Abstract
Background
Although the malaria burden in the Lao PDR has gradually decreased, the elimination of malaria by 2030 presents many challenges. Microscopy and malaria rapid diagnostic tests (RDTs) are used to diagnose malaria in the Lao PDR; however, some studies have reported the prevalence of sub-microscopic Plasmodium infections or asymptomatic Plasmodium carriers in endemic areas. Thus, highly sensitive detection methods are needed to understand the precise malaria situation in these areas.
Methodology/Principal findings
A cross-sectional malaria field survey was conducted in 3 highly endemic malaria districts (Xaysetha, Sanamxay, Phouvong) in Attapeu province, Lao PDR in 2015, to investigate the precise malaria endemicity in the area; 719 volunteers from these villages participated in the survey. Microscopy, RDTs and a real-time nested PCR were used to detect Plasmodium infections and their results were compared. A questionnaire survey of all participants was also conducted to estimate risk factors of Plasmodium infection. Numbers of infections detected by the three methods were microscopy: P. falciparum (n = 1), P. vivax (n = 2); RDTs: P. falciparum (n = 2), P. vivax (n = 3); PCR: Plasmodium (n = 47; P. falciparum [n = 4], P. vivax [n = 41], mixed infection [n = 2]; 6.5%, 47/719). Using PCR as a reference, the sensitivity and specificity of microscopy were 33.3% and 100.0%, respectively, for detecting P. falciparum infection, and 7.0% and 100.0%, for detecting P. vivax infection. Among the 47 participants with parasitemia, only one had a fever (≥37.5°C) and 31 (66.0%) were adult males. Risk factors of Plasmodium infection were males and soldiers, whereas a risk factor of asymptomatic Plasmodium infection was a history of ≥3 malaria episodes.
Conclusions/Significance
There were many asymptomatic Plasmodium carriers in the study areas of Attapeu province in 2015. Adult males, probably soldiers, were at high risk for malaria infection. P. vivax, the dominant species, accounted for 87.2% of the Plasmodium infections among the participants. To achieve malaria elimination in the Lao PDR, highly sensitive diagnostic tests, including PCR-based diagnostic methods should be used, and plans targeting high-risk populations and elimination of P. vivax should be designed and implemented.
Author summary
The Lao People’s Democratic Republic (Laos) is a country in the Greater Mekong Subregion. In Laos, the numbers of reported cases of malaria and deaths due to malaria have been gradually decreasing. Recently, the Lao government adopted a goal of eliminating malaria by 2030. To achieve this goal, we must understand the precise situation in each endemic area. With this background, we conducted a field survey in Attapeu, one highly endemic province, in 2015. We collected blood samples from 719 villagers, and most (98.1%, 705/719) had no fever (<37.5°C) at the time of survey. The samples were examined using three diagnostic methods: microscopy, rapid diagnostic test (RDT) and polymerase chain reaction (PCR). PCR revealed parasitemia in 6.5% of the villagers (47/719), and only one showed a fever (≥37.5°C) at the time of survey. The dominant species was Plasmodium vivax (87.2%, 41/47). Males and soldiers tended to be infected with malaria parasites whereas those with a history of multiple malaria episodes (≥3 times) tended to be asymptomatic malaria carriers. These findings suggest the need to implement targeted elimination plans for vivax malaria that include measures to protect males and soldiers, who are at high risk of malaria infection.
Introduction
The malaria burden in the Lao People’s Democratic Republic (PDR) has gradually decreased thanks to the efforts of the Lao government and the support of partners such as the World Health Organization (WHO), and the Global Fund to Fight AIDS, Tuberculosis and Malaria [1]. In 2015, the malaria-associated mortality rate (number of deaths/100,000 population) was 0.03, which was lower than the target in Millennium Development Goal 6 (MDG 6) (<0.20) [2]. However, in the same year, the malaria-associated morbidity rate (annual parasite incidence [API]: number of cases/1,000 population) was 4.9, which was higher than the target in MDG 6 (<0.6). Now, the Ministry of Health (MOH) of the Lao PDR and the WHO have adopted the goal of eliminating malaria by the year 2030 [3, 4].
In central, provincial and district hospitals in the Lao PDR, malaria is typically diagnosed by microscopy, whereas rapid diagnostic tests (RDTs) are used as a sub-standard diagnostic method at locations in rural areas, such as health centers and selected villages with high malaria endemicity (API ≥10). Recently, highly sensitive methods, such as polymerase chain reaction (PCR) [5], ultra-sensitive PCR [6–8] and loop-mediated isothermal amplification (LAMP) [9–11] are becoming available and being used to detect low-level malaria infections in research bases in endemic areas. Such methods can detect sub-microscopic malaria infections and asymptomatic Plasmodium carriers in the endemic areas.
We conducted a malaria field survey of 10 villages in Xepon district, Savannakhet province in the Lao PDR, where malaria was highly endemic from August to September in 2013 (the rainy season) [5]. A nested PCR using dried blood samples from healthy villagers revealed that there were many asymptomatic Plasmodium falciparum carriers who could be considered to be parasite reservoirs or a cryptic malaria group. In most cases, the parasite densities among the villagers were below the microscopic threshold, i.e., sub-microscopic malaria cases. Interestingly, these parasite carriers were likely to be grouped within a family [5]. An Oxford research team also reported similar results but for many P. vivax carriers from the Thapangthong and Nong districts, which are also located in Savannakhet province, from March to July in 2015 (dry season to the beginning of the rainy season) [8].
Recently, there has been a gradual decrease in the number of reported cases of malaria in Savannakhet province thanks to the extensive efforts of the provincial health office and the support of several partners. In contrast, the number of reported cases in southern provinces, such as Salavan, Sekong, Attapeu and Champasak, remain high. According to the national data collected by the Center of Malariology, Parasitology and Entomology (CMPE) of the MOH in 2013, the API of Attapeu province was 37.8, which was the highest among all provinces in the Lao PDR. Therefore, in the present study, a cross-sectional field survey was conducted in Attapeu province, which borders Vietnam and Cambodia in the southern part of the Lao PDR (Fig 1), to investigate the prevalence of asymptomatic Plasmodium infections among the people in malaria-endemic villages, the distribution of the species of malaria parasites, and the risk factors of Plasmodium infections. We also evaluated the performance of our PCR technique in comparison to microscopy and RDTs.
