Abstract
Spinocerebellar ataxia (SCA) is a progressive neurodegenerative disease that affects the cerebellum and spinal cord. Among the 40 types of SCA, SCA type 3 (SCA3), also referred to as Machado–Joseph disease, is the most common. In the present study, we investigated the therapeutic effects of intracranial transplantation of human olfactory ensheathing cells (hOECs) in the ATXN3-84Q mouse model of SCA3. Motor function begins to decline in ATXN3-84Q transgenic mice at approximately 13 weeks of age. ATXN3-84Q mice that received intracranial hOEC transplantation into the dorsal raphe nucleus of the brain exhibited significant improvements in motor function, as measured by the rotarod performance test and footprint pattern analysis. In addition, intracranial hOEC transplantation alleviated cerebellar inflammation, prohibited Purkinje cells from dying, and enhanced the neuroplasticity of cerebellar Purkinje cells. The protein levels of tryptophan hydroxylase 2, the rate-limiting enzyme for serotonin synthesis in the cerebellum, and ryanodine receptor (RYR) increased in mice that received intracranial hOEC transplantation. Because both serotonin and RYR can enhance Purkinje cell maturation, these effects may account for the therapeutic benefits mediated by intracranial hOEC transplantation in SCA3 mice. These results indicate that intracranial hOEC transplantation has potential value as a novel strategy for treating SCA3.
Keywords: human olfactory ensheathing cells, type 3 spinocerebellar ataxia, dorsal raphe nucleus, Purkinje cell
Introduction
Spinocerebellar ataxia (SCA) is an autosomal dominant, progressive neurodegenerative disease that typically affects the cerebellum and spinal cord.1 Among the 40 different types of SCA (SCA1-40), SCA type 3 (SCA3), also referred to as Machado–Joseph disease (MJD), is the most common. SCA3 represents approximately 20% of 50% of SCA cases worldwide.2
SCA3 is caused by a mutation in the gene encoding the ataxin 3 (ATXN3) protein. The mutation is characterized by the amplification of CAG trinucleotide repeats,3,4 and it results in the translation of a mutant ATXN3 protein with a large polyglutamine tract comprising up to 55 glutamine residues. The neurotoxic mutant protein promotes neuronal dysfunction and degeneration in specific regions of the brain.1,5,6 Patients with SCA3 present with severe neuropathy characterized by the loss of neurons in the cerebellar Purkinje cell layer and deep nuclei.7 In addition, protein aggregates in neurons of both affected and unaffected brain areas are observed.8–10 Although promising therapeutic results with gene silencing, autophagy activation, and proteolysis inhibition have been reported,11–14 there is still no effective treatment option available for patients with SCA3.
Purkinje cells are GABAergic neurons found in the Purkinje cell layer and located in the cerebellum. The Purkinje cells are repetitively firing cells whose firing frequency changes during directed movement.15 Previous studies have reported that decreased Purkinje cell number and intrinsic firing rate in SCA3 led to motor function disorder.7,13,16,17 It has also been reported that in an SCA3 animal model, dendritic development and metabotropic glutamate receptor signaling in Purkinje cells were disrupted.18 Therefore, dysfunction of Purkinje cells causes the motor function disorder, which is a factor for SCA3 pathogenesis. Furthermore, in order to determine the number of Purkinje cells in the SCA3 mouse model, the expression levels of calbindin, which was reported to be a reliable Purkinje cell marker,19,20 and cresyl violet staining, a staining process for recognizing the Purkinje cells in the cerebellum,21 were used.
Investigations into the potential use of stem cells, including human mesenchymal stem cells (hMSCs)22 and neural stem cells (NSCs),17 for the treatment of SCA have been reported. A previous study demonstrated that intravenous hMSC transplantation improved rotarod performance, delayed the onset of motor function deterioration, and inhibited cerebellar Purkinje cell death in a transgenic mouse model of SCA2. These neuroprotective effects might have been mediated by an increase in the secretion of neurotrophic factors or by direct contact with host cells. However, intracranial hMSC transplantation into the dorsal surface of the medulla failed to reproduce these effects. Compared with intravenous hMSC transplantation, intracranial hMSC transplantation rescued fewer Purkinje cells and did not improve motor function. The discrepancy between the 2 approaches is likely to be a result of differences in the injection site because hMSCs injected into the dorsal surface of the medulla have to migrate a longer distance to reach the affected brain regions compared with cells directly injected into the cerebellum.22 Another group reported that cerebellar NSCs transplanted into the cerebellum of adult mice with MJD were able to differentiate into neurons, astrocytes, and oligodendrocytes, indicating that cerebellar NSCs have therapeutic potential for the treatment of SCA3. The significant reduction in neuroinflammation and increase in the secretion of trophic factors in this context indicate that undifferentiated NSCs may also provide neuroprotective effects.17
Human olfactory ensheathing cells (hOECs) are specialized glial cells restricted to various regions of the primary olfactory system, including the olfactory mucosa, olfactory nerve, and the outer nerve layer of the olfactory bulb.23,24 The developmental study has shown that both olfactory ensheathing cells (OECs) and Schwann cells are from neural crest, providing an explanation as to why these 2 cell types share similar properties and characteristics.25 Previous studies demonstrated that 2 types of cells, including Schwann cell-like olfactory nerve glial cells and astrocyte-like olfactory nerve glial cells, were identified in the olfactory tissue.26–28 It has also been reported that p75+/S100+ OECs played a Schwann-like role in the olfactory epithelium.29 These studies demonstrated that the glial cells expressing Schwann cell markers (p75/S100) in the olfactory tissue are the OECs. hOECs used in the current study were derived from the human nasal polyp (hNP), belonging to the olfactory epithelium, following a previously published separation procedure.30 A purity of more than 95% hOECs was obtained, which was similar to the previous report.30 hOECs guide growing olfactory axons from neurons in the nasal cavity olfactory mucosa to the olfactory bulb where they form synapses.31 The ability of hOECs to guide axonal outgrowth and promote neural differentiation32 indicates that hOEC transplantation into sites of neuronal injury is a potential therapeutic approach for repairing neurons in patients with spinal cord injury and for treating neurodegenerative diseases such as Parkinson’s disease.33,34
Therefore, we sought to determine the therapeutic potential of hOECs in the treatment of the neurodegenerative disease SCA3. To this end, we investigated the effects of intracranial hOEC transplantation into the dorsal raphe nucleus (DRN) in a mouse model of SCA3.
