Abstract
Objective
Although both patients with schizophrenia and their caregivers report elevated levels of depression, anxiety, and stress (DASS), affective symptoms in patients and family members seldom constitute a primary treatment focus. The present study tested whether a culturally informed family therapy for schizophrenia (CIT-S) outperformed standard family psychoeducation (PSY-ED) not only in decreasing patient schizophrenia symptoms, but also in decreasing individual DASS. Because CIT-S fostered family cohesion throughout treatment, we predicted that increases in family cohesion would mediate treatment effects.
Method
Participants included 266 patients and family members nested within 115 families, randomized to the CIT-S or PSY-ED conditions. We specified a series of multilevel latent growth and latent change models to examine direct effects of CIT-S on patient schizophrenia symptoms, individual DASS, and family cohesion over time. Next, we employed parallel process growth models to test the indirect effect of CIT-S on decreasing patient and caregiver psychopathology over time via changes in family cohesion.
Results
The CIT-S treatment significantly reduced patient schizophrenia symptoms from baseline to follow-up, γ = −1.72, 95% CI [−2.83, −0.60], as well as individual DASS, γ = −4.39, [−6.44, −2.34] from baseline to termination. In line with treatment goals, CIT-S increased family cohesion from baseline to midpoint, γ = 0.93, [0.06, 1.80]. The CIT-S-related change in cohesion mediated changes in DASS, γ = −0.87, [−1.47, −0.27], but not patient symptoms.
Conclusions
By integrating the family’s cultural context into treatment, clinicians may foster family dynamics that enhance treatment outcomes and promote broad improvements in mental health.
Keywords: schizophrenia, latent growth modeling, multilevel mediation, family therapy, psychosis
Schizophrenia is a chronic and disabling psychiatric disorder that affects roughly one percent of the population (Minzenberg & Carter, 2012). The illness engenders pronounced distress in both patients and family members: Only 14 percent of patients with schizophrenia achieve sustained recovery within the first five years of a psychotic episode (Insel, 2010), and only 10 to 20 percent of patients are employed (Marwaha & Johnson, 2004). Consequently, family members often live with patients and assume the caregiving responsibility (Pitschel-Walz, Leucht, Bäuml, Kissling, & Engel, 2001). Because family members tend to spend significant amounts of time with patients in the caregiving role, transactional family relationships can constitute significant stressors for both patients and caregivers. Accordingly, family therapy is a natural candidate for psychosocial interventions targeting schizophrenia, as it addresses the familial stressors that affect both patient symptom trajectory and caregiver burden.
By targeting maladaptive communication patterns and expressed emotion (EE; Hooley, 2007), family therapy improves patient outcomes across symptom clusters and beyond the effects of antipsychotics alone (Falloon, Boyd, & McGill, 1984). Although positive symptoms of schizophrenia (e.g., hallucinations and delusions) cause acute deterioration in functioning, negative symptoms (e.g., avolition and anhedonia) are linked to long-term functional impairment (APA, 2013). Notably, comorbid anxiety disorders affect approximately a quarter of patients with schizophrenia, and up to half of patients with schizophrenia suffer from concurrent depression (Buckley, Miller, Lehrer, & Castle, 2009; Tsai & Rosenheck, 2013). Comorbid depression, in particular, relates to poorer quality of life and poorer clinical outcomes (Buckley et al., 2009). Consequently, along with addressing primary psychotic symptoms, therapies that target and monitor depression and anxiety symptoms may improve patient well-being more globally. Family therapy, for instance, produces improvements in negative and disorganized symptoms by enhancing non-hostile communication between patients and family members. Improvements in communication, in turn, help patients and family members to gain coping mechanisms, reduce stress, and compensate for social deficits that influence patients’ appraisal and response biases (Elis, Caponigro, & Kring, 2013).