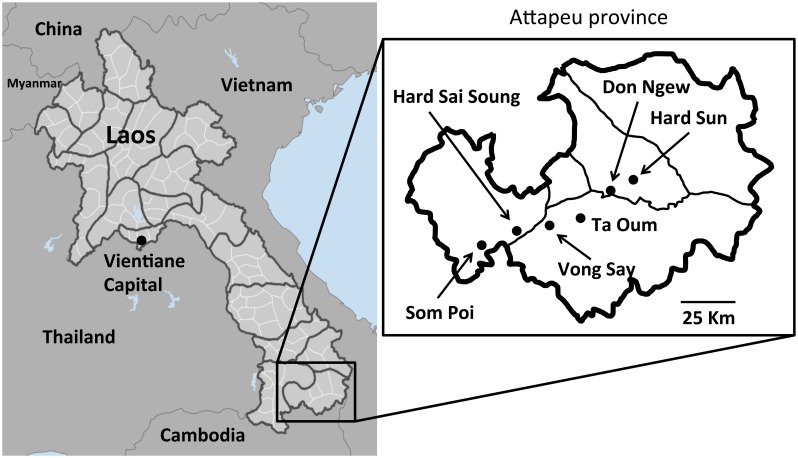
Fig 1. Study sites in Attapeu province, the Lao PDR.
Don Ngew and Hard Sun villages are located in Xaysetha district (API = 31.2); Vong Say and Ta Oum villages are located in Phouvong district (API = 103.4); and Hard Sai Soung and Som Poi villages are located in Sanamxay district (API = 59.9). API, annual parasite incidence (according to the API data from 2013). Map of Laos (left), Data source: CIESIN, CIAT, GPW, Available from: http://sedac.ciesin.columbia.edu/gpw. Map of Attapeu province (right) was created by the authors.
Methods
Ethics statement
The research proposal was reviewed and approved by the National Ethics Committee for Health Research, Ministry of Health, Lao PDR (No. 049 NIOPH/NECHR) in 2014. Written informed consent was obtained from all of the participants prior to the interview and the collection of blood for the diagnosis of malaria. The guardians of child participants (<18 years old) consented to their participation.
Sample collection
In May 2015, which was the beginning of rainy season, a cross-sectional field survey was conducted in the three districts that showed the greatest malaria endemicity in Attapeu province, Lao PDR: Xaysetha, Sanamxay and Phouvong (Fig 1). In 2013, the APIs of Xaysetha, Sanamxay and Phouvong were 31.2, 59.9 and 103.4, respectively. The populations of Xaysetha, Sanamxay and Phouvong were 32,888, 30,551 and 12,432, respectively, in 2014. The sample size of each district was calculated based on the malaria prevalences (API) in 2013 and the populations in 2014, with a confidence level of 95% and a confidence interval (CI) of 5%. The minimum sample sizes of Xaysetha, Sanamxay and Phouvong were 47, 87 and 143, respectively. Two malaria high endemic villages (strata 3; API ≥10) that were accessible by car were randomly selected in each district using village lists in district health offices.
Village leader or village health volunteer informed the villagers to join the malaria survey and asked not to go to their fields, forests or fishing on the day of the survey, if possible. The survey team was based at a temple, meeting place or health center in the villages and conducted an interview with and malaria blood test for the voluntary participants who came to us (convenience sampling). We accepted all voluntary participants who came to us regardless of their age, gender, occupation, ethnicity or health conditions. Information on demographics (age, gender, ethnicity, occupation, marital status, educational level), tympanic temperature, any current symptoms and signs (time first noticed), malaria treatment, number of previous malaria episodes (malaria history) and use of insecticide-treated bed nets was collected by using a questionnaire form.
An approximately 150–200 μL blood sample was collected from each participant by finger prick using a lancet. Three diagnostic methods were applied to detect Plasmodium infections in blood samples: microscopy (thin and thick blood smears), RDTs (SD Bioline Malaria Ag Pf/Pv, Standard Diagnostics, Inc., Gyeonggi-do, Republic of Korea) and PCR. The malaria RDTs were conducted on-site, whereas microscopy and the PCR were conducted at IPL in Vientiane after the survey. When Plasmodium infections were detected by the RDTs, an antimalarial medicine (Coartem; artemether + lumefantrine) was prescribed free of charge on-site by the staff members from the district hospital or health center that was conducting the survey in the village. For the PCR, blood samples were collected on filter papers (Whatman FTA Classic Cards, GE Healthcare Life Science, UK) in accordance with the manufacturer’s instructions.
DNA extraction and real-time PCR
DNA was extracted from the dried blood spots on the filter papers with a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions. Six punched-out circles (3.175 mm [1/8 inch]) from the dried blood spot on the filter paper were used for DNA extraction, which was equivalent to 30–40 μL of whole blood. The extracted DNA was eluted with 50 μL of elution buffer in the kit and preserved until use at -30°C. To identify malaria parasite infection, a real-time nested PCR was conducted using a primer set that was reported in a previous study (S1 Table) [12, 13]. In the primary real-time PCR, a universal primer set for amplifying the partial cytochrome b gene on the mitochondrial genome of all human malaria parasites was used. In the secondary real-time PCR, primer sets that were specific for P. falciparum and P. vivax were used to detect the two species. The real-time PCR was performed using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratory, Inc., USA) using 2 μL of the extracted DNA as a template, which was equivalent to 1.2–1.6 μL of whole blood. The primary PCR product was diluted 25 times with PCR-grade water, and 2 μL of the diluted primary PCR product was used as a template for the secondary real-time PCR. Serial diluted recombinant plasmid DNAs containing the cyt b region of P. falciparum or P. vivax were used as the positive control for each assay. PCR-grade water was used as the negative control for each assay. A sample was considered negative if there was no increase in the SYBR Green (fluorescent) signal after 35 cycles. The PCRs were performed independently, in triplicate. The sample was considered positive for Plasmodium DNA when positive results were obtained at least twice. As an external quality control for the PCR, some of the dried blood samples on the filter papers were sent to a laboratory in the National Center for Global Health and Medicine, Japan and examined by experienced researchers.
