence of dark red color.
In Vivo Cartilage Regeneration Induced by Fully Dedifferentiated Chondrocytes Cultured in MF
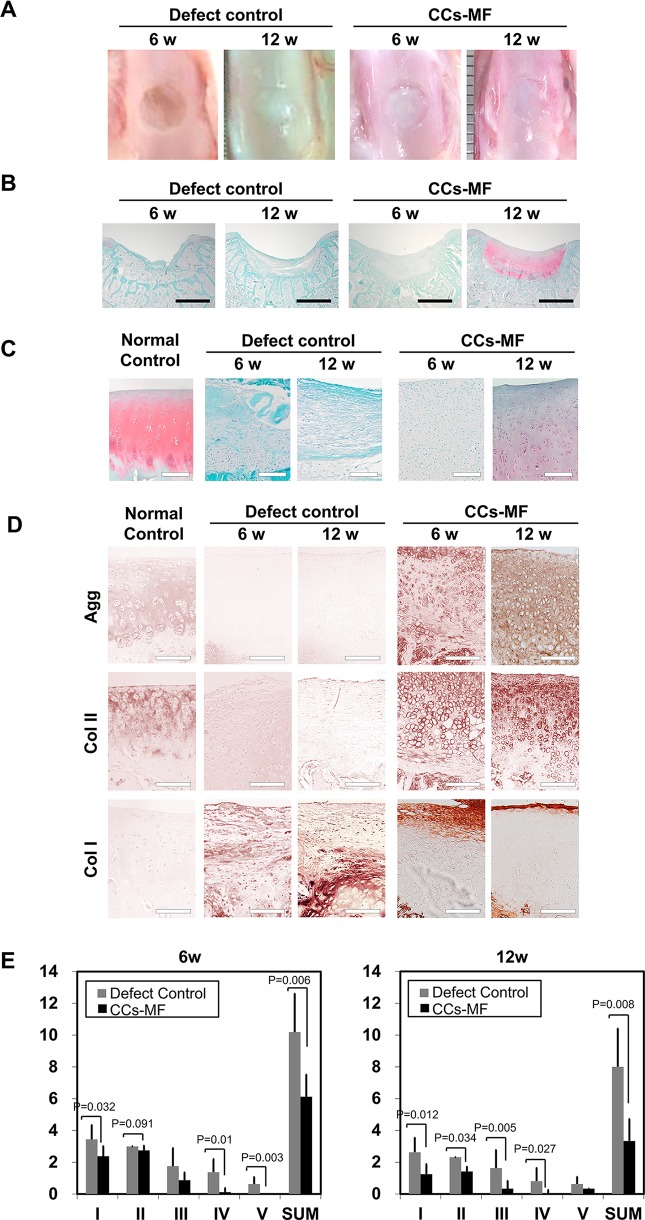
As CCs-MF induced superior in vitro chondrogenic differentiation and produced abundant GAG-containing cartilaginous matrix, we tested their ability to repair cartilage in a rabbit full-thickness cartilage defect model. For comparison, uninjured cartilage tissue and full-thickness-defect cartilage treated with FG served as normal and defect controls, respectively. The results are shown in Fig. 8. No postoperative complication or gross evidence of an inflammatory reaction was found in any of the transplanted animals. Six weeks after cartilage injury, analysis of macroscopic morphology (Fig. 8A) indicated that the injury sites of the defect controls were empty, whereas those of the CCs-MF-treated group exhibited regenerated tissues characterized by a turbid white color that differed from the translucence of the adjacent normal cartilage. After 12 wk, the injury sites of the defect controls had the turbid white appearance of regenerated tissues, whereas those of the CCs-MF-treated group had formed a translucent cartilage-like matrix that was difficult to differentiate from adjacent tissues (Fig. 8A).
Fig. 8.
