Abstract
Objective
To evaluate the adoption of HPV testing and recommended extended cervical cancer screening intervals in clinical practice, we described yearly uptake of Pap/HPV cotesting and estimated length of time between normal screens by patient characteristics.
Methods
We examined 55,575 Pap/HPV records from 27,035 women aged 30–65 years from the Johns Hopkins Hospital Pathology Data System between 2006–2013. Cotest uptake and median times to next screening test for cotests and cytology only were calculated. Adjusted hazard ratios were estimated using Cox proportional hazards models, with random effects adjustment for clustering within clinic.
Results
Cotest usage increased from <10% in 2006 to 78% in 2013. The median time to next screening test following normal cytology alone remained constant around 1.5 years. Screening intervals following a dual-negative cotest increased from 1.5 years in 2006/2007 to 2.5 years in 2010, coincident with increases in the proportion of women cotested. Intervals following a dual negative cotest were longer among Medicare patients (3 years) compared with privately insured women (2.5 years), and shorter among black (2 years) compared with white women (2.8 years).
Conclusion
By mid-2013 we observed broad adoption of Pap/HPV cotesting in routine screening in a large academic medical center. Increased screening intervals were observed only among cotested women, while those screened by cytology alone continued to be screened almost annually. The influence of different combinations of race and insurance on screening intervals should be further evaluated to ensure balance of screening risks and benefits in the U.S. population.
Keywords: cervical cancer screening, cytology, HPV cotest, screening guidelines, screening interval, implementation, dissemination
INTRODUCTION
Routine Pap smear screening programs in the United States have reduced rates of cervical cancer by over 50% in the last 30 years due to the high proportion (>80%) of women screened [1, 2]. These programs have proved effective despite the lack of uniformity in cervical cancer screening guidelines issued by professional organizations including the American Cancer Society (ACS), the American College of Obstetricians and Gynecologists (ACOG), the American Society for Colposcopy and Cervical Pathology (ASCCP), and the United States Preventive Services Task Force (USPSTF). Over the past decade and half, these guideline organizations have issued several revisions to their recommendations as scientific knowledge and screening technology advanced, with many emphasizing the incorporation of HPV DNA testing into screening. The guidelines and updates from each of these organizations are summarized in Table 1.
Table 1.
Summary of cervical cancer screening guidelines for women age 30–65 by organization over time
| ACS | ACOG | ASCCP** | USPSTF | |
|---|---|---|---|---|
|
| ||||
| Pre-2003 | Yearly Pap test, but after 3 consecutive normal exams, less frequently at the discretion of the doctor | Yearly Pap test | Annual or Bienniel Pap smears | Pap test at least every 3 years, but no benefit to annual Paps |
| [39] | [40] | [41] | [42] | |
|
| ||||
| 2003–2009 | Age 30+: after 3 normal Paps in a row- can move to every 2–3 years; OR screen every 3 years with Pap/HPV co-test | Age 30+: after 3 normal Paps in a row- can move to every 2–3 years; OR screen every 3 years with Pap/HPV co-test | 2004: Okay to co-test every 3 years | Pap test at least every 3 years, but no benefit to annual Paps, insufficient evidence to recommend co-testing |
| [14, 39] | [15] | [16] | [43] | |
|
| ||||
| 2009–2012 | No change | Age 30+: Pap every 2 years; OR after 3 consecutive negative Paps can be screened once every 3 years | No change | No change |
| [44] | ||||
|
| ||||
| 2012-present | Age 30–65: Pap test every 3 years or Pap/HPV co-test every 5 years* | Age 30–65: Pap test every 3 years or Pap/HPV co-test every 5 years* | Age 30–65: Pap test every 3 years or Pap/HPV co-test every 5 years* | Age 30–65: Pap test every 3 years or Pap/HPV co-test every 5 years |
| [6, 39] | [8] | [6] | [7] | |
Cytology only acceptable, Co-test preferred method
guidelines primarily for abnormal cytology until 2012
Previous evaluations of the adoption of Pap/HPV cotesting (simultaneous screening by both cytology and HPV DNA testing) in primary screening of women 30–65 years reported slow diffusion into routine clinical practice with reports of less than 40% uptake in 2010 [3–5]. In this study, we extend these evaluations in Johns Hopkins Hospital affiliated clinics to 2013, by which time consensus recommendations included HPV cotesting as an acceptable, if not preferred, screening method [6–8]. These evidence-based recommendations aimed to maximize the reduction in cervical cancer incidence while minimizing the harms incurred by over-screening and treatment of regressive disease. However, to achieve this balance, adoption of the guidelines as recommended by the expert panels of reviewers (fidelity) is critical [9]. A core component to the fidelity of cervical cancer screening using both cytology alone and HPV cotesting strategies is adherence to the recommended extended screening interval among women with normal, or negative, screening tests.
Although a few studies have explored the diffusion of HPV cotesting into routine screening, including a study in the Johns Hopkins Hospital system [3, 4, 10, 11], there are limited data evaluating the use of appropriate intervals in different cervical cancer screening strategies. Therefore, in this analysis, we calculate the length of time from a normal baseline screening test until the next screening test (i.e., “the screening interval”) for women aged 30–65 years screened with cytology alone or with Pap/HPV cotesting. We also compare the length of the screening interval by patient age, race, and insurance in order to evaluate factors associated with appropriate application of recommended screening intervals (i.e., adherence) in clinical practice over time.