The sensitivity and specificity of microscopy and the malaria RDTs for the diagnosis of P. falciparum and P. vivax were calculated using the results of the real-time nested PCR as a reference.
Statistical analysis
For bivariate analyses, the Chi-square test and Fisher’s exact test were used to evaluate an association between variables and Plasmodium infection. A P value less than 0.05 was considered statistically significant. Multivariate logistic regression analyses that adjusted for the effects of other variables were conducted to estimate the association between variables and Plasmodium infection and asymptomatic Plasmodium infection using SPSS version 18.0 (SPSS INC., Chicago, IL, USA). All variables with a P-value of 0.20 from univariate analysis were entered into a multivariate logistic regression analysis. Multicollinearity among all independent variables was tested before logistic regression.
Asymptomatic Plasmodium infection was defined by the following criteria: Plasmodium DNA was positive by PCR, tympanic temperature was less than 37.5°C (no fever) at the time of the survey and no history of any subjective symptoms and signs at the time of the survey and in the preceding 14 days.
Results
Socio-demographic data of the participants
A total of 719 villagers (male, n = 336; female, n = 383) participated in this survey (Table 1). The socio-demographic data of the participants are summarized in Table 2 and S2 Table. Most of the adult participants (n = 472; 65.6%) were farmers, and 160 of the participants (22.3%) were students. The study population included two major ethnic groups: Lao Loum (n = 189; 26.3%) and Lao Therng (n = 529; 73.6%). More than half of the participants (n = 410; 57.0%) had never been to primary school or had dropped out of primary school before graduation.
Table 1. The numbers of participants in the three districts of Attapeu province.
| District | Village Name (latitude and longitude) |
Date of Survey (Year, Month, Day) |
Population of villages (2014) |
No. of Participants | |
|---|---|---|---|---|---|
| Male | Female | ||||
| Xaysetha | Don Ngew | 2015 May 11 | 903 | 63 | 69 |
| (N 14° 44’ 46.7”, E 106° 57’ 15.0”) | |||||
| Hard Sun | 2015 May 12 | 1,053 | 50 | 70 | |
| (N 14° 47’ 45.0”, E 107° 01’ 06.7”) | |||||
| Phouvong | Vong Say | 2015 May 13 | 431 | 44 | 63 |
| (N 14° 39’ 45.0”, E 106° 41’ 57.7”) | |||||
| Ta Oum | 2015 May 14 | 330 | 47 | 57 | |
| (N 14° 40’ 53.2”, E 106° 49’ 36.0”) | |||||
| Sanamxay | Hard Sai Soung | 2015 May 15 | 280 | 39 | 63 |
| (N 14° 38’ 36.4”, E 106° 34’ 04.7”) | |||||
| Som Poi | 2015 May 16 | 1,020 | 94 | 60 | |
| (N 14° 33’ 29.3”, E 106° 27’ 59.3”) | |||||
| Total | 4,017 | 337 | 382 | ||
Table 2. The socio-demographic data of the participants (N = 719).
| Variable | Number | % | |
|---|---|---|---|
| Gender | Female | 383 | 53.3 |
| Male | 336 | 46.7 | |
| Age (years) | Median ± SD (Range) | 24 ± 16.6 (0–90) | |
| Occupation | Agriculture | 472 | 65.6 |
| Student | 160 | 22.3 | |
| Child | 54 | 7.5 | |
| Trader | 2 | 0.3 | |
| Soldier | 10 | 1.4 | |
| Housewife | 13 | 1.8 | |
| Teacher | 8 | 1.1 | |
| Education | No education or not completed* | 410 | 57.0 |
| Primary school | 240 | 33.4 | |
| Secondary school | 47 | 6.5 | |
| High school | 22 | 3.1 | |
| Marital status | Never married | 288 | 40.1 |
| Married | 425 | 59.1 | |
| Divorced | 4 | 0.6 | |
| Widowed | 1 | 0.1 | |
| No data | 1 | 0.1 | |
| Religion | Buddhism | 234 | 32.5 |
| Christianity | 3 | 0.4 | |
| Traditional Animism | 481 | 66.9 | |
| Other | 1 | 0.1 | |
| Ethnicity | Lao Loum | 189 | 26.3 |
| Lao Therng | 529 | 73.6 | |
| Lao Soung | 0 | 0.0 | |
| No data | 1 | 0.1 | |
| District | Xaysettha | 252 | 35.0 |
| Phouvong | 211 | 29.3 | |
| Sanamxay | 256 | 35.6 | |
*Not completed, Dropped out of primary school before graduation.
Plasmodium prevalence by the three diagnostic methods
Overall, Plasmodium DNA was detected in 47 (6.5%) participants by the PCR (S3 Table). Three of them were also detected by microscopy: P. falciparum (n = 1; 0.1%) and P. vivax (n = 2; 0.3%), and 5 of them were also detected by the RDTs: P. falciparum (n = 2; 0.3%) and P. vivax (n = 3; 0.4%) including the individuals who were microscopy-positive. Including all diagnostic tests, 5 of the 47 participants were P. falciparum, 41 of the 47 participants were P. vivax and 1 of the 47 participants was mixed infection with P. falciparum and P. vivax. Using the results of the real-time PCR as a reference, the sensitivity of microscopy for detecting P. falciparum and P. vivax was calculated as 16.7% (95% CI -13.2–46.5) and 4.7% (95% CI -1.6–10.9), respectively (Table 3). The sensitivity of the RDTs for P. falciparum and P. vivax was 33.3% (95% CI -4.4–71.1) and 7.0% (95% CI -0.6–14.6), respectively. The specificity of microscopy and the RDTs was 100.0% for both P. falciparum and P. vivax. With regard to gender, 41 of the 336 male participants were Plasmodium-positive, whereas only 6 of the 383 female participants were Plasmodium-positive (S4 Table).
