Abstract
Species may become invasive after introduction to a new range because phenotypic traits pre-adapt them to spread and become dominant. In addition, adaptation to novel selection pressures in the introduced range may further increase their potential to become invasive. The diploid Leucanthemum vulgare and the tetraploid L. ircutianum are native to Eurasia and have been introduced to North America, but only L. vulgare has become invasive. To investigate whether phenotypic differences between the two species in Eurasia could explain the higher abundance of L. vulgare in North America and whether rapid evolution in the introduced range may have contributed to its invasion success, we grew 20 L. vulgare and 21 L. ircutianum populations from Eurasia and 21 L. vulgare populations from North America under standardized conditions and recorded performance and functional traits. In addition, we recorded morphological traits to investigate whether the two closely related species can be clearly distinguished by morphological means and to what extent morphological traits have changed in L. vulgare post-introduction. We found pronounced phenotypic differences between L. vulgare and L. ircutianum from the native range as well as between L. vulgare from the native and introduced ranges. The two species differed significantly in morphology but only moderately in functional or performance traits that could have explained the higher invasion success of L. vulgare in North America. In contrast, leaf morphology was similar between L. vulgare from the native and introduced range, but plants from North America flowered later, were larger and had more and larger flower heads than those from Eurasia. In summary, we found litte evidence that specific traits of L. vulgare may have pre-adapted this species to become more invasive than L. ircutianum, but our results indicate that rapid evolution in the introduced range likely contributed to the invasion success of L. vulgare.
Introduction
Why only certain species become invasive when introduced into new ranges and others not is still a major question in invasion biology. Several studies focused on identifying traits that may pre-adapt plant species to become invasive after introduction to a new range (e.g. [1–3]). Studies comparing invasive with non-invasive introduced plant species revealed that traits associated with high plant performance such as a larger size or biomass [3–5], faster growth [6–8], or a higher specific leaf area (SLA) [5–7, 9, 10] may pre-adapt species to become invasive. Beside pre-adaptation, evolutionary changes in response to novel selection pressures in the introduced range may also play an important role in enabling introduced plants to become invasive [11–16]. The most common hypothesis regarding rapid evolution in the introduced range is the Evolution of Increased Competitive Ability (EICA) hypothesis [17], which proposes that certain plants may adapt to a reduced herbivore pressure in the introduced range by re-allocating resources from defense to growth and reproduction. However, introduced plants may also adapt to other biotic (e.g. plant competition) or abiotic (e.g. climate [11, 13, 18]) factors that differ from their native range. Numerous studies have investigated whether there are evolutionary changes in traits related to performance by growing plants from the native and introduced range under standardized conditions and these studies generally found evolution of increased size but only moderate or no support for the EICA hypothesis (reviewed by [14, 19]). In contrast, only a few studies have examined whether there is also rapid evolution in other plant traits [13, 18, 20–24]. For example, a recent study comparing herbarium specimens of species invasive to Australia showed that five out of 19 species have undergone significant changes in leaf shape through time [13].
The diploid Leucanthemum vulgare (Vaill.) Lam. (2n = 2x = 18) and the closely related tetraploid Leucanthemum ircutianum DC. (2n = 4x = 36) are perennial herbs native to Europe and western Asia. In the native range, both species are mainly found in meadows and pastures and can also be found in ruderal habitats such as roadside areas, but they usually do not persist on sites not mown for several years. Both species have been introduced as ornamentals and seed contaminants to North America and have become naturalized, but only the diploid L. vulgare has become invasive [25–27]. In its introduced range, L. vulgare invades pastures, meadows, roadside areas and forest openings where it reduces plant species diversity and hay or forage production [28, 29]. In North America, it was reported to be naturalized in Québec and in the north-eastern USA by the 18th century [25, 30]. Nowadays, it is common in the north-eastern and north-western states of the USA and in the south-eastern and south-western provinces of Canada [28, 29]. In a recent ploidy screening of 98 Leucanthemum populations collected across North America only two populations have been identified as L. ircutianum, all others were L. vulgare [27]. The higher invasion success of L. vulgare compared to L. ircutianum is unlikely caused by differences in the introduction rates as both species largely overlap in their native range distribution where L. ircutianum is more common than L. vulgare [31–33]. In addition, the analyses of 13 seed sources commercially sold under the name L. vulgare by twelve US and one Canadian seed company revealed that twelve contained L. ircutianum seeds and only one L. vulgare seeds (S1 Appendix). Moreover, the higher invasion success of the diploid L. vulgare is in contrast to recent studies that found a positive association between polyploidy and invasiveness in plants [34–36].
Due to their similar morphology, L. vulgare and L. ircutianum have often been treated as a species complex (sometimes referred to as “L. vulgare s.l.”), together with other morphologically similar species [37–39]. Leucanthemum ircutianum is an allopolyploid species with L. vulgare and possibly L. virgatum (Desr.) Clos as parental species [33]. Several attempts have been made to morphologically characterize L. vulgare and L. ircutianumin in their native range (e.g. [31, 40–43]). Differences in leaf shape such as the size of the teeth or lobes at the base of the stem leaves or the length to width ratio of the stem leaves have been identified that help to distinguish the two species from each other [31, 40–43], but phenotypic variation between and even within populations was also found to be high [42, 43].
In this study, we compared performance, functional and morphological traits of 20 native (Eurasian) L. vulgare populations with 21 native L. ircutianum populations and with 21 introduced (North American) L. vulgare populations under standardized conditions. We defined performance traits as traits that directly contribute to fitness (e.g. biomass, number of flower heads), functional traits as traits that impact fitness indirectly (e.g. specific leaf area, time to flowering [44]) and morphological traits as traits potentially important for species determination (e.g. leaf shape). With the comparison of native L. vulgare and L. ircutianum we aimed to investigate whether the two species differ in performance and functional traits that may explain the higher invasion success of L. vulgare in North America and whether they can be clearly distinguished by morphology. With the comparison of L. vulgare from the native and introduced range we aimed to determine whether L. vulgare from the two ranges differ in phenotypic traits, which would suggest that rapid evolutionary changes after the introduction to North America may have further increased the invasive potential of L. vulgare. We hypothesized that L. vulgare possesses performance and functional traits that made it better pre-adapted to become invasive in North America than L. ircutianum, and that some of these performance and functional traits have evolved post-introduction to further increase the invasiveness of L. vulgare. Within the restriction that little is known about evolutionary changes in morphological traits of invasive plants, we predicted that morphological traits have less significantly changed post-introduction than performance and functional traits, because the latter are likely to be under stronger selection.
