Abstract
As eukaryotic life evolved, so too did the need for a source of energy that meets the requirements of complex organisms. Oxygen provides this vast potential energy source, but the same chemical reactivity which provides this potential also can have detrimental effects. The lung evolved as an organ that can efficiently promote gas exchange for the entire organism but as such, the lung is highly susceptible to its external environment. Oxygen can be transformed through both enzymatic and non-enzymatic processes into reactive oxygen species (ROS) and reactive nitrogen species (RNS), which can lead to protein, lipid, and DNA damage. Under normal conditions ROS/RNS concentrations are minimized through the activity of antioxidants located both intracellularly and in the epithelial lining fluid of the lung. Oxidative stress in the lung results when the antioxidant capacity is overwhelmed or depleted through external exposures, such as altered oxygen tension or air pollution, or internally. Internal sources of oxidative stress include systemic disease and the activation of resident cells and inflammatory cells recruited in response to an exposure or systemic response. Pulmonary responses to oxidative stress include activation of oxidases, lipid peroxidation, increases in nitric oxide, and autophagy. These internal and external exposures with the subsequent pulmonary responses contribute to development of diseases directly linked to oxidative stress. These include asthma, COPD, and lung cancers. While the vulnerability of the lung to oxidative stress is acknowledged, few effective preventative strategies or therapeutics are currently available.
I. Paradox-O2 is essential but toxic
The reduction of O2 provides the greatest energy release of all of the stably accumulating gases in the Earth’s atmosphere, and the energy it generates through electron transport is believed to be a universal requirement for the evolution of complex multicellular organisms. As complexity increases, though, so too does the need for an organ that can efficiently provide gas exchange for delivery throughout the organism. The role of the lung, then, is to take in appropriate quantities of oxygen, exchanging it for carbon dioxide and other gaseous wastes within the alveoli and capillary bed. It is estimated that the surface area of the lung is approximately 80–100 m2, breathing 10,000–20,000 L of air per day. As such, the lung is highly susceptible to its external environment.
Contrary to the basic need for oxygen as an energy source, the high chemical reactivity of oxygen leads to its transformation, through both enzymatic and non-enzymatic processes, into reactive oxygen species (ROS), often with detrimental effects. Approximately 2% of inhaled O2 generates ROS, leading to protein, lipid, and DNA damage (1). ROS-induced oxidative stress in the lung was originally defined as an imbalance between pro-oxidants and anti-oxidants, making the lung vulnerable to injury (2). A more recent concept advances this definition to include a dynamic process involving discrete redox pathways within cells and disruption of redox signaling with much broader implications in pathophysiology (3). Under normal conditions ROS concentrations are minimized through the activity of antioxidant enzymes such as superoxide dismutases and catalase, and via direct antioxidants such as vitamin E, GSH, and ascorbate located both intracellularly and in the epithelial lining fluid of the lung (1,4). Oxidative stress in the lung results when the antioxidant capacity is depleted through external exposures, such as air pollution or altered oxygen tension, or internally by activation of resident cells or inflammatory cells recruited in response to an exposure or injury.