Table 3. The sensitivity and specificity of microscopy and the RDT in comparison to real-time PCR.
| Real-Time PCR | |||
|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | ||
| Microscopy | P. falciparum | 16.7% (-13.2–46.5) | 100.0% (100.0–100.0) |
| P. vivax | 4.7% (-1.6–10.9) | 100.0% (100.0–100.0) | |
| RDT | P. falciparum | 33.3% (-4.4–71.1) | 100.0% (100.0–100.0) |
| P. vivax | 7.0% (-0.6–14.6) | 100.0% (100.0–100.0) | |
RDT, rapid diagnostic test; CI, confidence interval.
The positive rate of the male participants (12.2%) was significantly higher than that of the female participants (1.6%) (P < 0.001). The positive rate of soldiers (50%) was the highest among the occupations (S4 Table). The prevalence of Plasmodium infections was heterogeneous among the districts: Xaysettha 0.40 (95% CI -0.38–1.18), Phouvong 7.11 (95% CI 3.61–10.60) and Sanamxay 12.11 (95% CI 8.09–16.13) (Table 4).
Table 4. Prevalence of Plasmodium infections.
| Place | No. of Participants | No. Positive | Prevalence (95% CI) |
|---|---|---|---|
| Xaysettha District | 252 | 1 | 0.40 (-0.38–1.18) |
| Don Ngew Village | 132 | 0 | 0.00 |
| Hard Sun Village | 120 | 1 | 0.83 (-0.82–2.48) |
| Phouvong District | 211 | 15 | 7.11 (3.61–10.60) |
| Vong Say Village | 107 | 8 | 7.48 (2.41–12.54) |
| Ta Oum Village | 104 | 7 | 6.73 (1.83–11.63) |
| Sanamxay District | 256 | 31 | 12.11 (8.09–16.13) |
| Hard Sai Soung Village | 102 | 5 | 4.90 (0.64–9.16) |
| Som Poi Village | 154 | 26 | 16.88 (10.90–22.87) |
CI, confidence interval.
Clinical data of the participants
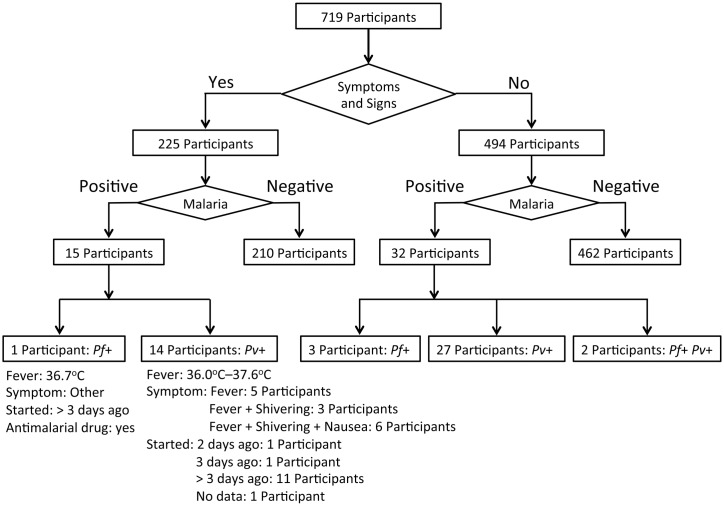
A flowchart summary of malaria screening based on the clinical and PCR data is shown in Fig 2. The average body temperature of the participants was 36.8°C (34.8°C–39.6°C), and only 14 participants (14/719: 1.9%) had a fever (>37.5°C) at the time of the survey. The average body temperature of the 47 PCR-positive participants was 36.7°C (36.0°C–37.6°C). A summary of the socio-demographic and clinical data of the 47 PCR-positive participants is shown in S3 Table. Only one of the 47 PCR-positive participants had a fever (>37.5°C) at the time of the survey. This febrile participant (ID: PT-015, 8 years of age, female) showed negative results for the blood smear and RDTs but was found to be infected with P. vivax using the real-time nested PCR. The fever started 2 days before the survey. This girl had used an insecticide-treated bet net while sleeping. The average age of all participants was 26.4 years (0–90 years, median age: 24 years), whereas that of the 47 PCR-positive participants was 29.0 years (6–75 years, median age: 25.0 years). Fifteen of the 47 PCR-positive participants answered that they had some symptoms or signs of health problems, such as fever, shivering and nausea, but only one participant had a fever (>37.5°C) at the time of the survey (S3 Table). Eleven of these 15 participants answered that the symptoms or signs started more than 3 days before the survey. In contrast, 32 of the 47 PCR-positive participants had no symptoms or signs of health problems at the time of and the preceding 14 days before the survey (S3 Table).
Fig 2. Flowchart summary of malaria screening based on the clinical and PCR data.
Risk factors associated with Plasmodium infections
Results of bivariate analyses to estimate an association between variables and Plasmodium infections are shown in S4 Table. Risk factors associated with Plasmodium infections were estimated by multiple logistic regression analyses. Three variables were independently associated with Plasmodium infections: being male (AOR: 6.04, 95% CI: 1.35–27.10), Soldier (AOR: 28.58, 95% CI: 2.89–282.60), and being Lao Loum (AOR: 2.71 95% CI: 1.12–6.59) (Table 5). The risk factor associated with asymptomatic (cryptic) Plasmodium infections was a history of 3 or more malaria episodes (multiple malaria infections) (Model 1, AOR: 12.66, 95% CI: 1.21–132.00) (S5 Table).
Table 5. Multivariate logistic regression analyses to identify risk factors for Plasmodium infections.
| Status | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | AOR | 95% CI | p | ||
| Village | Others | 1.00 | - | ||||
| Som Poi | 5.26 | 2.87–9.65 | <0.001 | - | - | - | |
| Gender | Female | 1.00 | 1.00 | ||||
| Male | 8.71 | 3.65–20.80 | <0.001 | 6.04 | 1.35–27.10 | 0.019 | |
| Occupation | Others | 1.00 | 1.00 | ||||
| Soldier | 15.88 | 4.42–57.00 | <0.001 | 28.58 | 2.89–282.60 | 0.004 | |
| Education | Lower than High School | 1.00 | - | ||||
| Equal to or higher than High School | 4.59 | 1.61–13.0 | 0.004 | - | - | - | |
| Religion | Not Buddhism | 1.00 | - | ||||
| Buddhism | 2.76 | 1.52–5.02 | 0.001 | - | - | - | |
| Ethnicity | Lao Theung | 1.00 | 1.00 | ||||
| Lao Loum | 3.85 | 2.11–7.03 | <0.001 | 2.71 | 1.12–6.59 | 0.027 | |
| ITBN use | Yes | 1.00 | - | ||||
| No | 0.49 | 0.18–1.31 | 0.156 | - | - | - | |
| Malaria past episode | ≤ 2 times | 1.00 | 1.00 | ||||
| ≥ 3 times | 3.35 | 1.48–7.61 | 0.004 | 2.35 | 0.93–5.96 | 0.071 | |
OR, odds ratio; CI, confidence interval; AOR, adjusted odds ratio; ITBN, insecticide-treated bed net.
