Abstract
Behavioral approach, defined as behavior directed toward a reward or novel stimulus, when elevated, may increase one’s vulnerability to substance use disorder. Behavioral approach has been associated with relatively greater left compared to right frontal activity; behavioral inhibition may be associated with relatively greater right compared to left frontal brain activity. We hypothesized that substance dependent individuals (SDI) would have higher behavioral approach than controls and greater prefrontal cortical activity during decision-making involving reward. We hypothesized that behavioral approach would correlate with left frontal activity during decision-making and that the correlation would be stronger in SDI than controls. 31 SDI and 21 controls completed the Behavioral Inhibition System/Behavioral Approach System (BIS/BAS) scales and performed a decision-making task during fMRI. Orbitofrontal (OFC) and dorsolateral prefrontal activity were correlated with BIS and BAS scores. Compared to controls, SDI had higher BAS Fun Seeking scores (p<0.001) and worse decision-making performance (p=0.004). BAS Fun Seeking correlated with left OFC activity during decision-making across group (r=0.444, p<0.003). The correlation did not differ by group. There was no correlation between BIS and right frontal activity. Left OFC may play a role in reward-related decision-making in substance use disorder especially in individuals with high behavioral approach.
Keywords: Substance dependence, behavioral approach, behavioral inhibition, orbital frontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), BIS/BAS
1. Introduction
Drug use is associated with novelty seeking and impulsivity, both of which play a role in Gray’s Reinforcement Sensitivity Theory (Gray, 1970, 1981, 1987; McNaughton and Corr, 2004). Gray proposed systems controlling behavior and emotion: a behavioral activation (approach) system that directs behavior toward a reward and is associated with novelty seeking, impulsivity, and positive emotions and a behavioral inhibition system that analyzes risk, resolves conflicting goals, and is associated with negative affect. Behavioral approach has been linked to the dopaminergic system (Depue and Collins, 1999) and genetic polymorphisms associated with high dopamine activity (Reuter et al., 2006). Individuals with high behavioral approach sensitivity are more likely to abuse alcohol and drugs which have rewarding properties involving dopamine function (Franken et al., 2006). In contrast, a highly active behavioral inhibition system has been associated with anxiety and anxiety-related disorders (Johnson et al., 2003; Maack et al., 2012) that are often treated with drugs that modulate serotonin (Ravindran and Stein, 2010).
To quantify individual differences in behavioral approach and inhibition, Carver and White developed the behavioral inhibition/behavioral approach (BIS/BAS) scale (Carver and White, 1994) which has shown validity in healthy and clinical populations (Jorm et al., 1998; Kasch et al., 2002; Leone et al., 2001). Alcohol, tobacco, and drug use is associated with high BAS scores (Balconi et al., 2014a; Franken et al., 2006; Johnson et al., 2003; Knyazev, 2004; Yen et al., 2009). The BAS Drive and Fun Seeking subscale scores, in particular, have been shown to be higher in heroin, cocaine, and amphetamine dependent subjects than controls (Franken et al., 2006; Perry et al., 2013). In contrast, high BIS scores have been weakly, if at all, associated with substance use (Franken and Muris, 2006; Knyazev, 2004).
EEG studies suggest that approach behaviors in controls are associated with greater left relative to right hemisphere activity (Coan and Allen, 2003; Harmon-Jones and Allen, 1998; Sutton and Davidson, 1997; Wacker et al., 2013), a finding supported by fMRI studies (Beaver et al., 2006; Krmpotich et al., 2013). Beaver et al. (2006) found an association between BAS scores and left orbitofrontal cortex (OFC) activity in response to rewarding food cues in controls. Krmpotich et al. (2013) reported a correlation between BAS scores and resting state left dorsolateral prefrontal cortex (DLPFC) activity in controls and stimulant dependent individuals. However, these prior fMRI studies did not investigate decision-making. Filling this gap in the literature is important because reward related decision-making is an important feature of substance use disorder (SUD) and may elucidate mechanisms by which behavioral approach increases the risk for substance use.
Dorsolateral prefrontal and orbitofrontal cortex have been implicated in impaired decision-making in SUD (Li et al., 2010; Olsen et al., 2015). The OFC is important for salience attribution of a stimulus, and may be overly active in drug users especially in response to drug cues (Chase et al., 2011; Schoenbaum and Shaham, 2008). The DLPFC is important for cognitive control and response inhibition. Ersche et al. (2005) found that during risky decision-making right DLPFC activity was higher in healthy controls while left OFC activity was increased in drug users, possibly reflecting lowered cognitive control (associated with DLPFC) and increased salience attribution (associated with OFC) of reward in drug users. The relationship between these nodes of the decision-making network and behavioral approach has not been well studied in SUD.
Given the evidence that behavioral approach is associated with substance use and greater left relative to right hemisphere activity, the present study sought to investigate relationships between BIS/BAS and right and left prefrontal brain activity during risky decision-making in substance dependent individuals (SDI). We hypothesized that i.) compared to controls, SDI would have higher behavioral approach, ii.) SDI would have greater OFC activity during risky decision-making, iii.) left OFC and DLPFC activity during decision-making would correlate with BAS scores, and iv.) right OFC and DLPFC activity would correlate with BIS scores. Finally, we hypothesized that the association between left frontal activity and BAS would be stronger in SDI than in healthy controls.
