Abstract
Typhoid fever remains a serious public health problem with a high impact on toddlers and young children. Vaccines against the Vi capsular polysaccharide are efficacious against typhoid fever demonstrating that antibodies against Vi confer protection. The currently licensed Vi typhoid vaccines have however limited efficacy and are manufactured by a complex process from wild-type bacteria. Due to these inherent issues with the current vaccines, an alternative vaccine based on an O-acetylated high molecular weight (HMW) polygalacturonic acid (GelSite-OAc™) was generated. The HMW polygalacturonic acid shares the same backbone as the Vi polysaccharide of Salmonella Typhi. The GelSite-OAc™ has a high molecular weight (>1 × 106 Da) and a high degree of O-acetylation (DOAc) (>5 μmole/mg), both exceeding the potency specifications of the current Vi vaccine. Studies in Balb/c mice demonstrated that GelSite-OAc™ was highly immunogenic, inducing a strong antigen-specific antibody response in a DOAc- and dose-dependent manner which was comparable to or higher than those induced by the licensed Vi vaccine. Importantly, the GelSite-OAc™ was shown to be fully protective in mice against lethal challenge with Salmonella Typhi. Furthermore, the GelSite-OAc™ demonstrated a boosting effect or memory response, exhibiting a >2-fold increase in antibody levels upon the second immunization with either GelSite-OAc™ or the Vi vaccine. This novel boosting effect is unique among polysaccharide antigens and potentially makes GelSite-OAc™ effective in people under 2 years old. Together these results suggest that the GelSite-OAc™ could be a highly effective vaccine against Salmonella Typhi.
Keywords: typhoid fever, vaccine, Salmonella Typhi, Vi polysaccharide, O-acetylation, polygalacturonic acid, animal model
Introduction
Typhoid fever is an acute and life-threatening febrile illness caused by Salmonella enterica serotype Typhi (S. Typhi). It is responsible for 16 – 33 million new typhoid fever cases and 500,000 – 600,000 deaths annually [1 – 3]. Contaminated food and water are the main sources of infection. The risk of infection is the highest in developing countries with poor sanitation including Asia, Africa, and Latin America. Several different antibiotics have been used to treat typhoid fever. However, multi-drug resistant strains of S. Typhi have emerged and rendered these costly treatments ineffective [4, 5]. Thus, the widespread use of low-cost efficacious vaccines is still the most effective way to reduce the impact of typhoid fever.
Currently, there are two types of vaccines available for controlling typhoid fever, a Vi polysaccharide vaccine administered parenterally in a single dose and an oral live attenuated Ty21a vaccine [6, 7]. The Vi vaccine is produced by costly and hazardous fermentation of S. Typhi wild type bacteria and elaborate purification processes. It is licensed for use in people ≥2 years and provides about 70% protection that lasts for two to three years [8]. An oral live vaccine in capsule form is currently licensed for persons over 5 years and provides a similar level of protection after four doses [8]. Thus, these vaccines provide limited protection and none are effective in people under 2 years.
Current Vi vaccines are based on the Vi capsular polysaccharide which is a linear alpha 1–4 linked polygalacturonic acid (PGA) that is N-acetylated at C2 and 60–90% O-acetylated at C3 of the galacturonic acid (Gal UA) residue. The O-acetylation at C3 and molecular weight are the two critical determinants of immunogenicity of the Vi polysaccharide and the potency indicators for the current Vi vaccines. Studies have shown that removal of the O-acetyl group at C3 reduces its immunogenicity [9–11]. Structural modeling of the Vi polysaccharide shows that the bulky nonpolar O-acetyl groups at C3 make up most of the surface of the polysaccharide molecule by protruding on both sides, whereas the carboxyl and N-acetyl (at C2) groups are mostly embedded or located close to the axis [12]. This is consistent with the O-acetyl group being the dominant immunogenicity determinant. Studies have also shown that the immunogenicity of the Vi polysaccharide decreases when its molecular weight is reduced [13–14]. The Vi vaccine, like other polysaccharide-based vaccines, acts as a T cell-independent antigen and does not elicit a booster response upon revaccination [15]. It is therefore not effective in infants or toddlers under 2 years. As a result, Vi polysaccharide-protein conjugate vaccines are being developed by covalent linking of Vi polysaccharide to a protein carrier [16–18].