Materials and Methods
Experimental Procedure
The aim of this study was to explore the feasibility of using hOECs as a therapeutic strategy for the neuronal degenerative disease, SCA3. Cells were transplanted into the DRN of SCA3 modeled mice at 13 weeks of age, followed by weekly behavior analysis until mice were 24 weeks old. Animals were then sacrificed for further histological analysis and molecular biology analysis. Three groups of the animals were evaluated, including an hOEC transplanted group, a vehicle group, and a wild-type (WT) group as a normal control.
Animals
SCA3/MJD transgenic mice on a C57BL/6 background were purchased from the Jackson Laboratory (ME, USA). The mice were bred in NAR Labs (Taipei, Taiwan) and relocated to the animal facilities in National Dong Hwa University (Hualien, Taiwan) when they reached 4 to 5 weeks of age. WT C57BL/6 mice were purchased from Biolasco (Taipei, Taiwan). All of the animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of National Dong Hwa University and were conducted in accordance with the animal experiment guidelines of National Dong Hwa University. At 13 weeks of age, SCA3 mice were randomly divided into the hOEC transplantation group or the vehicle group (n = 6 for each group). Age-matched, nontransgenic, WT C57BL/6 mice were used as WT controls (n = 6).
hOEC Culture and Characterization
hOECs were generated from human nasal polyps (hNP) samples as previously described.30 Then they were characterized by immunofluorescent staining using S100β- and p75 nerve growth factor receptor- (Abcam, Cambridge, UK) specific antibodies. Immunocytochemical staining of the hOECs was performed using different antibodies: p75 (Abcam) and S100 (Abcam). Cells were plated on a poly-l-lysine-coated chambered glass slide and allowed to grow at 37 °C in 5% CO2 for 24 h. Cells were than stained with p75 or S100. A fluorescence microscope was used to observe the expression of p75 and S100. The isolation of hOECs from human samples and the usage of hOECs for studying the therapeutic effect on neurodegenerative diseases were approved by the institutional review board (IRB) of China Medical University and Hospital, Taiwan (IRB: CMHU104-REC2-129). The cells were seeded at a density of 3 × 105 cells/mL in Dulbecco’s modified Eagle’s medium/F12 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (100 U/mL), and they were maintained in a 5% CO2 environment at 37 °C. To prepare the hOECs for cell transplantation, cells that reached 80% confluence were detached from the T-flask using trypsin and resuspended in phosphate-buffered saline (PBS) at a concentration of 1 × 105 cells/μL.
Intracranial hOEC Transplantation
SCA3 transgenic mice underwent intracranial hOEC (passage 8-11, not immortalized) transplantation at 13 weeks of age. The mice were anesthetized with 4% chlorhydrate (10 μL/g) and placed in a stereotaxic apparatus. The cranium was exposed by creating a sagittal incision in the skin, and a small burr hole was carefully made at 0 mm lateral and 5 mm caudal to the bregma. Then the tip of a 27-G Hamilton syringe (Hamilton, Reno, NV, USA) was inserted 2 to 3 mm through the dura into the meninges over the superior colliculus (into the caudal part of the DRN). hOECs (1 × 106 cells), suspended in 10 μL of PBS, were slowly injected at a rate of 10 μL/min.
Behavioral Assessments
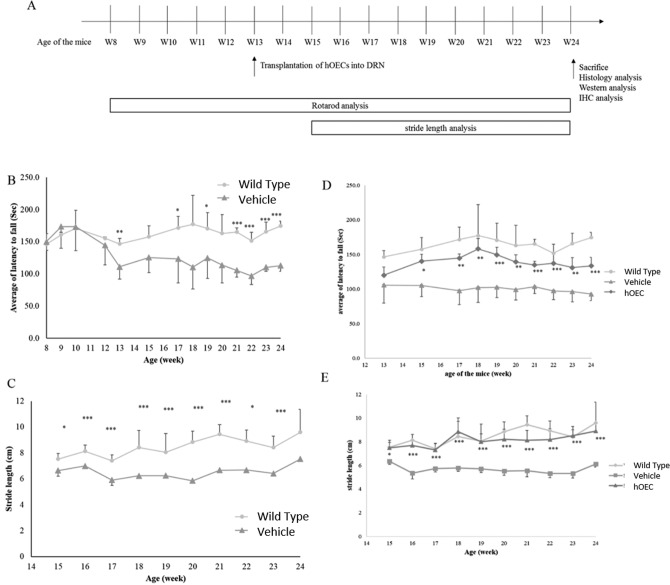
The rotarod performance tests and footprint pattern analysis were conducted 1 week prior to the transplantation surgery, and the recorded measurements were used as baseline values. After the transplantation procedure, the tests were conducted every 7 days for 11 weeks. The study design is illustrated in Fig. 1A.
Fig. 1.
Study flowchart and motor activity evaluation in wild-type mice and spinocerebellar ataxia type 3 (SCA3) transgenic mice. (A) Study design with transplantation and behavior analysis schedule. (B, D) Average of latency to fall (in seconds) was used to assess the rotarod performance of human olfactory ensheathing cell (hOEC)-transplanted SCA3 transgenic mice (hOEC group), wild-type C57BL/6 mice (wild-type group), and SCA3 transgenic mice injected with phosphate-buffered saline (PBS; vehicle group). (C, E) In footprint pattern analysis, the stride length (in centimeters) was measured to monitor the motor activities in the SCA3-hOEC group, wild-type group, and vehicle group. *P < 0.1, **P < 0.05, ***P < 0.01.
