Abstract
In most eukaryotic organisms, translation elongation requires two highly conserved elongation factors eEF1A and eEF2. Fungal systems are unique in requiring a third factor, the eukaryotic Elongation Factor 3 (eEF3). For decades, eEF3, a ribosome-dependent ATPase, was considered “fungal-specific”, however, recent bioinformatics analysis indicates it may be more widely distributed among other unicellular eukaryotes. In order to determine whether divergent eEF3-like proteins from other eukaryotic organisms can provide the essential functions of eEF3 in budding yeast, the eEF3-like proteins from Schizosaccharomyes pombe and an oomycete, Phytophthora infestans, were cloned and expressed in Saccharomyces cerevisiae. Plasmid shuffling experiments showed that both S. pombe and P. infestans eEF3 can support the growth of S. cerevisiae in the absence of endogenous budding yeast eEF3. Consistent with its ability to provide the essential functions of eEF3, P. infestans eEF3 possessed ribosome-dependent ATPase activity. Yeast cells expressing P. infestans eEF3 displayed reduced protein synthesis due to defects in translation elongation/termination. Identification of eEF3 in divergent species will advance understanding of its function and the ribosome specific determinants that lead to its requirement as well as contribute to the identification of functional domains of eEF3 for potential drug discovery.
Introduction
Translation is a highly conserved process during which proteins are synthesized from messenger RNA. This process is divided into the four phases of initiation, elongation, termination and ribosome recycling, each of which requires a specialized set of soluble protein factors (reviewed in [1]). During the initiation phase of translation, eukaryotic initiation factors facilitate the binding of an 80S ribosome at the start codon of the mRNA. This is followed by a repetitive cycle of aminoacyl-tRNA (aa-tRNA) delivery, peptide bond formation and ribosomal translocation during the elongation phase. In most eukaryotic organisms, translation elongation is catalyzed by two soluble elongation factors (eEFs), eEF1A and eEF2. eEF1A, the functional homolog of bacterial EF-Tu, is a G-protein that binds and recruits aa-tRNAs to the A-site of the ribosome. When a codon-anticodon match occurs, the ribosome stimulates eEF1A-mediated GTP hydrolysis resulting in the release of inactive GDP-bound eEF1A from the ribosome and accommodation of the aa-tRNA into the A-site. Following peptide bond formation, eEF2, the homolog of the bacterial GTPase EF-G, catalyzes the translocation of the peptidyl-tRNA from the A-site to the P-site of the ribosome thereby positioning the next codon for decoding. When a stop codon is encountered by the ribosome, release factors release the polypeptide from the ribosome and the ribosomal subunits are recycled for another round of protein synthesis.
Fungal translation elongation has long been considered unique among eukaryotes in its requirement for a third elongation factor, eEF3. eEF3 is a ribosome-dependent ATPase required for in vitro translation elongation assays when using ribosomes purified from yeast [2]. In contrast, Saccharomyces cerevisiae eEF1A and eEF2 alone catalyze translation elongation with rat liver ribosomes [3]. Therefore, it is likely a ribosome-specific determinant that establishes the need for eEF3. The reason why ribosomes from yeast require eEF3 and the function of eEF3 itself are not well understood. In one study, eEF3 facilitated the release of de-acetylated tRNA from the E-site of the ribosome [4]. eEF3 also stimulates eEF1A-mediated binding of cognate aa-tRNA to the A-site [5, 6] potentially through a direct interaction with eEF1A. [7, 8]. in vivo experiments confirm the important role of eEF3 in yeast protein synthesis. eEF3 is encoded by an essential gene in S. cerevisiae and strains harboring either temperature sensitive or mutant forms of eEF3 display protein synthesis and translation elongation defects [7–10]. eEF3 orthologues from other yeasts including Candida albicans, another ascomycete and Cryptococcus neoformans, a basidiomycete, can complement the loss of eEF3 in S. cerevisiae suggesting that the function of eEF3 is likely conserved among fungi [11, 12].
Structural studies of eEF3 identified five domains: an amino-terminal HEAT repeat followed by a four-helix bundle domain and two ATP-binding cassette (ABC) domains, the second of which is interrupted by a chromodomain insertion [13]. A cryo-EM reconstruction of the eEF3-ATP-post-translocation 80S ribosome complex demonstrated that eEF3 binds the ribosome near the E-site in agreement with its proposed function in E-site tRNA release [13]. The chromodomain insertion is proposed to interact with the ribosome and stabilize the ribosomal L1 stalk in an open conformation which may facilitate tRNA release. Mutations in this domain, however, show significant reduction in ATPase activity without affecting overall ribosome binding [10].
Recent bioinformatic analysis identified potential eEF3 orthologues in multiple non-fungal, lower eukaryotic species [14, 15]. These eEF3-like protein sequences are as similar to S. cerevisiae eEF3 as the functionally complementary C. neoformans eEF3, suggesting that these putative eEF3s are likely to maintain at least a subset of eEF3 functions. In this study, we present the first direct evidence suggesting that functional eEF3 orthologues exist outside the fungal kingdom. We have expressed the eEF3 orthologue from Schizosaccharomyes pombe and the oomycete Phytopthora infestans, the causative agent of late potato blight, in S. cerevisiae and showed that either can provide the essential functions of eEF3. in vitro studies demonstrated that P. infestans eEF3, like S. cerevisiae eEF3, possesses ribosome-stimulated ATPase activity.