Plant pectins share the same backbone with the Vi polysaccharide. They are alpha 1–4 linked polygalacturonic acid that is variably methylated. Those with a degree of methylation (DM) below 10% are considered as PGA [19, 20]. The commercial low-methoxyl (LM) pectin or PGA has been O-acetylated and the resulting acetylated product was found to share the same antigenicity with native Vi polysaccharide [11, 21]. However, it was not immunogenic in animals due to the low molecular weight (~4 × 105 Da) of the LM pectin or PGA used [18, 21].
We have developed a synthetic Vi antigen (GelSite-OAc™) by O-acetylation of a novel high-molecular-weight polygalacturonic acid (GelSite®) from the Aloe vera plant. GelSite® is uniquely characterized by a high molecular weight (>1 × 106 Da) and a very low degree of methylation (DM, <10%). These properties make it an ideal substrate for a synthetic Vi polysaccharide analog by O-acetylation (Supplementary figure 1). The GelSite® was successfully O-acetylated with a simple chemical reaction. We tested GelSite-OAc™ for its ability to immunize mice against S. Typhi. The results indicate that the GelSite-OAc™ can potentially serve as a typhoid Vi vaccine with distinct advantages over the current vaccines.
Materials and methods
GelSite polymer
All reagents used for GelSite-OAc™ manufacturing were obtained from Sigma Chemical Co (St. Louis, MO). High-molecular-weight polygalacturonic acid (HPGA or GelSite®) was isolated from Aloe vera L [22, 23]. Its chemical and physical properties are summarized in Table 1. GelSite® has been manufactured as a lyophilized sodium salt with a high purity (>99%) under cGMP at a kilogram scale.
Table 1.
Physical and chemical properties of GelSite®
| Molecular weight | > 1 × 106 Da (SEC; Dextran Standards) |
|---|---|
| Polydispersity (MW/Mn) | ≤1.8 |
| Anhydro-Gal UA content (w/w)% | 80–90% |
| Neutral sugar content (w/w)% | <5% |
| Degree of methylation (% mole/mole) | <6% |
| Sodium content (w/w) | 10–12% |
| Protein content (w/w) | <0.5% |
O-acetylation of GelSite polymer
The Schweiger method [24] was adopted with modifications for O-acetylation of GelSite® after comparison with another method [25] as described in Supplemental Materials and is outlined in Figure 1.
Fig. 1.
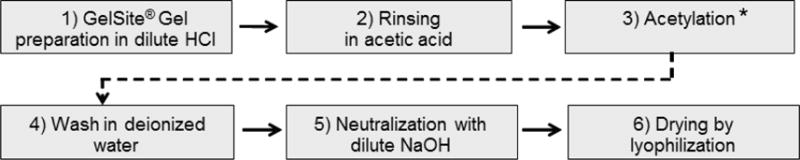
The O-acetylation process for production of O-acetylated polygalacturonic acid (GelSite-OAc™). The asterisk indicates the use of a small amount of perchloric acid as the catalyst.
Determination of Degree of O-acetylation
The method described by Hestrin [24] was used with modifications. The assay was conducted in 96-well plates. Samples were tested at ~0.2 mg/ml (w/v) in duplicate. Acetylcholine was used as the standard. DOAc was expressed as a molar ratio or percent of acetyl groups over Gal UA residues, or μM/mg - the unit used for DOAc specification for current Vi vaccine.
Determination of Molecular Weight
Molecular weight analysis was performed on a Waters 2695 HPLC with a Refractive Index detector and eight polysaccharide standards ranging from 5 kDa to 800 kDa (Shodex P-82 Pullulan Standards). Size exclusion separation was achieved using a mobile phase of 0.1 M ammonium acetate over a 90 min isocratic run at a flow rate of 0.4 mL/min.
All chromatograms were processed through Empower software. The observed retention time each standard was converted to elution volume.
Immunogenicity and protection
All animal studies were conducted with the approval of the Institutional Animal Use and Care Committee. The immunogenicity of GelSite-OAc™ was examined in 6–8 week old female Balb/c mice in comparison with a licensed Vi vaccine (Typhim Vi®; Sanofi-Pasteur). Groups of mice (n=10) were immunized with GelSite-OAc™ or Typhim Vi vaccine at 2.5 μg/mouse or the indicated doses by intramuscular injection in the right hind leg in 50 μl PBS. Animals were immunized twice, four weeks apart. Serum samples were collected every two weeks. For cross-boosting experiments, groups of mice (n=10) were immunized three times, four weeks apart with GelSite-OAc™ (with a DOAc of 155%) or Typhim Vi vaccine at 2.5 μg/mouse at the indicated times. Serum samples were collected every two weeks.