Rotarod performance test
The motor coordination of the mice was evaluated using a rotarod apparatus (IITC, BioLASCO, Taipei, Taiwan) under continuous acceleration (5-min trials at 4 to 40 rpm), and the time until the mouse fell (latency) was recorded.
Footprint pattern analysis
The footprint pattern test was used to analyze gait. For recording the stride length, the forelimbs and hind limbs of the mice were stained with different colors of nontoxic paint. Then, the mice walked along a 100 × 10 cm white sheet of paper. To evaluate gait uniformity, the distance between the center of the hind limb footprint and the center of preceding forelimb footprint was recorded over a sequence of 5 consecutive steps. The first and last footprints of the run were excluded from the analysis.
Histology and Cresyl Violet Staining
All mice were sacrificed at 24 weeks of age. Whole brain tissues from the mice were fixed in 3.7% formaldehyde overnight and subsequently embedded in paraffin. Sections (4 μm) were prepared and mounted onto microscope slides. After the sections were rehydrated and deparaffinized, they were stained with 0.5% crystal violet for 2 min. Then the slides were rinsed with water, counterstained with eosin Y, and mounted. The number of Purkinje cells was counted from 10 randomly chosen fields of the Purkinje layer in the cerebellar tissue sections using a microscope (Olympus IX71, Tokyo, Japan).
For the immunohistology analysis, cerebellar tissue sections (5 μm) were prepared as previously described. Antibodies including human-specific antimitochondrial (Merck Millipore, MA, USA) and anti–tumor necrosis factor α (TNF-α) antibodies were used. After the primary antibody application, a N-Histofine immunohistochemistry kit was utilized for recognizing the antigen-positive area, which appeared brown under the microscope.
Western Blot Analysis
Cerebellar tissues were incubated on ice in protein extraction buffer supplemented with protease inhibitors (iNTRON Biotechnology, Kyungki-Do, Korea). Then the protein samples were centrifuged and the supernatant was stored at −80 °C. The denatured proteins were separated using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and the separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Merck Millipore). The membranes were incubated with specific antibodies against TNF-α, interleukin 1β (IL-1β), postsynaptic density protein 95 (PSD-95), tryptophan hydroxylase 2 (TPH2), calbindin, and β-actin. All of the antibodies were purchased from Abcam. The immunoreactive bands were detected using horseradish peroxidase (HRP)–conjugated secondary antibodies (Merck Millipore) and enhanced chemiluminescence (ECL) reagents (Merck Millipore). The immunoreactive bands were analyzed using a luminescent image analyzer (Fujifilm LAS 3000, Tokyo, Japan).
Dot Blot Analysis
Dot blot analysis is a technique used to investigate the levels of high-molecular-weight proteins.35 Briefly, the protein samples were spotted on a PVDF membrane (Merck Millipore) and incubated for 1.5 h. Then ryanodine receptor (RYR) levels in the samples on the membrane were detected using a specific antibody against RYR (Abcam). The immunoreactive bands were detected using HRP-conjugated secondary antibodies (Merck Millipore) and ECL reagents (Merck Millipore). The immunoreactive bands were analyzed using a luminescent image analyzer (Fujifilm LAS 3000).
Statistical Analysis
Comparisons of different groups were analyzed using the Student t test (2 tailed, 2 sample). Values of P < 0.1 (*), P < 0.05 (**), and P < 0.01 (***) were labeled.
Results
SCA3 Mice Exhibited Severe Defects in Motor Function
The SCA3 transgenic mice used in this study carried a transgene (ATXN3-84Q) that simulates the progression of SCA3 in humans.36,37 As expected, SCA3 mice exhibited progressive declines in motor function. Motor function was evaluated using the rotarod performance test and footprint pattern analysis. Severe defects in motor function in SCA3 mice were first observed at approximately 13 weeks of age. The average length of time it took the 13-week-old mice in the SCA3 group to fall off the rotarod (latency) decreased compared with age-matched mice in the WT group (111.00 ± 19.07 s compared with 145 s, respectively; Fig. 1B). In addition, stride length in the SCA3 group (5.8-7.5 cm) significantly decreased compared with the WT group (7.5-9.6 cm; Fig. 1C). These findings confirmed that the ATXN3-84Q transgene induced motor function defects and symptoms characteristic of SCA3.
hOEC Transplantation Rescued Defects in Motor Function in SCA3 Mice
hOECs were generated from hNP samples as previously described.30 Immunofluorescent staining of S100β and p75 was performed to characterize the obtained hOECs. It was found that more than 95% of the cells were S100 and p75 positive (Fig. 2), which was similar to previous reports.30,38
Fig. 2.

Characterization of human olfactory ensheathing cells (hOECs). Immunofluorescent staining for characterizing hOECs using S100β and p75 markers. (A and B) p75 staining of hOECs: (A) nuclear staining of hOECs and (B) p75 staining. (C and D) S100β staining of hOECs: (C) nuclear staining of hOECs and (D) S100β staining. Scale bar: 200 µm.
To evaluate the therapeutic potential of hOECs in SCA3 mice, 1 × 106 cells were injected into the DRN brain regions of 13-week-old SCA3 mice. All the mice that underwent the procedure regained consciousness and resumed normal activities within 1 h after the surgery. No mice died as a result of the transplantation procedure. The defects in motor function observed in 13-week-old SCA3 mice exhibited signs of recovery 2 weeks after hOEC transplantation (week 15), and this effect was not observed in vehicle control SCA3 mice. At week 15, the average latency on the rotarod performance test was 119.95 ± 11.96 s in the hOEC transplantation group compared with 105.28 ± 16.36 s in the vehicle group (Fig. 1B). The average latency in the WT group was greater than 145 s. From weeks 15 through 24, the average latency in the hOEC transplantation group was 130 to 150 s, whereas the average latency in the vehicle group was 95 to 125 s. The difference in latency between the transplantation group and the vehicle group was statistically significant.