Materials and methods
Plasmid construction
S. cerevisiae eEF3: The sequence encoding an N-terminal 6x-His tagged S. cerevisiae eEF3 was cloned as a BamHI fragment from plasmid pTKB1142 into plasmid pTKB328 (CEN LEU2) where it is expressed from the S. cerevisiae TEF5 promoter (pTKB1263).
Schizzosaccharomyces pombe eEF3: The S. pombe tef3+ gene was amplified by PCR from fission yeast genomic DNA prepared using the Epicentre Masterpure Yeast DNA Purification Kit. The PCR was performed using the following primers: Forward Primer-5’ ATTGTTTGATCAATGCATCATCATCATCATCATTCTGCAAAGAGTGA-GAATAA-3’, Reverse Primer-5’CTATTAGGGCCCTTACAGGTCACTGACCTC-3’. The PCR product encoding N-terminal 6x-His tagged S. pombe eEF3 was cloned into the BamHI and ApaI restriction sites of the pTKB328 vector and is expressed from the S. cerevisiae TEF5 promoter (TKB1269).
P. infestans eEF3: The sequence encoding P. infestans eEF3 (NCBI Reference Sequence XP_002906761.1) was synthesized as two codon-optimized gblock gene fragments with an N-terminal 6x-His tag (Integrated DNA Technologies). These two gene fragments were cloned into plasmid pTKB328 using Gibson assembly (New England Biolabs) such that N-terminal 6x-His tagged P. infestans eEF3 is expressed from the S. cerevisiae TEF5 promoter (pTKB1264). The Sac1-XhoI fragment of pTKB1264 containing the TEF5 promoter and P. infestans eEF3 was cloned into pTKB372 (2μ LEU2) creating pTKB1268
Strains and growth conditions
S. cerevisiae strains used in this study are listed in Table 1. Yeast cells were grown in either yeast extract-peptone-dextrose (YEPD; 1% Bacto-yeast extract, 2% Bacto-tryptone, 2% dextrose) or defined synthetic complete medium (C) lacking the indicated amino acid and supplemented with 2% dextrose as a carbon source. Growth curves were generated by diluting exponentially growing cultures to an A600 of 0.1 and then measuring the A600 at the indicated time points.
Table 1. S. cerevisiae strains used in this study.
| Strain | Genotype | Reference |
|---|---|---|
| TKY1617 | MATα leu2-3, 112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15 yef3:HIS3 p S.cerevisiae YEF3 URA3 CEN | [10] |
| TKY1653 | MATαleu2-3,112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15 yef3::HIS3 p 6x-His YEF3 TRP1 CEN | [10] |
| TKY1786 | MATα leu2-3, 112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15 yef3:HIS3 pTEF5 6x-His S. pombe TEF3 LEU2 CEN | This work |
| TKY1787 | MATα leu2-3, 112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15 yef3:HIS3 pTEF5 6x-His P. infestans EF3 LEU2 CEN | This work |
| TKY1791 | MATα leu2-3, 112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15 yef3:HIS3 pTEF5 6xHis S. cerevisiae YEF3 LEU2 CEN | This work |
| TKY1792 | MATα leu2-3, 112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15 yef3:HIS3 pTEF5 6x-His P. infestans EF3 LEU2 2μ | This work |
Heterologous eEF3 proteins were expressed as the only form of eEF3 by plasmid shuffling in TKY1617. Briefly, TKY1617 was individually transformed with the plasmids described above using a lithium acetate method. Transformants were counterselected on media containing 5-fluoroorotic acid to select cells that have lost the complementing S. cerevisiae eEF3 expressing plasmid.
Immunoblotting
Yeast cells in mid-log phase growth were lysed with glass beads in 100 mM Tris-HCl pH 8.0, 20% glycerol, 1 mM dithiothreitol and 1 mM phenylmethylsulfonyl fluoride and cell debris was removed by centrifugation. Protein concentration in cell lysates was determined using the BioRad reagent according to the manufacturer’s instructions. Twenty-five micrograms of total protein were separated on a 7.8% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was stained with Ponceau S and immunoblotted with either a monoclonal anti-His antibody (BD Biosciences) or a monoclonal anti-PGK antibody (Novex). Quantitation of immunoblots was performed using ImageQuant software (GE Healthcare).
Antibiotic sensitivity assays
Yeast strains in exponential growth were diluted to an A600 of 0.6 and 200 μl was spread on YEPD plates. Sterile discs were placed on the plates and 10 μL of antibiotic at the indicated concentration was pipetted onto the disc. Plates were incubated at 30°C for 2d at which time the diameter of the zone of growth inhibition surrounding the discs was measured. Antibiotic concentrations were 1 mM cycloheximide, 25 mM hygromycin and 800 mg/mL paromomycin.
Translation assays
For in vivo [35S] methionine incorporation assays, yeast strains were grown in liquid culture in C-Met at 30°C to mid-log phase and assayed as previously described [16]. All time points were analyzed in triplicate. Polyribosome extracts were harvested and run on a 7–47% sucrose density gradient as previously described [17]. The areas under the 80S and polyribosome peaks were determined using Image J (National Institutes of Health).