Specific antibodies were measured by ELISA in 96-well plates as described previously [27] with modifications. Briefly, the plates were coated with the polysaccharide antigens at 2 μg/ml in PBS at 4 °C overnight. They were blocked with 1% BSA in PBS at room temperature for 2 hrs followed by incubation of serial dilutions of serum samples in 1% BSA in PBS for 2 hrs at room temperature. After washing, the plates were incubated with 1:3000 dilution of anti-mouse IgG-alkaline phosphatase conjugate (Sigma Aldrich Co. St. Louis, MO) at RT for 1 hr before washing and addition of pNPP substrate. The OD was measured at 410 nm. The Vi polysaccharides obtained from International Vaccine Institute and National Institute of Child Health and Human Development (NICHD) and GelSite-OAc™ were used as the antigens for coating the ELISA plates. The reference serum is a hyperimmune mouse serum with 40 ELISA units/ml obtained from NICHD. Antibody concentrations were determined using the CDC ELISA program (http://www.cdc.gov/ncidod/dbmd/bimb/elisa.htm) with the reference serum as the standard and expressed as units/ml.
The protective effect of the specific immune responses induced by GelSite-OAc™ was evaluated in challenge experiments with live S. Typhi as described previously [28]. Groups of 7 six-to eight-week-old female Balb/c mice (n=6) were immunized with GelSite-OAc™ with different DOAc (80%–155%) or Vi vaccine at 2.5 μg/mouse by intramuscular injection twice four weeks apart. The control received the buffer solution. At week 3 after the second immunization, mice from each group were challenged intraperitoneally with 100 LD50 (1,000 CPU/mouse) of the bacteria in 0.5 ml 5% porcine gastric mucin. Mice were then monitored daily for survival and body weight. The bacteria used were Salmonella enterica subsp. Enterica serovar Typhi (S. Typhi) obtained from ATCC (Item number 19430).
Statistics
Geometric mean concentration (GMC) and the standard deviation were determined for each group. The Student t test was used to analyze differences between groups. A p value ≤0.05 is considered significant. All statistical calculations were performed using Excel.
Results
Generation and characterization of O-acetylated GelSite polymer
The DOAc is a key parameter for GelSite-OAc™. Based on the basic process conditions described in Supplementary Materials and Methods, the parent molecule GelSite® could be readily O-acetylated to GelSite-OAc™ with a DOAc of 134% – 153%, which corresponds to 67% – 76% at either C2 or C3 position. The DOAc could be further increased by extending the acetylation reaction time to as high as 175% or 87.5% at either C2 or C3 position. GelSite-OAc with a lower DOAc (<100%) could also be generated by lowering the concentration of acetic anhydride from 50% to as low as 10% in the reaction mixture (Supplementary Materials and Methods).
A yield of 100% or higher for GelSite-OAc™ was consistently obtained, likely as the result of addition of acetyl groups and high process efficiency. Molecular weight comparisons showed that the molecular weight of GelSite-OAc™ increased by 11% when DOAc reached 142% (Supplementary Table 1 and Supplementary Figure 2). GelSite-OAc™ was found to be antigenic as determined with reference serum in a DOAc-dependent manner (Supplementary Figure 3).
Immunogenicity and protection
Effect of DOAc
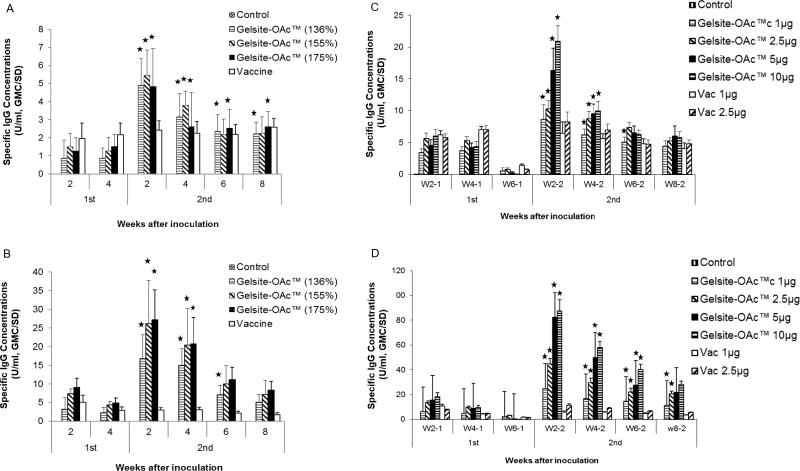
Three GelSite-OAc™ samples with different DOAc (136%, 155%, or 175% mole/mole) were initially tested. The results showed that levels of specific IgG responses were found to correlate with DOAc (Fig. 2). This was especially true after the 1st immunization no matter whether specific IgG was measured using Vi antigen (Fig. 2A) or synthetic antigen GelSite-OAc™ (Fig. 2B). The GelSite-OAc™ with the lowest DOAc (136%) consistently yielded the lowest antibody concentration at most time points. The GelSite-OAc™ samples with 155% or 175% DOAc exhibited very similar responses, suggesting that the GelSite-OAc™ with 155% and 175% DOAc could be equally effective. GelSite-OAc™ with 155% or 175% DOAc consistently induced comparable or higher antibody concentrations compared to Vi vaccine, except for time points after the first immunization when measured with the Vi antigen. When measured with GelSite-OAc™, the specific antibody concentrations induced by GelSite-OAc™ were consistently higher than those with the Vi vaccine after second immunization by as much as10 fold (p<0.05) (Fig. 2B).