Family therapy models connect improvements in family functioning and caregiver well-being with a decreased risk of patient relapse, with the idea that reducing blaming attributions and caregiver burden can improve patient outcomes (Koutra, Simos, Triliva, Lionis, & Vgontzas, 2016). By enriching family members’ understanding of their relative’s illness, family psychoeducation also improves illness management and treatment coordination between the family and treatment team. However, studies of family therapy for schizophrenia have conceptualized caregiver mood and anxiety symptoms almost exclusively as a vehicle for improving patient symptoms. Ethically, there is a need to consider caregiver mood and anxiety as outcomes of interest in their own right, independent of patient functioning. Caring for a relative with schizophrenia is a lifelong process associated with significant burden and distress (Madianos, Economou, Dafni, Koukia, Palli, & Rogakou, 2004; Suro and Weisman de Mamani, 2013). In a study on Mexican American caregivers of family members with schizophrenia, for example, 40 percent of the sample displayed clinically significant symptoms of depression (Magaña, Ramírez García, Hernández, & Cortez, 2007). Beyond reducing caregiver burden and patient symptom severity, family therapy has the potential to target caregiver mental health by encouraging familial unity, developing team-focused problem solving, and promoting participation in shared activities as a family.
Research on family caregivers in collectivistic cultures has illuminated the role of positive family factors in the maintenance of and risk for psychotic disorders, as well as the burden of care. For example, studies have linked family warmth, collectivism, and unity to lower patient symptoms (López et al., 2004), as well as lower levels of reported caregiver burden (Weisman, Rosales, Kymalainen, & Arnesto, 2005). For individuals who endorse high levels of family interdependence, caring for a relative may be a more normative or expected process, which is in line with empirical evidence of a relationship between exhibiting collectivistic attitudes and feeling a sense of obligation to care for an ill relative (Freeberg & Stein, 1996). Promoting positive factors such as familial warmth and cohesion may thus enhance treatment efficacy for both patients and caregivers (Bertrando et al., 1992; Gurak & Weisman de Mamani, 2016; Weisman et al., 2005).
Weisman de Mamani and colleagues (2005) drew from research on associations between cohesion, patient symptoms, and caregiver outcomes to develop a culturally-informed family therapy for schizophrenia (CIT-S; Weisman de Mamani et al., 2014). Grounding the culturally adapted components of CIT-S in theory on culture, expressed emotion, and psychiatric symptoms, the researchers proposed targeting family unity and cohesion to improve treatment outcomes. CIT-S is a 15-week treatment consisting of five modules, each lasting three sessions. The first module of CIT-S (Sessions 1 through 3), Family Collectivism, engages family members in dialogue about the family unit. Family members discuss individual contributions to the family, focusing on strengths of each player on the family team (Weisman de Mamani et al., 2014). The remaining four modules of CIT-S foster adaptive beliefs regarding the patient’s illness, improve communication, and promote unified problem solving. During the Psychoeducation module (Sessions 4 through 6), clinicians note the significant impact that critical comments and emotional over-involvement can have on patient outcomes, again underscoring the importance of working as a family team to treat the illness. In the Spiritual Coping module (Sessions 7 through 9), therapists draw from the family’s existing religious or spiritual beliefs to promote adaptive spiritual coping, encouraging family members to consider spiritual practices they can perform together, such as going to a service or praying. Family cohesion similarly serves as the foundation for the Communication Training module (Sessions 10 through 12), which helps the family to interact in a supportive manner. Family members foster relationships and confront problems in a style that reduces blaming attributions and EE. The final module, Problem Solving (Sessions 13 through 15), foments family members’ self-conceptions as part of a team working toward a common goal in order to address family issues.
Though informed by literature on ethnic differences in family functioning and predictors of relapse (Weisman et al., 2005), CIT-S has been demonstrated equally effective in reducing patient symptoms for Caucasian and ethnic minority families (Weisman de Mamani et al., 2014). In prior studies using the same data set as we use in the current study, CIT-S was found to reduce schizophrenia symptoms (Weisman de Mamani et al., 2014), as well as caregiver burden (Weisman de Mamani & Suro, 2016), beyond the effects of a standard three session family psychoeducation (PSY-ED) intervention. To date, however, no study has considered the efficacy of CIT-S on patient and caregiver mood or anxiety symptoms. Furthermore, despite the fact that the authors developed CIT-S with a focus on the empirically based, intermediate treatment target of family cohesion, mechanisms of the CIT-S treatment effects reported in prior studies remain elusive. In order to determine whether the cultural modifications were key ingredients of change, we propose testing whether changes in the theoretical contextual variables (i.e., family cohesion) explain the reduction in symptom severity over time observed with CIT-S.