To further evaluate motor function, we analyzed stride length and balance using footprint analysis. The average stride length in the hOEC transplantation group was similar to the WT group (range: 7.5-9.6 cm), and the average stride length in both groups was significantly greater compared with the vehicle group (range: 5.8-7.5 cm; P < 0.1 at week 15 and P < 0.01 at weeks 16-24; Fig. 1C). Together, these results indicate that hOEC cell transplantation improved motor function in SCA3 mice.
hOEC Transplantation Inhibited the Expression of Inflammation Markers in the Cerebellum
Neuronal inflammation is a key contributor to cell damage in neurodegenerative diseases.39 It has been reported that in SCA, astrocytes and glial cells were activated in the early stage of the disease, which would further release various inflammatory factors such as TNF-α and IL-1β. The inflammatory factors were reported to be neurotoxic in various instances.40 To explore the mechanism by which the transplanted hOECs improved motor function in SCA3 mice, we evaluated markers of inflammation in the cerebellum at week 24 using Western blot analysis. TNF-α and IL-1β levels increased in the vehicle group compared with those in the WT group, whereas TNF-α and IL-1β levels in the hOEC transplantation group were similar to those in the WT group (Fig. 3A). Further immunohistochemical studies of TNF-α also showed an increase in positive cells for the inflammatory marker in the sham group. However, the hOEC-treated group showed relatively very less positive cells for TNF-α (Fig. 4D-F).
Fig. 3.
Western blotting and dot blotting analysis of mouse cerebellum. The expression levels of anti-inflammation marker (tumor necrosis factor α [TNF-α] and interleukin 1β [IL-1β]) and Purkinje cell generation associated marker (calbindin, postsynaptic density protein 95 [PSD-95], tryptophan hydroxylase 2 [TPH2], and ryanodine receptor [RYR]) in the wild-type group, human olfactory ensheathing cell (hOEC)-transplanted group, and sham group at 24 weeks were evaluated by (A) Western blotting and (B) dot blot analysis.
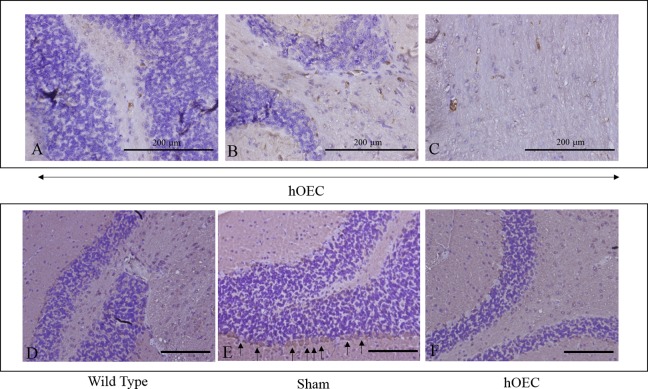
Fig. 4.
Immunohistochemistry staining of human mitochondria on human olfactory ensheathing cell (hOEC)-transplanted group (A-C) and tumor necrosis factor α (TNF-α) on wild-type, vehicle, and hOEC-transplanted group (D-F) in mouse cerebellum. The human mitochondrial positive cells, as indicated by the arrow, were found in (A, B) Purkinje cell layer and (C) granular layer of mouse cerebellar. The TNF-α immunohistochemistry for the tissue samples in the (D) wild-type, (E) vehicle, and (F) hOEC group (Scale bar: 200 µm in D, E, and F).
hOEC Transplantation Rescued Purkinje Cell Loss in the Cerebellum
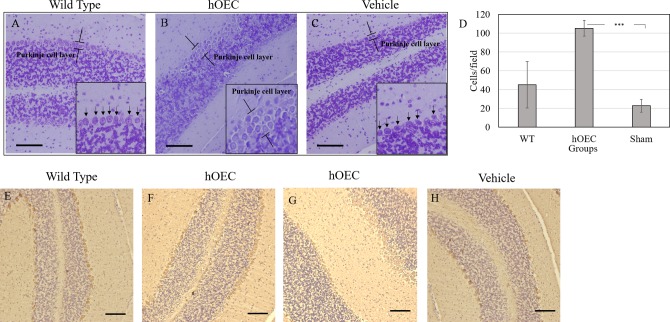
Purkinje cell degeneration is a key contributor to the onset of SCA3 disease. The number of cerebellar Purkinje cells in the WT, hOEC transplantation, and vehicle groups at week 24 was evaluated using cresyl violet staining and calbindin, a known marker of Purkinje cells, immunohistochemistry (IHC) staining. Consistent with previous reports, the number of Purkinje cells in the cerebellum constitutes a compact and integrated layer on the surface (Fig. 5A, E). In SCA3 transgenic mice, due to cell shrinkage and death, the Purkinje cell layer became fragmented (Fig. 5C, H).7,13,17 After hOEC transplantation, the number of Purkinje cells in SCA3 transgenic mice was increased and the Purkinje cell layer became compact again (Fig. 5B, F, G). These findings indicate that intracranial hOEC transplantation rescued the loss of Purkinje cells resulting from the onset of SCA3. To confirm this hypothesis, we evaluated calbindin levels using Western blot analysis. Consistent with the observed increase in the number of Purkinje cells, calbindin levels increased in the hOEC transplantation group compared with the vehicle group (Fig. 3A).
Fig. 5.
Recognizing Purkinje cells in the cerebellum. The Purkinje cells in the cerebellum were stained by crystal violet in (A) wild-type, (B) human olfactory ensheathing cell (hOEC)-transplanted, and (C) vehicle-treated groups. The number of Purkinje cells were quantified (D). The Purkinje cells in the cerebellum were recognized by their marker calbindin using immunohistochemistry (IHC) staining in (E) wild-type, (F and G) hOEC-transplanted, and (H) vehicle-treated groups. Scale bar: 100 µm. Arrows indicate the Purkinje cells.