Evaluation of in vivo cartilage tissue repair (n = 6). (A) Macroscopic appearance of patellar grooves. (B, C) Microscopic appearance of repaired tissues, as assessed with safranin O staining at 6- and 12-wk postsurgery. Images show a low-power magnification of the whole defect area (B) and a high-power magnification of central area of the defect (C). (D) Immunohistochemical staining for aggrecan, type I collagen, and type II collagen. (E) Comparison of the histological grading scores of regenerative tissues at 6 and 12 wk. Statistical analysis was performed using a t-test. The scoring categories were as follows: Category I, cell morphology; Category II, matrix staining (metachromasia); Category III, surface regularity; Category IV, thickness of the cartilage; and Category V, integration of donor with host adjacent cartilage. Sum of the point ranges between 0 (normal cartilage) and 14 (no repair tissue). The detailed grading scale is presented in Table 1. Dark scale bar, 2 mm and white scale bar, 100 μm.
When the regenerated tissues were histologically examined under safranin O staining (Fig. 8B and C), the defect control and CCs-MF-treated groups were both negative for safranin O staining at 6-wk posttransplantation, indicating that there had been little production of GAGs. Morphologically, the injured sites of CCs-MF-treated rabbits were fully filled with a matrix characterized by a cartilage-like morphology and many chondroblastic cells, whereas those of the defect controls were partially filled with disorganized fibrous tissue. At 12-wk posttransplantation, the defect areas of animals in the CCs-MF-treated group were covered with a relatively mature hyaline cartilage-like structure that was positive for safranin O staining, while those of the defect controls still lacked GAG positivity or any organized structure (Fig. 8B and C). Thus, while the defect control failed to generate any GAG-rich matrix during the course of the experiment, the CCs-MF-treated group had developed a GAG-rich cartilaginous matrix similar to normal cartilage by 12-wk postsurgery. In terms of the depth-dependent structure of the generated articular cartilage, the newly formed tissue of the CCs-MF-treated group at 12-wk posttransplantation resembled the normal control: Both had a fibrous layer at the surface and arrays of large lacunae in the deep layer of the cartilage.
To further confirm the composition of the newly formed matrix, tissue sections were subjected to IHC staining against type I collagen, type II collagen, and aggrecan (Fig. 8D). In the CCs-MF-treated group, expression of aggrecan and type II collagen was shown around the chondrocytes at 6-wk postsurgery, and their expression was detected throughout the cartilaginous matrix at 12 wk upon surgery, which is a similar expression pattern shown in normal control tissues. Type I collagen expression in the CCs-MF-treated group was mainly observed as a thick band on the superficial layer at 6-wk postsurgery, and it was reduced to thin layer localized at the most superficial layer at 12 wk. In the defect control group, the newly formed tissue was characterized by abundant expression of type I collagen, whereas type II collagen and aggrecan were barely detected at any time point (Fig. 8D). These results reveal that the expression patterns of the tested matrix molecules were nearly identical in the CCs-MF-treated and normal control groups at 12-wk postsurgery, indicating that transplantation of CCs-MF restored the characteristics of hyaline cartilage.
Using the modified histological scoring method described by Wakitani et al.24 as presented in Table 1, we compared the extent of cartilage repair between the defect control and CCs-MF-treated groups. At 6-wk postsurgery, compared to the control group, the CCs-MF-treated group scored significantly better (P < 0.05) in categories I (cell morphology), II (matrix staining), IV (thickness of cartilage), and V (integration of donor and host adjacent cartilage). At 12 wk, the CCs-MF-treated group significantly outperformed (P < 0.05) the control group in categories I (cell morphology), II (matrix staining), III (surface regularity), and IV (thickness of cartilage). The summed scores for each category were significantly different at each time point; a larger difference was seen at 12-wk postsurgery indicating that the transplantation of CCs-MF improved the restoration of hyaline cartilage in this model (Fig. 8E).