When testing theory-based causal mediation processes, there is a need to employ statistical methods that allow for the analysis of dynamic change processes (MacKinnon & Dwyer, 1993). Traditional pre-post analysis provides limited information regarding the relationships between mechanistic change processes and changes in outcomes of interest (Khoo, 2001). Fortunately, several statistical frameworks permit the flexibility of modeling slopes as outcomes within a multivariate, multilevel, longitudinal framework. In line with prior research on longitudinal mediation in clinical trials (e.g., Cheong, MacKinnon, & Khoo, 2003), we employ multilevel parallel process growth modeling to analyze changes in family cohesion, our theory-based mediating variable, on slopes of outcome variables. Beyond allowing the researcher to model the relationships between simultaneous change processes over time, multilevel latent growth models account for dependency in data due to nesting of patients within families. Ignoring dependency due to nesting can bias parameter estimates, standard errors, and degrees of freedom (Bauer, Gottfredson, Dean, & Zucker, 2013; Kenny & Judd, 1986; Tasca, Illing, Joyce, & Ogrodniczuk, 2009). Moreover, multivariate growth models allow for the examination of complex residual variance components that can be key to understanding stability and change (Curran et al., 2012). Unlike a pre-post analysis, a growth model provides flexibility in modeling heterogeneity in growth at the individual and/or family level. By estimating random intercepts and slopes, multilevel growth models capture the heterogeneity in psychotic and mood symptoms due to individual and family factors, which may relate to differential treatment response (Curran, Obeidat, & Losardo, 2010). Because parallel processes can be modeled with measurements occurring at different time points, growth models help establish the temporal precedence that provides greater confidence in a mediation effect (Cheong et al., 2003). Finally, latent growth models offer flexibility with missing data, which is often high due to elevated rates of treatment dropout in schizophrenia (Villeneuve, Potvin, Lesage, & Nicole, 2010).
Thus, in the present study, multilevel latent growth and latent change models were employed to assess whether CIT-S decreased patient schizophrenia symptoms, reduced patient and caregiver DASS, and increased family cohesion over time. By assessing treatment effects for schizophrenia symptoms at 6-month follow-up, we extend prior findings that CIT-S decreased patient symptoms from baseline to treatment termination (Weisman de Mamani et al., 2014). Also novel is our test of CIT-S effects on patient and caregiver DASS, as well as reports of family cohesion over time. Specifically, we assessed the indirect effects of the CIT-S treatment over time on patient and caregiver symptoms via changes in family cohesion, which was a major treatment target throughout all five modules of CIT-S. To establish temporal precedence of the mediator and outcome, we examined family cohesion at treatment midpoint, which was during the 7th session (after completing the psychoeducation module). The following hypotheses were tested: 1) Compared to the PSY-ED group, patients in the CIT-S group will display greater decreases in psychiatric symptom severity over time, and these effects will last through 6-month follow-up. 2) Compared to the PSY-ED group, patients and caregivers in the CIT-S group will display greater decreases in depression, anxiety, and stress over time. 3) Compared to the PSY-ED group, families in the CIT-S group will display greater increases in average family cohesion from baseline to midpoint. 4) Increases in family cohesion in the CIT-S group from baseline to midpoint will mediate the effect of CIT-S on reducing patient symptom severity and patient and caregiver DASS.