METHODS
Data source and data collection
This analysis used data from the Johns Hopkins Hospital (JHH) Pathology Data System (PDS), and included all Pap smear and HPV tests processed by the Department of Pathology between January 1, 2001 and May 28, 2013. PDS is an in-house clinical database used by the JHH Department of Pathology to record and store test results. It contains the results from any sample processed by the Department of Pathology, which receives samples from over 200 clinics in and around the Baltimore, Maryland area. Records were obtained through a data use agreement with Johns Hopkins Hospital, and a limited dataset was created to replace medical record numbers with unique patient identifiers. The dataset included a patient identifier, patient age, race, insurance type, date of sample collection, date of test, test result, diagnosis, ordering physician and clinic where the patient was seen. All study procedures were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Pap smear and HPV test results were extracted from the free text diagnosis variable by searching for expressions or strings of words using Stata version 13.1. For this analysis, all Pap smear test results were coded as normal (or no intraepithelial lesion or malignancy, NILM), atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells- cannot rule out high grade lesion (ASC-H), high-grade squamous intraepithelial lesion (HSIL), carcinoma (cancer), or atypical glandular cells (AGUS) based on the most severe diagnosis in the text variable field. HPV testing was performed using the Digene hc2 according to manufacturer’s instructions (Qiagen, Gaithersburg, MD) and results were coded as negative (relative light unit (RLU) <0.85), equivocal (0.85–3.0), or positive (>3.0).
Statistical Analysis
We received all Pap smear and HPV test results in PDS processed between January 1, 2001 and May 28, 2013, which totaled 306,722 records. We then applied several exclusions to the data (Supplemental Figure 1). In brief, for the initial data cleaning step, we eliminated records with male or unknown sex, samples collected prior to January 1, 2001, duplicate records, and records without a Pap smear result. We then restricted our dataset to include only samples collected from clinics likely to be performing routine screening (i.e., excluding specialty locations such as colposcopy and HIV clinics). We performed our analysis only among women of routine screening age who were eligible for HPV cotesting (ages 30–65 years), starting from January 1, 2006, when cotest prevalence reached at least 5% of the total screening tests in this population in order to allow sufficient sample size for comparisons. This resulted in a total of 55,575 records from 27,035 women. For the interval analysis, we restricted data through May 1, 2010 to allow for at least three years of follow-up. This resulted in a final sample size of 31,701 records from 18,048 women for that analysis.
Because of the observed correlation between race and insurance type, we examined race and insurance as a combined variable of mutually exclusive categories. Insurance was characterized by type of payer: private (including HMOs, PPOs, Blue Cross), Medicare, Medicaid, and Tricare (military). Any records without an insurance code or with an insurance code that could not be verified were excluded, as this was a key analytic variable associated with screening-related decisions. To eliminate any HPV testing as a follow-up to a prior abnormal result and thus not part of routine screening, we restricted our analyses to screening tests performed at least 300 days after a prior screen [12]. This restriction necessarily meant that only women with at least two records were included in this analysis. Additionally, because there was no consistent code in PDS to differentiate HPV tests ordered as part of routine cotesting versus triage, we restricted the analysis to normal Pap results, as there would be no other indication for HPV testing other than as a cotest. Thus, cotests were defined as any visit with a normal Pap smear that included an HPV test, which resulted in 3.6% of the records being excluded from this analysis due to an abnormal Pap result.
The proportion of all screening tests performed each year that included a cotest was calculated. Median times to next screening test, along with corresponding 95% confidence intervals, were calculated and graphed by cytology alone vs. cotest, year of screening, age, race, and insurance. Hazard ratios and 95% confidence intervals for time to next screening test for cytology alone and cotesting were estimated using Cox proportional hazards models. To account for the clustering of observations within clinics, these models were also run with the addition of a random effect term for clinic. All analyses were performed in Stata 13.1 (College Station, TX).
RESULTS
Population Characteristics
After exclusions, the dataset for this analysis included 55,575 records from 27,035 women who were eligible for HPV cotesting (30 to 65 years of age) and screened for cervical cancer between 2006 and mid-2013 (Supplemental Figure 1). These women had a median age of 47 years (interquartile range (IQR): 38–55). Overall, this group was 51% white, 36% black, and 13% other races. Seventy percent had private insurance, 8% had Medicare, 13% Medicaid, and 10% had Tricare (military) insurance. A similar distribution of these characteristics was also seen among the population in the interval analysis.
Uptake of Pap/HPV cotesting instead of cytology alone
Following the 2003 approval of HPV cotesting for use in routine screening by the US Food and Drug Administration, initial uptake of cotesting in the Johns Hopkins System remained below 5% through 2005. Over the following 6–7 years, cotesting increased almost 9-fold, from 8.9% in 2006 to 78.4% in mid-2013 (Table 2). To better understand the patterns of adoption of cotesting over time, we stratified our data into the following 3 time periods: (1) 2006–2008 when cotesting was first incorporated into guidelines and cotest use was 5–25%; (2) 2009–2011 when cotest use was 25–50%; and (3) 2012–2013 when cotest rates were >50%. The overall average percent of cotested samples during each of these 3 time periods were 12.6%, 42.2%, and 61.3%, respectively.
Table 2.