II. Sources of oxidative stress
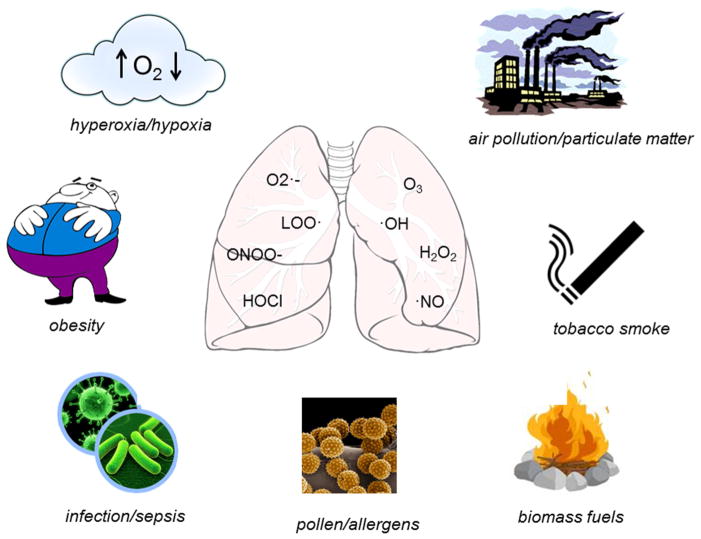
Altered oxygen tension
Increased fractional inspired oxygen (FIO2) is often administered clinically in situations of impaired lung function or poor perfusion. These conditions are often found in the context of sepsis, cardiac impairment, or prematurity and are associated with respiratory distress syndrome and/or bronchopulmonary dysplasia (5,6,7,8). While ambient oxygen levels precipitate oxidative reactions, raising the relative content of oxygen during inspiration can dramatically increase these reactions, especially when elevated oxygen therapy is accompanied by mechanical ventilation (9,10). The most vulnerable targets are the epithelium and endothelium of the lung which can be severely damaged by high concentrations of oxygen (11,12). Hyperoxia exposure causes direct oxidative injury to cells resulting in inflammatory and oxidative products and this injury induces an influx of inflammatory cells with their own capacity for ROS generation (see below) (13,14). Intriguingly, decreases in FIO2 can also lead to ROS generation through completely different mechanisms. Hypoxia precipitates uncoupling of mitochondrial respiration which subsequently causes the release of large amounts of O2·− (15). Mitochondrial SOD is available to convert O2·− to H2O2 but this system can be overwhelmed in the context of hypoxia. Furthermore, H2O2 is considered a ROS, is capable of generating oxidation reactions and is membrane permeable, allowing for migration to the cytoplasm or cell nucleus further oxidizing proteins and DNA.
Infection
Constant exposure to the external environment makes the lung especially vulnerable to infection. Whether bacterial, fungal or viral, infection generates an inflammatory response and subsequent accumulation of inflammatory cells such as macrophages (both resident alveolar and recruited), eosinophils, and neutrophils. As an immunological defense mechanism, immune cells respond by producing large quantities of ROS primarily from myeloperoxidases, eosinophil peroxidases, membrane-bound NADPH oxidases and mitochondrial sources, and products include superoxide (O2·−), nitric oxide (·NO), H2O2, and HOCl (16–18). Optimally, these reactive products are able to destroy invading organisms and upregulate appropriate signaling pathways to restore cellular homeostasis (19), but residual tissue damage often occurs. In addition, several common infective organisms produce their own ROS as part of their defense mechanisms (20).
Environmental inhalation exposure
Air pollution is a significant source of oxidative stress in the lung (21). The sources of air pollution are varied and include exhaust from combustion engines in high traffic areas and from refineries, which generate particulate matter (PM), gaseous pollutants such as ozone or nitrogen dioxides, tobacco smoke, biofuels (largely found in developing countries) and even pollen or sub-pollen grains, especially in the spring and fall. Large particles and coarse particulates (>PM10) are cleared by the nose and upper airways, but particles classified as fine (PM2.5) or ultrafine (<PM0.1) and gases enter the distal lungs, potentially causing injury and/or inflammatory responses (22,23). A single puff of tobacco smoke, aside from containing PM, has approximately 1015 radical molecules including epoxides, peroxides, semiquinones, poly aromatic hydrocarbons, hydroxyl radical, and H2O2, creating the “perfect storm” for cell injury (24). Inhaled irritants such as tobacco smoke also cause extensive lipid peroxidation, as evidenced by exhalation of pentane (25,26), as well as oxidation and nitration of proteins (27). Ozone (O3) is highly reactive and has been shown elevate superoxide levels (28) and other markers of oxidative stress such as 8-isoprostanes (29,30). Ultrafine PM0.1 has a unique pathology and one that has been difficult to characterize. Several studies have established that these particles can penetrate deep into the distal lung to cause localized inflammation, but may also pass through the surfactant layer of the lung to exert direct intracellular effects in lung cells and/or enter the vasculature (31–33). The exact mechanisms and signaling pathways leading to lung injury have not yet been elucidated.
Much of the world’s population still depends upon biomass fuels for cooking and heating. These fuels include wood, animal dung, and agricultural waste burned on open fires or traditional stoves (34). Burning biomass generates a variety of damaging chemicals including a large range of PM, carbon monoxide, nitrogen oxides and many organic species (35) (36). Indoor levels of PM in homes burning biomass fuel can range from 200 to 5000 mg/m3, while the World Health Organization guideline restricts exposure to ≤50μg/m3 (37,38). There is strong epidemiological evidence linking biomass fuel emissions to respiratory morbidity and mortality (39,40). Health concerns include respiratory infection, cancer of the trachea, bronchus or lung, ischemic heart disease, and chronic obstructive pulmonary disease (COPD) (41). Although the disease mechanisms are not fully understood, ROS generation and subsequent depletion of antioxidant enzymes following exposure is believed to be the primary event. Current hypotheses suggest this oxidative stress results in decreased immunity to infection and increased localized inflammation.