Fig. 2.
The effect of DOAc and dose-dependence of GelSite-OAc™ on immune response. Balb/c mice (n=10) were immunized twice with Vi vaccine or GelSite-OAc™ having different DOAc at 2.5 μg/mouse, 4 weeks apart. Specific IgG was measured using the Vi polysaccharide (A) or GelSite-OAc™ (B) as the antigen. For evaluation of antigen dose, Balb/c mice were immunized twice with GelSite-OAc™ (DOAc, 1.75) at the different doses, 6 weeks apart. Specific IgG was measured by ELISA using the Vi polysaccharide (C) or GelSite-OAc™ (D) as the antigen. The asterisks indicate significant difference (p<0.05) as compared to the antibody concentration at week 2 after the first immunization.
A unique boosting effect or memory response was observed with GelSite-OAc™ upon the second immunization. The antibody concentrations of all three GelSite-OAc™ groups as measured against the Vi or GelSite-OAc™ antigen increased after the second immunization by at least 2 fold and > 4 fold as compared to the highest concentration after the first immunization (p<0.05) (Fig. 2A and 2B). No such boosting effect was observed with Vi vaccine (Fig. 2A and 2B). This boosting effect is also shown by analyzing IgG subclasses (Supplemental Figure 4).
The specificity of the antibodies induced by GelSite-OAc™ was evaluated by reaction with its parental molecule (GelSite®) in comparison with Vi polysaccharide and GelSite-OAc™. No reaction with the GelSite® was observed at any dose levels of GelSite-OAc™ (data not shown).
Effect of antigen dose
The effect of GelSite-OAc™ antigen dose was also tested at four different dose levels (1, 2.5, 5, and 10 μg) in comparison with Vi vaccine at two different doses (1 and 2.5 μg). The antibody responses measured with either Vi or GelSite-OAc™ were antigen dose-dependent (Fig. 2C and 2D). The boosting effect was again only observed with GelSite-OAc™ after the second immunization.
Cross boosting
A cross-boosting experiment with GelSite-OAc™ and Vi vaccine was conducted in which animals were first immunized with one antigen followed by two boosting immunizations with the same or a different antigen (Fig. 3). The results showed that all animals primed with GelSite-OAc™ exhibited the boosting effect upon the second immunization with GelSite-OAc™ or Vi vaccine. The boosting level obtained with Vi vaccine was higher than that with GelSite-OAc™ when measured using Vi polysaccharide (Fig. 3A). This may reflect an additive effect based on the O-acetyl groups and induction of antibodies against other parts of the Vi polysaccharide.
Fig. 3.