Multivariate analysis was adjusted for gender, occupation, ethnicity and past malaria episodes.
Discussion
In the present study, all 719 participants resided in villages in the districts of Attapeu province that showed high malaria endemicity in the Lao PDR. We found 47 PCR-positive Plasmodium infections among the participants (6.5%), most (31/47, 66.0%) of whom were adult males (≥18 years). The national malaria data, which were summarized by the CMPE in 2015, revealed that P. falciparum, P. vivax and mixed infection accounted for 40%, 58% and 2% of malaria infections, respectively. The national data were based on the results obtained by microscopy (in hospitals) and RDTs (in health centers or villages) through the passive detection of symptomatic cases. In the present study, the proportions of P. falciparum (n = 2; 40%) and P. vivax (n = 3; 60%) infections detected by the RDTs were consistent with the national data from 2015.
However, the PCR analysis revealed that there were many P. vivax infections (41 P. vivax mono-infections and 2 P. falciparum and P. vivax mixed infections) among the participants. This finding suggests that malaria infections, particularly P. vivax infections, which can only be detected by microscopy or RDTs, represent the “tip of the iceberg”. Thus, a large portion of the malaria parasite population cannot be detected by the standard (microscopy) or sub-standard (RDTs) methods. To achieve the goal of eliminating malaria in the Lao PDR by 2030, a highly sensitive malaria detection method, such as PCR, should be implemented to detect the large portion of hidden malaria infections to prevent them from acting as reservoirs for the next outbreak of malaria.
In the present study, the logistic regression analyses revealed that the risk factors of Plasmodium infections were, being male, Soldier, and Lao Loum (Table 5). Moreover, the PCR revealed that 68.1% (32/47) of the participants with Plasmodium infections were asymptomatic or cryptic Plasmodium carriers (75% (3/4) of the P. falciparum infections, 65.9% (27/41) of the P. vivax infections, 100.0% (2/2) of the mixed infections with P. falciparum and P. vivax) in Attapeu province. The risk factor of cryptic Plasmodium infections was a history of 3 or more malaria episodes (S5 Table). Although male and soldier were the risk factors of Plasmodium infections, they were not the risk factors of cryptic Plasmodium infection. This might be associated with small sample size of the soldiers (only 10) or bias of the sample collection in this study (not a random sampling but a convenience sampling). Therefore, further investigations are requisite to understand the risk factor of soldier and male in Lao PDR. The prevalence of Plasmodium infection in Som Poi village was the highest among the 6 villages. Som Poi village is located near the border between the Lao PDR and Cambodia (Fig 1), and most residents were Lao Loum (98.7% 152/154), whose religion was Buddhism. The high prevalence of malaria in Som Poi village might be associated with their lifestyle in the village, environment including Anopheles mosquitoes or human migration between the Lao PDR and Cambodia.
There would be several reasons for this situation. In general, the adult populations in areas of high malaria endemicity might have acquired certain levels of immunity against malaria due to the frequent contraction of malaria parasites during their lives. Thus, the asexual growth of Plasmodium parasites in their blood would be suppressed, and the parasite might be maintained at a lower density, which could be lower than the threshold of detection by microscopy or rapid diagnostic testing. Indeed, the logistic regression analyses revealed that a history of multiple Plasmodium infections (>3 times) was significantly associated with the cryptic Plasmodium infections (S5 Table). Similar data was also reported from Cambodia [14]. Therefore, for effective control and elimination of malaria in the study area, special attention to and interventions for these high-risk populations are necessary. For example, active case detection for Plasmodium infection by highly sensitive methods such as PCR, LAMP or ultra-sensitive RDTs should be performed in high endemic villages or high-risk populations, such as males, soldiers or male farmers who stay overnight in the forest.
In the present study, 12.2% (41/336) of the males and 1.6% (6/383) of the females were infected with Plasmodium parasites. This discrepancy is probably associated with the occupational activities of the males. Most of the adult participants (65.6%, 472/719) in the present study were farmers. Both male farmers (205) and female farmers (267) worked in the fields, but only the males worked and sometimes stayed overnight in the forests with/without bed nets for the purpose of collecting food and logging. Another high-risk population was soldiers, who also work and stay overnight in the forest while training or on patrols, especially along the border of the country. In this sense, soldiers can be categorized as mobile and migrant populations that are considered to be high-risk populations for malaria [15]. In fact, 50% (5/10) of soldiers were infected with P. vivax, and 4 of the 5 soldiers had no symptoms at the time of the survey or in the preceding few days before the survey. Staying in the forest in the Greater Mekong Subregion is also considered to be associated with a high risk of malaria infection [16–21]. The difference between the prevalence of malaria among males and females has also been reported from the Thailand-Myanmar border, Cambodia and Ethiopia [22–24].
According to previous studies in low-transmission settings, the protective natural immunity to infection by P. vivax and P. falciparum is not understood [25]. However, there was a higher proportion of sub-microscopic and asymptomatic P. vivax infections in low-transmission settings, such as Cambodia, the Thailand-Myanmar border, Vietnam [26], the China-Myanmar border [27], Indonesia, Brazil [28], Colombia [29], Solomon Islands [30] and Ethiopia [31]. Our finding confirmed the situation in other low-transmission settings or pre-elimination settings in Southeast Asia, the Western Pacific, South America and Africa.