Purification of 6x-His-eEF3 proteins
The 6x-His tagged S. cerevisiae eEF3 was purified from TKY1653 as previously described [10]. The 6x-His tagged P. infestans eEF3 was purified from TKY1792 on a 1 mL HisTrap Column (GE Healthcare) in buffer A (50 mM potassium phosphate, pH 8.0, 1 M KCl, 0.1% Triton X-100, 10% glycerol, 1 mM DTT, and 0.2 mM PMSF plus Complete Protease Inhibitor (Roche)) with 20 mM imidazole. The protein was eluted using a gradient (0–100%) with buffer B (50 mM potassium phosphate, pH 8.0, 1 M KCl, 0.1% Triton X-100, 10% glycerol, 500 mM imidazole, 1 mM DTT, and 0.2 mM PMSF plus Complete Protease Inhibitor) over 20 column volumes. The protein was dialyzed into buffer C (20 mM HEPES, pH 7.5, 500 mM KCl, 0.1% Triton X-100, 0.1 mM EDTA, 10% glycerol, 1 mM DTT, and 0.2 mM PMSF)
ATP hydrolysis assay
ATP hydrolysis was analyzed using the PiColorLock Gold Phosphate Detection System (Innova Biosciences) according to the manufacturer’s instructions with the following specifications. The reactions were performed at room temperature for 30 min in 50 mM Tris, pH 7.5, 5 mM MgCl2, and 1 mM ATP with varying concentration of eEF3 in the presence or absence of 25 nM crude S. cerevisiae ribosomes prepared by the method of [18].
Phylogenetic analysis
A phylogenetic tree based on eEF3 sequences was generated using phylogeny.fr (http://www.phylogeny.fr) [19, 20]. Accession numbers for the sequences used were as follows: S. cerevisiae (NP_013350.1), S. pombe (NP_588285.1), Candida albicans (XP_711356.2), Aspergillus fumigatus (XP_748943.1), Blastomyces dermatitidis (EQL38330.1), Cryptococcus neoformans (XP_570261.1), P. infestans (XP_002906761.1), Saprolegnia diclina (XP_008620178.1), Ectocarpus siliculosus (CBJ25582.1), Fistulifera solaris (GAX16349.1), Chlamydomonas reinhardtii (XP_001692287.1), Volvox carteri (XP_002951155.1), and Coccomyxa subellipsoidea (EIE22864.1). Default setting were used except for the inclusion of bootstrapping.
Results
eEF3 activity is not unique to fungi
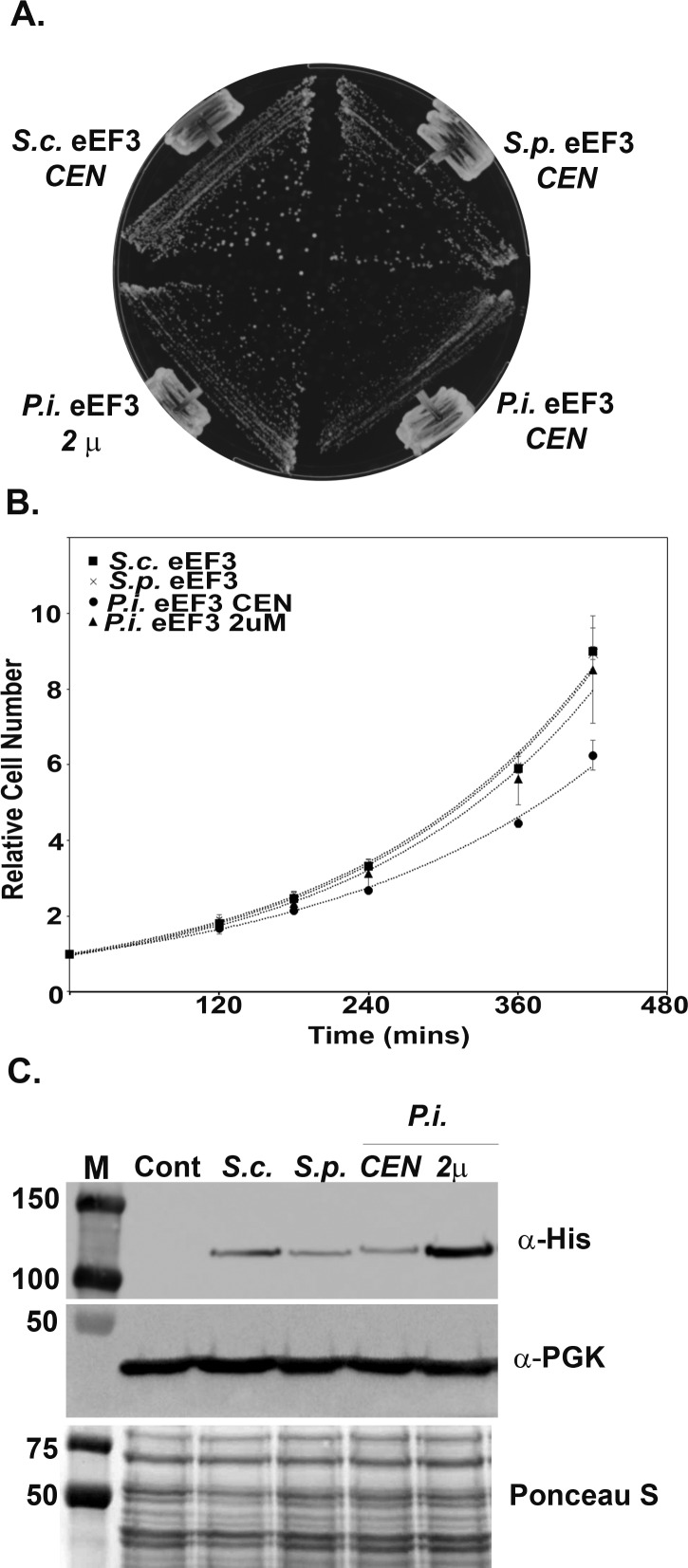
While originally designated as a fungal-specific elongation factor, recent bioinformatic analysis identified eEF3-like proteins in several lower eukaryotic species [14, 15]. The most significant regions of conservation between these proteins and S. cerevisiae eEF3 are found within the two ABC-type ATPase domains. Therefore, it is important to demonstrate that these proteins are not simply related ATPases but that they can provide the essential functions of eEF3. To address this question, the genes encoding eEF3 from an evolutionarily distant ascomycete, S. pombe, and the putative form of eEF3 from the oomycete, P. infestans, were cloned into a low copy S. cerevisiae expression vector with an N-terminal 6x-His tag (materials and methods). S. cerevisiae eEF3 is approximately 62% identical to S. pombe eEF3 and 45% identical to P. infestans eEF3. S. pombe eEF3 was included in the analysis to broaden the understanding of the evolution of eEF3. Both eEF3 proteins are expressed from the strong TEF5 promoter and the P. infestans eEF3 gene sequence was codon optimized for expression in budding yeast. TheYEF3 gene encoding S. cerevisiae eEF3 was also cloned into the same expression vector as a control. The eEF3 expression plasmids were individually transformed into a S. cerevisiae strain in which the only copy of YEF3 is untagged and present on a URA3 plasmid. The ability of S. pombe or P. infestans eEF3 to function as the only form of the protein was assessed by growth on 5-FOA containing media which only allows growth of cells that have lost the URA3 plasmid. Both S. pombe and P. infestans