Systemic diseases
Obesity leads to a state of chronic inflammation due to expression of inflammatory adipokines produced and excreted by adipocytes in fat stores (42,43). These metabolic changes, as well as other comorbidities, result in an increase in systemic and lung-specific biomarkers of oxidative stress, such as increases in 8-isoprostanes and malondialdehyde levels, and decreases in reduced glutathione concentrations in bronchoalveolar lavage. Obesity can cause or exacerbate numerous respiratory conditions including reduction in lung volume, hypoxia, dyspnea, and increased bronchial hyperresponsiveness, and can reduce the effectiveness of inhaled corticosteroids and increase susceptibility to asthma. The inflammatory responses and hypoxemia caused by obesity increase production of ROS, precipitate tissue injury, and disrupt mitochondrial respiratory chain control (44). Sepsis is a state of acute inflammation that affects the lung in an indirect manner. Systemic infection precipitates global inflammatory responses with elevations of circulating and tissue cytokines and chemokines as well as the recruitment and proliferation of leukocytic cells to affected tissues (18). The injury caused by the infectious agent combined with the body’s response to it creates massive oxidative stress, often leading to acute respiratory distress syndrome, hypoxic episodes and the need for respiratory support and elevated oxygen therapies.
III. Pulmonary responses to oxidative stress
Oxidative stress occurring within the lung induces an array of responses in an attempt to protect the lung from further injury. Unfortunately, these responses often propagate further damage and the release of additional reactive species. Damaging exposures can stimulate epithelial, endothelial and airway cells, as well as alveolar macrophages, to generate internally derived oxidative products, and can recruit inflammatory cells to the lung to generate additional oxidative stress. Oxidases are a major source of internally derived oxidative stress in the lung. The nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, or NOXs, are found on the cell membrane of most cells, and NOXs found on the membranes of leukocytes are activated in response to bacteria or foreign elements in the lung. NOXs are known to produce an abundance of oxidants to protect the lung (19,45). Hypoxia and ischemic injury in the lung, as well as airway inflammation, can irreversibly convert peroxisomal xanthine dehydrogenase into xanthine oxidase, where it serves as a major source of superoxide production. Lipid peroxidation is often a serious consequence of oxidative stress. Disregulation of iron metabolism can induce Fenton chemical reactions resulting in oxidation of lipid molecules. Oxidative stress resulting from high oxygen tension, asthma and ozone exposure can trigger nonenzymatic oxidation reactions that form lipid hydroperoxides. These highly potent electrophiles can react with amino acid residues and participate in signaling through receptor mediated pathways, or take part in lipid propagation reactions generating even more reactive products (46,47). Lipid peroxidation products are notoriously difficult to measure but evidence can be found in terminal products such as 8-isoprostanes, 4-hydroxynonenal, and malondialdehyde (1). Cyclooxygenases and lipoxygenases are endogenously produced in the lung and inflammation increases the activity of these enzymes, leading to increased levels of enzymatically derived lipid products that can adversely affect many signaling pathways. The lung endothelium can also upregulate nitric oxide (·NO) production through increases in nitric oxide synthase activity in response to inflammation. ·NO is a highly reactive species and quickly reacts with O2·− generating peroxynitrite (ONOO−) and other RNS. RNS are responsible for nitrosative modifications of proteins and lipids that can inactive or alter their function. Another response to oxidative stress is autophagy (16,45). Autophagy is a process by which eukaryotes degrade dysfunctional organelles or large cytosolic molecules and make the components available for reuse by the cell. Generally, decreases in autophagy are beneficial while increases are detrimental to lung homeostasis. Pulmonary insults such as PM exposure, asthma, and COPD can result in cellular injury severe enough to trigger autophagy in lung cells (48,49).