Another possible reason for the many P. vivax infections involves the treatment regimen that is administered for vivax malaria in the Lao PDR. The current guidelines recommend Coartem (artemether + lumefantrine) as the first-line drug for the treatment of vivax malaria, whereas previously, chloroquine had been recommended [1]. These antimalarial drugs can clear the asexual stage of P. vivax from the bloodstream of a patient. However, neither Coartem nor chloroquine can kill hypnozoites (a dormant stage of P. vivax) in human liver cells, which cause relapse. Presently, 8-aminoquinoline (or primaquine) is the only drug that shows efficacy against hypnozoites in the liver cells. A complete radical cure of vivax infection requires a 14-day course of primaquine [32]. However, although primaquine is included in the National Strategic Plan for Malaria Control and Elimination in the Lao PDR 2016–2020, availability of the drug is currently limited in hospitals in the 5 southern provinces because it causes acute hemolytic anemia among people with glucose 6-phosphate dehydrogenase (G6PD) deficiency. G6PD, which is one of the glycolysis enzymes in erythrocytes, protects erythrocytes from oxidative stress [33–35]. If the G6PD activity is lower than normal due to oxidative stress—such as the oxidative stress caused by taking primaquine—the erythrocytes will suddenly rupture and the patient will develop acute hemolytic anemia.
Although the prevalence of G6PD deficiency among populations in several countries—including the Greater Mekong Subregion—has been reported, the prevalence of G6PD deficiency among the Lao population is unknown [36]. Recently, the G6PD enzyme activity test (CareStart G6PD, Access Bio, Inc., Somerset, NJ, USA) and primaquine have become available at provincial and district hospitals in the 5 southern provinces in the Lao PDR. However, most of the vivax malaria patients in the Lao PDR reside in rural areas that are far from these hospitals. In such areas, malaria patients are likely to go to the nearest health center at which patients suspected of having malaria are diagnosed by malaria RDTs and treated with Coartem for both falciparum and vivax malaria. However, primaquine cannot be prescribed for vivax malaria patients at the health centers because in the treatment guideline of malaria in the Lao PDR, not only the G6PD test but also hematocrit and hemoglobin tests are required before prescribing primaquine for vivax malaria patients. Hematocrit and hemoglobin tests are not currently available at the health center level in the Lao PDR. Therefore, staff members at the health centers in the 5 southern provinces ask vivax malaria patients to go to district or provincial hospitals for radical treatment of vivax malaria, but many of them hesitate to go to these hospitals because they are far from their residence, i.e. they have to spend a lot of time and money on transportation. Thus, coverage with primaquine treatment for vivax malaria patients is still limited in the Lao PDR, and some vivax malaria patients may experience relapse several times (even within the same year). If the Lao MOH invests in facilities to provide blood tests such as hematocrit and hemoglobin at the health center level or simplify the treatment guideline for vivax malaria, elimination of vivax malaria would be accelerated.
The present study was conducted in May, which is the beginning of the rainy season in the Lao PDR. The predominance of P. vivax infection among the participants might have been influenced by this study period. According to a report from the CMPE in 2009–2015, the peak malaria incidence occurred between July to October in the Lao PDR. Generally, the prevalence of P. falciparum would be lower at the beginning of the rainy season because it is directly associated with the population density of Anopheles mosquitoes. If our field survey were conducted during July to October (the middle to the end of the rainy season), a greater number of P. falciparum-positive samples would have been detected in the same areas. In fact, in a previous study in Xepon district, Savannakhet province during August to September 2013, 78.8% (41/52) of Plasmodium infections comprised P. falciparum mono-infection [5], whereas in another study in Thapangthong and Nong districts, Savannakhet province during March to July 2015, 18.3% (32/175) of Plasmodium infections were P. falciparum mono-infection and 56.6% (99/175) were P. vivax mono-infection [8].
The current malaria elimination program in the Lao PDR only targets symptomatic cases. However, it has been shown that mosquitoes that feed on blood from people with sub-microscopic Plasmodium infections can transmit malaria [37, 38]. Thus, sub-microscopic Plasmodium carriers can contribute to malaria transmission [39, 40]. This fact must be kept in mind if the Lao PDR is to reach its ambitious goal of eliminating malaria by the year 2030.
Fever is one of the major symptoms of malaria. However, in the present study, only one of the 47 PCR-positive participants had a fever (>37.5°C) at the time of the survey. A similar result was reported from Africa. In low and unstable malaria transmission areas in Kenya, fever was a sensitive indicator of malaria only in children (<5 years) but not in older children and adults [41]. Thus, for screening malaria patients in low-transmission areas, fever may not be a useful indicator with which to detect malaria patients.
In the present study, there was no association between the use of insecticide-treated bed nets and Plasmodium infections. However, the villagers’ answers regarding the question about bed net use might be biased because some of our interviewers were the ones who distributed the bed nets to the villagers and asked them to use the nets when they sleep. Thus, some (or many) villagers were likely to answer “yes” to the interviewers even though they did not use the bed net at all or did not always use it.
One limitation of the present study was that we did not collect a history of sleeping away from home (e.g., sleeping in a farming hut) or in the forest or of working in the forest. Previous studies in the Greater Mekong Subregion including the Lao PDR showed that a history of sleeping in a farming hut or in the forest was associated with Plasmodium infections [8, 42, 43]. Forest workers in other Greater Mekong Subregion countries were also reported to be a high-risk group for malaria infections [16–21]. Nevertheless, if farmers use insecticide-treated bed nets properly while they sleep in the farming huts, Plasmodium infection might be preventable in the Lao PDR [44].
Another limitation of the present study was the exclusion of hard-to-access villages from the study area. Therefore, the villages selected in this study might not represent a complete picture of the districts. If a malaria survey is conducted in such hard-to-access villages in Attapeu province, there might be many malaria patients and asymptomatic Plasmodium carriers because in such villages, it is difficult for the villagers to access health care services even they have health problems. Thus, if there is a malaria patient or asymptomatic Plasmodium carrier and Anopheles mosquitos in one of these villages, the transmission would continue within the village, especially in the patient’s household and among the neighbors.