Proportion of cervical cancer screening tests that included a Pap/HPV cotest by year
| Year | Total N | % Co-tested | |
|---|---|---|---|
|
|
|||
| 2006 | 6059 | 8.85 |

|
| 2007 | 6772 | 11.68 | |
| 2008 | 7798 | 16.34 | |
| 2009 | 8594 | 33.33 |

|
| 2010 | 8412 | 46.21 | |
| 2011 | 8222 | 47.32 | |
| 2012 | 7864 | 57.01 |
|
| 2013* | 1954 | 78.35 | |
[2001–2005 co-testing <3% per year]
2013 includes visits through May 28, 2013
Temporal changes in screening intervals
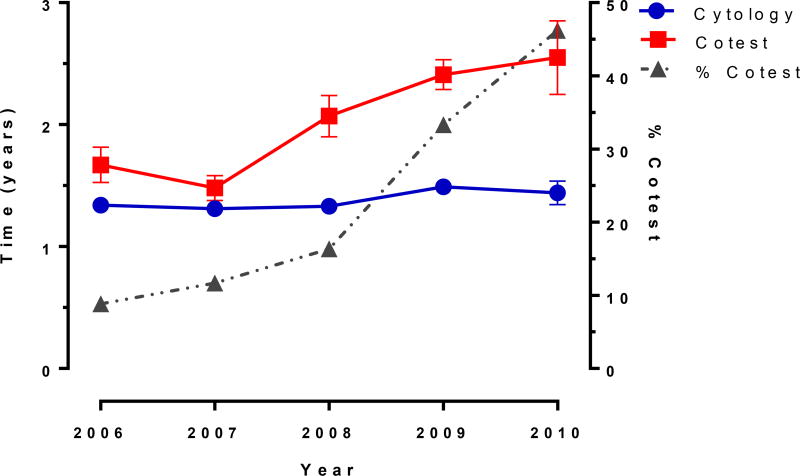
Screening intervals in women screened by cytology alone were relatively constant for women who screened negative from 2006–2010, with the median time to next screening test ranging from 1.3 years (95% CI: 1.3–1.4) to 1.5 years (95% CI: 1.3–1.5) (Figure 1). However, among women screened by cotest, the interval between screening tests increased significantly over time from 1.7 years (95% CI 1.5–1.8) to 2.5 years (95% CI: 2.2–2.9), concomitant with the temporal increases in overall cotest usage (Figure 1).
Figure 1. Median time to next screening test.
Vertical bars at each year represent 95% confidence intervals
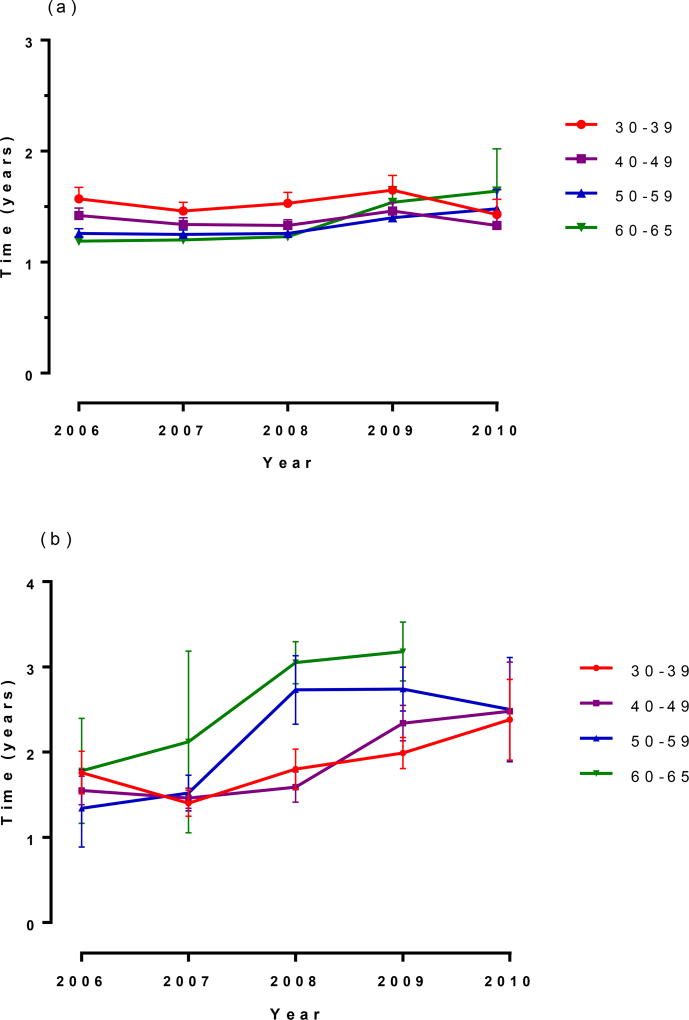
Age-specific screening intervals
Overall, screening intervals differed by no more than 3 months among the different age groups receiving cytology only screening over the study period (Figure 2a). In contrast, median screening intervals increased in all age groups receiving cotests between 2006 and 2010 (Figure 2b). Screening intervals among women aged 30–59 plateaued in 2010 at 2.5 years, with a longer interval plateau at 3.2 years observed in the 60–65 year old women. This increase in screening interval length among cotested women was more rapidly adopted among women aged 50–65 years compared with women aged 30–49 years.
Figure 2. Age-specific screening intervals (a) cytology alone, (b) cotest.