eEF3 could support growth of a yef3Δ strain (Fig 1A). While expression of S. pombe eEF3 could support growth at a level similar to S. cerevisiae eEF3, the strain expressing P. infestans eEF3 grew at a reduced rate (Fig 1A and 1B). The average doubling time was 137±4 min for the S.c. eEF3 expressing strain, 135±2 min for the S.p. eEF3 expressing strain and 162±9 min for the P.i. eEF3 (CEN) expressing strain. The ability of P. infestans eEF3 to complement the loss of YEF3 in S. cerevisiae suggests that it can perform the essential functions of eEF3 and is a bona fide eEF3 homologue.
Fig 1. S. pombe (S.p.) and P. infestans (P.i.) eEF3 complement the loss of S. cerevisiae (S.c.) eEF3.
(A) Yeast strains expressing eEF3 from the indicated species as the only form of eEF3 were streaked onto YEPD medium and incubated at 30°C for 2 d. CEN–low copy number plasmid/low expression. 2μ –high copy number plasmid/high expression. (B) Growth curves were generated from A600 measurements of exponentially growing cultures over the indicated time period. Error bars represent standard error. (C) Whole cell extracts were prepared from the indicated strains, separated by SDS-PAGE, and stained with Ponceau S (bottom panel). The membrane was then cut and immunoblotted with either an anti-His antibody to detect eEF3 or anti-Pgk1 antibody as a loading control. The control lane is an extract from a yeast strain (TKY1617) that does not express epitope tagged eEF3.
The reduced growth rate of cells expressing P. infestans eEF3 may arise from either reduced protein expression or reduced activity in a heterologous system. Cell lysates were collected and immunoblotted with an anti-His antibody to detect eEF3 expression levels in strains whose growth was supported solely by either S. cerevisiae eEF3, S. pombe eEF3 or P. infestans eEF3 (Fig 1C). The steady state levels of both S. pombe eEF3 and P. infestans eEF3 were comparable but lower than that of the native S. cerevisiae eEF3 protein. These data suggest that there is a threshold level of eEF3 activity required for wild-type growth. To determine if increasing the expression of P. infestans eEF3 would rescue the slow growth phenotype, 6x-His tagged P. infestans eEF3 expressed from the same promoter was cloned into a high copy expression plasmid and its expression level and ability to support growth of a yef3Δ strain were determined as described above. Over expression of P. infestans eEF3 (average of 2.2-fold over S. cerevisiae eEF3) could rescue the growth of yef3Δ cells to wild-type (S. cerevisiae eEF3) levels with an average doubling time of 138±14 min.(Fig 1B and 1C).
P. infestans eEF3 shows ribosome-stimulated ATPase activity
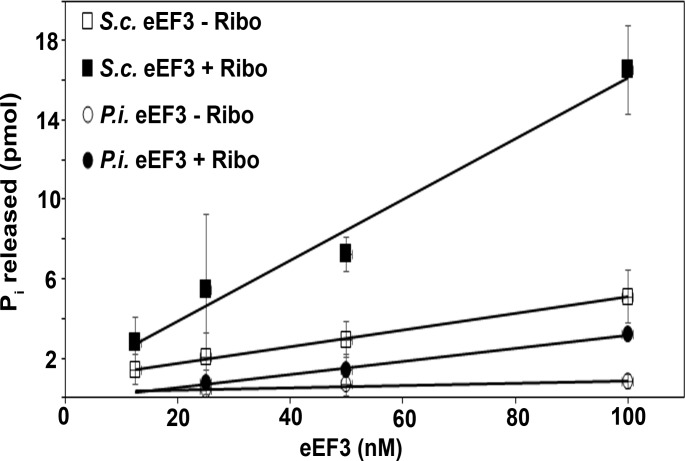
A key characteristic of S. cerevisiae eEF3 is its ribosome-stimulated ATPase activity. To determine if P. infestans eEF3 demonstrated similar activity, P. infestans eEF3 was expressed and purified from budding yeast and its activity was compared to S. cerevisiae eEF3. S. cerevisiae eEF3 has a low basal ATPase activity that was stimulated 3.2-fold at 100 nM eEF3 by the inclusion of ribosomes as reported previously (Fig 2, [10, 21, 22]). The activity of P. infestans eEF3 in the absence of ribosomes was very low compared to S. cerevisiae eEF3 (6.2-fold lower) at the same concentration. The inclusion of S. cerevisiae ribosomes could stimulate P. infestans eEF3 activity (3.9-fold) which is similar to the observed ribosome stimulation of S. cerevisiae eEF3 activity. While the ribosome-stimulated P. infestans eEF3 activity was concentration dependent, its activity was still 5.2-fold lower than S. cerevisiae eEF3.