Methods
Participants
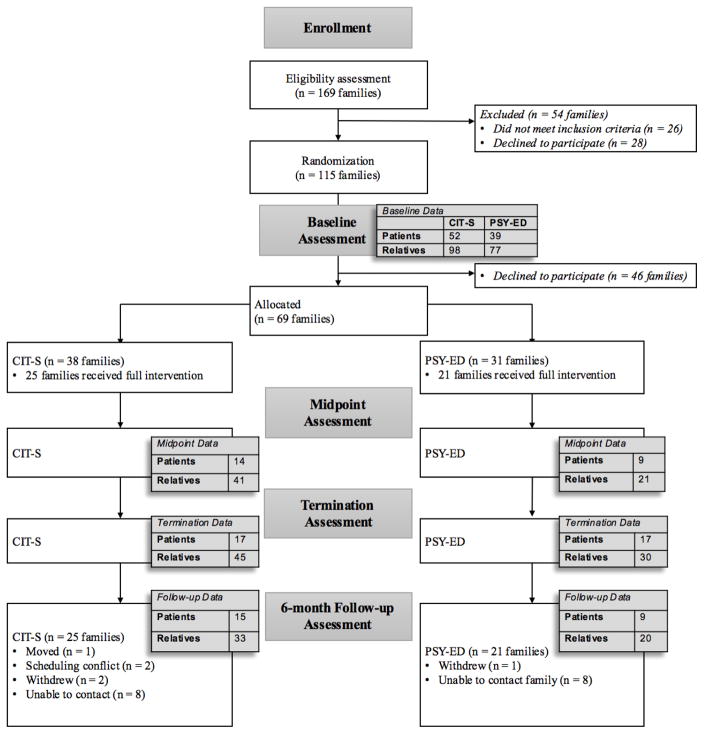
Demographic statistics of the full baseline sample are reported in Table 1, and Figure 1 contains a Consort Diagram of study participation. At baseline, 266 individuals (patients and family members) from 115 families were eligible to participate in family treatment for schizophrenia. Of these families, 64 were randomly assigned to CIT-S, a 15-week, culturally-informed family intervention. The other 51 families were assigned to PSY-ED, a 3-week standard family psychoeducation treatment (for additional details of both treatments, see Weisman de Mamani et al., 2014). Data from participants who dropped out of the study after randomization to treatment condition are included in the analyses. The sample included data from 36 families at treatment midpoint, 46 families at termination, and 41 families at 6-month follow-up. Prior to testing our models, we analyzed predictors of dropout for both the CIT-S and PSY-ED families.
Table 1.
Demographic statistics for patients and family members for CIT-S and PSY-ED
| CIT-S | PSY-ED | |||
|---|---|---|---|---|
| Patients (N = 52) | Family Members (N = 98) | Patients (N = 39) | Family Members (N = 77) | |
| Age | M = 37.24 SD = 13.4 |
M = 49.19 SD = 16.4 |
M = 38.72 SD = 11.69 |
M = 49.64 SD = 15.39 |
| Gender | 36.5% women | 54.1% women | 43.6% women | 58.4% women |
| Ethnicity | 44% Hispanic; 34% African American; 16% Caucasian; 6% Other | 57.7% Hispanic; 22.7% African American; 15.5% Caucasian; 1% Asian American; 3.1% Other | 53.8% Hispanic; 17.9% African American; 28.2% Caucasian | 46.8% Hispanic; 23.4% African American; 25.9% Caucasian; 3.9% Other |
| Education | 1.9% advanced degree; 15.7% college degree; 27.5% some college; 21.6% HS graduate; 12.6% some HS beyond grade 8; 5.9% grade 8 completed; 7.8% below grade 8 | 10.2% advanced degree; 28.6% college degree; 15.3% some college; 27.5% HS graduate; 12.2% some HS beyond grade 8; 3.1% grade 8 completed; 3.1% below grade 8 | 0% advanced degree; 10.5% college degree; 34.2% some college; 31.6% HS graduate; 21.1% some HS beyond grade 8; 0% grade 8 completed; 2.6% below grade 8 | 9.4% advanced degree; 29.7% college degree; 20.3% some college; 25.7% HS graduate; 9.5% some HS beyond grade 8; 4.0% grade 8 completed; 1.4% below grade 8 |
| Relationship to patient | NA | 45.9% parent; 17.3% partner; 12.2% sibling; 7.1% friend; 6.2% child; 11.3% extended family | NA | 37.7% parent; 22.1% partner; 16.9% sibling; 5.2% child; 2.6% friend; 15.5% extended family |
Figure 1.
Consort Diagram
Procedures
Participant recruitment occurred through referrals from hospitals and community health centers, as well as advertisements in newspapers and on Miami’s aboveground rail system. When individuals initially contacted the laboratory, they were administered a brief phone screen to determine eligibility, at which point participants who met criteria were scheduled for a baseline assessment. In total, 169 patients were assessed for eligibility to participate in family treatment. Participants meeting criteria for schizophrenia or schizoaffective disorder were included in the study, and some symptoms of psychosis were present in the majority of individuals participating in the treatment. Because extremely severe psychosis could interfere with a participant’s ability to understand the material covered in therapy or sustain attention for the 1.5-hour session, we excluded participants with scores of “6” (severe) or “7” (extremely severe) on the BPRS items of unusual thought content, suspiciousness, hallucinations, and conceptual disorganization, instead referring them to more comprehensive care. Additional exclusion criteria included having been incarcerated for violent crimes, current suicidality, a suicide attempt during the last year, and involuntary hospitalization within the past 3 months.