No median for women 60–65 years in 2010 because <50% had returned for their next visit as of that date
Vertical bars at each year represent 95% confidence intervals
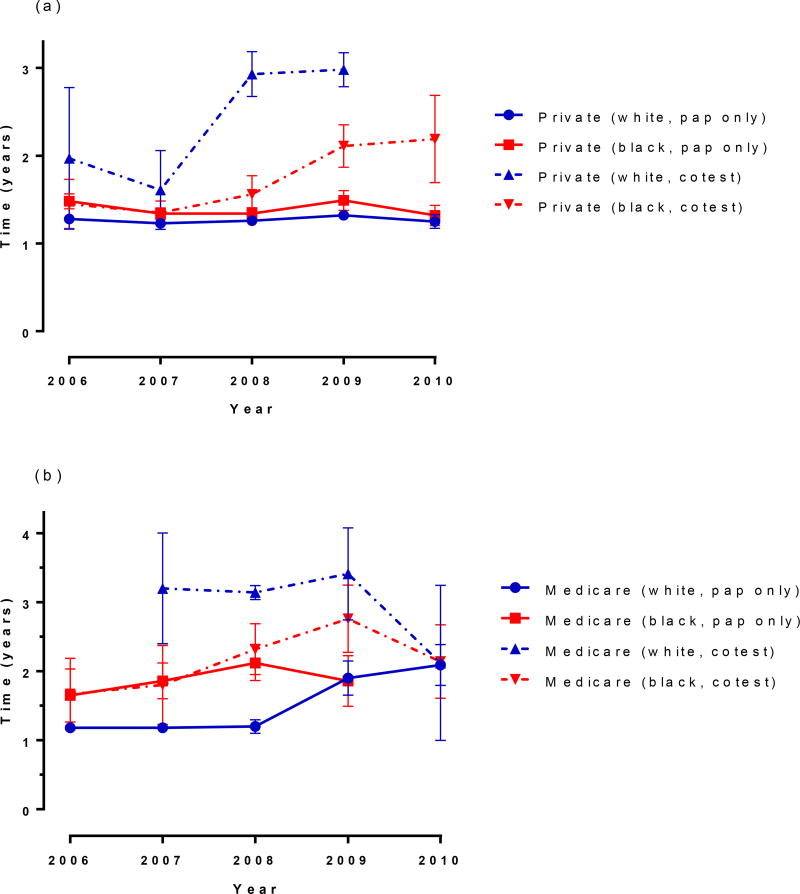
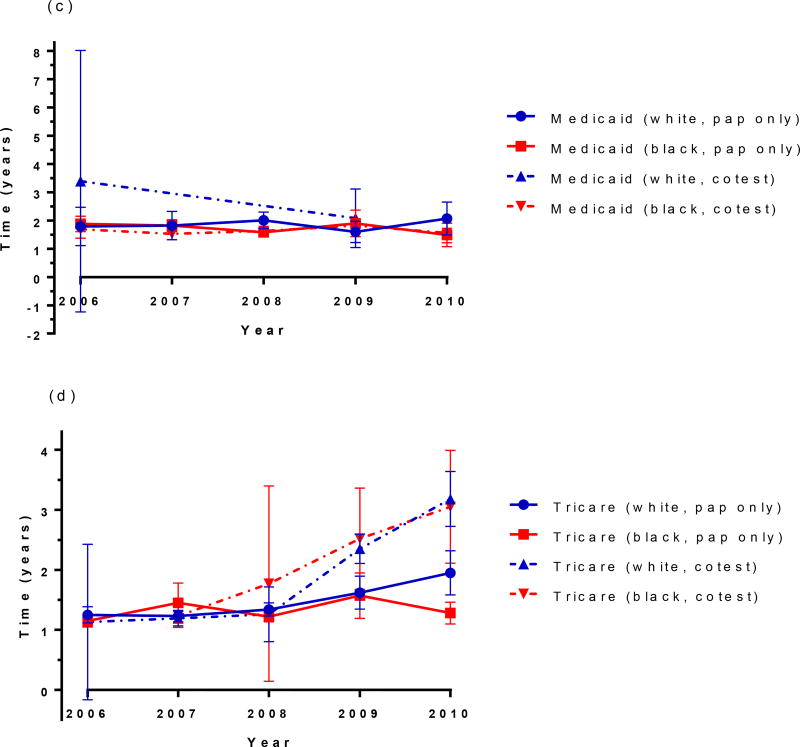
Race and insurance-specific screening intervals
When median time to next screen is compared by race within each insurance type, women of both races with private insurance and cytology only screening have almost identical median screening intervals. However, racial differences were observed over time in women with private insurance receiving cotesting. Black women had a shorter screening interval compared to white women, and this difference increased over time (Figure 3a). Among Medicare-insured women with cytology only testing, black women had a longer screening interval compared to white women until 2009, at which time the interval lengths were similar due to a trend of increasing interval length over time among white women. (Figure 3b). There were insufficient data to estimate median intervals for white women being cotested with Medicaid insurance, but for all other groups, the median screening interval fluctuated between 1.5 and 2 years with no notable trend over time for either cytology or cotesting (Figure 3c). In contrast, trends in the median screening interval among Tricare-insured women differed by screening method, but not by race. Specifically, intervals increased from 1.2 years to 3.1 years between 2006 and 2010 with cotesting, but only increased slightly from 1.2 years to 1.3 years over the same time period for cytology only, until 2010, when cytology intervals increased to 2.0 years for white women only (Figure 3d).