2. Materials and Methods
2.1. Subjects
Fifty-two subjects were recruited: 31 substance dependent individuals (SDI) and 21 controls. This sample has not been previously reported. All 52 subjects underwent diagnostic and drug dependence interviews and provided behavioral measures consisting of BIS/BAS and decision-making. One control did not have BIS/BAS data due to technical reasons; remaining subjects completed the entire BIS/BAS questionnaire. Seven subjects were excluded for excessive head motion (6 SDI) or claustrophobia (1 control). Hence, imaging data are reported on 45 subjects: 25 SDI (14M/11F) and 20 controls (11M/9F). Inclusion criteria: SDI met DSM-IV criteria for stimulant dependence. SDI were recruited from a residential treatment program at the University of Colorado Denver Addiction Research and Treatment Service (ARTS).
Average abstinence from drugs and alcohol was 13 months (range=2-31, standard deviation=7.9). Abstinence from drugs, nicotine, and alcohol was monitored by direct supervision and random drug screening at ARTS. Controls were recruited from the community and excluded if they met DSM-IV criteria for lifetime abuse or dependence on drugs or alcohol. Exclusion criteria for all subjects: neurological illness, schizophrenia, bipolar disorder, major depression within the last 2 months, head trauma with loss of consciousness >15 minutes, HIV, or IQ ≤80. All subjects provided written informed consent approved by the Colorado Multiple Institutional Review Board. All subjects but two were confirmed right-handed: One subject was left-handed and for one subject handedness data was not obtained.
2.2. Screening and Drug Dependence Assessments
All subjects received structured interviews administered by trained personnel. Lifetime drug dependence was assessed for stimulants, nicotine, alcohol, cannabis, opioids, club drugs, sedatives, and hallucinogens using the computerized Composite International Diagnostic Interview-Substance Abuse Module (CIDI-SAM) (Cottler et al., 1989). The Computerized Diagnostic Interview Schedule–Version IV (C-DIS-IV) was administered to exclude subjects with schizophrenia, bipolar disorder, and current major depression. Subjects were not excluded for antisocial personality disorder (ASPD) as comorbidity of ASPD with stimulant use is common in the U.S. (Compton et al., 2005) particularly in residential treatment programs. Thirty of 31 SDI and 5 of 21 controls met DSM-IV criteria for ASPD. IQ was estimated with matrix and verbal reasoning Wechsler Abbreviated Scale of Intelligence subtests (Psychological Corporation, 1999).
2.3. Behavioral Measures
2.3.1. Behavioral Inhibition System/Behavioral Activation System (BIS/BAS)
Behavioral inhibition and approach were measured using the Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) scales (Carver and White, 1994). The BIS scale assesses the tendency to respond with negative affect and behaviors that respond to goal conflict and generate anxiety (e.g., “I worry about making mistakes.”). The BAS scale assesses the tendency to respond with positive affect and behaviors that approach appetitive stimuli. BAS is divided into three subscales that focus on different aspects of approach behavior: (1) Drive, relating to persistent pursuit of desired goals (e.g., “I go out of my way to get things I want.”); (2) Fun-Seeking, the tendency to seek out novel rewards and act impulsively (e.g., “I often act on the spur of the moment.”), and (3) Reward Responsiveness, the tendency to experience a positive response to the occurrence or anticipation of reward (e.g., “It would excite me to win a contest.”). These scales have shown validity in assessing differences among individuals on emotional response to aversive or appetitive stimuli (Carver and White, 1994; Leone et al., 2001). Each subscale measures a different aspect of approach and were analyzed separately (Carver and White, 1994).
2.3.2. Decision-Making Task
Subjects played a modified version of the Iowa Gambling Task during fMRI scanning, as described previously (Cauffman et al., 2010; Thompson et al., 2012). Briefly, subjects began with a hypothetical $2,000, were presented four decks of cards, and instructed to earn as much money as possible. For each trial, the computer selected a deck and subject was asked to “Play” or “Pass” by pressing the appropriate button. “Play” resulted in a single positive or negative monetary value, along with the running total. “Pass” resulted in no change. Two decks resulted in a net gain (good) and two in a net loss (bad) when played over time. To perform well, subjects must learn to “Pass” on bad decks and “Play” on good decks. For each trial, the subject was given 2 seconds to make a decision followed immediately by feedback of 4 seconds duration. There were 50 trials of each deck (200 trials total). Decision-making trials were interspersed with 43 motor control trials and 59 fixation crosses in pseudorandom order to effectively jitter trial onsets. Motor control trials were identical to task trials except the subject was told which button to press. Task time was 26 minutes (two 13 minute runs). The current task differed from prior studies (Yamamoto et al., 2014, 2015) in that we included an explicit control for motor activity. Decision-making variables of interest were the number of passes on bad decks, plays on good decks, and motor control (instructed presses).
2.4. MRI Acquisition
Functional MR images were acquired on a 3T scanner with an 8-channel head coil using a GRE-EPI sequence (TR 2s, TE 30 ms, matrix 64 × 64, FOV 220 mm2, 3.4 × 3.4 mm2, slice thickness 3 mm, gap 1 mm, angled parallel to the AC-PC line).