Materials and methods
Cultivation of plant material
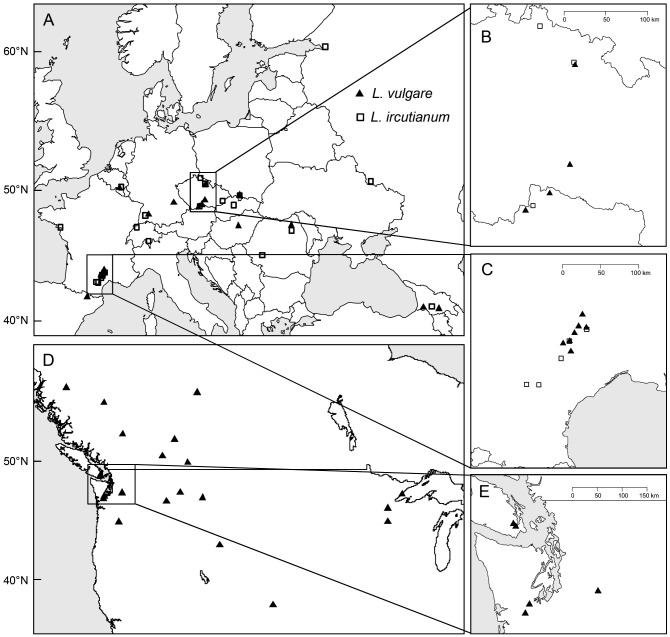
The seeds used in this experiment were collected from field populations from 2009 to 2013 and stored in paper bags at 2°C until sowing. To distinguish between the morphologically similar L. vulgare and L. ircutianum the ploidy level of each population used in the experiment was determined prior to the experiment either as part of a previous study [27] or as part of this study. For this purpose flow cytometric analyses (CyFlow SL Green 2P, Partec) were performed on one or two bulk samples per population each containing two seeds from up to five maternal plants per population (see [27] for more information on the methods). In one European population (PL3) diploid and tetraploid plants were detected and seeds from individual plants were analysed separately. Leucanthemum vulgare and L. ircutianum plants from this population were subsequently treated separately (PL3vul and PL3irc). In total, we grew potted plants of 20 L. vulgare and 21 L. ircutianum populations from the native range (Europe and western Asia) and 21 L. vulgare from the introduced range (USA and Canada) under standardized conditions in a common garden at CABI in Delémont, Switzerland (47.3732°N, 7.3261°E; 515 m) (Fig 1, S2 Appendix). Each population was represented by ten plants grown from seeds of five maternal plants.
Fig 1. Sampling locations of Leucanthemum vulgare (triangles) and L. ircutianum (squares) populations from the (A, B, C) native (Eurasia) and (D, E) introduced (North America) range.
From 4 to 8 November 2013, six seeds from five maternal plants per population were sown in 4 cm x 4 cm cells of seedling trays. Three seeds were sown per cell, resulting in a total of 620 cells. The seedling trays were filled with a mixture of garden soil (Selmaterra, Eric Schweizer AG, Switzerland), sand and vermiculite (14:3:1) with 1 g/L of slow-release NPK fertiliser (Hauert Tardit 6M) added. After germination, the numbers of seedlings were reduced to one per cell, i.e. two per maternal plant. To ensure that each population was represented by ten seedlings, missing seedlings (n = 13) were replaced by seedlings of the corresponding population. The seedlings were kept in a heated greenhouse with artificial light (16h photoperiod, temperature constantly set at 20°C for four weeks and then decreased to 15°C during the night) until the beginning of January 2014 and then moved to a colder greenhouse (no artificial light, temperatures maintained above 0°C) for vernalization in order to promote flowering in the first year. At the beginning of February, all 620 plants were potted in 1 L pots filled with the same mixture of soil as described above and kept in the same greenhouse for another two weeks. Afterwards, all plants were moved outside in a garden bed (6 m x 6 m), where they were embedded in sawdust and arranged in a random design (distance between pots: 6 cm). The plants were exposed to precipitation and watered during dry periods. All plants survived throughout the experiment and all except six plants flowered during the summer.
A collection permit was obtained to collect L. vulgare seeds in the Banff National Park of Canada (Permit Number: BAN-2011-9993). No specific permissions were needed for seed collections at other sites since they were not in protected areas. No permits were necessary to introduce the seeds to Switzerland. The study was carried out on private land with the permission of the owner.
Phenotypic measurements
From November 2013 to July 2014, a total of 17 phenotypic traits were measured, recorded or calculated (Table 1; for reasons for inclusion see S3 Appendix). Beside seven traits directly related to vegetative and reproductive performance (germination rate, number of leaves and the length of the blade of the longest leaf at the seedling stage as well as the total biomass, the length of the longest shoot and the number of shoots and flower heads at the end of the flowering stage) and four functional traits (days to germination, days to flowering, specific leaf area (SLA) and leaf dry matter content (LDMC)) we also recorded six morphological traits potentially important to distinguish L. vulgare and L. ircutianum as suggested by previous authors [32, 40, 43] and our own field observations (the length to width ratio of the leaf blade at the seedling stage and of a mid-stem leaf at the flowering stage, the perimeter to area ratio of rosette and mid-stem leaves, the width of the undivided middle part at the base devided by the total width at the base of a randomly chosen stem leaf and flower head diameter). Although we classified flower head diameter as a morphological trait we acknowledge that it may potentially also be correlated with pollination and seed production and could therefore also be classified as a performance trait.
Table 1. List of phenotypic traits recorded for Leucanthemum vulgare and L. ircutianum.
| Performance traits |
| Germination rate |
| Length of leaf blade of longest leaf of three-month old seedlings |
| Number of leaves longer than 1 cm of three-month old seedlings |
| Number of shoots at the end of the flowering stage |
| Number of flower heads at the end of the flowering stage |
| Length of longest shoot at the end of the flowering stage |
| Dry weight of above ground biomass at the end of the flowering stage |
| Functional traits |
| Days to germination |
| Days to flowering |
| Leaf dry matter content: ratio of dry to fresh weight of a randomly chosen rosette leaf |
| Specific leaf area: ratio of area to dry weight of a randomly chosen rosette leaf |
| Morphological traits |
| Length to width ratio of leaf blade of longest leaf of three-month old seedlings |
| Perimeter to area ratio of randomly chosen rosette leaf |
| Perimeter to area ratio of randomly chosen mid-stem leaf |
| Length to width ratio of leaf blade of longest leaf of three-month old seedlings |
| Width of undivided middle part at the base devided by the total width at the base of a randomly chosen stem leaf |
| Flower head diameter (average taken from three flower heads per plant) |
From 11 November to 4 December, germination was monitored two to three times a week and the date when the first seedling emerged was recorded for each cell of the seedling trays. The germination rate was then calculated per population as the number of seeds that germinated, divided by the number of seeds that had been sown. In early February, just before transplanting the seedlings into the 1 L pots, the number of leaves longer than 1 cm were recorded and the length of the longest leaf as well as the length and width of its leaf blade were measured using a calliper with 1 mm-precision. At the end of April, when all plants were still in the rosette stage, one fully expanded and undamaged leaf was collected from each plant and immediately weighed. For subsequent measurements a binary image was taken from each leaf using a flatbed scanner. Afterwards, all scanned leaves were dried at 80°C for 24h and weighed. The dry to fresh weight ratio of the rosette leaves was used to calculate LDMC and the leaf area to dry weight ratio was used to calculate SLA. From April to July the onset of flowering, defined as the date when the first flower head of a plant was completely open, was recorded two to three times per week. Once at least three principal flower heads of a plant were completely open (typically 1–2 weeks after the first flower head opened) the diameter of three principal flower heads was measured using a calliper with 0.1 mm-precision. At the same time, an undamaged mid-stem leaf of a fully developed stem was removed and scanned with a flatbed scanner for subsequent morphological measurements. As soon as more than half of the flower heads of an individual plant started to senesce, which corresponded with the date when all or the majority of the flower heads were completely open and no new shoots were produced, the total number of stems and flower heads as well as the length of the longest stem were recorded. At the same time, all above ground biomass was harvested, dried for at least 48h at 80°C and then weighed.