Figure 3. Race and insurance-specific screening intervals. (a) private insurance, (b) Medicare, (c) Medicaid, (d) Tricare.
For (a)- not enough data to estimate private white cotest in 2010; (b) not enough data to estimate Medicare white cotest in 2006 (c), not enough data to estimate Medicaid white cotest in 2010; Vertical bars at each year represent 95% confidence intervals
DISCUSSION
Cervical cancer screening recommendations evolved over the last fifteen years in parallel with increasing knowledge and technology. First, there was the addition of ViraPap to conventional cytology, then the transition to liquid-based cytology, and more recently the incorporation of HPV DNA testing, as either a reflex or cotest [13–16]. While screening guidelines are an important driver of clinical practice, they are not a mandate. Despite the strong evidence base that supports the consensus screening guidelines [3, 4], providers often desire supplementary “real-world” evidence of the harms and benefits of alternative screening strategies before deciding to change a long-standing practice [17, 18]. To meet this demand, it is critical to conduct surveillance studies to describe the adoption and impact of screening practice changes at a population level (e.g., within large health systems).
As such, we have been tracking the uptake of cotesting within the Johns Hopkins Hospital Pathology Data System since 2001. Our prior analysis showed a traditional pattern of diffusion as described by Rogers [19] with innovators and early adopters starting to use HPV tests in the first few years following FDA approval. Consistent with the typical sigmoidal curve describing diffusion of innovations theory [19], we observed a rapid increase in cotesting in women 30 years and older beginning in 2009, with a reasonably constant increase in adoption through 2012. A second wave of cotest increase beginning in 2012 to a mid-year height of 78% in 2013, indicates diffusion to the late adopters [4]
Adoption of cotesting is only one of the criteria for evaluation of the successful implementation of cotesting in real world practice; the second element of implementation fidelity [9] is the length of time between a negative cotest and the next screen. Recommendations for longer screening intervals are based on the higher negative predictive value following a negative cotest versus a single negative cytology test result, enabling a safe extension of screening intervals [20–22]. Recognizing that both cytology alone and cotesting balance benefits and harms of screening only when applied at the recommended extended screening interval, we have expanded our adoption surveillance to estimation of time to next screen following a normal cotest. We found no change in screening frequency among women screened by cytology alone, but an increase among most women screened by cotesting.
During our observation period, most guidelines recommended that women 30 years or older with a normal screening test be re-screened in 2–3 years if screened by cytology alone, or in 3 years if screened by cotesting. Under those guidelines, we found screening intervals approached the recommended length for cotesting, but remained shorter than recommended for cytology by 2010. The shorter intervals following cytology mirror those described by Weinmann et al. across four different US healthcare systems through 2007 [11]. In 2012, guidelines further increased screening intervals for co-testing from 3- to 5-years, and including explicit recommendations against annual screening in any circumstance. Continued surveillance of this health system will be necessary to examine the rate and determinants of adoption of these most recent, and future, guideline changes.
Effective translation of evidenced-based interventions into clinical practice involves complex interactions between multiple layers of health services delivery [23]. While the large sample size of pathology records afforded by evaluation of co-testing practices at a health systems level has advantages, the data by nature are limited in scope. This does not afford the opportunity to examine individual provider and patient level factors associated with screening practice (e.g., provider specialty, provider age, patient request, etc.); however, our analysis provides a framework for the design of future studies to more fully explore the population-level variability in HPV co-testing.
Though our descriptive analysis of practice within a large health system is unable to determine the cause of the differences in adherence to recommended screening intervals between cytology and cotesting, we highlight important themes that could be considered in future evaluations. First, adoption of new extended screening intervals was higher among women screened by cotesting compared with cytology alone. Stakeholders willing to adopt new technology in screening, such as HPV testing, may also be more willing to implement a practice change (i.e., longer screening intervals) compared with those using a longstanding standard of care. Second, Medicare-insured women had significantly longer screening intervals compared with other insurers, suggesting different levels or rates of adoption of comprehensive practice change at the payer-level. For example, providers may continue to use cytology alone due to reimbursement issues, but may remain concerned about false-negative Pap results and feel more comfortable with more frequent testing. Both observations point to an element of general stakeholder (provider/patient/health system) readiness or capacity to change that could be evaluated in future studies.
Furthermore, differences in reaching the recommended 3-year cotesting interval length by age, race, and insurance after mutual adjustment and accounting for clinic-level differences suggest that the fidelity of evidence-based screening interventions is not uniform across populations. There has been ample discussion in the literature regarding the disparities in cervical cancer by race in the United States, showing later stage of diagnosis and higher incidence and mortality rates for black women compared to white women [24, 25] [26, 27]. Correction for these disparities should not come at a cost of increased harms of screening. Identifying the reasons for the significantly shorter screening intervals observed among privately-insured dual negative cotested black women is a clear priority to ensure optimal and equitable cervical cancer prevention across the entire US population.