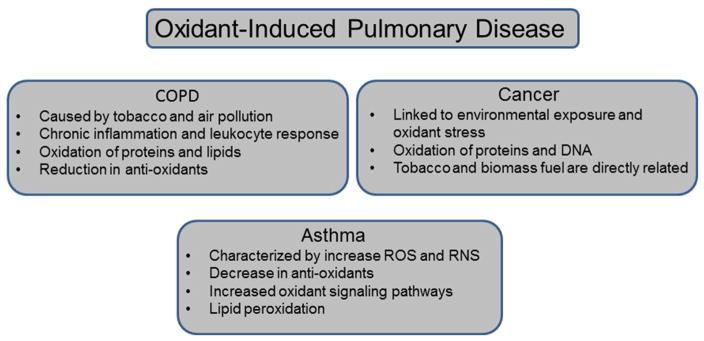
IV. Pulmonary diseases associated with oxidative stress (Figure 2)
Figure 2.
COPD in characterized by progressive persistent airflow limitation and hyperinflation, with both systemic and localized inflammation (1). The leading cause of COPD worldwide is tobacco smoke, followed by exposure to high levels of air pollution, and these exposures generate a state of chronic inflammation with persistent tissue injury and inflammatory responses (50). In addition, inhalation of these irritants leads to inactivation of essential protective proteases in the lung. The initiation and progression of COPD is strongly associated with the oxidation of essential proteins and lipids in the airway epithelium and sputum, preventing normal function (51) and with the reduction of antioxidants such as glutathione and superoxide dismutases (22,52).
Asthma is a chronic inflammatory disorder of the airways characterized by airway hyperreponsiveness and airflow limitations (53). It is the most common chronic lung disease, affecting 8–10% of the US population and approximately 300 million people worldwide (54). Clinically, asthma is associated with smooth muscle hypertrophy, goblet cell hyperplasia, and deposition of matrix proteins causing physiological and functional changes in the lung. Asthma is characterized by the presence of increased levels of ROS and RNS in sputum and breath condensates, which further increase mucus secretion, enhance epithelial permeability (55) (56) and induce smooth muscle contraction (57). This increase in oxidative potential is accompanied by a global decrease in antioxidants (58–60). ROS and RNS can modulate transcription through activation of proteins such as NFkB or AP-1 (61,62) and can induce expression of adhesion molecules CD11b and CD18 (63). Additionally, greater levels of nitrated proteins (through reaction with RNS) are present in asthmatics (64,65). Oxidative stress in asthmatics is also associated with substantial increases in lipid peroxidation with subsequent increases in pentane exhalation and elevated levels of 8-isoprostanes in bronchoalveolar lavage fluids during exacerbations (66,67). Asthma can be exacerbated by exposure to ozone or allergens, causing an even further increase in oxidative stress (68,69). Reconsidering asthma as a disease of disrupted redox balance may open new concepts for identification of pathogenesis.
Lung cancer is highly linked to environmental exposures and oxidant stress (31). While oxidants are not necessarily responsible for cellular transformation per se, they induce cell injury and changes in signaling pathways which are linked to neoplastic changes. Specifically, PM is considered a Group 1 carcinogen by the International Agency for Research on Cancer. As described above, PM can originate from smoking, biomass fuels or air pollution, and ultrafine PM can reach the distal areas of the lung to damage fragile epithelial cells and induce inflammatory responses with their subsequent ROS production. Oxidized and nitrated proteins have been linked to lung cancers and DNA damage occurs in response to intracellular oxidant production but the exact mechanism by which ROS contributes to cellular transformation is still unknown (27). Since smoking remains prevalent and biomass fuels are still frequently used in China, epidemiological studies in that country have identified strong links between these exposures and cancer risks, indicating that 75% of lung cancer deaths are attributable to combined exposure (70,71). Interestingly, transformed cancer cells express high levels of antioxidants, which contribute to their accelerated cell division, and have elevated overall redox capacity. Thus cancer cells are more sensitive to various chemotherapeutic oxidants than non-cancerous cells (72).
V. Conclusions
Oxygen is essential for all eukaryotic life but oxidative stress generated from ROS is associated with development of pulmonary diseases. Many sources of ROS/RNS, both external and internal, are largely associated with societal advancements without appropriate assessment of the risks and consequences. As antioxidant therapies have been largely ineffective in treating diseases associated with oxidative stress, there is a clear need for a better understanding of the chemistry of oxidation within biological systems as complex as the lung and implementing strategies that would reduce exposures.