Doctoral level clinical psychology students under the supervision of the study’s principal investigator, a licensed clinical psychologist, conducted the CIT-S and PSY-ED intervention programs. Data on individual depression, anxiety, and stress were collected at baseline, treatment midpoint, and termination (3 time points). Patient symptom severity on the BPRS was measured at baseline, termination, and 6-month follow-up (3 time points). Data on family cohesion, the proposed mediator, were obtained at baseline and again at midpoint, following the conclusion of the Family Collectivism module.
Measures
Patient Diagnosis
Patient diagnosis of schizophrenia or schizoaffective disorder was confirmed using the Structured Clinical Interview for DSM-IV Axis I Disorders, Version 2.0, patient edition (SCID-I/P; First, Spitzer, Gibbon, & Williams, 2002). All interviewers watched six videotapes of SCID-I/P interviews and independently rated each video to determine whether a diagnosis of schizophrenia or schizoaffective disorder was present or absent, with strong interrater reliability (Cohen’s k = 1.0). Because the majority of patients with schizophrenia have a comorbid diagnosis of a substance use, mood, or anxiety disorder (Tsai & Rosenheck, 2013), comorbid diagnoses were not excluded.
Psychotic Symptom Severity
The Brief Psychiatric Rating Scale (BPRS; Lukoff, Liberman, & Nuechterlein, 1986) was used to measure patient symptom severity across four domains: positive, negative, affective (depression/anxiety), and manic symptoms. The BPRS is a 24-item measure, with each question rated on a 7-point Likert scale. Clinicians code some items based on observed behavior and speech, and others on patient self-report. After extensive training with the principal investigator of the study, interviewers coded six BPRS training tapes (Ventura, Green, Shaner, & Liberman, 1993). Intraclass correlations between interviewer ratings and consensus ratings from Ventura et al. (1993) ranged from .79 to .98 for all items.
Depression, Anxiety, and Stress
The Depression Anxiety Stress Scale (DASS; Lovibond & Lovibond, 1995) measured general emotional distress in patients and caregivers. The DASS consists of 42 questions answered on a rating scale of 0 (Did not apply to me at all) to 3 (Applied to me very much, or most of the time). The scale contains three factors (depression, anxiety and stress), with 14 items per factor, and a total score can also be calculated by summing the 42 items. The reliability for the DASS in the present sample was strong (Cronbach’s alpha = 0.96).
Family Cohesion
Family unity was measured with the Family Cohesion subscale of the Family Environment Scale (FES; Moos & Moos, 1981). The Family Cohesion subscale of the FES contains 9 items rated true or false, all of which assess the degree of support, commitment, and assistance family members provide one another. A total score is obtained by summing the 9 items, with higher scores indicative of greater cohesion. The FES demonstrated good reliability in the present sample (Cronbach’s alpha = 0.79).