2.5. Pre-Processing and Model Specification
Data were pre-processed and analyzed with Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). The first three image volumes from each run were excluded for saturation effects. Functional data were realigned using rigid body transforms to the mean volume. Subjects were excluded for head motion >3 mm. Realigned images were normalized to Montreal Neurological Institute (MNI) space. Data were smoothed with a 6-mm full-width-half-maximum Gaussian kernel. Final smoothness of the data after pre-processing was 8.2 × 8.4 × 7.9 mm3. Time series data were filtered using a high pass filter and corrected for temporal autocorrelation. The stimulus functions were convolved with a canonical hemodynamic response function.
Eleven conditions were modeled: decision and outcome for each of the four decks (i.e., 8) and the control condition (i.e., 2) plus fixation trials (i.e., 1). Session was modeled as a nuisance variable. Within-subject contrast maps of decision>motor control were calculated after adjusting for education and six motion parameters (three translation and three rotation parameters from the rigid-body realignment). These within-subject maps were brought to the second level for analysis.
2.6. Region-of-Interest (ROI) Definitions
Two bilateral ROIs were created based on a priori predictions of their relevance in risky decision-making: OFC and DLPFC. OFC ROIs were based on the meta-analytic framework of Neurosynth (http://neurosynth.org) which identifies neuroimaging studies reporting significant BOLD activity associated with a given construct (Yarkoni et al., 2011). Among 336 studies showing significant activity associated with “decision-making” (queried 04/10/2015), a coordinate localized to the LOFC (MNI: −22, 34, −16; z-score 5.82) was used to construct a 6-mm diameter sphere. The ROFC mirrored the LOFC (MNI: 22, 34, −16). Bilateral DLPFC ROIs consisted of 6 mm diameter spheres centered at MNI coordinates ±34, 32, 38 taken from a previous decision-making study using a similar task but on a completely independent group of subjects (Yamamoto et al., 2015). The DLPFC coordinates from the 2015 study were also obtained from Neurosynth (http://neurosynth.org). Beta estimates were extracted for each ROI for each subject from the first-level contrast maps using the Marsbar (Brett et al., 2002) toolbox in SPM8.
2.7. Statistical Analyses
Whole brain analyses were conducted in SPM8. Whole brain cluster correction was determined using Monte Carlo simulation in AFNI (http://afni.nimh.nih.gov/afni/). All other analyses were carried out in SPSS (SPSS, 2013).
2.7.1. Demographic and Behavioral Data
Normally distributed continuous variables were analyzed with 2-tailed t-tests or analysis of covariance (ANCOVA) with group as between-subjects factor after adjusting for education. Categorical variables were analyzed with chi-squared tests. Significance was set at p<0.05.
2.7.2. Group Differences in Brain Activity
2.7.2.1. ROI
Beta estimates in each ROI were analyzed using ANCOVA with group as a between-subjects factor after adjusting for education. Significance was set at p<0.05 using Bonferroni correction for 4 comparisons (i.e., p≤0.0125).
2.7.2.2. Whole Brain
Whole brain group analysis was conducted in SPM8 using ANCOVA after adjusting for education. Multiple testing corrections were applied with family-wise error correction using AlphaSim Monte-Carlo simulations and a voxel-level threshold of p<0.001, resulting in required cluster-level threshold of 117 contiguous voxels to achieve a whole-brain significance of p<.05.
2.8. Correlations Between BIS/BAS and Brain Activity
Across group, BIS and BAS subscales were correlated with brain activity from each ROI using Pearson’s r. Bonferroni correction for multiple comparisons was used. We used methods of Preacher to test whether the correlation coefficients differed between groups (Preacher, 2002).
3. Results
3.1. Demographics
SDI and controls were similar in age (35.2±6.9 vs. 33.2±8.6 years, p=0.37) and sex (14M/17F vs. 12M/9F, p=0.397). SDI had fewer years of education (12.6±1.9 vs.15.0±1.4, p<0.001) and lower IQ (102.5±10.5 vs. 114.4±11.2, p<0.001) than controls (Table 1). Because education and IQ were highly correlated (r=0.707, p<0.001), only education was used as a covariate in subsequent analyses.
Table 1.
Demographics and behavior
| Demographics | Control (21) | SDI (31) | p-value |
|---|---|---|---|
| Sex* | 12M/9F | 14M/17F | 0.397 |
| Age (years) | 33.2±8.6 | 35.2±6.9 | 0.371 |
| Education (years) | 15.0±1.4 | 12.6±1.9 | <0.001 |
| IQ | 114.4±11.2 | 102.5±10.5 | <0.001 |
| Behavioral tests | |||
| BIS | 18.9±3.9 | 21.3±3.1 | 0.011 |
| BAS | |||
| Drive | 9.8±1.2 | 12.7±2.3 | <0.001 |
| Fun-Seeking | 11.1±1.8 | 13.3±1.6 | <0.001 |
| Reward Responsiveness | 15.9±2.5 | 17.4±2.1 | 0.059 |
| Performance | |||
| Passbad | 42.5±19.9 | 25.5±11.9 | 0.004 |
| Playgood | 73.6±14.2 | 70.6±11.1 | 0.810 |
| Motor control (instructed presses) | 39.7±5.3 | 35.1±9.9 | 0.372 |
| Total money earned ($) | 3,634±1,682 | 2,690±785 | 0.102 |
chi-squared test for group and sex
3.2. Drug Characteristics
SDI were recruited for stimulant-dependence; however, most SDI exhibited dependence on other drugs. The number of SDI with specific drug dependence diagnoses were: stimulants, 31 (100%); nicotine, 22 (71%); alcohol, 18 (58%); cannabis, 13 (42%); opioids, 8 (26%); club drugs, 4 (13%); sedatives, 3 (10%); and hallucinogens, 1 (3%). Three (14%) of the 21 controls met criteria for nicotine dependence.