The connection between cerebellum and parallel neuron fibres is formed during the maturation of Purkinje cells.41 PSD-95 is a membrane-associated guanylate kinase scaffolding protein that localizes to neural postsynaptic densities, and the PSD-95 expression level is directly associated with neuroplasticity, implying the maturation of Purkinje cells.42 Western blot analysis revealed that PSD-95 protein levels in the cerebellum increased in the cell transplantation group compared with the vehicle group (Fig. 3A). These findings suggest that intracranial hOEC transplantation enhanced neuronal complexity and neuroplasticity in the cerebellum of SCA3 mice.
hOEC Transplantation Upregulated Serotonin Levels and RYR Levels in the Cerebellum
Serotonin plays a key role in the regulation of cerebellar development43 as it can control Purkinje cell maturation and the elimination of climbing fibers.44,45 Western blot analysis revealed that levels of TPH2, the rate-limiting enzyme for serotonin synthesis, were upregulated in the cerebellum of mice in the hOEC transplantation group, and this effect was not observed in the vehicle group (Fig. 3A). In addition, immunohistochemistry staining of human mitochondria revealed that the transplanted hOECs were present in the cerebellum of mice in the hOEC transplantation group (Fig. 4A-C). Previous studies have demonstrated that RYR plays an important role in Purkinje cell maturation.44,46,47 Dot blot analysis revealed that RYR levels were also upregulated in the hOEC transplantation group (Fig. 3B). Together, these findings indicate that the upregulation of serotonin and RYR contributed to the observed improvements in motor function in the hOEC transplantation group.
In summary, these data indicate that improvements in motor function in SCA3 mice that underwent hOEC transplantation were mediated by the inhibition of Purkinje cell degeneration, the upregulation of serotonin levels, and the upregulation of RYR-induced Purkinje cell maturation.
Discussion
SCA3 is a neurodegenerative disease with no effective treatment options available. In the present study, we demonstrated that hOEC transplantation provided improvements in motor function in a mouse model of SCA3. SCA3 mice exhibited defects in motor function at 13 weeks of age, indicating that Purkinje cells in the cerebellum were degenerated. The transplanted hOECs rescued the observed defects in motor function by maintaining the number of Purkinje cells in the cerebellum and restoring their function.It is speculated that the decreased levels of TNF-α and IL-1β (Fig. 3A) was a sign of improvement in the microenvironment of the mouse cerebellum. This improvement might protect the Purkinje cells from degeneration. On the other hand, it has been reported that increased TPH2 and RYR 44,46,47 were associated with the maturation of Purkinje cells, thus indicating that the factors might have aided in the maturation of many progenitor or immature cells. We further observed that the number of Purkinje cells increased posttransplantation with hOECs (Fig. 5), and the cell number was even higher than that in the WT mice, which suggested that some of the cells were newly proliferated. Human mitochondrial immune-positive cells were not observed in the Purkinje cell layer after hOEC transplantation, indicating that these newly proliferated Purkinje cells were from SCA3 transgenic mice themselves.
We propose 3 potential mechanisms to account for the hOEC-mediated improvement in motor behavior in the SCA3 mouse model. Firstly, the decrease in inflammation markers indicated that hOECs inhibited inflammation, thereby improving the microenvironment in the cerebellum. This hypothesis is further supported by previous studies which demonstrated that transplanted hOECs modulate the immune response.48,49 The observed changes in the microenvironment might promote Purkinje cell survival, thereby rescuing the defects in motor function.
Secondly, hOECs promoted Purkinje cell differentiation and maturation. A previous report demonstrated that cocultured NSCs and hOECs strongly induced NSC differentiation and promoted the formation of markedly long neural processes.32 Other studies have reported that hOEC transplantation into spinal cord injury sites facilitates neural regeneration by promoting neurite outgrowth.50,51 Furthermore, RYR, which plays an important role in Purkinje cell differentiation and maturation,44,46,47 was upregulated in SCA3 mice that underwent hOEC transplantation. It has been reported that in the cerebellum, RYR1 and RYR2 were expressed in Purkinje cells, while the RYR2 was expressed in granule cells. Either downregulation of RYR1 expression in Purkinje cells or downregulation of RYR2 expression in granule cells led to the interruption of Purkinje cell branching, suggesting that both RYR1 and RYR2 played important roles in promoting the dendritic differentiation of Purkinje cells.47 The antibody utilized in this study is specific for both types of RYRs. Although it was not clearly defined which subtypes of RYRs were regulated by the transplantation of hOECs, the upregulated RYR expression in the cerebellum suggested the improvement in Purkinje cell differentiation and maturation (Fig. 3). Based on these data, we speculate that the transplanted hOECs triggered neural differentiation in cerebellar stem cells, thereby maintaining the number of Purkinje cells and restoring their function.