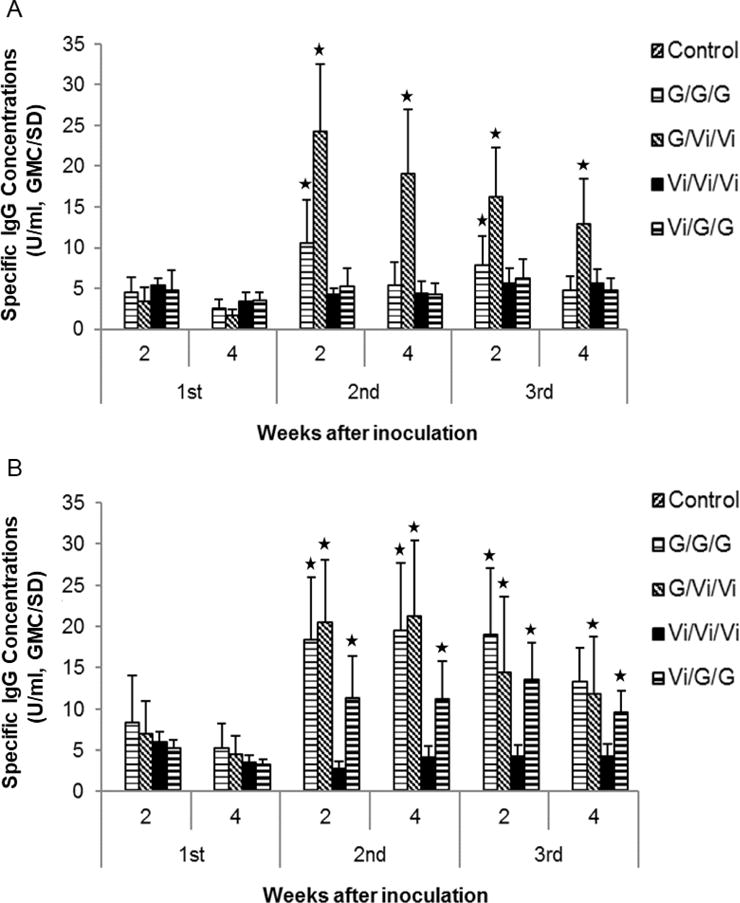
The evaluation of cross-boosting effect. Balb/c mice (n=10) were first immunized with Vi vaccine (Vi) or GelSite-OAc™ (G) followed by the same or a different vaccine for the 2nd and third immunization. Mice were immunized three times and four weeks apart. Specific IgG antibodies were measured with Vi polysaccharide (A) or GelSite-OAc™(B). G, GelSite-OAc™; Vi, Vi vaccine. The asterisks indicate significant difference (p<0.05) as compared to the antibody concentration at week 2 after the first immunization.
Animals primed and boosted only with Vi vaccine did not show a boosting effect. Those primed with Vi vaccine and boosted with GelSite-OAc™ did show a boosting effect, although to a less extent and only detectable when antibodies were measured against GelSite-OAc™ (Fig. 3B). No additional boosting effect was observed at the third immunization.
Protection
The protective effect of vaccination with GelSite-OAc™ was evaluated in challenge experiments using live S. Typhi as described previously [26]. A series of GelSite-OAc™ with a broad range of DOAc (80 – 155%) were generated and used to establish the relationship of DOAc with antibody induction and protection. The levels of antibody concentrations (Figure 4A and 4B) and protection (Figure 4C and 4D) increased in direct correlation with DOAc. The unacetylated GelSite® (0% DOAc) did not induce any detectable antibodies against either Vi polysaccharide or GelSite-OAc™, nor provided any protection. GelSite-OAc™ with an 80% DOAc exhibited the lowest antibody concentration and no boosting effect after the second immunization, and provided only a partial protection, while others with a higher DOAc (≥100%) generated much higher antibody concentrations (>10 fold; p<0.05) with boosting effect (Figure 4A and 4B) and provided the full protection (Figure 4C and 4D). Complete protection was also observed with GelSite-OAc™ having a DOAc above 100% in a separate experiment (data not shown).
Fig. 4.