Fig 2. P. infestans eEF3 possesses ribosome-stimulated ATPase activity.
ATPase activity of S. cerevisiae (■) and P. infestans (●) eEF3 was assayed in the presence (filled) or absence (empty) of S. cerevisiae ribosomes. The Pi released was measured using the PiColorLock Gold Phosphate Detection System. Reactions included varying amounts of eEF3, 25 nM ribosomes and 1 mM ATP and were carried out at room temperature for 30 min.
Cells expressing P. infestans eEF3 exhibit translation defects
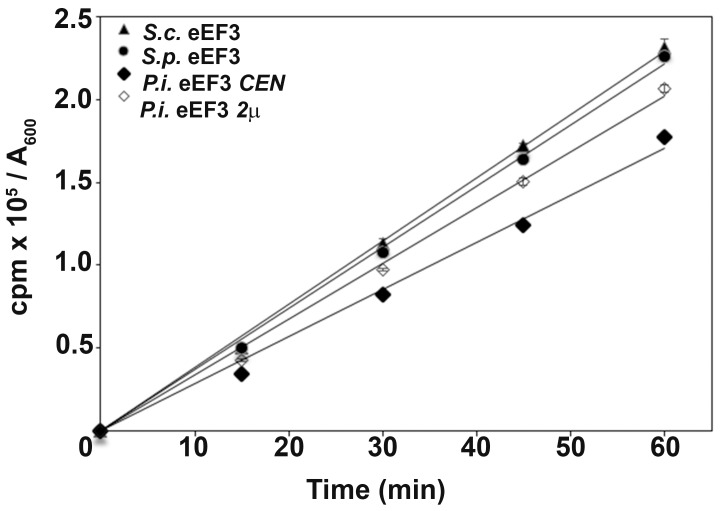
The slow growth of P. infestans eEF3 cells compared to S. pombe expressing cells suggests that the function of P. infestans eEF3 is also compromised in the heterologous system. To determine whether the slow growth was due to a defect in translation, total protein synthesis was measured by [35S] methionine incorporation in exponentially growing cells expressing the indicated forms of eEF3. Strains expressing either S. cerevisiae or S. pombe eEF3 had similar rates of protein synthesis over the course of the experiment while a strain with low P. infestans eEF3 expression showed a 23–31% reduction in total protein synthesis (Fig 3). This defect was rescued to near wild-type (S. cerevisiae and S. pombe) levels by increasing the amount of P. infestans eEF3 protein with a high copy expression vector.
Fig 3. Strains expressing P. infestans eEF3 exhibit a reduction in protein synthesis.
S. cerevisiae strains expressing eEF3 from the indicated species (S. cerevisiae (S.c.), S. pombe (S.p.), and P. infestans (P.i.) were grown to log phase in C-MET at 30°C. [35S] methionine was added and total protein synthesis was measured by tricholoroacetic acid precipitation. Incorporation (counts per min) is expressed per A600 unit. Each time point was performed in triplicate and error bars represent standard error. A representative experiment is shown.
To further probe the nature of the translation defect in the low P. infestans eEF3 expression strain, sensitivity to three antibiotics known to inhibit translation elongation was measured. The low P. infestans eEF3 expression strain showed an increase in sensitivity to paromomycin, hygromycin and cycloheximide compared to wild-type cells in an assay measuring the zone of growth inhibition (Fig 4A). Increased expression of P. infestans eEF3 could either fully (hygromycin) or partially rescue (cycloheximide and paromomycin) the level of sensitivity. In these assays, S. pombe eEF3 also demonstrated a small increase in sensitivity to all three elongation inhibitors suggesting that its reduced expression level compared to S. cerevisiae eEF3 does result in a mild translation defect that does not significantly impact growth rates or total protein synthesis. To examine more specifically which steps of protein synthesis were affected in the low P. infestans eEF3 strain, polyribosome profiles were analyzed for each of the indicated strains. The polyribosome profiles of both S. cerevisiae eEF3 and S. pombe eEF3 expressing strains were similar in the absence of cycloheximide while the low P. infestans eEF3 expressing strain showed an increase in the number of polyribosomes and a concomitant decrease in the 80S ribosome peak (Fig 4B). The polyribosome profile observed in a strain expressing high levels of P. infestans eEF3, however, did not significantly differ from that seen in a strain expressing low levels of P. infestans eEF3. These results are consistent with a defect in translation elongation/termination in P. infestans eEF3 expressing strains.
Fig 4. Strains expressing P. infestans eEF3 show defects in translation elongation and/or termination.