Thirdly, our results showed that hOECs regulated the expression levels of TPH2, implying increased levels of serotonin. Previous studies demonstrated that hOEC transplantation increases the serotonergic fibers in the central nervous system of an SCI model.52–56 Serotonergic fibers are the third largest population of afferent fibers extending into the cerebellum, after mossy fibers and climbing fibers.43 These serotonergic fibers form a dense network in the granular layer and are found around the somata of Purkinje cells and in the overlying molecular layer that contains the dendrites of Purkinje cells.43 It has been reported that serotonin influences the physiological maturation of Purkinje cells.43,54,55 Although the number of serotonergic fibers was not directly analyzed, the expression level of TPH2, the rate-limiting enzyme of serotonin synthesis, was measured. We showed that an increased TPH2 level was detected after hOEC transplantation (Fig. 3), which might be correlated with the increased Purkinje cells in the cerebellum (Fig. 5). These results indicate that the serotonin may play a key role in the therapeutic effect of hOECs. On the other hand, it is speculated that this inhibitory signal from serotonin might also play a role in rescuing the overactivated Purkinje cells in the SCA3 mouse model. Previous reports indicated that increased intrinsic excitability was found in the Purkinje cells of the SCA3 mouse model. This overexcitation results in the blockage of depolarization and the loss of the ability to sustain spontaneous repetitive firing, which may cause the degeneration of Purkinje cells.16 Further studies will be conducted to understand how hOECs affect serotonin in rescuing the SCA3 disease model. The site of cell transplantation might also be a key factor associated with the success of hOEC transplantation in the treatment of SCA3. Intracranial transplantation of hMSCs into the dorsal surface of the medulla failed to provide neuroprotective effects in a mouse model of SCA2, potentially because the distance between the injection site and the cerebellum was too long.22 In contrast, NSC transplantation into the cerebellum of SCA3 mice improved motor coordination and neuropathological defects by inducing the upregulation of neurotrophic factors and the downregulation of proinflammatory factors.17 In this study, hOECs were intracranially transplanted into the DRN, a site that is close to the cerebellum. The transplanted cells were observed in the Purkinje cell layer and the granular layer of the cerebellum, indicating that they had migrated to the intended location. Furthermore, the DRN is the site of the largest serotonergic nucleus in the brain, and TPH2 levels were upregulated in the cerebellum of SCA3 mice that underwent hOEC transplantation. As previously mentioned, serotonin plays an important role in cerebellar development,43 Purkinje cell maturation,44,45 and the inhibition of Purkinje cell excitation. hOEC-induced increases in serotonergic fibers have been observed at sites of spinal cord injury.57,58 In the present study, improvements in motor function were observed in SCA3 mice that underwent hOEC transplantation into the DRN, and these effects were not observed in the vehicle control group that received intracranial injections of PBS into the DRN. These results suggest that the DRN might be a suitable site for hOEC transplantation in the treatment of SCA3.
We also observed an increase in RYR levels in the cerebellum in SCA3 mice that underwent hOEC transplantation, indicating that the transplanted cells promoted Purkinje cell maturation. These effects might have been mediated by the activity of serotonergic fibers in the DRN. In summary, intracranial hOEC transplantation into the DRN represents an alternative delivery strategy for the treatment of SCA.
Footnotes
Ethical Approval: The isolation of hOECs from human samples and the usage of hOECs for studying the therapeutic effect on neurodegenerative diseases were approved by the institutional review board (IRB) of China Medical University and Hospital. The approval of number is CMHU104-REC2-129. IACUC: All procedures in the animal experiment described in the study were reviewed and approved by the institutional animal care and use committee (IACUC) of National Dong-Hwa University. The approval of number is 103-015.
Statement of Human and Animal Rights: Human Rights: The procedures and the experiments we've performed respected the ethical standards in the Helsinki Declaration of 1975, as revised in 2000, as well as the national law. Animal Rights: All animal experiments should comply with the ARRIVE guidelines and should be carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments, or the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). We followed such guidelines in the manuscript.
Statement of Informed Consent: The human nasal polyps were obtained with donors' written informed consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Coutinho P, Andrade C. Autosomal dominant system degeneration in Portuguese families of the Azores Islands. A new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology. 1978;28(7):703–709. [DOI] [PubMed] [Google Scholar]
- 2. Bird TD. Hereditary ataxia overview In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, et al., editors. Seattle (WA): GeneReviews(R); 1993. [Google Scholar]
- 3. Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, Kawakami H, Nakamura S, Nishimura M, Akiguchi I, et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994;8(3):221–228. [DOI] [PubMed] [Google Scholar]
- 4. Sun YM, Lu C, Wu ZY. Spinocerebellar ataxia: relationship between phenotype and genotype—a review. Clin Genet. 2016;90(4):305–314. [DOI] [PubMed] [Google Scholar]
- 5. Paulson HL. Dominantly inherited ataxias: lessons learned from Machado-Joseph disease/spinocerebellar ataxia type 3. Semin Neurol. 2007;27(2):133–142. [DOI] [PubMed] [Google Scholar]
- 6. Pedroso JL, Franca MC, Jr, Braga-Neto P, D’Abreu A, Saraiva-Pereira ML, Saute JA, Teive HA, Caramelli P, Jardim LB, Lopes-Cendes I, et al. Nonmotor and extracerebellar features in Machado-Joseph disease: a review. Mov Disord. 2013;28(9):1200–1208. [DOI] [PubMed] [Google Scholar]