Discussion
Here we show that expanded chondrocytes can show different characteristics depending on the culture media used for their in vitro expansion, with some media inducing premature senescence while others enabled the chondrocytes to retain their functionality during proliferation. Previous studies have used various media for the in vitro expansion of chondrocytes, including DMEM,17,30 Coon’s modified Ham’s F12 medium,31 DMEM/F12,32 and α-MEM (Minimum essential media).32,33 Supplementation of the medium with FBS or a patient’s autologous serum (2% to 10%), which have high content of nutrients and growth factors, was found to clearly facilitate the in vitro expansion of chondrocytes.34,35 Several specific growth factors have been reported to influence the characteristics of chondrocytes during in vitro culture. For example, FGF2, TGF-β1, and PDGF were found to enhance the differentiation potential of adult human articular chondrocytes toward chondrogenesis, osteogenesis, and adipogenesis.33 In addition, TGF-β1, IGF-1, BMP-2, and BMP-7 were shown to potentiate the differentiation of chondrocytes during in vitro chondrogenesis by maximizing matrix production.36,37 The use of specific growth factors has been shown to increase the extent of cell dedifferentiation during chondrocyte expansion, as well as the capacity of such cells to redifferentiate in response to chondrogenic stimuli. FGF2 was found to act as a potent mitogen for various connective tissue–forming cells including chondrocytes.18,21–23 In other reports, human articular chondrocytes in vitro expanded without growth factor for 6 passages failed to form cartilage tissue in high-density 3-dimensional culture, whereas those grown in serum-free media supplemented with TGF-β exhibited cartilage tissue formation.18,37,38 We previously transplanted chondrocytes expanded with FGF2 and found that they showed superior differentiation potential in vivo even compared with chondrogenesis-stimulated constructs.39 Moreover, the addition of FGF2 during in vitro expansion was found not only to stimulate proliferation but also to improve the chondrogenic differentiation of adipose-derived stem cells.40,41
In the present study, we set out to determine the effect of basal media on characteristics of in vitro–expanded chondrocytes. Our results revealed that the addition of FGF2 accelerated both cell expansion and dedifferentiation during early passages. Despite this accelerated dedifferentiation, however, chondrocytes cultured in the presence of FGF2 (CCs-DF and CCs-MF) showed more potent chondrogenic differentiation potential in vitro and in vivo compared to cells cultured in the same media without FGF2. In addition to these growth factor–related differences, we found that the choice of basal medium affected several aspects of expansion culture. First, the number of senescent cells increased more rapidly in CCs-D compared to CCs-M. Second, the in vitro and in vivo chondrogenic potentials were significantly higher in CCs-M and CCs-MF compared with CCs-D and CCs-DF, respectively. Third and most important, CCs-DF showed more plasticity in terms of their differentiation potential, as evidenced by their ability to undergo osteogenesis, which was barely detected among CCs-MF. Consistent with the findings of a previous report42 examining surface marker expression, our data showing acquisition of in vitro adipogenic and in vivo osteogenic differentiation potential further showed that CCs-DF took on an MSC-like phenotype. In terms of stromal/stem cell marker expression, cells grown in the FGF2-containing media exhibited stronger CD29 expression at passage 8, indicating that FGF2 induced a stromal cell character. Meanwhile, the decreased expression of α-SMA in such cells appears to suggest that the FGF2-treated chondrocytes were in a highly proliferating state.43,44
Although we cannot rule out the possibility that tissue-specific stem cells or more primitive chondrocytes included in our cultured CCs had medium-specific reactions during in vitro expansion culture, it is clear that the utilized growth medium had tremendous influences on the characteristics of the expanded chondrocytes. Many researchers have sought to induce the stable chondrogenic differentiation of MSCs in the hopes of utilizing MSCs for cartilaginous regeneration.1,45,46 On the contrary, we herein show that chondrocytes obtained from a non-load-bearing site can be efficiently expanded and induced to an MSC-like state by using the right medium/growth factor combination (Figs. 4 and 6). Tissues formed by transplantation of CCs-DF with HA-TCPs in an ectopic bone model contained mostly hypertrophic chondrocytes and mineralized bone tissues.