Conclusion
The present PCR examination revealed 47 asymptomatic Plasmodium infections (6.5%) among the 719 voluntary participants from malaria-endemic villages in Attapeu province in May 2015. Most of them (66.0%; 31/47) were adult males (≥18 years old), and the dominant parasite species was P. vivax. Males and soldiers were associated with Plasmodium infections whereas a history of 3 or more malaria episodes was associated with cryptic Plasmodium infections. Of the 32 asymptomatic Plasmodium carriers, 30 (93.8%) had infections that were below the detection threshold of microscopy, and 28 (87.5%) had infections that were below the detection threshold of the RDTs. To achieve the goal of malaria elimination in the Lao PDR by 2030, sensitive diagnostic methods—such as a PCR- or LAMP-based method—should be utilized, and plans targeting high-risk populations (males and soldiers) and the elimination especially of P. vivax should be designed and implemented.
Supporting information
(DOC)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors would like to thank Dr. Mixay Phommakhod, chief of the Station of Malariology, Parasitology and Entomology, Attapeu Provincial Health Office for supporting the field survey. We also gratefully acknowledge the staff members in the Attapeu Provincial Health Office, the three Districts Health Offices, and the Health Centers, and the chiefs of the villages and all of the people who participated in the field survey.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was financially supported by a JICA/AMED SATREPS project for “the development of innovative research technique in genetic epidemiology of malaria and other parasitic diseases in the Lao PDR for containing their expanding endemicity”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (WHO). World Malaria Report. Geneva: WHO; 2016. http://www.who.int/malaria/publications/world-malaria-report-2016/en/.
- 2.The Government of the Lao PDR and the United Nations. The millennium development goals progress report for the Lao PDR 2013. The Government of the Lao PDR and the United Nations; 2013.
- 3.Ministry of Health PDR Laos. National strategy for malaria control and pre-elimination 2011–2015. National Report; 2010.
- 4.Malaria Policy Advisory Committee Meeting WHO. Malaria elimination strategy in the greater Mekong subregion. Geneva: World Health Organization; 2015. http://www.who.int/malaria/mpac/mpac-march2015-gms-briefing-note.pdf.
- 5.Pongvongsa T, Nonaka D, Iwagami M, Nakatsu M, Phongmany P, Nishimoto F, et al. Household clustering of asymptomatic malaria infections in Xepon district, Savannakhet province, Lao PDR. Malar J. 2016;15(1): 508 doi: 10.1186/s12936-016-1552-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu W, Morris U, Aydin-Schmidt B, Msellem MI, Shakely D, Petzold M, et al. SYBR Green real-time PCR-RFLP assay targeting the plasmodium cytochrome B gene—a highly sensitive molecular tool for malaria parasite detection and species determination. PLoS One. 2015;10(3): e0120210 doi: 10.1371/journal.pone.0120210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med. 2015;12(3): e1001788 doi: 10.1371/journal.pmed.1001788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phommasone K, Adhikari B, Henriques G, Pongvongsa T, Phongmany P, von Seidlein L, et al. Asymptomatic Plasmodium infections in 18 villages of southern Savannakhet Province, Lao PDR (Laos). Malar J. 2016;15(1): 296 doi: 10.1186/s12936-016-1336-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polley SD, González IJ, Mohamed D, Daly R, Bowers K, Watson J, et al. Clinical evaluation of a loop-mediated amplification kit for diagnosis of imported malaria. J Infect Dis. 2013;208(4): 637–644. doi: 10.1093/infdis/jit183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins H, González IJ, Polley SD, Angutoko P, Ategeka J, Asiimwe C, et al. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis. 2013;208(4): 645–652. doi: 10.1093/infdis/jit184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallejo AF, Martínez NL, González IJ, Arévalo-Herrera M, Herrera S. Evaluation of the loop mediated isothermal DNA amplification (LAMP) kit for malaria diagnosis in P. vivax endemic settings of Colombia. PLoS Negl Trop Dis. 2015;9(1): e3453 doi: 10.1371/journal.pntd.0003453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Putaporntip C, Buppan P, Jongwutiwes S. Improved performance with saliva and urine as alternative DNA sources for malaria diagnosis by mitochondrial DNA-based PCR assays. Clin Microbiol Infect. 2011;17(10): 1484–1491. doi: 10.1111/j.1469-0691.2011.03507.x [DOI] [PubMed] [Google Scholar]
- 13.Tanizaki R, Ujiie M, Kato Y, Iwagami M, Hashimoto A, Kutsuna S, et al. First case of Plasmodium knowlesi infection in a Japanese traveller returning from Malaysia. Malar J. 2013;12: 128 doi: 10.1186/1475-2875-12-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peto TJ, Kloprogge SE, Tripura R, Nguon C, Sanann N, Yok S, et al. History of malaria treatment as a predictor of subsequent subclinical parasitaemia: a cross-sectional survey and malaria case records from three villages in Pailin, western Cambodia. Malar J. 2016; 15: 240 doi: 10.1186/s12936-016-1284-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kounnavong S, Gopinath D, Hongvanthong B, Khamkong C, Sichanthongthip O. Malaria elimination in Lao PDR: the challenges associated with population mobility. Infect Dis Poverty. 2017;6(1): 81 doi: 10.1186/s40249-017-0283-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pongvongsa T, Ha H, Thanh L, Marchand RP, Nonaka D, Tojo B, et al. Joint malaria surveys lead towards improved cross-border cooperation between Savannakhet Province, Laos and Quang Tri province, Vietnam. Malar J. 2012;11: 262 doi: 10.1186/1475-2875-11-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thanh PV, Hong NV, Van N, Van Malderen C, Obsomer V, Rosanas- Urgell A, et al. Epidemiology of forest malaria in Central Vietnam: the hidden parasite reservoir. Malar J. 2015;14: 86 doi: 10.1186/s12936-015-0601-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourée P. Malaria in forests (in French). Med Sante Trop. 2014;24: 147 [PubMed] [Google Scholar]