- 7. Scherzed W, Brunt ER, Heinsen H, de Vos RA, Seidel K, Burk K, Schols L, Auburger G, Del Turco D, Deller T, et al. Pathoanatomy of cerebellar degeneration in spinocerebellar ataxia type 2 (SCA2) and type 3 (SCA3). Cerebellum. 2012;11(3):749–760. [DOI] [PubMed] [Google Scholar]
- 8. Bichelmeier U, Schmidt T, Hubener J, Boy J, Ruttiger L, Habig K, Poths S, Bonin M, Knipper M, Schmidt WJ, et al. Nuclear localization of ataxin-3 is required for the manifestation of symptoms in SCA3: in vivo evidence. J Neurosci. 2007;27(28):7418–7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paulson HL, Perez MK, Trottier Y, Trojanowski JQ, Subramony SH, Das SS, Vig P, Mandel JL, Fischbeck KH, Pittman RN. Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron. 1997;19(2):333–344. [DOI] [PubMed] [Google Scholar]
- 10. Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rub U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124(1):1–21. [DOI] [PubMed] [Google Scholar]
- 11. Nascimento-Ferreira I, Nobrega C, Vasconcelos-Ferreira A, Onofre I, Albuquerque D, Aveleira C, Hirai H, Deglon N, Pereira de Almeida L. Beclin 1 mitigates motor and neuropathological deficits in genetic mouse models of Machado-Joseph disease. Brain. 2013;136(pt 7):2173–2188. [DOI] [PubMed] [Google Scholar]
- 12. Nascimento-Ferreira I, Santos-Ferreira T, Sousa-Ferreira L, Auregan G, Onofre I, Alves S, Dufour N, Colomer Gould VF, Koeppen A, Deglon N, et al. Overexpression of the autophagic beclin-1 protein clears mutant ataxin-3 and alleviates Machado-Joseph disease. Brain. 2011;134(pt 5):1400–1415. [DOI] [PubMed] [Google Scholar]
- 13. Nobrega C, Nascimento-Ferreira I, Onofre I, Albuquerque D, Hirai H, Deglon N, de Almeida LP. Silencing mutant ataxin-3 rescues motor deficits and neuropathology in Machado-Joseph disease transgenic mice. PLoS One. 2013;8(1):e52396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simoes AT, Goncalves N, Koeppen A, Deglon N, Kugler S, Duarte CB, Pereira de Almeida L. Calpastatin-mediated inhibition of calpains in the mouse brain prevents mutant ataxin 3 proteolysis, nuclear localization and aggregation, relieving Machado-Joseph disease. Brain. 2012;135(pt 8):2428–2439. [DOI] [PubMed] [Google Scholar]
- 15. Thach WT. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968;31(5):785–797. [DOI] [PubMed] [Google Scholar]
- 16. Shakkottai VG, do Carmo Costa M, Dell’Orco JM, Sankaranarayanan A, Wulff H, Paulson HL. Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. J Neurosci. 2011;31(36):13002–13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mendonca LS, Nobrega C, Hirai H, Kaspar BK, Pereira de Almeida L. Transplantation of cerebellar neural stem cells improves motor coordination and neuropathology in Machado-Joseph disease mice. Brain. 2015;138(pt 2):320–335. [DOI] [PubMed] [Google Scholar]
- 18. Konno A, Shuvaev AN, Miyake N, Miyake K, Iizuka A, Matsuura S, Huda F, Nakamura K, Yanagi S, Shimada T, et al. Mutant ataxin-3 with an abnormally expanded polyglutamine chain disrupts dendritic development and metabotropic glutamate receptor signaling in mouse cerebellar Purkinje cells. Cerebellum. 2014;13(1):29–41. [DOI] [PubMed] [Google Scholar]
- 19. Whitney ER, Kemper TL, Rosene DL, Bauman ML, Blatt GJ. Calbindin-D28k is a more reliable marker of human Purkinje cells than standard Nissl stains: a stereological experiment. J Neurosci Methods. 2008;168(1):42–47. [DOI] [PubMed] [Google Scholar]
- 20. Laure-Kamionowska M, Maslinska D. Calbindin positive purkinje cells in the pathology of human cerebellum occurring at the time of its development. Folia Neuropathol. 2009;47(4):300–305. [PubMed] [Google Scholar]
- 21. Kayakabe M, Kakizaki T, Kaneko R, Sasaki A, Nakazato Y, Shibasaki K, Ishizaki Y, Saito H, Suzuki N, Furuya N, et al. Motor dysfunction in cerebellar Purkinje cell-specific vesicular GABA transporter knockout mice. Front Cell Neurosci. 2013;7:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang YK, Chen MH, Chiang YH, Chen YF, Ma WH, Tseng CY, Soong BW, Ho JH, Lee OK. Mesenchymal stem cell transplantation ameliorates motor function deterioration of spinocerebellar ataxia by rescuing cerebellar Purkinje cells. J Biomed Sci. 2011;18:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raisman G. Specialized neuroglial arrangement may explain the capacity of vomeronasal axons to reinnervate central neurons. Neuroscience. 1985;14(1):237–254. [DOI] [PubMed] [Google Scholar]
- 24. Doucette R. PNS-CNS transitional zone of the first cranial nerve. J Comp Neurol. 1991;312(3):451–466. [DOI] [PubMed] [Google Scholar]
- 25. Barraud P, Seferiadis AA, Tyson LD, Zwart MF, Szabo-Rogers HL, Ruhrberg C, Liu KJ, Baker CV. Neural crest origin of olfactory ensheathing glia. Proc Natl Acad Sci U S A. 2010;107(49):21040–21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barnett SC, Hutchins AM, Noble M. Purification of olfactory nerve ensheathing cells from the olfactory bulb. Dev Biol. 1993;155(2):337–350. [DOI] [PubMed] [Google Scholar]
- 27. Higginson JR, Barnett SC. The culture of olfactory ensheathing cells (OECs)--a distinct glial cell type. Exp Neurol. 2011;229(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pixley SK. The olfactory nerve contains two populations of glia, identified both in vivo and in vitro. Glia. 1992;5(4):269–284. [DOI] [PubMed] [Google Scholar]
- 29. Barnett SC, Chang L. Olfactory ensheathing cells and CNS repair: going solo or in need of a friend? Trends Neurosci. 2004;27(1):54–60. [DOI] [PubMed] [Google Scholar]
- 30. Shyu WC, Liu DD, Lin SZ, Li WW, Su CY, Chang YC, Wang HJ, Wang HW, Tsai CH, Li H. Implantation of olfactory ensheathing cells promotes neuroplasticity in murine models of stroke. J Clin Invest. 2008;118(7):2482–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chou RH, Lu CY, Wei L, Fan JR, Yu YL, Shyu WC. The potential therapeutic applications of olfactory ensheathing cells in regenerative medicine. Cell Transplant. 2014;23(4-5):567–571. [DOI] [PubMed] [Google Scholar]