Several morphological measurements were taken on the scanned rosette and stem leaves using the software ImageJ [45]. For each of the scanned rosette and stem leaves the area and perimeter were measured and their ratio was calculated. In addition, the total leaf length, the leaf width (not including teeth and lobes) at the leaf length midpoint, the total leaf width (including teeth and lobes) at the leaf base and the width of the undivided middle part (excluding lobes and teeth) at the leaf base were measured on the stem leaves as pictured in S4 Appendix. These measurements were then used to calculate the leaf length to width ratio and the ratio of the undivided middle part to the total width at the leaf base (a measurement to describe the relative length of the teeth and lobes at the base of the stem leaf).
Statistical analyses
In a first step, two principal component analyses (PCAs) based on the correlation matrices of individual plants and population means of all recorded traits (Table 1) were performed to visualize the relationships among native and introduced L. vulgare as well as native L. ircutianum plants and populations. Plants with missing values for one of the measured traits were excluded from the PCA based on individual plants. This reduced the number of plants included in this PCA to 556 (192 L. ircutianum plants, 168 L. vulgare plants from Eurasia and 196 L. vulgare plants from North America).
In a second step, linear mixed models (for continuous variables) and generalized linear mixed models (for discrete variables) were used to test for differences in individual plant traits between L. vulgare and L. ircutianum from the native range and between L. vulgare from the native and introduced range. For each plant trait, separate analyses were conducted to compare native L. vulgare with native L. ircutianum and native with introduced L. vulgare populations. Continuous variables were transformed as required to address normality assumptions. Maternal plant nested within population was considered as a random factor. To analyse differences in germination rates a quasi-binomial generalized linear model was used. To assess whether differences in storage time affected germination rate the year when the seeds had been collected was included in the model. Since storage time did not have an influence on the germination rate (t = 0.9, P = 0.4), it was subsequently removed from the analyses. To analyse whether the biomass at the end of the flowering stage was correlated with the days to flowering and whether there was a correlation between latitude of population origin and days to flowering or biomass we conducted linear regression analyses on population means. These analyses were done separately for native and introduced L. vulgare populations.
All analyses were performed with the software R version 3.2.3 [46]. The PCAs were done using the function dudi.pca in the ade4 package [47], linear mixed models were done using the function lme in the nlme package [48] and generalized linear mixed models were done using the function glmer in the lme4 package [49].
Results
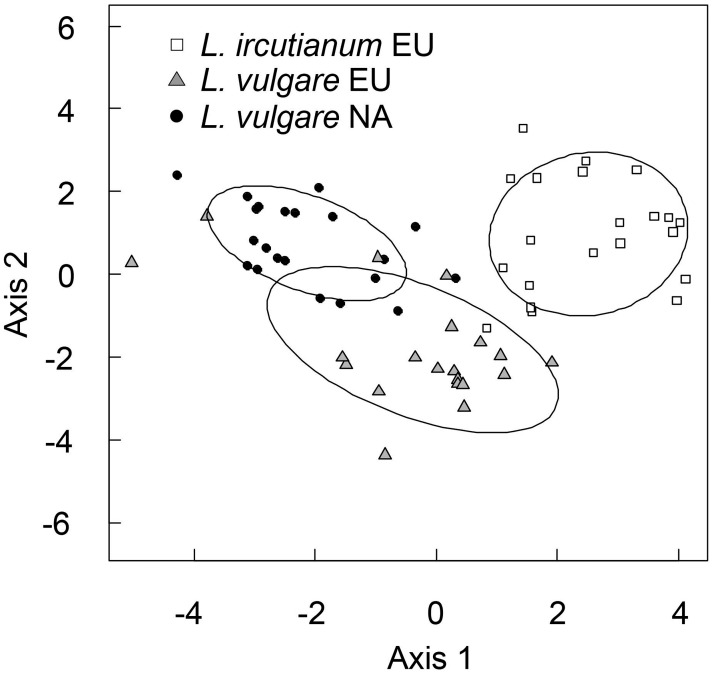
The PCA based on individual plants revealed a relatively clear separation of L. vulgare and L. ircutianum although the variability was large and some L. vulgare plants grouped with L. ircutianum and vice versa (S5 Appendix). Native and introduced L. vulgare plants were only partly separated (S5 Appendix). The PCA based on population means revealed a clearer separation of L. vulgare and L. ircutianum populations while the phenotypic spaces of native and introduced L. vulgare populations were still partially overlapping (Fig 2). Leucanthemum vulgare and L. ircutianum populations were mostly separated along the second axis while Eurasian and North American L. vulgare populations were separated along the first and second axis. The first axis of the PCA based on population means was most strongly positively correlated with the relative length of the teeth or lobes at the base of the stem leaf and negatively correlated with the length to width ratio of the stem leaf, the number of flower heads per plant, the biomass, the number of shoots and the perimeter to area ratio of the mid-stem leaves and the second axis was most strongly positively correlated with the length of the longest shoot at the end of the flowering stage, the blade length of the longest seedling leaf, the days to flowering and the diameter of the flower heads and negatively correlated with the perimeter to area ratio of the rosette leaf and the perimeter to area ratio of the stem leaf (S6 Appendix).
Fig 2. Principal Component Analysis (PCA) plot based on population mean values of 17 traits measured from ten plants from 62 populations of Leucanthemum vulgare and L. ircutianum from the native (Eurasia, EU) and introduced (North America, NA) range grown under standardized conditions.
To show the phenotypic space occupied by each species from the native and introduced range confidence ellipses defined by the gravity center (centroid) of the cloud and 1.5 times the standard deviation were constructed. The first axis explains 31.7% of the total variation in the dataset; the second axis explains 17.9%.
Comparison of Leucanthemum vulgare and L. ircutianum from the native range
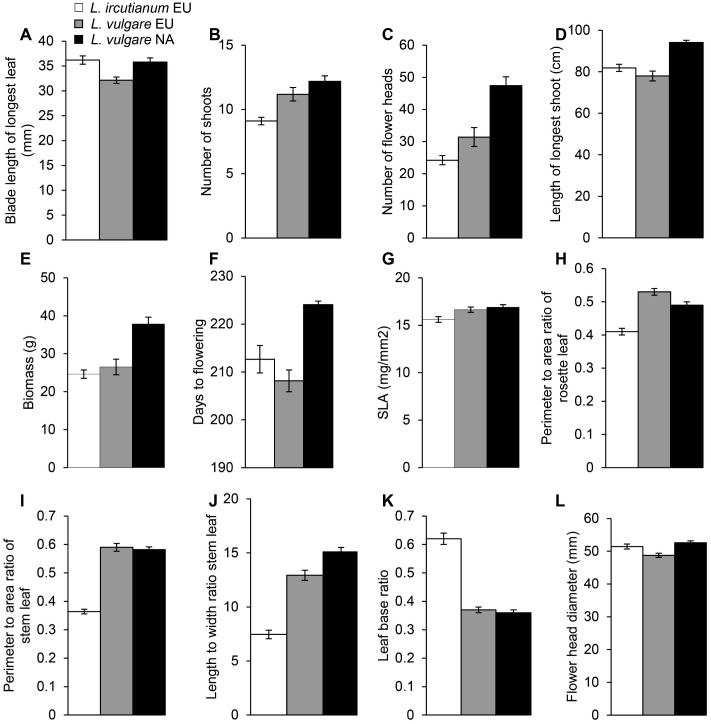
With regard to performance traits, germination rate was slightly lower for L. vulgare seeds than for those of L. ircutianum (t = 2.2, P = 0.04, S7 Appendix). The three-month old L. vulgare seedlings had shorter leaf blades (t = 3.9, df = 39, P < 0.001, Fig 3A), but the seedlings of the two species did not differ in their number of leaves (z = 0.1, P = 0.9, S7 Appendix). Leucanthemum vulgare produced on average 20% more shoots and flower heads than L. ircutianum (z = 3.6, P = 0.004 and z = 2.2, P = 0.03, Fig 3B and 3C) but, maximum height (t = 1.4, df = 39, P = 0.2, Fig 3D) and biomass at the end of the flowering stage (t = 0.7, df = 39, P = 0.5, Fig 3E) were similar for both species.