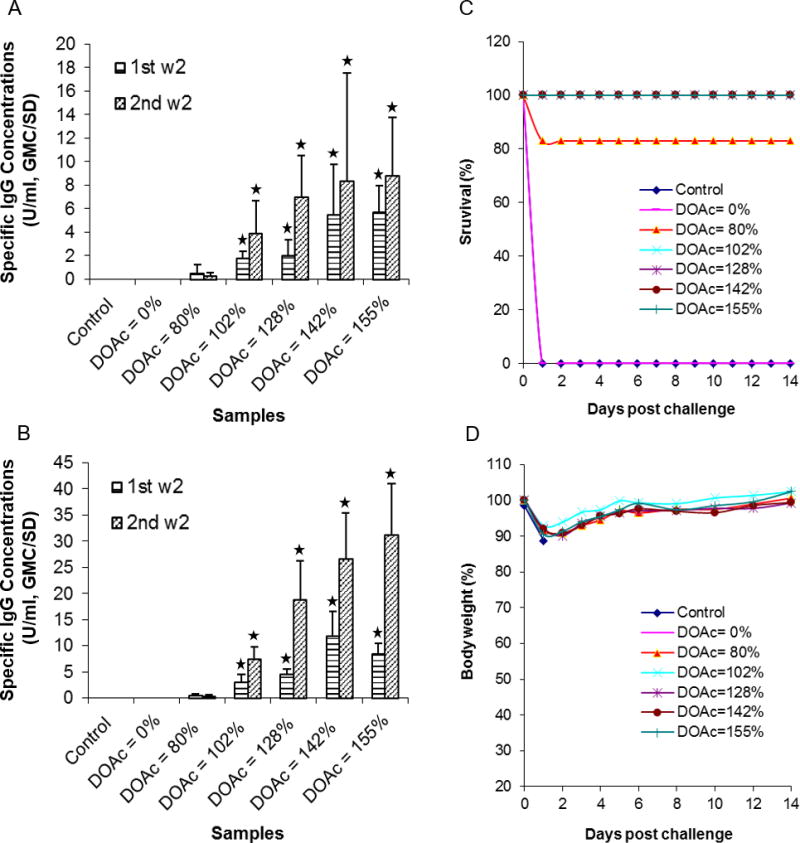
The correlation of DOAc with immunogenicity and protection of GelSite-OAc™. Balb/c mice (n=6) were immunized with GelSite-OAc™ having different DOAc at 2.5 μg/mouse twice, 4 weeks apart. Specific IgG in individual serum samples was measured using the Vi polysaccharide (A) or GelSite-OAc™ (B) as the antigen. Mice were challenged with 100 LD50 of S. Typhi at week 3 following the 2nd immunization. C, % survival; D, Mean body weight. The asterisks indicate significant difference (p<0.05) as compared to the group with 80% DOAc.
Discussion
The results described herein demonstrate that GelSite-OAc™ is structurally similar to Vi polysaccharide, shares the same antigenicity with Vi polysaccharide, and is highly immunogenic and protective in mice. It exceeds the potency standards of currently licensed Vi vaccines. It also uniquely exerts a boosting effect (memory response), which potentially makes it effective in people under 2 years who are not covered by current vaccines.
Polysaccharide antigens are known to be T cell-independent and as a result lack the immune memory or boosting effect. However, they can become T cell-dependent and induce memory responses and are effective in people under 2 years old when they are conjugated to a protein carrier. This is the basis for current commercial polysaccharide-protein conjugate vaccines [29, 30]. As a pure polysaccharide, GelSite-OAc™ is unique in possessing the boosting effect. Furthermore, the boosting effect can be induced not only with the GelSite-OAc™, but also using Vi vaccine as the second dose. This further confirms this boosting effect as well as the structural similarity between GelSite-OAc™ and Vi polysaccharide. Importantly, this boosting effect potentially makes it effective in people under 2 years without the need for conjugation to a protein carrier. These results also suggest that GelSite-OAc™ may be used together with the current Vi vaccine as the second dose. This could potentially extend the protective period after each follow-on immunization. Current Vi vaccines are recommended to be given every 3 – 7 years in the endemic areas and every 1 – 7 years for tourists [8].
The boosting effect of GelSite-OAc™ is unlikely to be due to the presence of the proteins or pre-existing polysaccharide-protein complex or conjugate in the GelSite-OAc™, since GelSite® contains less than 0.5% (w/w) proteins and did not induce any detectable antibodies against either Vi polysaccharide or GelSite-OAc™. Currently, only one polysaccharide antigen, a structurally unusual zwitterionic capsular polysaccharide from Bacteroides fragilis with alternating charges on adjacent monosaccharide units, has been demonstrated to be T cell - dependent [31, 32]. Thus, the distribution pattern of the O-acetyl groups on GelSite-OAc™ could be an important factor for the observed boosting effect with GelSite-OAc™. The IgG subclass profile of the mouse response suggests that a Th2-biased T cell activation might be involved in the response against GelSite-OAc™. It is similar to the one obtained with the Vi-conjugate vaccine [33] and Streptococcus pneumonia polysaccharide conjugate vaccine [34]. Future studies will further evaluate the cellular responses with GelSite-OAc™ to better characterize it as a potentially new T cell-dependent polysaccharide antigen.