- 32. Sethi R, Sethi R, Redmond A, Lavik E. Olfactory ensheathing cells promote differentiation of neural stem cells and robust neurite extension. Stem Cell Rev. 2014;10(6):772–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Agrawal AK, Shukla S, Chaturvedi RK, Seth K, Srivastava N, Ahmad A, Seth PK. Olfactory ensheathing cell transplantation restores functional deficits in rat model of Parkinson’s disease: a cotransplantation approach with fetal ventral mesencephalic cells. Neurobiol Dis. 2004;16(3):516–526. [DOI] [PubMed] [Google Scholar]
- 34. Kato T, Honmou O, Uede T, Hashi K, Kocsis JD. Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia. 2000;30(3):209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oprandy JJ, Olson JG, Scott TW. A rapid dot immunoassay for the detection of serum antibodies to eastern equine encephalomyelitis and St. Louis encephalitis viruses in sentinel chickens. Am J Trop Med Hyg. 1988;38(1):181–186. [DOI] [PubMed] [Google Scholar]
- 36. Cemal CK, Carroll CJ, Lawrence L, Lowrie MB, Ruddle P, Al-Mahdawi S, King RH, Pook MA, Huxley C, Chamberlain S. YAC transgenic mice carrying pathological alleles of the MJD1 locus exhibit a mild and slowly progressive cerebellar deficit. Hum Mol Genet. 2002;11(9):1075–1094. [DOI] [PubMed] [Google Scholar]
- 37. Chen X, Tang TS, Tu H, Nelson O, Pook M, Hammer R, Nukina N, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J Neurosci. 2008;28(48):12713–12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sasaki M, Lankford KL, Zemedkun M, Kocsis JD. Identified olfactory ensheathing cells transplanted into the transected dorsal funiculus bridge the lesion and form myelin. J Neurosci. 2004;24(39):8485–8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khansari PS, Sperlagh B. Inflammation in neurological and psychiatric diseases. Inflammopharmacology. 2012;20(3):103–107. [DOI] [PubMed] [Google Scholar]
- 40. Cvetanovic M, Ingram M, Orr H, Opal P. Early activation of microglia and astrocytes in mouse models of spinocerebellar ataxia type 1. Neuroscience. 2015;289:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McMahon SA, Diaz E. Mechanisms of excitatory synapse maturation by trans-synaptic organizing complexes. Curr Opin Neurobiol. 2011;21(2):221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meyer D, Bonhoeffer T, Scheuss V. Balance and stability of synaptic structures during synaptic plasticity. Neuron. 2014;82(2):430–443. [DOI] [PubMed] [Google Scholar]
- 43. Oostland M, van Hooft JA. The role of serotonin in cerebellar development. Neuroscience. 2013;248:201–212. [DOI] [PubMed] [Google Scholar]
- 44. Roegge CS, Morris JR, Villareal S, Wang VC, Powers BE, Klintsova AY, Greenough WT, Pessah IN, Schantz SL. Purkinje cell and cerebellar effects following developmental exposure to PCBs and/or MeHg. Neurotoxicol Teratol. 2006;28(1):74–85. [DOI] [PubMed] [Google Scholar]
- 45. Oostland M, Buijink MR, van Hooft JA. Serotonergic control of Purkinje cell maturation and climbing fibre elimination by 5-HT3 receptors in the juvenile mouse cerebellum. J Physiol. 2013;591(pt 7):1793–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaya L, Meissner B, Riedl MC, Muik M, Schwarzer C, Ferraguti F, Sarg B, Lindner H, Schweigreiter R, Knaus HG, et al. Direct association of the reticulon protein RTN1A with the ryanodine receptor 2 in neurons. Biochim Biophys Acta. 2013;1833(6):1421–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ohashi R, Sakata S, Naito A, Hirashima N, Tanaka M. Dendritic differentiation of cerebellar Purkinje cells is promoted by ryanodine receptors expressed by Purkinje and granule cells. Dev Neurobiol. 2014;74(4):467–480. [DOI] [PubMed] [Google Scholar]
- 48. Roet KC, Eggers R, Verhaagen J. Noninvasive bioluminescence imaging of olfactory ensheathing glia and schwann cells following transplantation into the lesioned rat spinal cord. Cell Transplant. 2012;21(9):1853–1865. [DOI] [PubMed] [Google Scholar]
- 49. Wewetzer K, Grothe C, Claus P. In vitro expression and regulation of ciliary neurotrophic factor and its alpha receptor subunit in neonatal rat olfactory ensheathing cells. Neurosci Lett. 2001;306(3):165–168. [DOI] [PubMed] [Google Scholar]
- 50. Takeoka A, Jindrich DL, Munoz-Quiles C, Zhong H, van den Brand R, Pham DL, Ziegler MD, Ramon-Cueto A, Roy RR, Edgerton VR, et al. Axon regeneration can facilitate or suppress hindlimb function after olfactory ensheathing glia transplantation. J Neurosci. 2011;31(11):4298–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ziegler MD, Hsu D, Takeoka A, Zhong H, Ramon-Cueto A, Phelps PE, Roy RR, Edgerton VR. Further evidence of olfactory ensheathing glia facilitating axonal regeneration after a complete spinal cord transection. Exp Neurol. 2011;229(1):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28(8):1611–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kubasak MD, Jindrich DL, Zhong H, Takeoka A, McFarland KC, Munoz-Quiles C, Roy RR, Edgerton VR, Ramon-Cueto A, Phelps PE. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain. 2008;131(pt 1):264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Darrow EJ, Strahlendorf HK, Strahlendorf JC. Response of cerebellar purkinje cells to serotonin and the 5-HT1A agonists 8-OH-DPAT and ipsapirone in vitro. Eur J Pharmacol. 1990;175(2):145–153. [DOI] [PubMed] [Google Scholar]
- 55. Kerr CW, Bishop GA. Topographical organization in the origin of serotoninergic projections to different regions of the cat cerebellar cortex. J Comp Neurol. 1991;304(3):502–515. [DOI] [PubMed] [Google Scholar]
- 56. Dieudonne S. Serotonergic neuromodulation in the cerebellar cortex: cellular, synaptic, and molecular basis. Neuroscientist. 2001;7(3):207–219. [DOI] [PubMed] [Google Scholar]
- 57. Stamegna JC, Felix MS, Roux-Peyronnet J, Rossi V, Feron F, Gauthier P, Matarazzo V. Nasal OEC transplantation promotes respiratory recovery in a subchronic rat model of cervical spinal cord contusion. Exp Neurol. 2011;229(1):120–131. [DOI] [PubMed] [Google Scholar]
- 58. Lu J, Feron F, Ho SM, Mackay-Sim A, Waite PM. Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic adult rats. Brain Res. 2001;889(1-2):344–357. [DOI] [PubMed] [Google Scholar]