Figure 1.
Highlights.
Oxygen is essential for higher life forms but contributes to formation of ROS/RNS
The lung is especially vulnerable to environmental exposures
Pulmonary responses to oxidative stress can cause further injury
Asthma, COPD, and cancers are linked to oxidative stress in the lung
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD--implications and relevance for treatment. International journal of chronic obstructive pulmonary disease. 2014;9:1207–1224. doi: 10.2147/COPD.S51226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sies H, Cadenas E. Oxidative stress: damage to intact cells and organs. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1985;311:617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- 3.Jones DP. Redefining oxidative stress. Antioxidants & redox signaling. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 4.Zhu S, Manuel M, Tanaka S, Choe N, Kagan E, Matalon S. Contribution of reactive oxygen and nitrogen species to particulate-induced lung injury. Environmental health perspectives. 1998;106(Suppl 5):1157–1163. doi: 10.1289/ehp.98106s51157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saugstad OD. Oxygen and oxidative stress in bronchopulmonary dysplasia. J Perinat Med. 2010;38:571–577. doi: 10.1515/jpm.2010.108. [DOI] [PubMed] [Google Scholar]
- 6.Karapetsa M, Pitsika M, Goutzourelas N, Stagos D, Tousia Becker A, Zakynthinos E. Oxidative status in ICU patients with septic shock. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2013;61:106–111. doi: 10.1016/j.fct.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Lang JD, McArdle PJ, O’Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest. 2002;122:314S–320S. doi: 10.1378/chest.122.6_suppl.314s. [DOI] [PubMed] [Google Scholar]
- 8.Ware LB, Matthay MA. The acute respiratory distress syndrome. The New England journal of medicine. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 9.Nagato AC, Bezerra FS, Lanzetti M, Lopes AA, Silva MA, Porto LC, Valenca SS. Time course of inflammation, oxidative stress and tissue damage induced by hyperoxia in mouse lungs. International journal of experimental pathology. 2012;93:269–278. doi: 10.1111/j.1365-2613.2012.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chacon-Cabrera A, Rojas Y, Martinez-Caro L, Vila-Ubach M, Nin N, Ferruelo A, Esteban A, Lorente JA, Barreiro E. Influence of mechanical ventilation and sepsis on redox balance in diaphragm, myocardium, limb muscles, and lungs. Transl Res. 2014;164:477–495. doi: 10.1016/j.trsl.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol. 1986;48:721–731. doi: 10.1146/annurev.ph.48.030186.003445. [DOI] [PubMed] [Google Scholar]
- 12.Crapo JD, Barry BE, Foscue H, Shelburne J. Structural and Biochemical Changes in Rat Lungs Ocurring During Exposures to Lethal and Adaptive Doses of Oxygen. Am Rev Res Dis. 1980;122:123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- 13.Turrens JF, Freeman BA, Crapo JD. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Archives of biochemistry and biophysics. 1982;217:411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- 14.Huang D, Fang F, Xu F. Hyperoxia induces inflammation and regulates cytokine production in alveolar epithelium through TLR2/4-NF-kappaB-dependent mechanism. European review for medical and pharmacological sciences. 2016;20:1399–1410. [PubMed] [Google Scholar]
- 15.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Experimental physiology. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 16.Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox biology. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo RF, Ward PA. Role of oxidants in lung injury during sepsis. Antioxidants & redox signaling. 2007;9:1991–2002. doi: 10.1089/ars.2007.1785. [DOI] [PubMed] [Google Scholar]