Fig 3. Mean and standard errors for (A) blade length of longest leaf at the seedling stage, (B) number of shoots per plant, (C) number of flower heads per plant, (D) length of longest shoot, (E) biomass at the end of the flowering stage, (F) days to flowering, (G) specific leaf area (SLA) of a rosette leaf, (H) perimeter to area ratio of a rosette leaf, (I) perimeter to area ratio of a stem leaf, (J) length to width ratio of a stem leaf (K) width of undivided middle part at the base devided by the total width at the base of a stem leaf and (L) flower head diameter for 21 native (Eurasia, EU) Leucanthemum ircutianum populations and 20 native and 21 invasive (North America, NA) L. vulgare populations grown under standardized conditions.
With regard to functional traits, days to germination (z = 0.4, P = 0.7, S7 Appendix) and days to flowering (z = 1.2, P = 0.2, Fig 3F) did not differ between native L. vulgare and L. ircutianum. SLA was 6% higher for L. vulgare than for L. ircutianum (t = 2.9, df = 39, P = 0.006, Fig 3G), but LDMC was similar for both species (t = 0.6, df = 39, P = 0.5, S7 Appendix).
In contrast to the moderate differences in performance and functional traits, the two species clearly differed in all of the measured leaf morphological traits, except for the length to width ratio of the leaf blades of the three-month old seedlings (t = 0.0, df = 39, P = 1.0, S7 Appendix): The perimeter to area ratio of the rosette and stem leaves was higher for L. vulgare compared to L. ircutianum and the stem leaves of L. vulgare had a larger length to width ratio and longer teeth and lobes at their base than those of L. ircutianum (all P < 0.001, Fig 3H and 3K). In addition, the flower heads of L. vulgare were slightly smaller compared to those of L. ircutianum (t = 2.6, df = 39, P = 0.01, Fig 3L).
Comparison of native and introduced Leucanthemum vulgare populations
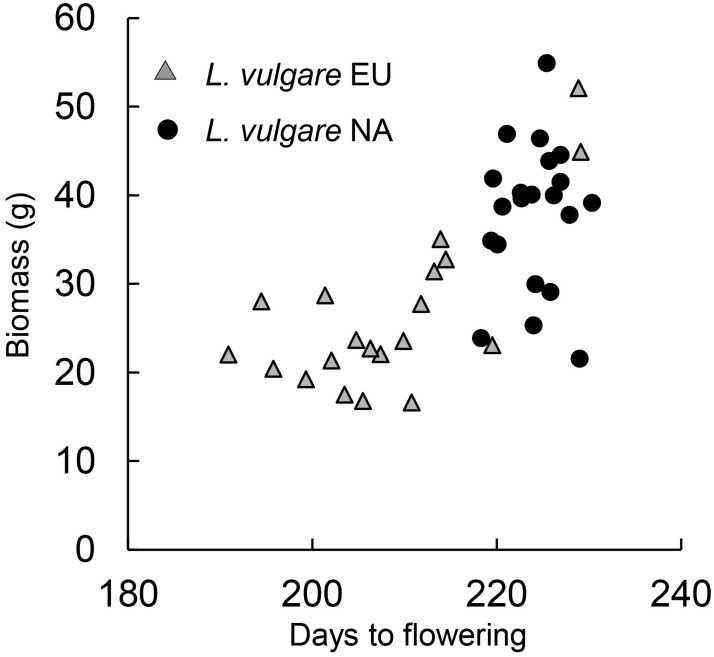
With regard to performance traits, germination rate and the number of leaves of three-month old seedlings were similar for native and introduced L. vulgare (P > 0.1, S7 Appendix), but the leaf blade of the longest leaf of the seedlings of introduced L. vulgare was on average longer than those of native L. vulgare (t = 3.5, df = 39, P = 0.001, Fig 3A) indicating that they had a higher biomass. At the end of the flowering stage, native and introduced L. vulgare had a similar number of shoots (z = 1.6, P = 0.1, Fig 3B), but total biomass of L. vulgare from the introduced range was 43% higher (Fig 3E), their longest shoot was 21% longer and they had 50% more flower heads (all P < 0.001, Fig 3C and 3D). With regard to functional traits, days to germination, LDMC and SLA did not differ between native and introduced L. vulgare (all P > 0.1, S7 Appendix, Fig 3G) but L. vulgare from the introduced range flowered on average 16 days later than those from the native range (z = 6.9, P < 0.001, Fig 3F). We found a positive correlation between biomass and days to flowering for native (t = 3.7, df = 18, P = 0.002) but not for introduced (t = 0.3, df = 19, P = 0.8) L. vulgare populations (Fig 4). The L. vulgare populations from the introduced range were collected from slightly more northern latitudes than those from the native range (t = 1.9, df = 39, P = 0.07). In the introduced range, biomass was negatively but not significantly correlated with latitude (t = 1.9, df = 19, P = 0.07), but there was no correlation between latitude and days to flowering (t = 1.3, df = 19, P = 0.2). Biomass and days to flowering of native L. vulgare populations were not correlated with latitude (both P > 0.1).
Fig 4. Relationship between days to flowering and biomass for native (Eurasia, EU, t = 3.7, df = 18, P = 0.002) and introduced (North America, NA, t = 0.3, df = 19, P = 0.8) Leucanthemum vulgare populations.
With regard to morphological traits, L. vulgare from the introduced range had larger flower heads (t = 5.4, df = 19, P < 0.001, Fig 3L) and their mid-stem leaves had a larger length to width ratio (t = 4.0, df = 19, P < 0.001, Fig 3J) than those from the native range, but they did not differ in any of the other leaf morphological traits (all P > 0.1).
Discussion
Our common garden experiment revealed clear phenotypic differences between L. vulgare and L. ircutianum populations from the native range as well as between native and introduced L. vulgare populations. Native L. vulgare and L. ircutianum differed in morphological traits but not or only moderately in performance or functional traits that could explain the higher abundance of L. vulgare in its introduced range. In contrast, Leucanthemum vulgare populations from the introduced range flowered significantly later, were taller and had a higher biomass and more flower heads than those from the native range, suggesting that rapid evolution post-introduction may have contributed to the invasion success of L. vulgare. The leaf morphological traits, however, were similar for L.vulgare populations from the native and introduced range.
Differences between Leucanthemum vulgare and L. ircutianum from the native range
We found that native L. vulgare had on average 20% more shoots and flower heads than L. ircutianum, but this did not result in a higher biomass, potentially due to their thinner stems, more dissected leaves and smaller flower heads. Similar to these results obtained under standardized conditions, a higher number of shoots and flower heads of L. vulgare compared to L. ircutianum was also observed in field populations [27]. The higher number of flower heads of native L. vulgare compared to L. ircutianum plants might have contributed to its higher invasion success if the number of flower heads per plant is closely related to reproductive output. However, since we did not record the number of seeds per plant it remains unclear whether the two species differ in the total number of seeds or whether the slightly smaller flower heads of L. vulgare resulted in similar numbers of seeds per plant of both species. Although it has been reported that field populations of L. vulgare would flower 1–2 weeks earlier than L. ircutianum [31], we found no difference in days to flowering between the two species under common garden conditions. We found that L. vulgare had a significantly higher SLA but similar LDMC compared to L. ircutianum. High SLA values have been associated with fast growth rates [50] and invasive species have repeatedly been found to have up to 20 to 30% higher SLAs than closely related non-invasive introduced species [5–7, 9, 10]. However, the relative difference in SLA between L. vulgare and L. ircutianum was only 6% and it is therefore questionable that this small difference is biologically relevant.