(A) Antibiotic sensitivity was determined by measuring the diameter of the zone of growth inhibition around a disk containing 10 μL of the indicated drug: Paromomycin (800 mg/ml), hygromycin (25 mM), and cycloheximide (1 mM). The graph represents the average of three experiments and error bars representing the standard error are shown. (B) Ribosome extracts were prepared from the indicated strains in the absence of cycloheximide. Extracts were analyzed by 7–47% sucrose density gradient centrifugation and representative A254 traces are shown. The area under the 80S and polyribosome peaks was analyzed using Image J and the ratio of polyribosomes to monosomes (p/m) is indicated for each strain. S. cerevisiae (S.c.), S. pombe (S.p.), and P. infestans (P.i.).
Bioinformatic analysis shows divergence in the chromodomain of P.infestans eEF3
To help identify the regions of eEF3 important for its activity, a domain-specific comparison of sequence identity was performed for all three eEF3 proteins (Fig 5A). As expected, the ABC1 and ABC2 domains involved in ATP binding have a high level of identity in both S. pombe eEF3 and P. infestans eEF3 when compared to S. cerevisiae eEF3. The four-helix bundle domain, whose function is currently unknown, has the lowest level of conservation across all three proteins. Outside of this domain, P. infestans eEF3 shows significantly less sequence conservation in the HEAT repeat domain and the chromodomain region, both of which have been shown to contact the ribosome in cryo-EM reconstructions [13]. The largest difference between S. pombe and P. infestans eEF3 in comparison to S. cerevisiae eEF3 is found in the chromodomain region. S. pombe eEF3 is 79.1% identical to S. cerevisiae eEF3 in this region while P. infestans eEF3 is only 36.1% identical. This region disrupts the second ABC domain and has been shown to be important for ribosome-dependent ATPase activity [10]. A sequence alignment of the chromodomain of all three eEF3s is shown in Fig 5B. Notably, P. infestans eEF3 is lacking 13-amino acids compared to both S. cerevisiae eEF3 and S. pombe eEF3. These data suggest that the chromodomain may be a species-specific determinant of ATPase activity either independently or through its role in ribosome binding.
Fig 5. Domain specific differences in the conservation of eEF3.
(A) Individual domains of S.cerevisiae (S.c.), S. pombe (S.p.), and P. infestans (P.i.) eEF3 were aligned using Clustal Omega and the percentage identity to S. cerevisiae eEF3 is shown [23, 24]. The amino acids comprising each domain in S. cerevisiae are indicated. (B) Alignment of the chromodomain of S. cerevisiae, S. pombe, and P. infestans eEF3 was performed using Clustal Omega and shading based on identity was done using Jalview [25]. Dark gray represents identity in all three species and light gray represents identity in two species. (C) Maximum likelihood tree with 500 bootstrap replicates created using phylogeny.fr.
Discussion
The reason fungal ribosomes require eEF3 and how mammalian ribosomes have evolved to synthesize proteins without it have been long-standing questions in the field of protein synthesis. In this report, we present the first evidence that eEF3 activity exists outside the fungal kingdom. P. infestans is a filamentous eukaryotic microorganism of the oomycetes class. Oomycetes were originally grouped with fungi based on their phenotypic characteristics and are still often referred to as fungi despite genetic evidence to the contrary. Sequencing efforts have in fact shown that oomycetes are phylogenetically distinct from fungi and are instead classified with brown algae and diatoms [26, 27]. Phylogenetic comparison of another translation elongation factor, eEF1A, from P. infestans with other taxonomic groups supports the hypothesis that oomycetes evolved independently of fungi [28] and in a phylogenetic tree constructed using eEF3 sequences from a variety of unicellular eukaryotes, P. infestans eEF3 is located in the same clade as green algae and brown algae/diatoms, separate from fungi (Fig 5C). Therefore, P. infestans eEF3 is a non-fungal eEF3 which can perform the essential function of the yeast protein in vivo.
Despite its ability to complement the loss of S. cerevisiae eEF3, P. infestans eEF3 exhibits lower activity in the S. cerevisiae system. S. pombe eEF3 and P. infestans eEF3 are expressed at a similar level, both lower than the S. cerevisiae eEF3, but only the expression of P. infestans eEF3 is associated with an effect on growth rate. Furthermore, increasing the expression of P. infestans eEF3 using a high copy expression plasmid rescues this effect on growth. These observations correlate with what was observed in vitro. P. infestans eEF3 had a lower ATPase activity in the absence and presence of ribosomes. However, P. infestans eEF3 ATPase activity displayed a similar fold stimulation of activity upon the addition of ribosomes as S. cerevisiae eEF3. One explanation for these results is that it may not be an inability to interact with S. cerevisiae ribosomes but an overall low ATPase activity which causes the growth defect observed in vivo. Similar effects on growth rate were observed with C. neoformans eEF3 complementation. However, in this study, purified C. neoformans eEF3 activity showed weak stimulation by addition of S. cerevisiae ribosomes and an altered eEF3-ribosome interaction was suspected as the cause of the low activity [11].
The slow growth phenotype observed in P. infestans eEF3 is likely due to defects in protein synthesis. Cells with low P. infestans eEF3 expression showed a defect in [35S] methionine incorporation that was rescued to near wild-type levels by increasing its levels. However, increased expression of P. infestans eEF3 did not complement the polyribosome profile defect seen in cells expressing this form of eEF3. Previous experiments have shown that altered translation elongation can exist even when total protein synthesis is apparently normal [29] and may be due to the different aspects of translation being examined in each assay. These results suggest that there is still a minor elongation defect when P. infestans eEF3 is overexpressed which is consistent with the mild antibiotic sensitivities observed in this strain.