Statistical Analyses
Preliminary Analyses
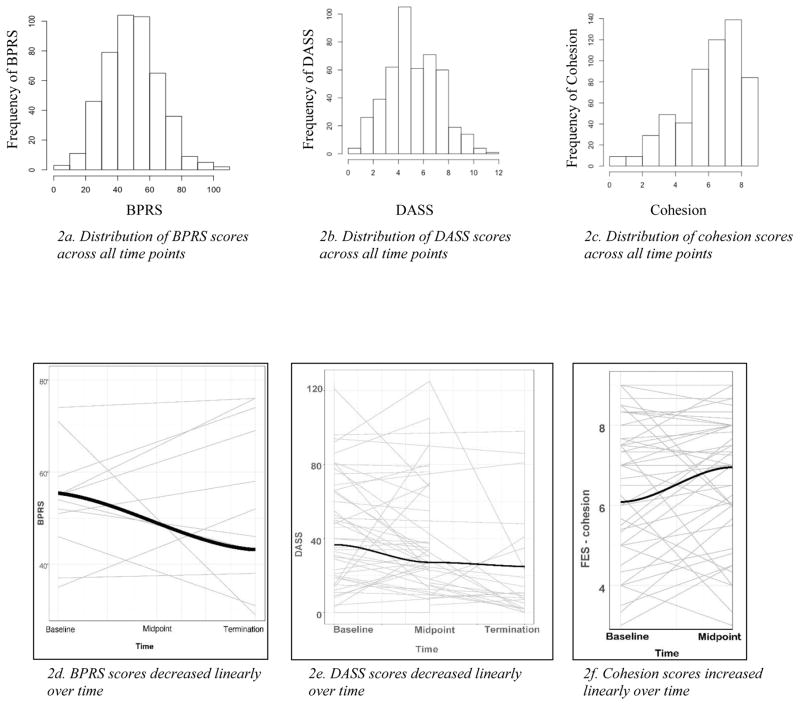
Preliminary data analyses were conducted in RStudio. A visual inspection of the variables included in the models indicated no violations of the assumptions of normality and homoscedasticity. BPRS, DASS, and FES scores were normally distributed, with skew and kurtosis values within normal limits (skew < +/−2, kurtosis < +/−7; Kline, 2015), and therefore, no transformations were executed (see Figure 2a, b, c). The trajectories of change of the variables were plotted to determine the most appropriate functional form (i.e., shape of trajectory over time). The plots indicated linear trends for all three variables of interest (Figure 2d, e, f), with notable variability in individual intercepts and growth trajectories. Residuals and random effects were plotted to assess homoscedasticity, normality, and homogeneity of variance, and we found no evidence of violations of regression assumptions. Table 2 contains means and correlation values for cohesion, BPRS, and DASS at each time point.
Figure 2.
Histograms of BPRS(2a), DASS(2b), and cohesion(2c); functional form of BPRS(2d), DASS (2e), and cohesion(2f)
Table 2.
Means, standard deviations, and correlation matrix for cohesion, DASS, and BPRS at each time point
| COH 0 | COH 2 | DASS 0 | DASS 2 | DASS 4 | BPRS 0 | BPRS 4 | BPRS 10 | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | 5.89 | 5.95 | 37.94 | 32.87 | 27.15 | 53.10 | 48.12 | 50.55 |
| (SD) | (1.80) | (2.40) | (11.40) | (11.18) | (20.35) | (15.13) | (18.40) | (16.22) |
| COH 0 | 1 | |||||||
| COH 2 | 0.713 | 1 | ||||||
| DASS 0 | −0.725 | −0.797 | 1 | |||||
| DASS 2 | −0.568 | −0.944 | 0.798 | 1 | ||||
| DASS 4 | −0.607 | −0.621 | 0.762 | 0.674 | 1 | |||
| BPRS 0 | −0.109 | −0.116 | -- | -- | -- | 1 | ||
| BPRS 4 | −0.024 | −0.186 | -- | -- | -- | 0.292 | 1 | |
| BPRS 10 | −0.191 | −0.267 | -- | -- | -- | 0.334 | 0.703 | 1 |
Attrition
Before testing models, we conducted an analysis of treatment attrition for the CIT-S and PSY-ED groups. Several demographic variables were related to treatment dropout. We found that ethnicity was a significant predictor of treatment dropout for CIT-S families, (F(5,57) = 7.27, p<.001), with families where the patient identified as Black completing fewer treatment sessions on average (B = −9.86, p<.001, 95% CI [−14.9, −4.82]). In addition, education was a significant predictor of treatment dropout for CIT-S families (F(1,61) = 32.81, p<.001), such that greater education was associated with more sessions completed (B = 3.34, p<.001, 95% CI [2.18, 4.51]. For PSY-ED families, dropout was not significantly predicted by ethnicity (F(4,43) = 1.56, p=.201) nor education (F(1,46) = 2.04, p=.16). None of the primary outcome variables were associated with patient dropout in either group. Patient symptom severity on the BPRS was not a significant predictor of dropout for CIT-S ((F(1,58) = 3.64, p=.061) or PSY-ED ((F(1,42) = 0.12, p=.73) families, in line with reports by Weisman de Mamani et al. (2014). Similarly, DASS did not predict dropout for CIT-S ((F(1,59) = .19, p=.67)) or PSY-ED ((F(1,42) = .04, p=.85), nor did family cohesion (CITS: F(1,59) = .88, p=.35; PSY-ED: F(1,42) = .03, p=.87). Further details regarding predictors of attrition in the CIT-S group are provided by Gurak, Weisman de Mamani, and Ironson (2017).