3.3. BIS/BAS
SDI scored higher than controls on BIS (21.3±3.1 vs. 18.9±3.9; F=6.9, p=0.011), BAS Drive (12.7±2.3 vs. 9.8±1.2; F=22.7, p<0.001), and BAS Fun Seeking (13.3±1.6 vs 11.1±1.8; F=16.0, p<0.001), with a trend for higher scores on BAS Reward Responsiveness (17.4±2.1 vs. 15.9±2.5; F=3.7, p=0.059) (Table 1).
3.4. Decision-Making
SDI passed on bad decks significantly fewer times than controls (SDI: 25.5±11.9, controls: 42.5±19.9; F=9.3, p=0.004), consistent with less risk avoidance or worse negative reinforcement learning. There were no group differences in playing on good decks (SDI: 70.6±11.1, controls: 73.6±14.2; F=0.058, p=0.81), suggesting that decision-making involving positive reinforcement learning did not differ across group. Motor control responses (computer instructed a press) did not differ between SDI and controls, indicating that general attention to the task and motor function was the same across group (SDI: 35.1±9.9, controls: 39.7±5.3; F=0.81, p=0.372). Total money earned was less in SDI vs controls, but not significantly (SDI: $2690±$785; controls: $3634±$1682; F=2.77, p=0.1) (Table 1).
3.5. Group Differences in Brain Activity
3.5.1. ROI
SDI showed greater activity than controls in left OFC (p<0.003) during decision-making. No other comparisons survived Bonferroni corrections (Figure 1).
Figure 1.
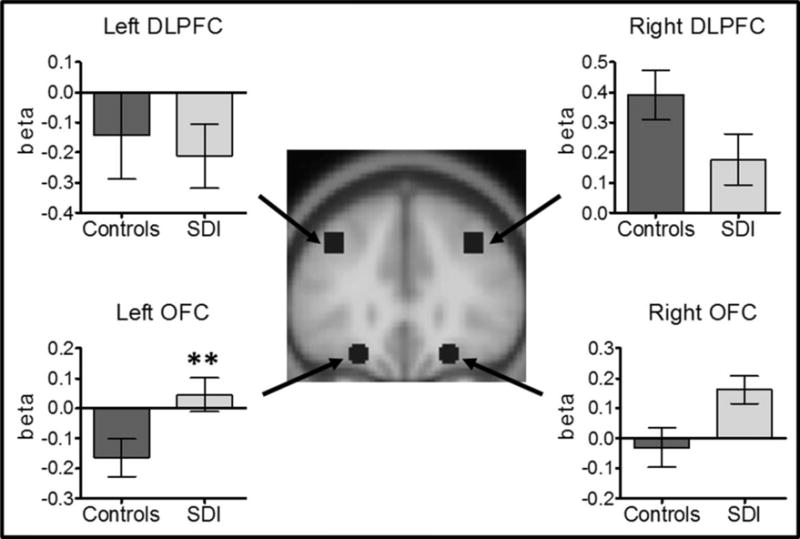
BOLD signal in SDI and controls during decision-making. SDI showed higher activity than controls in left OFC, **p<0.003, surviving Bonferroni correction for multiple comparisons. Images are in neurological convention. Graphs mean ± SEM
3.5.2. Whole Brain
There were no significant clusters surviving a multiple comparisons correction for group differences of SDI>control or control>SDI.
3.6. Correlations Between BIS/BAS and Brain Activity
Left OFC activity correlated with BAS Fun Seeking across groups (r=0.444, p<0.003; Figure 2 and Table 2). Correlation within groups (SDI: r=0.526, p=0.007; controls: r=0.056, p=0.821) did not survive multiple comparisons correction. Contrary to our hypothesis, there was no group difference in correlation coefficients between left OFC activity and BAS Fun Seeking (z=1.609, p=0.11). There were no correlations between BAS Drive or BAS Reward and brain activity or between BIS and right OFC or DLPFC activity.
Figure 2.
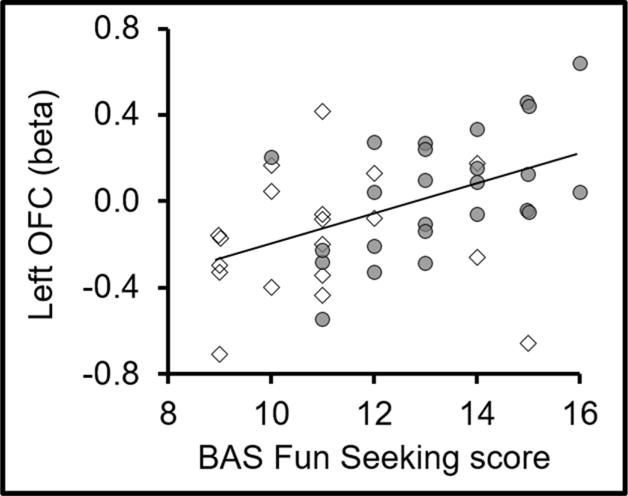
Left OFC activity correlated positively with BAS Fun Seeking across groups (r=0.444, p<0.003), surviving Bonferroni correction for multiple comparisons. SDI (solid gray circles), controls (open diamonds)
Table 2.