In contrast to the moderate differences in performance and functional traits between L. vulgare and L. ircutianum we found significant morphological differences between populations of the two species. In agreement with other studies (e.g. [40, 43]), L. vulgare had on average more dissected leaves with a higher leaf perimeter to area ratio, longer teeth or lobes at the base of the mid-stem leaves and a larger leaf length to width ratio than L. ircutianum. In addition, L. vulgare had slightly smaller flower heads than L. ircutianum. However, even though L. vulgare and L. ircutianum differed in several morphological traits, the perimeter to area ratio of the stem leaves was the only trait that clearly separated the two species at the population level and all of the traits measured showed overlapping values between the two species at the level of individual plants. This is in agreement with other studies that stated that multiple characters need to be considered in order to clearly distinguish the two species [42, 43].
Theoretically, the differences in leaf morphology found between L. vulgare and L. ircutianum may have contributed to the unequal invasion success of the two species. Highly dissected and narrow leaves, as found for L. vulgare, tend to have more effective heat loss and higher evaporation and assimilation rates than broad, undivided leaves, and this may be beneficial in warm, sun-exposed environments [51, 52], but it is unlikely that these differences in leaf shape played an important role in the invasion success of L. vulgare since L. vulgare is also invasive in regions with high precipitation, such as western parts of Washington and Oregon and in regions with low temperatures, such as Alberta and British Columbia.
The observed phenotypic differences between the diploid L. vulgare and the tetraploid L. ircutianum may potentially be due to their differences in ploidy level. Polyploidy has often been associated with larger plants that produce larger leaves and fewer but larger flowers and seeds [53–56]. In addition, later germination, slower growth rates and later flowering as well as increased early growth rates have been found for polyploids compared to diploids [53–56]. Most of these phenotypic traits were similar for L. vulgare and L. ircutianum and only the larger flower heads of L. ircutianum are in line with these general effects of polyploidy. In agreement with our results, a similar study which compared diploid and allotetraploid cytotypes of Centaurea stoebe under standardized conditionsfound that the tetraploid cytotype had less dissected leaves and a lower SLA compared to the diploid cytotype [24]. However, because of the allopolyploid origin of L. ircutianum [33], hybridization or post-polyploidization evolution may have also contributed to the phenotypic differences between L. vulgare and L. ircutianum, potentially masking proposed phenotypic consequences of polyploidization.
Our common garden study provides little evidence that innate phenotypic traits pre-disposed L. vulgare to become more abundant in North America than L. ircutianum. However, the results of common garden studies can vary with the location and environmental conditions of the study [11, 57]. The environmental conditions experienced in our study are likely to be different than those in the introduced range and phenotypic traits may therefore be expressed differently than in the introduced range. To further elucidate whether L. vulgare possesses traits that particularly contribute to its invasion success common garden studies at multiple sites in the native and introduced range or common garden experiments that are more closely mimicking the environmental conditions encountered in the invaded habitats (e.g. regarding soil type and interacting species) need to be conducted.
Differences between native and introduced Leucanthemum vulgare
We found that L. vulgare plants from the introduced range flowered significantly later, were taller and had a higher biomass and more flower heads than those from the native range. These results are in agreement with those found in a field study comparing L. vulgare populations in the US and in Europe [27]. Because of a potential trade-off between size and age of reproduction [58, 59], the higher biomass of introduced L. vulgare may be a consequence of their later flowering. In line with this, we found a positive correlation between days to flowering and biomass for native L. vulgare populations, but not for introduced ones. A few other common garden studies also found a shift in days to flowering between native and introduced populations. For example, similar to our study, introduced North American populations of Centaurea diffusa Lam. flowered later and plants were larger than native European ones [16] and introduced populations of Ambrosia artemisiifolia L flowered later compared to native conspecifics [60]. In contrast, earlier flowering of introduced compared to native populations was found for Centaurea stoebe L., C. solstitialis L. and Silene latifolia Poir. [21, 61–64].
Differences in the days to flowering may evolve as an adaptation to differences in land use practices. Several plant species have been found to adapt their flowering phenology according to the time of mowing or grazing [65–67]. For instance, van Tienderen and van der Toorn [65] conducted a reciprocal-transplant experiment with Plantago lanceolata L. and found that plants from an early-mown hayfield flowered earlier than those from a late-mown hayfield and that plants from a pasture population flowered last across all transplantation sites. Similarly, Reisch and Poschlod [66] found that under common garden conditions Scabiosa columbaria L. populations from mown sites flowered on average four weeks earlier than those from grazed sites. Leucanthemum vulgare is generally avoided by cattle [28], but the mowing regime may highly affect their flowering time. Based on observations made during field surveys to study the herbivore communities associated with L. vulgare in Europe and North America [27], the mowing regime of sites with L. vulgare appears to differ between the two ranges. In Europe, L. vulgare and L. ircutianum mainly occur on meadows and pastures, most of which are generally mown for a first time in June and are not commonly found on sites that are not regulary mown. In North America, however, L. vulgare is often found on sites such as waste areas, landfills and forests, which are not mown, or on meadows, pastures and roadsides (S2 Appendix) that are mown later in the season. Consequently, in Europe, there is likely a strong selection pressure for early flowering while in North America there may be selection for late flowering plants as they have the advantage to grow larger and produce more seeds. Interestingly, two L. vulgare populations from the native range (DE8 from Germany and GE1 from Georgia) flowered later and had a higher biomass compared to all other native populations and also compared to the average introduced population. The seeds from these two populations had been collected from ruderal habitats that had not been mown in the past while most of the other seeds from the native range had been collected from sites that were mown at least once per year.
Differences in the days to flowering between the native and introduced range could potentially also result from an adaptation to differences in climate. For several native and invasive plant species grown under standardized conditions, populations collected from more northern latitudes were found to flower earlier and at a smaller size than those collected from southern locations (e.g. [11, 68–74]). Hence, phenotypic differences between ranges can be confounded with latitudinal clines [75]. Leucanthemum vulgare populations from the introduced range were collected from slightly more northern latitudes than those from the native range and no correlation between days to flowering or biomass and latitude was found, neither for L. vulgare from the native nor for those from the introduced range. Therefore, the later flowering of North American L. vulgare is unlikely caused by climatic differences between the two ranges. In addition, differences in the days to flowering between the native and introduced range might hypothetically also be the result of different selection pressures imposed by pre-dispersal seed predators or by pollinators between the two ranges. Both groups of organisms have been found to impose selection pressure on the time of flowering of plants and generally, pollinators tend to promote early flowering whereas seed predators tend to promote late flowering [76].
In contrast to traits affecting performance, most of the morphological traits did not differ between L. vulgare populations from the native and introduced range. The only two morphological traits that significantly differed between native and introduced populations of L. vulgare were a larger length to width ratio of the mid-stem leaves and a larger size of the flower heads. The larger length to width ratio of mid-stem leaves of introduced compared to native L. vulgare was due to an increase by 17% in leaf length at a similar leaf width and is therefore, together with the larger size of the flower heads, likely a consequence of their overall larger size.