Bioinformatic analysis of existing genome databases has identified eEF3 orthologues in at least 18 different non-fungal species, including multiple green algae [14]. Since molecular genetic techniques have not been developed for many of these organisms, complementation of the loss of S. cerevisiae eEF3 may be the most expedient way to assay eEF3-like functions for these proteins, however, this technique has its limitations. For example, as noted above, heterologous eEF3 proteins are often expressed at lower levels than endogenous S. cerevisiae eEF3 from the same expression construct even when codon-optimized. We have performed similar complementation experiments using a codon-optimized construct to express Chlamydomonas reinhardtii eEF3 and were unable to make any conclusion on functionality because expression levels were very low even when expressed from a high copy plasmid (unpublished observations). In S. cerevisiae, it has been observed that point mutations in key Walker ATP binding motifs and the chromodomain affect the expression of eEF3 resulting in little or no protein (unpublished observations, [10]). While an effect on RNA stability cannot be excluded, these data suggest that eEF3 expression levels may be regulated by either proper folding or protein activity. In addition, lack of complementation in this system does not preclude the possibility that the heterologous eEF3 proteins retain eEF3-like function in its native organism as negative results may result from ribosomal or other divergence within the protein synthesis machinery.
The identification of eEF3 in non-fungal species will facilitate the utilization of phylogenetics to gain a greater mechanistic understanding of the role of eEF3 in translation and will provide important information on the evolution of protein synthesis. For example, the analysis of the conservation of domains among the three eEF3 proteins analyzed here, indicates that the chromodomain insertion in ABC2 maybe an important species-specific functional domain. Previous studies suggesting that this domain is important for ATPase activity [10] and the observations detailed here point to possible unique aspects of ribosome association and ATPase activation of eEF3 proteins.
Since its initial discovery as an essential, fungal-specific translation elongation factor, eEF3 has been put forth as a potential therapeutic target for the treatment of fungal infections. This work identifies a factor with eEF3 activity in another important class of pathogens. Oomycetes represent a significant threat to global food security through effects on both agriculture and fish farming [30]. If eEF3 is shown to play an essential role in oomycetes, as it does in yeast, then targeting eEF3 activity in these organisms may prove to be an effective mechanism to control these pathogens.
Acknowledgments
We thank Kia Bourdot for assistance with the analysis of C. reinhardtii eEF3 and Dr. Paul Copeland and the members of the Copeland, Kinzy and Dunaway labs for helpful comments and discussions.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the New Jersey Health Foundation (njhealthfoundation.org) grant number PC 11-17 to T.G.K., and by Novo Nordisk (novonordisk-us.com) grant number 10307 to S.D. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dever TE, Kinzy TG, Pavitt GD. Mechanism and Regulation of Protein Synthesis in Saccharomyces cerevisiae. Genetics. 2016;203(1):65–107. Epub 2016/05/18. doi: 10.1534/genetics.115.186221 ; PubMed Central PMCID: PMCPMC4858804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skogerson L, Wakatama E. A ribosome-dependent GTPase from yeast distinct from elongation factor 2. Proc Natl Acad Sci U S A. 1976;73(1):73–6. ; PubMed Central PMCID: PMCPMC335841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skogerson L, Engelhardt D. Dissimilarity in protein chain elongation factor requirements between yeast and rat liver ribosomes. J Biol Chem. 1977;252(4):1471–5. [PubMed] [Google Scholar]
- 4.Triana-Alonso FJ, Chakraburtty K, Nierhaus KH. The elongation factor 3 unique in higher fungi and essential for protein biosynthesis is an E site factor. J Biol Chem. 1995;270(35):20473–8. . [DOI] [PubMed] [Google Scholar]
- 5.Uritani M, Miyazaki M. Role of yeast peptide elongation factor 3 (EF-3) at the AA-tRNA binding step. J Biochem. 1988;104(1):118–26. Epub 1988/07/01. . [DOI] [PubMed] [Google Scholar]
- 6.Kamath A, Chakraburtty K. Role of yeast elongation factor 3 in the elongation cycle. J Biol Chem. 1989;264(26):15423–8. Epub 1989/09/15. . [PubMed] [Google Scholar]
- 7.Anand M, Balar B, Ulloque R, Gross SR, Kinzy TG. Domain and nucleotide dependence of the interaction between Saccharomyces cerevisiae translation elongation factors 3 and 1A. J Biol Chem. 2006;281(43):32318–26. doi: 10.1074/jbc.M601899200 . [DOI] [PubMed] [Google Scholar]
- 8.Anand M, Chakraburtty K, Marton MJ, Hinnebusch AG, Kinzy TG. Functional interactions between yeast translation eukaryotic elongation factor (eEF) 1A and eEF3. J Biol Chem. 2003;278(9):6985–91. doi: 10.1074/jbc.M209224200 . [DOI] [PubMed] [Google Scholar]
- 9.Qin S, Xie A, Bonato MCM, McLaughlin CS. Sequence analysis of the translational elongation factor 3 from Saccharomyces cerevisiae. J Biol Chem. 1990;265:1903–12. [PubMed] [Google Scholar]