A primary strength of this study was the utilization of ‘real world’ clinical practice data from a large academic cytology laboratory. However, with this clinical dataset, our analysis was limited to visits when patients were seen in an affiliated clinic and had a Pap smear and/or HPV test performed and tested at JHH. As a result, we lack a true population denominator, and do not know the extent of in and out migration from the cohort, which may influence the validity of our inference. However, a recent evaluation of time to next screening test following a normal cytology or dual negative cotest from a population-based screening registry in New Mexico reported similar results, with a median time to follow-up of 1.5–2.0 years for cytology alone and >3.0 years for cotesting [12] over the 2007–2012 time period. We also lack information on long-term screening history, and so cannot rule out that some of the differences seen are due to true risk differences where different follow-up algorithms may have been appropriate. This is especially relevant when interpreting the data from the women screened by cytology alone, as the recommendations during this time period included annual screening until three normal cytology results were observed. We attempted to limit this impact by restricting our analysis to women with at least one prior normal test result. However, we would expect this to result in shorter screening intervals among the youngest women with the highest risk of cytological abnormality [28, 29], yet in our population screening intervals were in general longer for the youngest age groups (30–39 year olds).
Importantly, this study utilized clinical practice data from a large medical system that includes a diversity of clinics and patients and results were relatively similar to a state-wide registry in New Mexico, suggesting our results may be generalizable to a larger population. An important strength of our study, however, was that we were able to provide direct evidence about how screening is occurring in a real-world setting, rather than collecting self-report or vignette data which only reflect practice intentions not actual practice [4, 30–36].
In summary, our results demonstrate that in this medical system, HPV/cytology cotesting among 30–65 year old women follows a typical diffusion of innovations sigmoidal cumulative adoption over time, with clearly defined early and late adopters of the evidence-based practice. Studies to better understand the patient, provider, and payer-level differences driving the behavior between early and late adopters may be instrumental in accelerating adoption of future changes in evidence-based cervical cancer screening practices. For example, continued updates of this and similar studies will be important to determine whether the ultimate consensus recommendations across multiple professional organizations reached in 2012 [6–8] will influence provider behavior by reducing uncertainty in best practice. In addition, in 2014 the FDA approved an algorithm of HPV testing with HPV16/18 genotyping using the Roche cobas HPV test for primary screening of women aged 25–65 years [37]. Some professional organizations have already issued interim guidance for this strategy [38], and surveillance of the adoption of this screening practice will be critical to generate a solid population-based evidence base for guideline updates, particularly as population-uptake of the HPV vaccine increases. Given the call for more evaluation of ‘real-world’ data from clinical practice in the United States [18], the present analysis and future evaluations are critical to ensure broad stakeholder adoption of recommended screening guidelines.
Supplementary Material
Acknowledgments
The authors wish to thank Amy Tatsas, John Boitnott and the Johns Hopkins Hospital Department of Pathology. They also thank Nicole A. Patterson for assistance with the graphics.
Funding: This work was supported by the Agency for Healthcare Research and Quality (R36 HS022199 to MIS), the National Cancer Institute (Specialized Program of Research Excellence in Cervical Cancer- 2P50-CA098252-11 to AFR and R01 CA123467 to PEG). This work was also supported by the Maryland Cigarette Restitution Fund Research Grant to the Johns Hopkins Medical Institutions (FY15 to AFR) and the Johns Hopkins Individualized Health Initiative (Hopkins inHealth to DFP).
Footnotes
Author Contributions:
Conceptualization: M Silver, P Gravitt
Methodology: M Silver, A Rositch, D Phelan-Emrick, P Gravitt
Software: M Silver
Validation: M Silver, P Gravitt
Formal Analysis: M Silver
Investigation: M Silver
Resources: M Silver, D Phelan-Emrick, P Gravitt
Data Curation: M Silver
Writing- original draft: M Silver
Writing- review & editing: M Silver, A Rositch, D Phelan-Emrick, P Gravitt
Visualization: M Silver, P Gravitt
Supervision: P Gravitt
Project Administrator: M Silver
Funding Acquisition: M Silver, P Gravitt
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Howlader NNA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2011, National Cancer Institute. Bethesda, MD: 2014. http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014. [Google Scholar]