- 18.Bar-Or D, Carrick MM, Mains CW, Rael LT, Slone D, Brody EN. Sepsis, oxidative stress, and hypoxia: Are there clues to better treatment? Redox report: communications in free radical research. 2015;20:193–197. doi: 10.1179/1351000215Y.0000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free radical biology & medicine. 2011;51:1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rada B, Lekstrom K, Damian S, Dupuy C, Leto TL. The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. Journal of immunology. 2008;181:4883–4893. doi: 10.4049/jimmunol.181.7.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occupational and environmental medicine. 2003;60:612–616. doi: 10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holguin F. Oxidative stress in airway diseases. Annals of the American Thoracic Society. 2013;10(Suppl):S150–157. doi: 10.1513/AnnalsATS.201305-116AW. [DOI] [PubMed] [Google Scholar]
- 23.Krishna MT, Chauhan AJ, Frew AJ, Holgate ST. Toxicological mechanisms underlying oxidant pollutant-induced airway injury. Reviews on environmental health. 1998;13:59–71. [PubMed] [Google Scholar]
- 24.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Annals of the New York Academy of Sciences. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. discussion 27–18. [DOI] [PubMed] [Google Scholar]
- 25.Euler DE, Dave SJ, Guo H. Effect of cigarette smoking on pentane excretion in alveolar breath. Clin Chem. 1996;42:303–308. [PubMed] [Google Scholar]
- 26.Habib MP, Clements NC, Garewal HS. Cigarette smoking and ethane exhalation in humans. Am J Respir Crit Care Med. 1995;151:1368–1372. doi: 10.1164/ajrccm.151.5.7735586. [DOI] [PubMed] [Google Scholar]
- 27.Pignatelli B, Li CQ, Boffetta P, Chen Q, Ahrens W, Nyberg F, Mukeria A, Bruske-Hohlfeld I, Fortes C, Constantinescu V, Ischiropoulos H, Ohshima H. Nitrated and oxidized plasma proteins in smokers and lung cancer patients. Cancer research. 2001;61:778–784. [PubMed] [Google Scholar]
- 28.Tsukagoshi H, Haddad EB, Sun J, Barnes PJ, Chung KF. Ozone-induced airway hyperresponsiveness: role of superoxide anions, NEP, and BK receptors. J Appl Physiol (1985) 1995;78:1015–1022. doi: 10.1152/jappl.1995.78.3.1015. [DOI] [PubMed] [Google Scholar]
- 29.Montuschi P, Nightingale JA, Kharitonov SA, Barnes PJ. Ozone-induced increase in exhaled 8-isoprostane in healthy subjects is resistant to inhaled budesonide. Free radical biology & medicine. 2002;33:1403–1408. doi: 10.1016/s0891-5849(02)01084-5. [DOI] [PubMed] [Google Scholar]
- 30.Wiegman CH, Li F, Clarke CJ, Jazrawi E, Kirkham P, Barnes PJ, Adcock IM, Chung KF. A comprehensive analysis of oxidative stress in the ozone-induced lung inflammation mouse model. Clinical science. 2014;126:425–440. doi: 10.1042/CS20130039. [DOI] [PubMed] [Google Scholar]
- 31.Kim HJ, Choi MG, Park MK, Seo YR. Predictive and Prognostic Biomarkers of Respiratory Diseases due to Particulate Matter Exposure. Journal of cancer prevention. 2017;22:6–15. doi: 10.15430/JCP.2017.22.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li R, Kou X, Xie L, Cheng F, Geng H. Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats. Environmental science and pollution research international. 2015;22:20167–20176. doi: 10.1007/s11356-015-5222-z. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Zhao J, Jiang R, Song W. Rat lung response to ozone and fine particulate matter (PM2.5) exposures. Environmental toxicology. 2015;30:343–356. doi: 10.1002/tox.21912. [DOI] [PubMed] [Google Scholar]
- 34.Rehfuess E, Mehta S, Pruss-Ustun A. Assessing household solid fuel use: multiple implications for the Millennium Development Goals. Environmental health perspectives. 2006;114:373–378. doi: 10.1289/ehp.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mudway IS, Duggan ST, Venkataraman C, Habib G, Kelly FJ, Grigg J. Combustion of dried animal dung as biofuel results in the generation of highly redox active fine particulates. Particle and fibre toxicology. 2005;2:6. doi: 10.1186/1743-8977-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, Smith KR. Woodsmoke health effects: a review. Inhalation toxicology. 2007;19:67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- 37.Ezzati M, Kammen DM. The health impacts of exposure to indoor air pollution from solid fuels in developing countries: knowledge, gaps, and data needs. Environmental health perspectives. 2002;110:1057–1068. doi: 10.1289/ehp.021101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO. WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide; Global updat 2005. Geneva, Switzerland: 2006. [Google Scholar]