Region of Interest correlations with BIS/BAS
| All subjects | Left OFC |
Right OFC |
Left DLPFC |
Right DLPFC |
|---|---|---|---|---|
| BIS | 0.29 | 0.311 | 0.01 | −0.084 |
| BAS Drive | 0.14 | 0.06 | −0.17 | −0.232 |
| BAS Fun Seeking | 0.444** | 0.28 | 0.04 | −0.050 |
| BAS Reward Responsiveness | −0.04 | 0.18 | 0.08 | −0.009 |
p<0.003, survives Bonferroni correction for multiple comparisons
4. Discussion
The current study found that SDI have higher BAS and BIS scores than controls and that left OFC activity during decision-making correlated with BAS Fun Seeking across group. Contrary to our hypotheses, we did not find that the association between left OFC activity and BAS was stronger in SDI than controls, nor did BIS correlate with right frontal activity during decision-making across or within group. Taken together, our findings suggest that left OFC is associated with behavioral characteristics that are relevant to substance use and could play a role in risky decision-making.
Higher BAS scores in SDI compared to controls is consistent with prior studies investigating personality predictors of drug and alcohol use (Franken and Muris, 2006; Knyazev, 2004). In a large sample of 4,501 Russian youths, Knyazev found that BAS was positively related to substance use and risky behavior. Franken and Muris reported that BAS correlated positively with drug and alcohol use in 276 non-clinical college students. Among the subscales, we found that BAS Fun Seeking and Drive were higher in SDI compared to controls, replicating the results of Perry et al. (2013) and supporting the hypothesis that greater behavioral approach may increase vulnerability to drug use disorders (Franken et al., 2006; Johnson et al., 2003). BAS Drive has been linked to cue-induced craving in alcoholics (Franken, 2002). Whether the relationship between the subscales and drug use differs by drug classes requires further study.
In addition to predicting substance use, BAS Fun Seeking correlates with worse decision-making in healthy controls (Suhr and Tsanadis, 2007). Suhr and Tsanadis found a significant relationship of high BAS Fun Seeking and worse decision-making on the Iowa Gambling Task (IGT) in controls. BAS does not assess decision-making per se, but rather sensitivity toward reward, novelty and risk taking. Our interpretation is that differences in sensitivity to these factors influence decision-making behavior involving novelty or reward. BAS Fun Seeking scores combined with poor decision-making performance provide evidence that SDI may be more impulsive and less risk avoidant than controls. Decision-making is a highly complex process involving sensitivity to positive feedback, negative feedback, expectation, and, according to the somatic theory of the IGT, affect (Bechara and Damasio, 2002). We did not model these factors separately, but instead focused on risky decision-making in general.
Increased behavioral approach was associated with greater left OFC activity during decision-making. This is consistent with an fMRI study by Barros-Loscertales that found behavioral approach in healthy controls correlated with left prefrontal activity while subjects viewed erotic pictures (Barrós-Loscertales et al., 2010). An EEG study by Balconi et al. observed higher BAS Reward Responsiveness and BAS Drive in cocaine users compared to controls. In addition, there was relatively increased left compared to right alpha power when participants made disadvantageous decisions on the IGT (Balconi et al., 2014b), suggesting that behavioral approach was associated with left relative to right hemisphere activity. However, this study did not correlate EEG with behavioral approach. Our study extends that of Balconi et al. in that we correlated behavioral approach and brain activity and found that left OFC activity correlated with BAS Fun Seeking. Both BAS Fun Seeking and Reward subscales measure willingness to approach a reward; however, the Fun Seeking subscale incorporates impulsivity, a trait associated with the OFC (Berlin et al., 2004; Winstanley et al., 2004) and addiction (Moeller et al., 2002; Perry et al., 2013). Further, the BAS Fun Seeking component of willingness to approach a rewarding event or monetary incentive fits well with the purported role of the OFC in associating a stimulus and reward, in tracking the value of a predicted reward, and in behavioral reinforcement, all of which can be affected in substance use disorder (Schoenbaum and Shaham, 2008).
A large body of evidence indicates that drugs of abuse, especially stimulants, alter OFC signals and OFC-dependent behaviors (Schoenbaum et al., 2006). Specifically, the OFC is thought to play a role in signaling outcome expectancies. Our findings of increased OFC activity during decision-making in SDI compared to controls is consistent with previous imaging studies in abstinent stimulant-dependent individuals (Bolla et al., 2003; Ersche et al., 2005; Yamamoto et al., 2014). Greater OFC activity in SDI may reflect greater salience of possible reward cues during decision-making. Animal studies support the idea that the OFC measures incentive value of prior outcomes and uses this information to update decisions during a task (Schoenbaum et al., 2006). Another possible explanation for increased OFC activity in SDI is that it may reflect an inability to devalue a stimulus that no longer predicts reward. For example, in a Pavlovian devaluation experiment, rats exposed to cocaine were unable to appropriately devalue a conditioned reinforcer, mimicking the behavior of rats who have received OFC lesions (Schoenbaum et al., 2004). Thus, the OFC appears to be critical for signaling outcome expectancies and may be disrupted by drug exposure. Taken together, we hypothesize that there are alterations in OFC signaling during decision-making in SDI and that trait behavioral approach might be a related factor.