Besides selection in the introduced range, genetic drift due to founder effects could also be responsible for the observed differences between native and introduced populations of L. vulgare. However, L. vulgare has been introduced multiple times to North America as ornamental and as seed contaminant [28] and it is very unlikely that the introduced plants exclusively consisted of late-flowering genotypes. Therefore, we suggest that natural selection in the introduced range more likely explains the observed pattern than founder effects. Since late-flowering L. vulgare populations were also found in the native range, they were potentially also among the plants introduced to North America, but late flowering genotypes may also have evolved in the introduced range independently from the native range. To discriminate between these hypotheses comprehensive molecular studies would be necessary.
Because we grew plants from field-collected seeds, environmental maternal effects could also have contributed to the observed differences between native and introduced L. vulgare populations [77]. Hence, their influence on the performance of L. vulgare populations collected in different biogeographic regions should be considered in future studies. However, maternal effects tend to mainly affect early development and their effects often decrease later in the life cycle [77–81]. Hence, while maternal effects may play a role in explaining the differences in leaf size of three-month old seedlings between native and introduced L. vulgare, they are unlikely responsible for the large differences in days to flowering and biomass at the end of flowering.
Conclusions
Our study did not provide clear evidence that L. vulgare was better pre-disposed to become invasive than L. ircutianum and we suggest that future studies should focus on identifying alternative explanations for the higher invasion success of L. vulgare compared to L. ircutianum. However, our results support our hypothesis that rapid evolution in the introduced range has likely contributed to the invasion success of L. vulgare in North America. One possible explanation for the increased performance of introduced compared to native L. vulgare populations is a release from a trade-off between early flowering and size due to a less intensive mowing regime of L. vulgare habitats in the introduced range. Our study contributes to the growing evidence showing that rapid evolution in the introduced range is common and that adaptation to altered environmental conditions may contribute to the invasion success of introduced plants [13, 14].
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(TXT)
Acknowledgments
We are grateful to M. Elsby, O. Rais, D. Sjolie and F. Willemin for their technical help with the experiment and to A.-C. Cossy-Pasche, B. Šingliarová and V. Renevey for their help with flow cytometric analyses. We would also like to thank the following people for collecting seeds or assistance in locating field sites: A. Barre, A. Bischoff, L. Blonski, J.-L. Boevé, M. Braun, J. Carder, S. Cesselli, A. Cortilet, G. Davidyan, G. Deisadze, A. Diaconu, M. Dolgovskaya, Z. Dorner, B. Drinkwater, M. Eylar, A. Foster, J. Gaskin, H. Hanson, J. Hardin, D. Kikodze, P. Kotanen, F. Krahulec, J. Littlefield, C. MacRae, K. Mann, A. Martinson, A. McClay, A. de Meij, V. Mrázová, A. Norton, E. Olsen, A. Pena Umana, V. Renevey, S. Reznik, M. Rutishauser, L. Sauvain, B. Šingliarová, U. Studer, S. Töpfer, S. Turner, C. Weber and P. Woods.
Data Availability
All relevant data are within the Supporting Information files.
Funding Statement
This study was supported by the Ministry of Forests, Lands and Natural Resource Operations, British Columbia, the Canadian Agricultural Adaptation Program, through the Agriculture and Food Council of Alberta, the Montana Noxious Weed Trust Fund, through Montana State University, the Wyoming Biological Control Steering Committee, and the USDA Forest Service. CABI is an international intergovernmental organisation, and we gratefully acknowledge the core financial support from our member countries (and lead agencies) including the United Kingdom (Department for International Development), China (Chinese Ministry of Agriculture), Australia (Australian Centre for International Agricultural Research), Canada (Agriculture and Agri-Food Canada), Netherlands (Directorate-General for International Cooperation) and Switzerland (Swiss Agency for Development and Cooperation). See http://www.cabi.org/about-cabi/who-we-work-with/key-donors/ for full details. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pyšek P, Richardson DM. Traits associated with invasiveness in alien plants: Where do we stand? In: Nentwig W, editor. Biol Invasions. Ecological Studies. 193: Springer; Berlin Heidelberg; 2007. p. 97–125. [Google Scholar]
- 2.Pyšek P, Jarošík V, Pergl J, Randall R, Chytrý M, Kühn I, et al. The global invasion success of Central European plants is related to distribution characteristics in their native range and species traits. Divers Distrib. 2009;15(5):891–903. doi: 10.1111/j.1472-4642.2009.00602.x [Google Scholar]
- 3.van Kleunen M, Weber E, Fischer M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett. 2010;13(2):235–45. doi: 10.1111/j.1461-0248.2009.01418.x [DOI] [PubMed] [Google Scholar]
- 4.Schlaepfer DR, Glaettli M, Fischer M, van Kleunen M. A multi-species experiment in their native range indicates pre-adaptation of invasive alien plant species. New Phytol. 2010;185(4):1087–99. doi: 10.1111/j.1469-8137.2009.03114.x [DOI] [PubMed] [Google Scholar]
- 5.Gallagher RV, Randall RP, Leishman MR. Trait differences between naturalized and invasive plant species independent of residence time and phylogeny. Conserv Biol. 2015;29(2):360–9. doi: 10.1111/cobi.12399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grotkopp E, Rejmánek M. High seedling relative growth rate and specific leaf area are traits of invasive species: Phylogenetically independent contrasts of woody angiospernis. Am J Bot. 2007;94(4):526–32. doi: 10.3732/ajb.94.4.526 [DOI] [PubMed] [Google Scholar]
- 7.Grotkopp E, Rejmánek M, Rost TL. Toward a causal explanation of plant invasiveness: Seedling growth and life-history strategies of 29 pine (Pinus) species. Am Nat. 2002;159(4):396–419. doi: 10.1086/338995 [DOI] [PubMed] [Google Scholar]
- 8.van Kleunen M, Johnson SD. South African Iridaceae with rapid and profuse seedling emergence are more likely to become naturalized in other regions. J Ecol. 2007;95(4):674–81. [Google Scholar]
- 9.Lake JC, Leishman MR. Invasion success of exotic in natural ecosystems: the role of disturbance, plant attributes and freedom from herbivores. Biol Conserv. 2004;117(2):215–26. doi: 10.1016/s0006-3207(03)00294-5 [Google Scholar]
- 10.Hamilton MA, Murray BR, Cadotte MW, Hose GC, Baker AC, Harris CJ, et al. Life-history correlates of plant invasiveness at regional and continental scales. Ecol Lett. 2005;8(10):1066–74. doi: 10.1111/j.1461-0248.2005.00809.x [Google Scholar]
- 11.Maron JL, Vilà M, Bommarco R, Elmendorf S, Beardsley P. Rapid evolution of an invasive plant. Ecol Monogr. 2004;74(2):261–80. doi: 10.1890/03-4027 [Google Scholar]