Given the known shortcoming of MSCs in cartilage regeneration (e.g., calcification upon in vivo incubation), we did not test CCs-DF in our rabbit in vivo cartilage defect regeneration experiment. However, we speculate that these MSC-like chondrocytes might prove useful for the construction of osteochondral tissue that could support a well-integrated cartilage–bone interface.
In contrast to CCs-DF, CCs-MF formed abundant cartilage tissues in our ectopic bone model, with little in vivo osteogenic differentiation observed. In the rabbit cartilage defect model, the cartilage newly formed by CCs-MF at 12-wk postinjury retained safranin O–positivity at the osteochondral interface, indicating that CCs-MF retained superior chondrogenic potential and produced cartilaginous matrix even in highly osteogenic in vivo environment. The discrepancy of the osteogenic differentiation potentials obtained in our in vitro and in vivo experiments supports the consensus that in vitro osteogenesis assays cannot represent the true osteogenic differentiation potential found in the in vivo microenvironment. In contrast, we obtained consistent results in our in vitro and in vivo assays of chondrogenic differentiation.
We found that CCs-DF acquired a hypertrophic morphology even when undergoing in vitro chondrogenesis. Compared with CCs-MF, the chondrocytes in CCs-DF pellets were consistently bigger at weeks 2 and 3 of in vitro chondrogenesis (Fig. 5A and B). Ironically, the MSC-specific culture medium supplemented with FGF2-triggered potent chondrogenesis, whereas the conventional chondrocyte culture medium supplemented with FGF2-yielded MSC-like cells. In addition to forming ectopic bone tissues, CCs-DF supported the formation of bone marrow in ectopic bone model in vivo, as evidenced by CD41 marker expression. Compared with the CD41(+) cells in the BM-MSC-transplanted group, the CD41(+) cells in the CCs-DF-transplanted group were somewhat smaller and appeared immature, possibly indicating that the CCs-DF induced belated hematopoiesis compared with the BM-MSCs.47
In summary, we herein show that CCs-MF exhibited the best proliferation rate and chondrogenic differentiation potential, whereas CCs-DF showed the highest plasticity in terms of multidifferentiation potential. These findings indicate that the rational design of a chondrocyte expansion protocol could critically support the use of such cells for the reconstruction of both cartilage-related surrounding tissues and the cartilage itself.
Footnotes
Author Contributions: Jungsun Lee contributed to conception and design of the study, collection and assembly of the data, analysis and interpretation of the data, and drafting the manuscript. Jin-Yeon Lee, Byung-Chul Chae, and Jeongho Jang contributed to collection and assembly of the data. EunAh Lee contributed to analysis and interpretation of the data, drafting the manuscript, and gave final approval of the manuscript. Youngsook Son contributed to conception and design of the study, analysis and interpretation of the data, drafting the manuscript, and gave final approval of the manuscript.
Ethical Approval: All procedures were performed in accordance with animal study protocols approved by the institutional animal care and use committee of Biosolution Research Institute (MCTTISCUC ASP 09-002).
Statement of Human and Animal Rights: All animal studies were conducted according to NIH guidelines.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP, 2015R1A5A1037656) and by the Korean Health Technology R&D Project funded by the Korean Ministry of Health & Welfare (HI16C1010; HI15C0963). The funding sources had no involvement in the design of the study or the collection, analysis, or interpretation of the data.