It should be noted that while BAS Fun Seeking correlated with left OFC activity during decision-making across our entire sample, correlations did not survive multiple comparisons correction in either group when analyzed separately. The lack of within-group correlation was somewhat unexpected and most likely due to low power. In SDI, the correlation was strongly positive (r=0.526), but did not survive multiple comparison correction. In controls, if we removed one outlier, the BAS correlation with left OFC became positive and stronger (r=0.364), but not significant. Another explanation is that the association between approach behavior and left frontal activity may reflect a dimensional, rather than diagnostic, construct as proposed by the National Institute of Mental Health RDoC (http://www.nimh.nih.gov/research-priorities/rdoc) where behavioral constructs are measured on a continuum. Approach is modelled within the positive valence domain (Johnson et al., 2016). In this case SDI may lie more at the upper end of the behavioral approach spectrum. A third possible explanation, as proposed by Spielberg et al. (2011), involves DLPFC modulation of OFC activity. DLPFC could provide attentional focus by inhibiting left OFC thus preventing interference from competing or distracting stimuli. In that study, controls showed a negative correlation between BAS score and left OFC activity during a Stroop interference task. Another group found that while controls showed a negative correlation of Stroop scores with OFC activity, drug users showed the opposite (Goldstein et al., 2001). Our results cannot be compared directly to these studies because of the different tasks used, nevertheless our results are consistent with an association between approach and left OFC, but one that may be influenced by top down cognitive control or modulation by DLPFC.
With regards to the BIS scale, which measures sensitivity to aversive stimuli or conflict, SDI scored higher than controls. High BIS scores have been related to anxiety and depression (Johnson et al., 2003; Maack et al., 2012), although a few studies reported a positive association of BIS scores with alcohol intake (Keough and O’Connor, 2014; Wardell et al., 2011, 2013). In those studies, elevated drinking was associated with both high BIS and high BAS, suggesting that the two behavioral systems may influence alcohol consumption in an additive or synergistic manner. Accordingly, Wardell et al. (2011) found that high BIS predicted alcohol use only when BAS was high. A longitudinal study revealed that over time negative mood-related problems due to alcohol use were found only in subjects high on both BIS and BAS (Wardell et al., 2013). It was postulated that high BIS or negative mood combined with high BAS approach behavior may promote drinking to reduce bad feelings, suggesting that the combined effect of BIS and BAS is important for behavior. Approximately 60% of our SDI met criteria for alcohol dependence, another possible explanation for the observed high BIS.
We did not find a correlation between right OFC or DLPFC activity and BIS. The literature on an association of BIS with right hemisphere has been mixed. Our results are consistent with studies that did not find an association between BIS and relatively greater right compared to left hemisphere activity on EEG (Amodio et al., 2008; Coan and Allen, 2003; Hewig et al., 2006).
Strengths of this study include replication of prior results showing greater activation in the OFC using the modified gambling task in SDI compared to controls (Yamamoto et al., 2014) in an independent data set. We used a reproducible unbiased definition of ROIs derived from a large, publicly available dataset (https://neurosynth.org). Whereas a prior EEG study in cocaine users found higher BAS scores and, separately, relative left asymmetric activity during decision-making (Balconi et al., 2014b), we extend the literature in reporting for the first time in an fMRI study a direct correlation between BAS and left OFC activity across individuals.
4.1 Limitations
A limitation of this study is that although SDI were recruited for stimulant dependence, most were polysubstance users. Hence, our findings cannot be attributed to the action of a single drug class. Nonetheless, the study is relevant as polysubstance use is quite prevalent in the United States (Abuse, 2012; Barrett et al., 2006). Secondly, all but one SDI met DSM-IV criteria for ASPD. However, comorbidity of ASPD with stimulant use is common in the U.S. (Compton et al., 2005) and could even be considered as one facet of this complex disorder (Hicks et al., 2004). While decision-making deficits and approach behavior may be related to clinical symptoms of ASPD, they also may be part of the symptomatology of substance use disorders. We cannot definitively state whether our results relate specifically to substance dependence, ASPD or their interaction. Third, in addition to BIS and BAS, a third behavioral system, the fight-flight-freeze system (FFFS) (Pickering and Corr, 2008) is hypothesized to mediate reactions to aversive stimuli and involves fear and avoidance. FFFS could not be measured separately as the BIS scale contains some elements of both FFFS and BIS. Fourth, the sample size is small and may have reduced our power to detect group differences.
In conclusion, compared to controls, SDI had higher BAS and BIS scores and worse decision-making performance, consistent with the hypothesis that high behavioral approach may be a risk factor for substance use disorder. SDI exhibited significantly higher left OFC activity during decision-making compared to controls. Left OFC activity correlated with BAS Fun Seeking across groups. These results suggest that left OFC may play a role in poor decision-making in substance use especially in individuals with elevated approach behavior.
Highlights.
We examined behavioral inhibition/behavioral approach (BIS/BAS) association with orbitofrontal cortex (OFC) and dorsolateral prefrontal cortex (DLPFC) activity during decision-making.
BAS scores were higher in substance dependent individuals (SDI) than controls
Left OFC activity during decision making was greater in SDI than controls.
Higher BAS Fun Seeking was related to greater left OFC decision-making activity.
Acknowledgments
The authors would like to acknowledge the staff at Addiction Research Treatment Services (ARTS) and Debra Singel, RT.