- 12.Prentis PJ, Wilson JRU, Dormontt EE, Richardson DM, Lowe AJ. Adaptive evolution in invasive species. Trends Plant Sci. 2008;13(6):288–94. doi: 10.1016/j.tplants.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 13.Buswell JM, Moles AT, Hartley S. Is rapid evolution common in introduced plant species? J Ecol. 2011;99(1):214–24. [Google Scholar]
- 14.Felker-Quinn E, Schweitzer JA, Bailey JK. Meta-analysis reveals evolution in invasive plant species but little support for Evolution of Increased Competitive Ability (EICA). Ecology and Evolution. 2013;3(3):739–51. doi: 10.1002/ece3.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn MA, Buckley YM, Müller-Schärer H. Increased population growth rate in invasive polyploid Centaurea stoebe in a common garden. Ecol Lett. 2012;15(9):947–54. doi: 10.1111/j.1461-0248.2012.01813.x [DOI] [PubMed] [Google Scholar]
- 16.Turner KG, Hufbauer RA, Rieseberg LH. Rapid evolution of an invasive weed. New Phytol. 2014;202(1):309–21. doi: 10.1111/nph.12634 [DOI] [PubMed] [Google Scholar]
- 17.Blossey B, Nötzold R. Evolution of increased competitive ability in invasive nonindigenous plants—a hypothesis. J Ecol. 1995;83(5):887–9. doi: 10.2307/2261425 [Google Scholar]
- 18.Colomer‐Ventura F, Martínez‐Vilalta J, Zuccarini P, Escolà A, Armengot L, Castells E. Contemporary evolution of an invasive plant is associated with climate but not with herbivory. Funct Ecol. 2015;29(11):1475–85. [Google Scholar]
- 19.Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia. 2005;144(1):1–11. doi: 10.1007/s00442-005-0070-z [DOI] [PubMed] [Google Scholar]
- 20.Zou J, Rogers WE, Siemann E. Differences in morphological and physiological traits between native and invasive populations of Sapium sebiferum. Funct Ecol. 2007;21(4):721–30. doi: 10.1111/j.1365-2435.2007.01298.x [Google Scholar]
- 21.Mráz P, Bourchier RS, Treier UA, Schaffner U, Müller-Schärer H. Polyploidy in phenotypic space and invasion context: A morphometric study of Centaurea stoebe s.l. Int J Plant Sci. 2011;172(3):386–402. doi: 10.1086/658151 [Google Scholar]
- 22.Eriksen RL, Desronvil T, Hierro JL, Kesseli R. Morphological differentiation in a common garden experiment among native and non-native specimens of the invasive weed yellow starthistle (Centaurea solstitialis). Biol Invasions. 2012;14(7):1459–67. doi: 10.1007/s10530-012-0172-6 [Google Scholar]
- 23.Guo W-Y, Lambertini C, Loc Xuan N, Li X-Z, Brix H. Preadaptation and post-introduction evolution facilitate the invasion of Phragmites australis in North America. Ecology and Evolution. 2014;4(24):4567–77. doi: 10.1002/ece3.1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henery ML, Bowman G, Mráz P, Treier UA, Gex-Fabry E, Schaffner U, et al. Evidence for a combination of pre-adapted traits and rapid adaptive change in the invasive plant Centaurea stoebe. J Ecol. 2010;98(4):800–13. doi: 10.1111/j.1365-2745.2010.01672.x [Google Scholar]
- 25.Fernald ML. Chrysanthemum leucanthemum and the American white weed. Rhodora. 1903;5:177–81. [Google Scholar]
- 26.Mulligan GA. Chromosome races in the Chrysanthemum leucanthemum complex. Rhodora. 1958:122–5. [Google Scholar]
- 27.Stutz S, Štajerová K, Hinz HL, Müller-Schärer H, Schaffner U. Can enemy release explain the invasion success of the diploid Leucanthemum vulgare in North America? Biological Invasions. 2016;18(7):2077–91. [Google Scholar]
- 28.Olson BE, Wallander RT. Oxeye daisy (Chrysanthemum leucanthemum L.) In: Sheley RL, Petroff JK, editors. Biology and Management of Noxious Rangeland Weeds. Corvallis, Oregon: Oregon State University Press; 1999. p. 282–9. [Google Scholar]
- 29.Clements DR, Cole DE, Darbyshire S, King J, McClay A. The biology of Canadian weeds. 128. Leucanthemum vulgare Lam. Canadian Journal of Plant Science. 2004;84(1):343–63. [Google Scholar]
- 30.Lavoie C, Saint-Louis A, Guay G, Groeneveld E, Villeneuve P. Naturalization of exotic plant species in north-eastern North America: trends and detection capacity. Divers Distrib. 2012;18(2):180–90. doi: 10.1111/j.1472-4642.2011.00826.x [Google Scholar]
- 31.Zelený V. Kopretina bílá (Leucanthemum vulgare Lam. complex) v Československu Prague: Academia; 1982. [Google Scholar]
- 32.Vogt R. Die Gattung Leucanthemum Mill. (Compositae-Anthemideae) auf der Iberischen Halbinsel Madrid: Ruiza; 1991. 1–261 p. [Google Scholar]
- 33.Oberprieler C, Eder C, Meister J, Vogt R. AFLP fingerprinting suggests an allopolyploid origin of two members of the Leucanthemum vulgare aggregate (Compositae, Anthemideae) in central Europe. Nord J Bot. 2011;29(3):370–7. doi: 10.1111/j.1756-1051.2011.01025.x [Google Scholar]
- 34.Pandit MK, Pocock MJO, Kunin WE. Ploidy influences rarity and invasiveness in plants. J Ecol. 2011;99(5):1108–15. doi: 10.1111/j.1365-2745.2011.01838.x [Google Scholar]
- 35.Pandit MK, White SM, Pocock MJO. The contrasting effects of genome size, chromosome number and ploidy level on plant invasiveness: a global analysis. New Phytol. 2014;203(2):697–703. doi: 10.1111/nph.12799 [DOI] [PubMed] [Google Scholar]
- 36.te Beest M, Le Roux JJ, Richardson DM, Brysting AK, Suda J, Kubešová M, et al. The more the better? The role of polyploidy in facilitating plant invasions. Ann Bot. 2012;109(1):19–45. doi: 10.1093/aob/mcr277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hegi G, editor. Illustrierte Flora von Mitteleuropa Spermatophyta Band IV. Angiospermae, Dicotyledones 4. Berlin—Hamburg: Paul Parey; 1987. [Google Scholar]
- 38.Greuter W. Compositae (pro parte majore). Euro+Med Plantbase: the information resource for Euro-Mediterranean plant diversity. http://ww2.bgbm.org/EuroPlusMed/ 2006+ [cited 2017 14 August].
- 39.Heywood VH. Leucanthemum In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, et al. , editors. Flora Europaea Vol 4 Cambridge, U.K: Cambridge University Press; 1976. [Google Scholar]
- 40.Böcher TW, Larsen K. Cytotaxonomical studies in the Chrysanthemum leucanthemum complex. Watsonia. 1957;4(1):11–6. [Google Scholar]
- 41.Przywara L, Schmager J. Cytotaxonomical studies on Chrysanthemum leucanthemum L. sensu lato. Acta Biol Cracov, Ser Bot. 1968;11:105–16. [Google Scholar]
- 42.Pearson PL. An experimental taxonomic study of Chrysanthemum leucanthemum L. [Doctoral Thesis]: University of Durham; 1967.
- 43.Scholz C, Uhlemann I. Leucanthemum ircutianum DC. und Leucanthemum vulgare Lam. in Sachsen-Anhalt. Mitt florist Kart Sachsen-Anhalt. 2001;6:43–8. [Google Scholar]
- 44.Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, et al. Let the concept of trait be functional! Oikos. 2007;116(5):882–92. [Google Scholar]
- 45.Rasband W. ImageJ. Bethesda, MD, USA: US National Institutes of Health 1997–2014. http://imagej.nih.gov/ij/.
- 46.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. http://www.R-project.org. [Google Scholar]
- 47.Dray S, Dufour A-B. The ade4 package: implementing the duality diagram for ecologists. Journal of statistical software. 2007;22(4):1–20. [Google Scholar]
- 48.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. Linear and nonlinear mixed effects models 2015. http://CRAN.R-project.org/package=nlme.