References
- 1. Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54(10):3254–3266. [DOI] [PubMed] [Google Scholar]
- 2. Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212–234. [DOI] [PubMed] [Google Scholar]
- 4. Vanlauwe J, Almqvist F, Bellemans J, Huskin JP, Verdonk R, Victor J. Repair of symptomatic cartilage lesions of the knee: the place of autologous chondrocyte implantation. Acta Orthop Belg. 2007;73(2):145–158. [PubMed] [Google Scholar]
- 5. Lee J, Lee E, Kim HY, Son Y. Comparison of articular cartilage with costal cartilage in initial cell yield, degree of dedifferentiation during expansion and redifferentiation capacity. Biotechnol Appl Biochem. 2007;48(Pt. 3):149–158. [DOI] [PubMed] [Google Scholar]
- 6. Eslaminejad MB, Taghiyar L, Falahi F. Costal versus articular chondrocytes in alginate three-dimensional cultures. Iran J Biotechnol. 2009;7(3):129–136. [Google Scholar]
- 7. Johnson TS, Xu JW, Zaporojan VV, Mesa JM, Weinand C, Randolph MA, Bonassar LJ, Winograd JM, Yaremchuk MJ. Integrative repair of cartilage with articular and nonarticular chondrocytes. Tissue Eng. 2004;10(9–10):1308–1315. [DOI] [PubMed] [Google Scholar]
- 8. Kitaoka E, Satomura K, Hayashi E, Yamanouchi K, Tobiume S, Kume K, Obinata M, Nagayama M. Establishment and characterization of chondrocyte cell lines from the costal cartilage of SV40 large T antigen transgenic mice. J Cell Biochem. 2001;81(4):571–582. [DOI] [PubMed] [Google Scholar]
- 9. Popko J, Szeparowicz P, Sawicki B, Wołczyński S, Wojnar J. Rabbit articular cartilage defects treated with cultured costal chondrocytes (preliminary report). Folia Morphol (Warsz). 2003;62(2):107–112. [PubMed] [Google Scholar]
- 10. Saadeh PB, Brent B, Mehrara BJ, Steinbrech DS, Ting V, Gittes GK, Longaker MT. Human cartilage engineering: chondrocyte extraction, proliferation, and characterization for construct development. Ann Plast Surg. 1999;42(5):509–513. [PubMed] [Google Scholar]
- 11. Szeparowicz P, Popko J, Sawicki B, Wołczyński S. Is the repair of articular cartilage lesion by costal chondrocyte transplantation donor age-dependent? An experimental study in rabbits. Folia Histochem Cytobiol. 2006;44(3):201–206. [PubMed] [Google Scholar]
- 12. Tay AG, Farhadi J, Suetterlin R, Pierer G, Heberer M, Martin I. Cell yield, proliferation, and postexpansion differentiation capacity of human ear, nasal, and rib chondrocytes. Tissue Eng. 2004;10(5–6):762–770. [DOI] [PubMed] [Google Scholar]
- 13. Barbero A, Grogan S, Schäfer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12(6):476–484. [DOI] [PubMed] [Google Scholar]
- 14. Dozin B, Malpeli M, Camardella L, Cancedda R, Pietrangelo A. Response of young, aged and osteoarthritic human articular chondrocytes to inflammatory cytokines: molecular and cellular aspects. Matrix Biol. 2002;21(5):449–459. [DOI] [PubMed] [Google Scholar]
- 15. Rosen F, McCabe G, Quach J, Solan J, Terkeltaub R, Seegmiller JE, Lotz M. Differential effects of aging on human chondrocyte responses to transforming growth factor beta: increased pyrophosphate production and decreased cell proliferation. Arthritis Rheum. 1997;40(7):1275–1281. [DOI] [PubMed] [Google Scholar]
- 16. von der Mark K, Gauss V, von der Mark M, Muller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267(5611):531–532. [DOI] [PubMed] [Google Scholar]
- 17. Dell’Accio F, De Bari C, Luyten FP. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 2001;44(7):1608–1619. [DOI] [PubMed] [Google Scholar]