The O-acetyl group is the most critical determinant of immunogenicity. Structural modeling showed that the bulky nonpolar O-acetyl groups at C3 make up most of the surface of the Vi polysaccharide molecule by protruding in rows on both sides [12]. When measured with Vi polysaccharide, the actual concentrations induced by GelSite-OAc™ could be much higher than those by Vi vaccine for the O-acetyl group-specific antibodies as the total antibodies induced by Vi vaccine likely include those against other less important parts of the Vi polysaccharide [12]. Evaluation of the effect of DOAc on immunogenicity and protection conducted in the present studies has identified a tentative DOAc threshold for induction of memory response and full protection. GelSite-OAc™ with an 80% DOAc exhibited no boosting effect and provided only a partial protection, while others with a higher DOAc (≥100%) generated the boosting effect and provided the full protection. Thus, the DOAc could likely be characterized with a lower limit of 100%. Together, these results suggest that a GelSite-OAc™ with DOAc of 100% or higher could be fully protective along with the boosting effect, thus forming a solid foundation to establish a DOAc specification for the vaccine.
The production process for GelSite-OAc™ is simple with a very high yield (>100%). The GelSite-OAc™ can be produced at very large quantities at a lower cost (1 kilogram GelSite-OAc™ = 20–40 million vaccine doses at 25–50 μg/dose), thus greatly increasing the availability and affordability of the Vi vaccines worldwide. Thus, GelSite-OAc™ can potentially be developed as a new synthetic typhoid vaccine with significant advantages over the current Vi vaccines with respect to safety, effectiveness, and cost.
Supplementary Material
Acknowledgments
This work was supported by a grant from National Institute of Allergy and Infectious Diseases (1R43AI081394-1A2). The authors would also like to thank Nanotherapeutics for their continued support of this research.
Footnotes
Potential conflicts of interest
The authors declare no conflicts of interest.
References cited
- 1.DeRoeck D, Jodar L, Clemens J. Putting typhoid vaccination on the global health agenda. The New England journal of medicine. 2007;357:1069–71. doi: 10.1056/NEJMp078144. [DOI] [PubMed] [Google Scholar]
- 2.Crump JA, Luby SP, Mintz ED, Crump JA, Mintz ED. Global trends typhoid and paratyphoid fever. Clinical infectious diseases. 2010;50:241–245. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wain J, Hendriksen RS, Mikoleit ML, Keddy KH, Ochiai RL. Typhoid fever. Lancet. 2015;385:1136–1145. doi: 10.1016/S0140-6736(13)62708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kariuki S, Gordon MA, Feasey N, Parry CM. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine. 2015;33(Suppl 3):C21–9. doi: 10.1016/j.vaccine.2015.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirza SH, Beeching NJ, Hart CA. Multi-drug resistant typhoid: a global problem. Journal of medical microbiology. 1996;44:317–9. doi: 10.1099/00222615-44-5-317. [DOI] [PubMed] [Google Scholar]
- 6.Guzman CA, Borsutzky S, Griot-Wenk M, Metcalfe IC, Pearman J, Collioud A, et al. Vaccines against typhoid fever. Vaccine. 2006;24:3804–11. doi: 10.1016/j.vaccine.2005.07.111. [DOI] [PubMed] [Google Scholar]
- 7.Levine MM. Typhoid fever vaccines. In: Plotkin SA, Orentstein WA, editors. Vaccines. 3rd. Philadelphia: Sounders; 1999. pp. 781–815. [Google Scholar]
- 8.Typhoid vaccines: WHO position paper. WHO Weekly Epidemiological Record. WHO; Geneva: 2008. pp. 49–60. [Google Scholar]
- 9.Jarvis FG, Mesenko MT, Martin DG, Perrine TD. Physiochemical properties of the Vi antigen before and after mild alkaline hydrolysis. Journal of bacteriology. 1967;94:1406–10. doi: 10.1128/jb.94.5.1406-1410.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rijpkema S, Durrani Z, Lemercinier X, Jones C. Detection of O-acetylated Vi polysaccharide of Salmonella enterica subspecies typhi by enzyme immunoassay. Biologicals: journal of the International Association of Biological Standardization. 2004;32:11–6. doi: 10.1016/j.biologicals.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Szewczyk B, Taylor A. Immunochemical properties of Vi antigen from Salmonella typhi Ty2: presence of two antigenic determinants. Infection and immunity. 1980;29:539–44. doi: 10.1128/iai.29.2.539-544.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szu SC, Li XR, Stone AL, Robbins JB. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infection and immunity. 1991;59:4555–61. doi: 10.1128/iai.59.12.4555-4561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Fernandez A, Faro J, Fernandez C. Immune responses to polysaccharides: lessons from humans and mice. Vaccine. 2008;26:292–300. doi: 10.1016/j.vaccine.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Martin DG, Jarvis FG, Milner KC. Physicochemical and biological properties of sonically treated Vi antigen. Journal of bacteriology. 1967;94:1411–6. doi: 10.1128/jb.94.5.1411-1416.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Report of the Ad-hoc consultation on typhoid vaccine introduction and typhoid surveillance. WHO IVB/12.02. 2012 [Google Scholar]