- 49.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical software. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01 [Google Scholar]
- 50.Wright IJ, Westoby M. Understanding seedling growth relationships through specific leaf area and leaf nitrogen concentration: generalisations across growth forms and growth irradiance. Oecologia. 2001;127(1):21–9. doi: 10.1007/s004420000554 [DOI] [PubMed] [Google Scholar]
- 51.Schuepp PH. Tansley review No. 59 Leaf boundary layers. New Phytol. 1993;125(3):477–507. doi: 10.1111/j.1469-8137.1993.tb03898.x [DOI] [PubMed] [Google Scholar]
- 52.Nicotra AB, Leigh A, Boyce CK, Jones CS, Niklas KJ, Royer DL, et al. The evolution and functional significance of leaf shape in the angiosperms. Funct Plant Biol. 2011;38(7):535–52. [DOI] [PubMed] [Google Scholar]
- 53.Levin DA. Polyploidy and novelty in flowering plants. Am Nat. 1983;122(1):1–25. doi: 10.1086/284115 [Google Scholar]
- 54.Ramsey J, Schemske DW. Neopolyploidy in flowering plants. Annu Rev Ecol Syst. 2002;33:589–639. [Google Scholar]
- 55.Levin DA. The role of chromosomal change in plant evolution. New York, USA: Oxford University Press; 2002. [Google Scholar]
- 56.Gottschalk W. Die Bedeutung der Polyploidie für die Evolution der Pflanzen. Stuttgart, Germany: Fischer; 1976. [Google Scholar]
- 57.Moloney KA, Holzapfel C, Tielborger K, Jeltsch F, Schurr FM. Rethinking the common garden in invasion research. Perspectives in Plant Ecology Evolution and Systematics. 2009;11(4):311–20. doi: 10.1016/j.ppees.2009.05.002 [Google Scholar]
- 58.Stearns SC. The evolution of life histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- 59.Roff DA. Evolution of life histories: theory and analysis. New York, USA: Chapman & Hall; 1992. [Google Scholar]
- 60.Hodgins K, Rieseberg L. Genetic differentiation in life‐history traits of introduced and native common ragweed (Ambrosia artemisiifolia) populations. J Evol Biol. 2011;24(12):2731–49. doi: 10.1111/j.1420-9101.2011.02404.x [DOI] [PubMed] [Google Scholar]
- 61.Hahn MA, Müller-Schärer H. Cytotype differences modulate eco-geographical differentiation in the widespread plant Centaurea stoebe. Ecology. 2013;94(5):1005–14. [DOI] [PubMed] [Google Scholar]
- 62.Dlugosch KM, Cang FA, Barker BS, Andonian K, Swope SM, Rieseberg LH. Evolution of invasiveness through increased resource use in a vacant niche. Nature plants. 2015;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolfe LM, Elzinga JA, Biere A. Increased susceptibility to enemies following introduction in the invasive plant Silene latifolia. Ecol Lett. 2004;7(9):813–20. doi: 10.1111/j.1461-0248.2004.00649.x [Google Scholar]
- 64.Blair AC, Wolfe LM. The evolution of an invasive plant: an experimental study with Silene latifolia. Ecology. 2004;85(11):3035–42. [Google Scholar]
- 65.van Tienderen PH, van der Toorn J. Genetic differentiation between populations of Plantago lanceolata. I. Local adaptation in three contrasting habitats. J Ecol. 1991;79(1):27–42. [Google Scholar]
- 66.Reisch C, Poschlod P. Land use affects flowering time: seasonal and genetic differentiation in the grassland plant Scabiosa columbaria. Evol Ecol. 2009;23(5):753–64. [Google Scholar]
- 67.Völler E, Auge H, Bossdorf O, Prati D. Land use causes genetic differentiation of life-history traits in Bromus hordeaceus. Global Change Biol. 2013;19(3):892–9. doi: 10.1111/gcb.12087 [DOI] [PubMed] [Google Scholar]
- 68.Weber E, Schmid B. Latitudinal population differentiation in two species of Solidago (Asteraceae) introduced into Europe. Am J Bot. 1998;85(8):1110–21. [PubMed] [Google Scholar]
- 69.Clevering OA, Brix H, Lukavska J. Geographic variation in growth responses in Phragmites australis. Aquat Bot. 2001;69(2–4):89–108. doi: 10.1016/s0304-3770(01)00132-2 [Google Scholar]
- 70.Olsson K, Ågren J. Latitudinal population differentiation in phenology, life history and flower morphology in the perennial herb Lythrum salicaria. J Evol Biol. 2002;15(6):983–96. doi: 10.1046/j.1420-9101.2002.00457.x [Google Scholar]
- 71.Kollmann J, Bañuelos MJ. Latitudinal trends in growth and phenology of the invasive alien plant Impatiens glandulifera (Balsaminaceae). Divers Distrib. 2004;10(5–6):377–85. doi: 10.1111/j.1366-9516.2004.00126.x [Google Scholar]
- 72.Stinchcombe JR, Weinig C, Ungerer M, Olsen KM, Mays C, Halldorsdottir SS, et al. A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4712–7. doi: 10.1073/pnas.0306401101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montague JL, Barrett SCH, Eckert CG. Re-establishment of clinal variation in flowering time among introduced populations of purple loosestrife (Lythrum salicaria, Lythraceae). J Evol Biol. 2008;21(1):234–45. doi: 10.1111/j.1420-9101.2007.01456.x [DOI] [PubMed] [Google Scholar]
- 74.Novy A, Flory SL, Hartman JM. Evidence for rapid evolution of phenology in an invasive grass. J Evol Biol. 2013;26(2):443–50. doi: 10.1111/jeb.12047 [DOI] [PubMed] [Google Scholar]
- 75.Colautti RI, Maron JL, Barrett SCH. Common garden comparisons of native and introduced plant populations: latitudinal clines can obscure evolutionary inferences. Evolutionary Applications. 2009;2(2):187–99. doi: 10.1111/j.1752-4571.2008.00053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elzinga JA, Atlan A, Biere A, Gigord L, Weis AE, Bernasconi G. Time after time: flowering phenology and biotic interactions. Trends Ecol Evol. 2007;22(8):432–9. doi: 10.1016/j.tree.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 77.Roach DA, Wulff RD. Maternal effects in plants. Annu Rev Ecol Syst. 1987;18:209–35. doi: 10.1146/annurev.es.18.110187.001233 [Google Scholar]
- 78.Agrawal AA. Herbivory and maternal effects: Mechanisms and consequences of transgenerational induced plant resistance. Ecology. 2002;83(12):3408–15. doi: 10.2307/3072089 [Google Scholar]
- 79.Hereford J, Moriuchi KS. Variation among populations of Diodia teres (Rubiaceae) in environmental maternal effects. J Evol Biol. 2005;18(1):124–31. doi: 10.1111/j.1420-9101.2004.00797.x [DOI] [PubMed] [Google Scholar]
- 80.Bischoff A, Müller-Schärer H. Testing population differentiation in plant species—how important are environmental maternal effects. Oikos. 2010;119(3):445–54. [Google Scholar]
- 81.Weiner J, Martinez S, Müller-Schärer H, Stoll P, Schmid B. How important are environmental maternal effects in plants? A study with Centaurea maculosa. J Ecol. 1997:133–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(TXT)
Data Availability Statement
All relevant data are within the Supporting Information files.