- 18. Jakob M, Démarteau O, Schäfer D, Hintermann B, Dick W, Heberer M, Martin I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81(2):368–377. [DOI] [PubMed] [Google Scholar]
- 19. Schulze-Tanzil G, de Souza P, Villegas Castrejon H, John T, Merker HJ, Scheid A, Shakibaei M. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002;308(3):371–379. [DOI] [PubMed] [Google Scholar]
- 20. Giovannini S, Diaz-Romero J, Aigner T, Mainil-Varlet P, Nesic D. Population doublings and percentage of S100-positive cells as predictors of in vitro chondrogenicity of expanded human articular chondrocytes. J Cell Physiol. 2010;222(2):411–420. doi: 10.1002/jcp.21965. [DOI] [PubMed] [Google Scholar]
- 21. Claus S, Mayer N, Aubert-Foucher E, Chajra H, Perrier-Groult E, Piperno M, Damour O, Mallein-Gerin F. Cartilage-characteristic matrix reconstruction by sequential addition of soluble factors during expansion of human articular chondrocytes and their cultivation in collagen sponges. Tissue Eng Part C Methods. 2012;18(2):104–112. [DOI] [PubMed] [Google Scholar]
- 22. Martin I, Vunjak-Novakovic G, Yang J, Langer R, Freed LE. Mammalian chondrocytes expanded in the presence of fibroblast growth factor 2 maintain the ability to differentiate and regenerate three-dimensional cartilaginous tissue. Exp Cell Res. 1999;253(2):681–688. [DOI] [PubMed] [Google Scholar]
- 23. Yoo GS, Lee E, Jang J, Lee EA, Son Y. The chondrogenic stimulating effect of fibroblast growth factor-2 on adipose-derived stem cells. Tissue Eng Regen Med. 2010;7(1):93–100. [Google Scholar]
- 24. Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76(4):579–592. [DOI] [PubMed] [Google Scholar]
- 25. Cicione C, Díaz-Prado S, Muiños-López E, Hermida-Gómez T, Blanco FJ. Molecular profile and cellular characterization of human bone marrow mesenchymal stem cells: donor influence on chondrogenesis. Differentiation. 2010;80(2–3):155–165. [DOI] [PubMed] [Google Scholar]
- 26. Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim S, Cho H, Lee E, Won Y, Kim C, Ahn W, Lee E, Son Y. Osteogenic stimulation of human adipose-derived stem cells by pre-treatment with fibroblast growth factor 2. Cell Tissue Res. 2016;364(1):137–147. [DOI] [PubMed] [Google Scholar]
- 28. Rosen F, McCabe G, Quach J, Solan J, Terkeltaub R, Seegmiller JE, Lotz M. Differential effects of aging on human chondrocyte responses to transforming growth factor beta: increased pyrophosphate production and decreased cell proliferation. Arthritis Rheum. 1997;40(7):1275–1281. [DOI] [PubMed] [Google Scholar]
- 29. Yang KG, Saris DB, Geuze RE, Helm YJ, Rijen MH, Verbout AJ, Dhert WJ, Creemers LB. Impact of expansion and redifferentiation conditions on chondrogenic capacity of cultured chondrocytes. Tissue Eng. 2006;12(9):2435–2447. [DOI] [PubMed] [Google Scholar]
- 30. Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, Luyten FP, TIG/ACT/01/2000&EXT Study Group. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(1 Suppl):10S–19S. [DOI] [PubMed] [Google Scholar]
- 31. Dozin B, Malpeli M, Cancedda R, Bruzzi P, Calcagno S, Molfetta L, Priano F, Kon E, Marcacci M. Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med. 2005;15(4):220–226. [DOI] [PubMed] [Google Scholar]
- 32. Filip A, Bianchi A, Mainard D, Lacolley P, Magdalou J, Mercier N. A simple two dimensional culture method to study the hypertrophic differentiation of rat articular chondrocytes. Biomed Mater Eng. 2015;25(1 Suppl):87–102. [DOI] [PubMed] [Google Scholar]
- 33. Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48(5):1315–1325. [DOI] [PubMed] [Google Scholar]