- 19.Thang ND, Erhart A, Speybroeck N, le Hung X, le Thuan K, Hung CT, et al. Malaria in central Vietnam: analysis of risk factors by multivariate analysis and classification tree models. Malar J. 2008;7: 28 doi: 10.1186/1475-2875-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erhart A, Ngo DT, Phan VK, Ta TT, Van Overmeir C, Speybroeck N, et al. Epidemiology of forest malaria in central Vietnam: a large scale cross-sectional survey. Malar J. 2005;4: 58 doi: 10.1186/1475-2875-4-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erhart A, Thang ND, Hung NQ, le Toi V, le Hung X, Tuy TQ, et al. Forest malaria in Vietnam: a challenge for control. Am J Trop Med Hyg. 2004;70(2): 110–118. [PubMed] [Google Scholar]
- 22.Golassa L, Enweji N, Erko B, Aseffa A, Swedberg G. Detection of a substantial number of sub-microscopic Plasmodium falciparum infections by polymerase chain reaction: a potential threat to malaria control and diagnosis in Ethiopia. Malar J. 2013;12: 352 doi: 10.1186/1475-2875-12-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steenkeste N, Rogers WO, Okell L, Jeanne I, Incardona S, Duval L, et al. Sub-microscopic malaria cases and mixed malaria infection in a remote area of high malaria endemicity in Rattanakiri province, Cambodia: implication for malaria elimination. Malar J. 2010;9: 108 doi: 10.1186/1475-2875-9-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golassa L, Baliraine FN, Enweji N, Erko B, Swedberg G, Aseffa A. Microscopic and molecular evidence of the presence of asymptomatic Plasmodium falciparum and Plasmodium vivax infections in an area with low, seasonal and unstable malaria transmission in Ethiopia. BMC Infect Dis. 2015;15: 310 doi: 10.1186/s12879-015-1070-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22(1): 13–36. doi: 10.1128/CMR.00025-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imwong M, Nguyen TN, Tripura R, Peto TJ, Lee SJ, Lwin KM, et al. The epidemiology of subclinical malaria infections in South-East Asia: findings from cross-sectional surveys in Thailand-Myanmar border areas, Cambodia, and Vietnam. Malar J. 2015;14: 381 doi: 10.1186/s12936-015-0906-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Zhao Y, Lv Y, Liu F, Wang Q, Li P, et al. Comparison of methods for detecting asymptomatic malaria infections in the China-Myanmar border area. Malar J. 2017;16(1): 159 doi: 10.1186/s12936-017-1813-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng Q, Cunningham J, Gatton ML. Systematic review of sub-microscopic P. vivax infections: prevalence and determining factors. PLoS Negl Trop Dis. 2015;9(1): e3413 doi: 10.1371/journal.pntd.0003413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallejo AF, Chaparro PE, Benavides Y, Álvarez Á, Quintero JP, Padilla J, et al. High prevalence of sub-microscopic infections in Colombia. Malar J. 2015;14: 201 doi: 10.1186/s12936-015-0711-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris I, Sharrock WW, Bain LM, Gray KA, Bobogare A, Boaz L, et al. A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J. 2010;9: 254 doi: 10.1186/1475-2875-9-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golassa L, Enweji N, Erko B, Aseffa A, Swedberg G. Detection of a substantial number of sub-microscopic Plasmodium falciparum infections by polymerase chain reaction: a potential threat to malaria control and diagnosis in Ethiopia. Malar J. 2013;12: 352 doi: 10.1186/1475-2875-12-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO. Malaria Treatment Guideline 2015 World Health Organization (WHO). Geneva: WHO; 2015. http://www.who.int/malaria/publications/atoz/9789241549127/en/. [Google Scholar]
- 33.WHO Working Group. Glucose-6-phosphate dehydrogenase deficiency. Bull World Health Organ. 1989;67(6): 601–611. [PMC free article] [PubMed] [Google Scholar]
- 34.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371(9606): 64–74. doi: 10.1016/S0140-6736(08)60073-2 [DOI] [PubMed] [Google Scholar]
- 35.Luzzatto L. Glucose 6-phosphate dehydrogenase deficiency: from genotype to phenotype. Haematologica. 2006;91(10): 1303–1306. [PubMed] [Google Scholar]
- 36.Ong KI, Kosugi H, Thoeun S, Araki H, Thandar MM, Iwagami M, et al. Systematic review of the clinical manifestations of glucose-6-phosphate dehydrogenase deficiency in the Greater Mekong Subregion: implications for malaria elimination and beyond. BMJ Global Health. 2017;2: e000415 doi: 10.1136/bmjgh-2017-000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boudin C, Olivier M, Molez JF, Chiron JP, Ambroise-Thomas P. High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am J Trop Med Hyg. 1993;48(5): 700–706. [DOI] [PubMed] [Google Scholar]
- 38.Coleman RE, Kumpitak C, Ponlawat A, Maneechai N, Phunkitchar V, Rachapaew N, et al. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J Med Entomol. 2004;41(2): 201–208. [DOI] [PubMed] [Google Scholar]
- 39.Schneider P, Bousema JT, Gouagna LC, Otieno S, van de Vegte-Bolmer M, Omar SA, et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg. 2007;76(3): 470–474. [PubMed] [Google Scholar]
- 40.Muirhead-Thomson RC. Low gametocyte thresholds of infection of Anopheles with Plasmodium falciparum; a significant factor in malaria epidemiology. Br Med J. 1954;1(4583): 68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutanda AL, Cheruiyot P, Hodges JS, Ayodo G, Odero W, John CC. Sensitivity of fever for diagnosis of clinical malaria in a Kenyan area of unstable, low malaria transmission. Malar J. 2014;13: 163 doi: 10.1186/1475-2875-13-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somboon P, Aramrattana A, Lines J, Webber R. Entomological and epidemiological investigations of malaria transmission in relation to population movements in forest areas of north-west Thailand. Southeast Asian J Trop Med Public Health. 1998;29(1): 3–9. [PubMed] [Google Scholar]
- 43.Erhart A, Ngo DT, Phan VK, Ta TT, Van Overmeir C, Speybroeck N, et al. Epidemiology of forest malaria in central Vietnam: a large scale cross-sectional survey. Malar J. 2005;4: 58 doi: 10.1186/1475-2875-4-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nonaka D, Laimanivong S, Kobayashi J, Chindavonsa K, Kano S, Vanisaveth, et al. Is staying overnight in a farming hut a risk factor for malaria infection in a setting with insecticide-treated bed nets in rural Laos? Malar J. 2010;9: 372 doi: 10.1186/1475-2875-9-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.