- 39.Guarnieri M, Diaz E, Pope D, Eisen EA, Mann J, Smith KR, Smith-Sivertsen T, Bruce NG, Balmes JR. Lung Function in Rural Guatemalan Women Before and After a Chimney Stove Intervention to Reduce Wood Smoke Exposure: Results From the Randomized Exposure Study of Pollution Indoors and Respiratory Effects and Chronic Respiratory Effects of Early Childhood Exposure to Respirable Particulate Matter Study. Chest. 2015;148:1184–1192. doi: 10.1378/chest.15-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith KR. Indoor air pollution in developing countries: recommendations for research. Indoor air. 2002;12:198–207. doi: 10.1034/j.1600-0668.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- 41.Kurmi OP, Dunster C, Ayres JG, Kelly FJ. Oxidative potential of smoke from burning wood and mixed biomass fuels. Free radical research. 2013;47:829–835. doi: 10.3109/10715762.2013.832831. [DOI] [PubMed] [Google Scholar]
- 42.Couillard C, Ruel G, Archer WR, Pomerleau S, Bergeron J, Couture P, Lamarche B, Bergeron N. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J Clin Endocrinol Metab. 2005;90:6454–6459. doi: 10.1210/jc.2004-2438. [DOI] [PubMed] [Google Scholar]
- 43.Fujita K, Nishizawa H, Funahashi T, Shimomura I, Shimabukuro M. Systemic oxidative stress is associated with visceral fat accumulation and the metabolic syndrome. Circulation journal: official journal of the Japanese Circulation Society. 2006;70:1437–1442. doi: 10.1253/circj.70.1437. [DOI] [PubMed] [Google Scholar]
- 44.Shore SA. Environmental perturbations: Obesity. Comprehensive Physiology. 2011;1:263–282. doi: 10.1002/cphy.c100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paredi P, Kharitonov SA, Leak D, Shah PL, Cramer D, Hodson ME, Barnes PJ. Exhaled ethane is elevated in cystic fibrosis and correlates with carbon monoxide levels and airway obstruction. Am J Respir Crit Care Med. 2000;161:1247–1251. doi: 10.1164/ajrccm.161.4.9906122. [DOI] [PubMed] [Google Scholar]
- 47.Paredi P, Kharitonov SA, Leak D, Ward S, Cramer D, Barnes PJ. Exhaled ethane, a marker of lipid peroxidation, is elevated in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:369–373. doi: 10.1164/ajrccm.162.2.9909025. [DOI] [PubMed] [Google Scholar]
- 48.Aggarwal S, Mannam P, Zhang J. Differential regulation of autophagy and mitophagy in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 2016;311:L433–452. doi: 10.1152/ajplung.00128.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen ZH, Wu YF, Wang PL, Wu YP, Li ZY, Zhao Y, Zhou JS, Zhu C, Cao C, Mao YY, Xu F, Wang BB, Cormier SA, Ying SM, Li W, Shen HH. Autophagy is essential for ultrafine particle-induced inflammation and mucus hyperproduction in airway epithelium. Autophagy. 2016;12:297–311. doi: 10.1080/15548627.2015.1124224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. 2001;429:195–207. doi: 10.1016/s0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- 51.Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013;144:266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 52.Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, MacNee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60:293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fitzpatrick AM, Jones DP, Brown LA. Glutathione redox control of asthma: from molecular mechanisms to therapeutic opportunities. Antioxidants & redox signaling. 2012;17:375–408. doi: 10.1089/ars.2011.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szefler SJ. Advancing asthma care: the glass is only half full! The Journal of allergy and clinical immunology. 2011;128:485–494. doi: 10.1016/j.jaci.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoshino T, Okamoto M, Takei S, Sakazaki Y, Iwanaga T, Aizawa H. Redox-regulated mechanisms in asthma. Antioxidants & redox signaling. 2008;10:769–783. doi: 10.1089/ars.2007.1936. [DOI] [PubMed] [Google Scholar]
- 56.Jarjour NN, Calhoun WJ. Enhanced production of oxygen radicals in asthma. The Journal of laboratory and clinical medicine. 1994;123:131–136. [PubMed] [Google Scholar]