- 34. Hu DN, Yang PY, Ku MC, Chu CH, Lim AY, Hwang MH. Isolation and cultivation of human articular chondrocytes. Kaohsiung J Med Sci. 2002;18(3):113–120. [PubMed] [Google Scholar]
- 35. Müller S, Acevedo L, Wang X, Karim MZ, Matta A, Mehrkens A, Schaeren S, Feliciano S, Jakob M, Martin I, Barbero A, Erwin WM. Notochordal cell conditioned medium (NCCM) regenerates end-stage human osteoarthritic articular chondrocytes and promotes a healthy phenotype. Arthritis Res Ther. 2016;18(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee J, Son Y. TGF beta stimulates conversion of fibrocartilage phenotype of costal chondrocytes. Tissue Eng Regen Med. 2004;1(2):171–177. [Google Scholar]
- 37. Shi S, Mercer S, Eckert GJ, Trippel SB. Growth factor transgenes interactively regulate articular chondrocytes. J Cell Biochem. 2013;114(4):908–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yaeger PC, Masi TL, de Ortiz JL, Binette F, Tubo R, McPherson JM. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997;237(2):318–325. [DOI] [PubMed] [Google Scholar]
- 39. Lee J, Lee JY, Lee E, Son Y. FGF-2-expanded costal chondrocytes regenerate hyaline cartilage in rabbit osteochondral defects. Tissue Eng Regen Med. 2011;8(2):200–207. [Google Scholar]
- 40. Inoue S, Hori Y, Hirano Y, Inamoto T, Tabata Y. Effect of culture substrate and fibroblast growth factor addition on the proliferation and differentiation of human adipo-stromal cells. J Biomater Sci Polym Ed. 2005;16(1):57–77. [DOI] [PubMed] [Google Scholar]
- 41. Yuasa M, Yamada T, Taniyama T, Masaoka T, Xuetao W, Yoshii T, Horie M, Yasuda H, Uemura T, Okawa A, Sotome S. Dexamethasone enhances osteogenic differentiation of bone marrow- and muscle-derived stromal cells and augments ectopic bone formation induced by bone morphogenetic protein-2. PLoS One. 2015;10(2):e0116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diaz-Romero J, Nesic D, Grogan SP, Heini P, Mainil-Varlet P. Immunophenotypic changes of human articular chondrocytes during monolayer culture reflect bona fide dedifferentiation rather than amplification of progenitor cells. J Cell Physiol. 2008;214(1):75–83. [DOI] [PubMed] [Google Scholar]
- 43. Khouw IM, van Wachem PB, Plantinga JA, Vujaskovic Z, Wissink MJ, de Leij LF, van Luyn MJ. TGF-beta and bFGF affect the differentiation of proliferating porcine fibroblasts into myofibroblasts in vitro. Biomaterials. 1999;20(19):1815–1822. [DOI] [PubMed] [Google Scholar]
- 44. Li Q, Liu T, Zhang L, Liu Y, Zhang W, Liu W, Cao Y, Zhou G. The role of bFGF in down-regulating α-SMA expression of chondrogenically induced BMSCs and preventing the shrinkage of BMSC engineered cartilage. Biomaterials. 2011;32(21):4773–4781. [DOI] [PubMed] [Google Scholar]
- 45. Amin HD, Brady MA, St-Pierre JP, Stevens MM, Overby DR, Ethier CR. Stimulation of chondrogenic differentiation of adult human bone marrow-derived stromal cells by a moderate-strength static magnetic field. Tissue Eng. 2014;20(11–12):1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bornes TD, Jomha NM, Mulet-Sierra A, Adesida AB. Hypoxic culture of bone marrow-derived mesenchymal stromal stem cells differentially enhances in vitro chondrogenesis within cell-seeded collagen and hyaluronic acid porous scaffolds. Stem Cell Res Ther. 2015;6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mitjavila-Garcia MT, Cailleret M, Godin I, Nogueira MM, Cohen-Solal K, Schiavon V, Lecluse Y, Le Pesteur F, Lagrue AH, Vainchenker W. Expression of CD41 on hematopoietic progenitors derived from embryonic hematopoietic cells. Development. 2002;129(8):2003–2013. [DOI] [PubMed] [Google Scholar]