- 16.Kossaczka Z, Lin FY, Ho VA, Thuy NT, Van Bay P, Thanh TC, et al. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infection and immunity. 1999;67:5806–10. doi: 10.1128/iai.67.11.5806-5810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon R, Levine MM. Glycoconjugate vaccine strategies for protection against invasive Salmonella infections. Human vaccines & immunotherapeutics. 2012;8:494–8. doi: 10.4161/hv.19158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szu SC, Taylor DN, Trofa AC, Clements JD, Shiloach J, Sadoff JC, et al. Laboratory and preliminary clinical characterization of Vi capsular polysaccharide-protein conjugate vaccines. Infection and immunity. 1994;62:4440–4. doi: 10.1128/iai.62.10.4440-4444.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May CD. Industrial pectins: sources, production, and applications. Carbohydrate Polymers. 1990;12:79–99. [Google Scholar]
- 20.Voragne AGJ, Pilnik W, Thilbault JF, Axelrose MAV, Renard CMGC. AM Stephen ed Food Polysaccharides and Their Applications. New York: Marcel Dekker, Inc; 1995. Pectins; pp. 287–339. [Google Scholar]
- 21.Szu SC, Bystricky S, Hinojosa-Ahumada M, Egan W, Robbins JB. Synthesis and some immunologic properties of an O-acetyl pectin [poly(1–>4)-alpha-D-GalpA]-protein conjugate as a vaccine for typhoid fever. Infection and immunity. 1994;62:5545–9. doi: 10.1128/iai.62.12.5545-5549.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Springer MJ, Ni Y, Finger-Baker I, Ball JP, Hahn J, DiMarco AV, et al. Preclinical dose-ranging studies of a novel dry powder norovirus vaccine formulation. Vaccine. 2016;34:1452–8. doi: 10.1016/j.vaccine.2016.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velasquez LS, Shira S, Berta AN, Kilbourne J, Medi BM, Tizard I, et al. Intranasal delivery of Norwalk virus-like particles formulated in an in situ gelling, dry powder vaccine. Vaccine. 2011;29:5221–31. doi: 10.1016/j.vaccine.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schweiger RG. Acetyl pectates and their reactivity with polyvalent metal ions. Journal of Organic Chemistry. 1964;29:2973–5. [Google Scholar]
- 25.Carson JF, Maclay WD. The acylation of polyuronides with formamide as a dispersing agent. Journal of the American Chemical Society. 1946;68:1015–7. doi: 10.1021/ja01210a034. [DOI] [PubMed] [Google Scholar]
- 26.Hestrin S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. The Journal of biological chemistry. 1949;180:249–61. [PubMed] [Google Scholar]
- 27.Szu SC, Bystricky S. Physical, chemical, antigenic, and immunologic characterization of polygalacturonan, its derivatives, and Vi antigen from Salmonella typhi. Methods in enzymology. 2003;363:552–67. doi: 10.1016/S0076-6879(03)01079-6. [DOI] [PubMed] [Google Scholar]
- 28.Park JH, Hong JJ, Choi ES, Lee JW, Park JH. Efficacy of purified Vi polysaccharide typhoid vaccine. Journal of veterinary science. 2002;3:67–70. [PubMed] [Google Scholar]
- 29.Goldblatt D. Conjugate vaccines. Clinical Experimental Immunology. 2000;119:1–3. doi: 10.1046/j.1365-2249.2000.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavallari M, De Libero G. From immunologically archaic to neoteric glycovaccines. Vaccines. 2017;5:E4. doi: 10.3390/vaccines5010004. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–87. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surana NK, Kasper DL. The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunological reviews. 2012;245:13–26. doi: 10.1111/j.1600-065X.2011.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An SJ, Yoon YK, Kothari S, Kim DR, Kim JA, Kothari N, et al. Immune suppression induced by Vi capsular polysaccharide is overcome by Vi-DT conjugate vaccine. Vaccine. 2012;30:1023–8. doi: 10.1016/j.vaccine.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 34.Safari D, Dekker HA, de Jong B, Rijkers GT, Kamerling JP, Snippe H. Antibody- and cell-mediated immune responses to a synthetic oligosaccharide conjugate vaccine after booster immunization. Vaccine. 2011;29:6498–504. doi: 10.1016/j.vaccine.2011.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.