Role of Funding Source
Funding for this study was provided by the National Institute of Drug Abuse (NIDA) grants DA024104 (JT), DA027748 (JT); Sakai’s time on this study was supported by DA031761. NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Yamamoto: design, analysis, data interpretation, manuscript; Banich: data interpretation, manuscript; Regner: data interpretation, manuscript; Sakai: data interpretation, manuscript; Tanabe: design, analysis, data interpretation, manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors report no potential conflicts of interest.
References
- Abuse S. Mental Health Services Administration (2012) Results from the 2011 national survey on drug use and health: summary of national findings. NSDUH Ser H-44 HHS Publ No SMA 12-4713 2012 [Google Scholar]
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: implications for theories of self-regulation. Psychophysiology. 2008;45:11–19. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Balconi M, Finocchiaro R, Campanella S. Reward sensitivity, decisional bias, and metacognitive deficits in cocaine drug addiction. J Addict Med. 2014a;8:399–406. doi: 10.1097/ADM.0000000000000065. [DOI] [PubMed] [Google Scholar]
- Balconi M, Finocchiaro R, Canavesio Y. Reward-system effect (BAS rating), left hemispheric “unbalance” (alpha band oscillations) and decisional impairments in drug addiction. Addict Behav. 2014b;39:1026–1032. doi: 10.1016/j.addbeh.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Balconi M, Mazza G. Brain oscillations and BIS/BAS (behavioral inhibition/activation system) effects on processing masked emotional cues. ERS/ERD and coherence measures of alpha band. Int J Psychophysiol Off J Int Organ Psychophysiol. 2009;74:158–165. doi: 10.1016/j.ijpsycho.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Darredeau C, Pihl RO. Patterns of simultaneous polysubstance use in drug using university students. Hum Psychopharmacol. 2006;21:255–263. doi: 10.1002/hup.766. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Ventura-Campos N, Sanjuán-Tomás A, Belloch V, Parcet MA, Ávila C. Behavioral activation system modulation on brain activation during appetitive and aversive stimulus processing. Soc Cogn Affect Neurosci. 2010;5:18–28. doi: 10.1093/scan/nsq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, Ditzhuijzen J, van Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/S0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain J Neurol. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox (Abstract 10511. In Presented at the 8th international conference on functional mapping of the human brain, June 2-6, 2002, Sendai/Japan)[available on CDROM] NeuroImage. 2002;16 [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol. 1994;67:319–333. doi: 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham S, Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Dev Psychol. 2010;46:193–207. doi: 10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: An activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40:106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Compton WM, Conway KP, Stinson FS, Colliver JD, Grant BF. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2005;66:677–685. doi: 10.4088/jcp.v66n0602. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: A comprehensive substance abuse interview. Br J Addict. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22:491–517. doi: 10.1017/s0140525x99002046. doi:null. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJG, Clark L, Stocks-Gee G, London M, Deakin JB, Robbins TW, Sahakian BJ. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl) 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IHA. Behavioral approach system (BAS) sensitivity predicts alcohol craving. Personal Individ Differ. 2002;32:349–355. doi: 10.1016/S0191-8869(01)00030-7. [DOI] [Google Scholar]
- Franken IHA, Muris P. BIS/BAS personality characteristics and college students’ substance use. Personal Individ Differ. 2006;40:1497–1503. doi: 10.1016/j.paid.2005.12.005. [DOI] [Google Scholar]
- Franken IHA, Muris P, Georgieva I. Gray’s model of personality and addiction. Addict Behav. 2006;31:399–403. doi: 10.1016/j.addbeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: Involvement in response inhibition. Neuroreport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA. The Psychology of Fear and Stress. CUP Archive 1987 [Google Scholar]
- Gray JA. A Critique of Eysenck’s Theory of Personality. In: Eysenck PHJ, editor. A Model for Personality. Springer; Berlin Heidelberg: 1981. pp. 246–276. [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion. Behav Res Ther. 1970;8(70):249–266. 90069–0. doi: 10.1016/0005-7967. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. J Pers Soc Psychol. 1998;74:1310–1316. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Hewig J, Hagemann D, Seifert J, Naumann E, Bartussek D. The relation of cortical activity and BIS/BAS on the trait level. Biol Psychol. 2006;71:42–53. doi: 10.1016/j.biopsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Arch Gen Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Carver CS, Joormann J, Cuccaro ML. Genetic polymorphisms related to behavioral approach and behavioral inhibition scales. Personal Individ Differ. 2016;88:251–255. doi: 10.1016/j.paid.2015.09.024. [DOI] [Google Scholar]
- Johnson SL, Turner RJ, Iwata N. BIS/BAS levels and psychiatric disorder: An epidemiological study. J Psychopathol Behav Assess. 2003;25:25–36. doi: 10.1023/A:1022247919288. [DOI] [Google Scholar]