- 2.Services DoHaH, editor. CDC. Behavioral Risk Factor Surveillane System Survey Data. Atlanta, Georgia: Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 3.Phelan DF, Boitnott J, Clark DP, Dubay L, Gravitt P. Trends of Human Papillomavirus Testing in Cervical Cancer Screening at a Large Academic Cytology Laboratory. Obstetrics & Gynecology. 2011;118(2):289–295. doi: 10.1097/AOG.0b013e3182253c33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatsas AD, Phelan DF, Gravitt PE, Boitnott JK, Clark DP. Practice Patterns in Cervical Cancer Screening and Human Papillomavirus Testing. American Journal of Clinical Pathology. 2012;138(2):223–229. doi: 10.1309/AJCPPVX91HQMNYZZ. [DOI] [PubMed] [Google Scholar]
- 5.Bekker JB, John TS, Leiman G. Confirming Suboptimal Adherence to HPV Cotesting Guidelines in an Academic Center in Vermont. American Journal of Clinical Pathology. 2013;139(2):259–260. doi: 10.1309/AJCP41LLYKYUGXVD. [DOI] [PubMed] [Google Scholar]
- 6.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FAR, Moriarty AT, Waxman AG, Wilbur DC, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the Prevention and Early Detection of Cervical Cancer. American Journal of Clinical Pathology. 2012;137(4):516–542. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 7.Moyer VA. Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine. 2012;156(12):880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 8.ACOG. Practice Bulletin No. 131: Screening for Cervical Cancer. Obstetrics & Gynecology. 2012;120(5):1222–1238. doi: 10.1097/aog.0b013e318277c92a. http://1210.1097/AOG.1220b1013e318277c318292a. [DOI] [PubMed] [Google Scholar]
- 9.Proctor E, Brownson R. Chapter 13: Measurement issues in dissemination and implementation research. In: Brownson R, Colditz G, Proctor E, editors. Dissemination and Implementation Research in Health: Translating Science to Practice. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 10.Zhao C, Li Z, Nayar R, Levi AW, Winkler BA, Moriarty AT, Barkan GA, Rao J, Miller F, Fan F, et al. Prior High-Risk Human Papillomavirus Testing and Papanicolaou Test Results of 70 Invasive Cervical Carcinomas Diagnosed in 2012: Results of a Retrospective Multicenter Study. Archives of Pathology & Laboratory Medicine. 2014 doi: 10.5858/arpa.2014-0028-OA. [DOI] [PubMed] [Google Scholar]
- 11.Weinmann S, Williams AE, Kamineni A, Buist DSM, Masterson EE, Stout NK, Stark A, Ross TR, Owens CL, Field TS, et al. Cervical cancer screening and follow-up in 4 geographically diverse US health care systems, 1998 through 2007. Cancer. 2015 doi: 10.1002/cncr.29445. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuzick J, Myers O, Hunt WC, Saslow D, Castle PE, Kinney W, Waxman A, Robertson M, Wheeler CM on behalf of the New Mexico HPVPRSC. Human papillomavirus testing 2007–2012: Co-testing and triage utilization and impact on subsequent clinical management. International Journal of Cancer. 2014 doi: 10.1002/ijc.29337. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogarth S, Hopkins MM, Rodriguez V. A molecular monopoly? HPV testing, the Pap smear and the molecularisation of cervical cancer screening in the USA. Sociology of Health & Illness. 2012;34(2):234–250. doi: 10.1111/j.1467-9566.2011.01411.x. [DOI] [PubMed] [Google Scholar]
- 14.Saslow D, Runowitz C, Solomon D, Moscicki A-B, Smith R, Eyre H, Cohen C. American Cancer Society Guideline for the Early Detection of Cervical Neoplasia and Cancer. CA Cancer J Clin. 2002;52(6):342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 15.ACOG. ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists. Number 45, August 2003. Cervical cytology screening (replaces committee opinion 152, March 1995) Obstet Gynecol. 2003;102(2):417–427. doi: 10.1016/s0029-7844(03)00745-2. [DOI] [PubMed] [Google Scholar]
- 16.Wright TCJ, Schiffman M, Solomon D, Cox JT, Garcia F, Goldie S, Hatch K, Noller KL, Roach N, Runowicz C, et al. Interim Guidance for the Use of Human Papillomavirus DNA Testing as an Adjunct to Cervical Cytology for Screening. Obstetrics & Gynecology. 2004;103(2):304–309. doi: 10.1097/01.AOG.0000109426.82624.f8. 310.1097/1001.AOG.0000109426.0000182624.f0000109428. [DOI] [PubMed] [Google Scholar]
- 17.Smith-McCune K. Choosing a screening method for cervical cancer: Papanicolaou testing alone or with human papillomavirus testing. JAMA Internal Medicine. 2014 doi: 10.1001/jamainternmed.2014.1368. [DOI] [PubMed] [Google Scholar]
- 18.Feldman S. CAn the new cervical cancer screening and management guidelines be simplified? JAMA Internal Medicine. 2014;174(7):1029–1030. doi: 10.1001/jamainternmed.2014.576. [DOI] [PubMed] [Google Scholar]
- 19.Rogers E. Diffusion of Innovataions. 5. New York: The Free Press; 2003. [Google Scholar]
- 20.Gage JC, Schiffman M, Katki HA, Castle PE, Fetterman B, Wentzensen N, Poitras NE, Lorey T, Cheung LC, Kinney WK. Reassurance Against Future Risk of Precancer and Cancer Conferred by a Negative Human Papillomavirus Test. Journal of the National Cancer Institute. 2014;106(8) doi: 10.1093/jnci/dju153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katki HA, Kinney WK, Fetterman B, Lorey T, Poitras NE, Cheung L, Demuth F, Schiffman M, Wacholder S, Castle PE. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. The Lancet Oncology. 2011;12(7):663–672. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dillner J, Rebolj M, Birembaut P, Petry K-U, Szarewski A, Munk C, de Sanjose S, Naucler P, Lloveras B, Kjaer S, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337 doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damschroder L, Aron D, Keith R, Kirsh S, Alexander J, Lowery J. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation Science. 2009;4(1):50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheppard CS, El-Zein M, Ramanakumar AV, Ferenczy A, Franco EL. Assessment of mediators of racial disparities in cervical cancer survival in the United States. International Journal of Cancer. 2016;138(11):2622–2630. doi: 10.1002/ijc.29996. [DOI] [PubMed] [Google Scholar]