- 57.Stewart RM, Weir EK, Montgomery MR, Niewoehner DE. Hydrogen peroxide contracts airway smooth muscle: a possible endogenous mechanism. Respiration physiology. 1981;45:333–342. doi: 10.1016/0034-5687(81)90016-5. [DOI] [PubMed] [Google Scholar]
- 58.Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. The Journal of allergy and clinical immunology. 2003;111:72–78. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 59.Dohlman AW, Black HR, Royall JA. Expired breath hydrogen peroxide is a marker of acute airway inflammation in pediatric patients with asthma. The American review of respiratory disease. 1993;148:955–960. doi: 10.1164/ajrccm/148.4_Pt_1.955. [DOI] [PubMed] [Google Scholar]
- 60.Henricks PA, Nijkamp FP. Reactive oxygen species as mediators in asthma. Pulmonary pharmacology & therapeutics. 2001;14:409–420. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- 61.Adcock IM, Brown CR, Kwon O, Barnes PJ. Oxidative stress induces NF kappa B DNA binding and inducible NOS mRNA in human epithelial cells. Biochemical and biophysical research communications. 1994;199:1518–1524. doi: 10.1006/bbrc.1994.1403. [DOI] [PubMed] [Google Scholar]
- 62.Haddad JJ, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1alpha and NF-kappa B redox sensitivity. Evidence for inhibition by glutathione oxidation in alveolar epithelial cells. J Biol Chem. 2000;275:21130–21139. doi: 10.1074/jbc.M000737200. [DOI] [PubMed] [Google Scholar]
- 63.Fraticelli A, Serrano CV, Jr, Bochner BS, Capogrossi MC, Zweier JL. Hydrogen peroxide and superoxide modulate leukocyte adhesion molecule expression and leukocyte endothelial adhesion. Biochim Biophys Acta. 1996;1310:251–259. doi: 10.1016/0167-4889(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 64.Hanazawa T, Kharitonov SA, Barnes PJ. Increased nitrotyrosine in exhaled breath condensate of patients with asthma. Am J Respir Crit Care Med. 2000;162:1273–1276. doi: 10.1164/ajrccm.162.4.9912064. [DOI] [PubMed] [Google Scholar]
- 65.MacPherson JC, Comhair SA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, Samoszuk MK, Hazen SL. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. Journal of immunology. 2001;166:5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- 66.Barreto M, Villa MP, Olita C, Martella S, Ciabattoni G, Montuschi P. 8-Isoprostane in exhaled breath condensate and exercise-induced bronchoconstriction in asthmatic children and adolescents. Chest. 2009;135:66–73. doi: 10.1378/chest.08-0722. [DOI] [PubMed] [Google Scholar]
- 67.Dworski R, Murray JJ, Roberts LJ, 2nd, Oates JA, Morrow JD, Fisher L, Sheller JR. Allergen-induced synthesis of F(2)-isoprostanes in atopic asthmatics. Evidence for oxidant stress. Am J Respir Crit Care Med. 1999;160:1947–1951. doi: 10.1164/ajrccm.160.6.9903064. [DOI] [PubMed] [Google Scholar]
- 68.Holz O, Jorres RA, Timm P, Mucke M, Richter K, Koschyk S, Magnussen H. Ozone-induced airway inflammatory changes differ between individuals and are reproducible. Am J Respir Crit Care Med. 1999;159:776–784. doi: 10.1164/ajrccm.159.3.9806098. [DOI] [PubMed] [Google Scholar]
- 69.Peden DB, Setzer RW, Jr, Devlin RB. Ozone exposure has both a priming effect on allergen-induced responses and an intrinsic inflammatory action in the nasal airways of perennially allergic asthmatics. Am J Respir Crit Care Med. 1995;151:1336–1345. doi: 10.1164/ajrccm.151.5.7735583. [DOI] [PubMed] [Google Scholar]
- 70.Guan WJ, Zheng XY, Chung KF, Zhong NS. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016;388:1939–1951. doi: 10.1016/S0140-6736(16)31597-5. [DOI] [PubMed] [Google Scholar]
- 71.Lin HH, Murray M, Cohen T, Colijn C, Ezzati M. Effects of smoking and solid-fuel use on COPD, lung cancer, and tuberculosis in China: a time-based, multiple risk factor, modelling study. Lancet. 2008;372:1473–1483. doi: 10.1016/S0140-6736(08)61345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu J, Wang Z. Increased Oxidative Stress as a Selective Anticancer Therapy. Oxidative medicine and cellular longevity. 2015;2015:294303. doi: 10.1155/2015/294303. [DOI] [PMC free article] [PubMed] [Google Scholar]