- Jorm AF, Christensen H, Henderson AS, Jacomb PA, Korten AE, Rodgers B. Using the BIS/BAS scales to measure behavioural inhibition and behavioural activation: Factor structure, validity and norms in a large community sample. Personal Individ Differ. 1998;26:49–58. doi: 10.1016/S0191-8869(98)00143-3. [DOI] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol. 2002;111:589–597. doi: 10.1037/0021-843X.111.4.589. [DOI] [PubMed] [Google Scholar]
- Keough MT, O’Connor RM. Clarifying the Measurement and the role of the behavioral inhibition system in alcohol misuse. Alcohol Clin Exp Res. 2014;38:1470–1479. doi: 10.1111/acer.12387. [DOI] [PubMed] [Google Scholar]
- Knyazev GG. Behavioural activation as predictor of substance use: Mediating and moderating role of attitudes and social relationships. Drug Alcohol Depend. 2004;75:309–321. doi: 10.1016/j.drugalcdep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Krmpotich TD, Tregellas JR, Thompson LL, Banich MT, Klenk AM, Tanabe JL. Resting-state activity in the left executive control network is associated with behavioral approach and is increased in substance dependence. Drug Alcohol Depend. 2013;129:1–7. doi: 10.1016/j.drugalcdep.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone L, Perugini M, Bagozzi RP, Pierro A, Mannetti L. Construct validity and generalizability of the Carver–White behavioural inhibition system/behavioural activation system scales. Eur J Personal. 2001;15:373–390. doi: 10.1002/per.415. [DOI] [Google Scholar]
- Li X, Lu ZL, D’Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI Images. Hum Brain Mapp. 2010;31:410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maack DJ, Tull MT, Gratz KL. Examining the incremental contribution of behavioral inhibition to generalized anxiety disorder relative to other Axis I disorders and cognitive-emotional vulnerabilities. J Anxiety Disord. 2012;26:689–695. doi: 10.1016/j.janxdis.2012.05.005. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 2002;68:105–111. doi: 10.1016/s0376-8716(02)00106-0. [DOI] [PubMed] [Google Scholar]
- Olsen VV, Lugo RG, Sütterlin S. The somatic marker theory in the context of addiction: Contributions to understanding development and maintenance. Psychol Res Behav Manag. 2015;8:187–200. doi: 10.2147/PRBM.S68695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RI, Krmpotich T, Thompson LL, Mikulich-Gilbertson SK, Banich MT, Tanabe J. Sex modulates approach systems and impulsivity in substance dependence. Drug Alcohol Depend. 2013;133:222–227. doi: 10.1016/j.drugalcdep.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering A, Corr PJ. JA Gray’s reinforcement sensitivity theory (RST) of personality. SAGE Handb Pers Theor Assess. 2008;1:239–257. [Google Scholar]
- Preacher KJ. Calculation for the test of the difference between two independent correlation coefficients [Computer software] 2002 [Google Scholar]
- Reuter M, Schmitz A, Corr P, Hennig J. Molecular genetics support Gray’s personality theory: The interaction of COMT and DRD2 polymorphisms predicts the behavioural approach system. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP. 2006;9:155–166. doi: 10.1017/S1461145705005419. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: A review of preclinical studies. Biol Psychiatry. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML. Laterality index in functional MRI: Methodological issues. Magn Reson Imaging. 2008;26:594–601. doi: 10.1016/j.mri.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhr JA, Tsanadis J. Affect and personality correlates of the Iowa Gambling Task. Personal Individ Differ. 2007;43:27–36. doi: 10.1016/j.paid.2006.11.004. [DOI] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychol Sci. 1997;8:204–210. doi: 10.1111/j.1467-9280.1997.tb00413.x. [DOI] [Google Scholar]
- Thompson LL, Claus ED, Mikulich-Gilbertson SK, Banich MT, Crowley T, Krmpotich T, Miller D, Tanabe J. Negative reinforcement learning is affected in substance dependence. Drug Alcohol Depend. 2012;123:84–90. doi: 10.1016/j.drugalcdep.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Mueller EM, Pizzagalli DA, Hennig J, Stemmler G. Dopamine-d2-receptor blockade reverses the association between trait approach motivation and frontal asymmetry in an approach-motivation context. Psychol Sci. 2013;24:489–497. doi: 10.1177/0956797612458935. [DOI] [PubMed] [Google Scholar]
- Wardell JD, O’Connor RM, Read JP, Colder CR. Behavioral approach system moderates the prospective association between the behavioral inhibition system and alcohol outcomes in college students. J Stud Alcohol Drugs. 2011;72:1028. doi: 10.15288/jsad.2011.72.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell JD, Read JP, Colder CR. The role of behavioral inhibition and behavioral approach systems in the associations between mood and alcohol consequences in college: A longitudinal multilevel analysis. Addict Behav. 2013;38:2772–2781. doi: 10.1016/j.addbeh.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci Off J Soc Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto DJ, Reynolds J, Krmpotich T, Banich MT, Thompson L, Tanabe J. Temporal profile of fronto-striatal-limbic activity during implicit decisions in drug dependence. Drug Alcohol Depend. 2014;136:108–114. doi: 10.1016/j.drugalcdep.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto DJ, Woo CW, Wager TD, Regner MF, Tanabe J. Influence of dorsolateral prefrontal cortex and ventral striatum on risk avoidance in addiction: A mediation analysis. Drug Alcohol Depend. 2015;149:10–17. doi: 10.1016/j.drugalcdep.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen JY, Ko CH, Yen CF, Chen CS, Chen CC. The association between harmful alcohol use and Internet addiction among college students: Comparison of personality. Psychiatry Clin Neurosci. 2009;63:218–224. doi: 10.1111/j.1440-1819.2009.01943.x. [DOI] [PubMed] [Google Scholar]