- 25.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: A report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecologic Oncology. 2014;133(2):353–361. doi: 10.1016/j.ygyno.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017 doi: 10.1002/cncr.30507. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 27.Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120(13):2032–2038. doi: 10.1002/cncr.28548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuzick J, Myers O, Hunt WC, Robertson M, Joste NE, Castle PE, Benard VB, Wheeler CM. A Population-Based Evaluation of Cervical Screening in the United States: 2008–2011. Cancer Epidemiology Biomarkers & Prevention. 2014;23(5):765–773. doi: 10.1158/1055-9965.EPI-13-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gage JC, Katki HA, Schiffman M, Fetterman B, Poitras NE, Lorey T, Cheung LC, Castle PE, Kinney WK. Age-stratified 5-year risks of cervical precancer among women with enrollment and newly detected HPV infection. International Journal of Cancer. 2015;136(7):1665–1671. doi: 10.1002/ijc.29143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roland KB, Soman A, Benard VB, Saraiya M. Human papillomavirus and Papanicolaou tests screening interval recommendations in the United States. American Journal of Obstetrics and Gynecology. 2011;205(5):447.e441–447.e448. doi: 10.1016/j.ajog.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Saraiya M, Berkowitz Z, Yabroff KR, Wideroff L, Kobrin S, Benard V. Cervical Cancer Screening With Both Human Papillomavirus and Papanicolaou Testing vs Papanicolaou Testing Alone: What Screening Intervals Are Physicians Recommending? Arch Intern Med. 2010;170(11):977–986. doi: 10.1001/archinternmed.2010.134. [DOI] [PubMed] [Google Scholar]
- 32.Yabroff KR, Saraiya M, Meissner HI, Haggstrom DA, Wideroff L, Yuan G, Berkowitz Z, Davis WW, Benard VB, Coughlin SS. Specialty Differences in Primary Care Physician Reports of Papanicolaou Test Screening Practices: A National Survey, 2006 to 2007. Annals of Internal Medicine. 2009;151(9):602–611. doi: 10.7326/0003-4819-151-9-200911030-00005. [DOI] [PubMed] [Google Scholar]
- 33.Cooper CP, Saraiya M, Mclean TA, Hannan J, Liesmann JM, Rose SW, Lawson HW. Report from the CDC. Pap Test Intervals Used by Physicians Serving Low-Income Women through the National Breast and Cervical Cancer Early Detection Program. J Womens Health. 2005;14(8):670–678. doi: 10.1089/jwh.2005.14.670. [DOI] [PubMed] [Google Scholar]
- 34.Berkowitz Z, Saraiya M, Benard V, Yabroff KR. Common Abnormal Results of Pap and Human Papillomavirus Cotesting: What Physicians Are Recommending for Management. Obstetrics & Gynecology. 2010;116(6):1332–1340. doi: 10.1097/AOG.0b013e3181fae4ca. 1310.1097/AOG.1330b1013e3181fae1334ca. [DOI] [PubMed] [Google Scholar]
- 35.Meissner HI, Tiro JA, Yabroff KR, Haggstrom DA, Coughlin SS. Too Much of a Good Thing? Physician Practices and Patient Willingness for Less Frequent Pap Test Screening Intervals. Medical Care. 2010;48(3):249–259. doi: 10.1097/MLR.0b013e3181ca4015. [DOI] [PubMed] [Google Scholar]
- 36.Lee JW-Y, Berkowitz Z, Saraiya M. Low-Risk Human Papillomavirus Testing and Other Nonrecommended Human Papillomavirus Testing Practices Among U.S. Health Care Providers. Obstetrics & Gynecology. 2011;118(1):4–13. doi: 10.1097/AOG.0b013e3182210034. [DOI] [PubMed] [Google Scholar]
- 37.FDA approves first human papillomavirus test for primary cervical cancer screening. [ http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm]
- 38.Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FAR, Kinney WK, Massad LS, Mayeaux EJ, Saslow D, et al. Use of Primary High-Risk Human Papillomavirus Testing for Cervical Cancer Screening: Interim Clinical Guidance. Obstetrics & Gynecology. 2015 doi: 10.1097/AOG.0000000000000669. Publish Ahead of Print: 10.1097/AOG.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 39.Chronological History of ACS Recommendations for the Early Detection of Cancer in People Without Cancer Symptoms. [ http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/chronological-history-of-acs-recommendations]
- 40.ACOG. ACOG practice bulletin. Diagnosis and treatment of cervical carcinomas. Number 35, May 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;78(1):79–91. doi: 10.1016/s0020-7292(02)90092-5. [DOI] [PubMed] [Google Scholar]
- 41.Davey DD, Austin RM, Birdsong G, Buck HW, Cox JT, Darragh TM, Elgert PA, Hanson V, Henry MR, Waldman J. ASCCP Patient Management Guidelines: Pap Test Specimen Adequacy and Quality Indicators. American Journal of Clinical Pathology. 2002;118(5):714–718. doi: 10.1309/6GBF-EGH8-WXDE-ANGX. [DOI] [PubMed] [Google Scholar]
- 42.USPSTF. Guide to Clinical Preventive Services: Report of the U.S. Preventive Services Task Force. 2. Baltimore, MD: WIlliams & WIlkins; 1996. [Google Scholar]
- 43.USPSTF. U.S. Preventive Services Task Force: Recommendations and Rationale-Screening for Cervical Cancer: Recommendations and Rationale. Am Fam Physician. 2003;67(8):1759–1766. [PubMed] [Google Scholar]
- 44.ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol. 2009;114(6):1409–1420. doi: 10.1097/AOG.0b013e3181c6f8a4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.