- 10.Sasikumar AN, Kinzy TG. Mutations in the chromodomain-like insertion of translation elongation factor 3 compromise protein synthesis through reduced ATPase activity. J Biol Chem. 2014;289(8):4853–60. doi: 10.1074/jbc.M113.536201 ; PubMed Central PMCID: PMCPMC3931047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blakely G, Hekman J, Chakraburtty K, Williamson PR. Evolutionary divergence of an elongation factor 3 from Cryptococcus neoformans. J Bacteriol. 2001;183(7):2241–8. doi: 10.1128/JB.183.7.2241-2248.2001 ; PubMed Central PMCID: PMCPMC95130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colthurst DR, Schauder BS, Hayes MV, Tuite MF. Elongation factor 3 (EF-3) from Candida albicans shows both structural and functional similarity to EF-3 from Saccharomyces cerevisiae. Mol Microbiol. 1992;6(8):1025–33. . [DOI] [PubMed] [Google Scholar]
- 13.Andersen CB, Becker T, Blau M, Anand M, Halic M, Balar B, et al. Structure of eEF3 and the mechanism of transfer RNA release from the E-site. Nature. 2006;443(7112):663–8. doi: 10.1038/nature05126 . [DOI] [PubMed] [Google Scholar]
- 14.Mateyak MK, Sasikumar AN, Dunaway S, Kinzy TG. The Unique Evolutionary Distribution of Eukaryotic Elongation Factor 3 In: Hernandez GJ R, editor. Evolution of the Protein Synthesis Machinery and Its Regulation. Switzerland: Springer International; 2016. p. 313–26. [Google Scholar]
- 15.Ebstrup T, Saalbach G, Egsgaard H. A proteomics study of in vitro cyst germination and appressoria formation in Phytophthora infestans. Proteomics. 2005;5(11):2839–48. doi: 10.1002/pmic.200401173 . [DOI] [PubMed] [Google Scholar]
- 16.Carr-Schmid A, Valente L, Loik VI, Williams T, Starita LM, Kinzy TG. Mutations in elongation factor 1beta, a guanine nucleotide exchange factor, enhance translational fidelity. Mol Cell Biol. 1999;19(8):5257–66. Epub 1999/07/20. ; PubMed Central PMCID: PMCPMC84369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esposito AM, Mateyak M, He D, Lewis M, Sasikumar AN, Hutton J, et al. Eukaryotic polyribosome profile analysis. J Vis Exp. 2010;(40). Epub 2010/06/23. doi: 10.3791/1948 ; PubMed Central PMCID: PMCPMC3149985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin BS, Dever TE. Molecular genetic structure-function analysis of translation initiation factor eIF5B. Methods Enzymol. 2007;429:185–201. Epub 2007/10/05. doi: 10.1016/S0076-6879(07)29009-3 . [DOI] [PubMed] [Google Scholar]
- 19.Dereeper A, Audic S, Claverie JM, Blanc G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol Biol. 2010;10:8 Epub 2010/01/14. doi: 10.1186/1471-2148-10-8 ; PubMed Central PMCID: PMCPMC2821324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36(Web Server issue):W465–9. Epub 2008/04/22. doi: 10.1093/nar/gkn180 ; PubMed Central PMCID: PMCPMC2447785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uritani M, Miyazaki M. Characterization of the ATPase and GTPase activities of elongation factor 3 (EF-3) purified from yeasts. J Biochem. 1988;103(3):522–30. Epub 1988/03/01. . [DOI] [PubMed] [Google Scholar]
- 22.Dasmahapatra B, Chakraburtty K. Protein synthesis in yeast. I. Purification and properties of elongation factor 3 from Saccharomyces cerevisiae. J Biol Chem. 1981;256(19):9999–10004. Epub 1981/10/10. . [PubMed] [Google Scholar]
- 23.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539 Epub 2011/10/13. doi: 10.1038/msb.2011.75 ; PubMed Central PMCID: PMCPMC3261699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, et al. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010;38(Web Server issue):W695–9. Epub 2010/05/05. doi: 10.1093/nar/gkq313 ; PubMed Central PMCID: PMCPMC2896090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25(9):1189–91. Epub 2009/01/20. doi: 10.1093/bioinformatics/btp033 ; PubMed Central PMCID: PMCPMC2672624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levesque CA. Fifty years of oomycetes-from consolidation to evolutionary and genomic exploration. Fungal Divers. 2011;50(1):35–46. doi: 10.1007/s13225-011-0128-7. WOS:000295329300003. [Google Scholar]
- 27.Gunderson JH, Elwood H, Ingold A, Kindle K, Sogin ML. Phylogenetic relationships between chlorophytes, chrysophytes, and oomycetes. Proc Natl Acad Sci U S A. 1987;84(16):5823–7. Epub 1987/08/01. ; PubMed Central PMCID: PMCPMC298955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van't Klooster JW, van den Berg-Velthuis G, van West P, Govers F. tef1, a Phytophthora infestans gene encoding translation elongation factor 1alpha. Gene. 2000;249(1–2):145–51. Epub 2000/06/01. . [DOI] [PubMed] [Google Scholar]
- 29.Carr-Schmid A, Durko N, Cavallius J, Merrick WC, Kinzy TG. Mutations in a GTP-binding motif of eukaryotic elongation factor 1A reduce both translational fidelity and the requirement for nucleotide exchange. J Biol Chem. 1999;274(42):30297–302. Epub 1999/10/09. . [DOI] [PubMed] [Google Scholar]
- 30.Derevnina L, Petre B, Kellner R, Dagdas YF, Sarowar MN, Giannakopoulou A, et al. Emerging oomycete threats to plants and animals. Philos Trans R Soc Lond B Biol Sci. 2016;371(1709). Epub 2017/01/13. doi: 10.1098/rstb.2015.0459 ; PubMed Central PMCID: PMCPMC5095538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.