Model specification
The first step in model specification involved testing independent latent growth or latent change models for the outcomes of interest, with treatment included as a predictor in all models. Mplus takes a multivariate approach to account for dependency due to repeated measures, such that a standard latent growth model in Mplus represents a two-level model in a standard multilevel modeling framework (Muthén & Muthén, 2012). Since BPRS was a family-level variable, we assessed BPRS effects with a standard latent growth model. Because DASS was measured at the individual level, we specified DASS growth models using a two-level latent growth model in Mplus, which accounted for the nesting of time points within individuals, and of individuals within families. In line with standard growth modeling practices (Muthén & Muthén, 2012), loadings for the intercept latent variable were constrained at one, and loadings for the slope latent variable were set equal to the number of months after baseline at which the measurements were taken (0, 4, and 10 months for BPRS; 0, 2, and 4 months for DASS). Because family cohesion scores were obtained from two time points (at baseline and midpoint, after the Family Collectivism module), and latent growth modeling requires a minimum of three indicators per latent construct, the trajectory of family cohesion was modeled using a latent change score. A latent change score model is theoretically similar to a latent growth model but can accommodate change at just two time points (Coman, Picho, McArdle, Villagra, Dierker, & Iordache, 2013; McArdle, 2009). To fit the latent change model, we first created a latent variable representing change in cohesion, specified with the single indicator of cohesion at midpoint. The latent change variable was regressed on baseline family cohesion, with the loading of both cohesion indicators constrained at 1. The estimate of the latent variable regressed on the treatment variable provided a measure of the treatment-related change in family cohesion from baseline to the end of the Family Collectivism module.
In the next step of the model testing process, the latent change score model for the mechanistic variable (cohesion) and the latent growth models for the outcome (BPRS or DASS) were entered into parallel process latent growth/change models, which allowed us to model relationships between the treatment-related change in cohesion and treatment-related growth in the outcomes (i.e., indirect effects). For all models, model fit was assessed according to the following criteria suggested by Kline (2015): χ2 > .05, root mean square error of approximation (RMSEA) < .06, comparative fit index (CFI) > .95, and standardized root mean square residual (SRMR) < .08. Effect sizes on growth parameters were calculated in accordance with the recommendations of Feingold (2013; 2015), using the formula d = (b*duration)/SD, where b is the treatment effect on the slope of the outcome and SD is the pooled within-group standard deviation of the outcome variable. The result of this formula represents a standardized mean difference (Cohen’s d) between groups after treatment, with values above 0.2, 0.5, and 0.8 indicating small, medium, and large effect sizes, respectively.
Results
Treatment effects on individual growth models
We fit individual latent growth or latent change models to test direct treatment effects and ensure model fit was adequate before combining them into parallel process models and examining indirect effects. The latent change model testing the treatment effect on family cohesion from baseline to midpoint indicated a time by treatment interaction. The CIT-S group exhibited an average increase of roughly one unit on the FES from baseline to midpoint (γ = 0.93, SE = 0.44, p = 0.03, d = 0.82), whereas cohesion levels did not change for the PSY-ED group (see Table 3 for full results of the latent change model, including 95% CIs). CIT-S outperformed PSY-ED in decreasing patient BPRS scores from baseline to 6-month follow-up (γ = −1.72, SE = 0.57, p <.001, d = 0.63), and in decreasing patient and caregiver DASS scores from baseline to termination (γ = −4.39, SE = 1.05, p <.001, d = 0.87). Tables 3 and 4 contain full results of fixed and random effects, as well as 95% CIs and model fit statistics, for treatment effects on BPRS and DASS. In other words, all CIT-S effects over time were significant in the expected direction, and the linear latent growth models had good fit, suggesting that it was appropriate to examine indirect effects as parallel processes.
Table 3.
Latent change model for family cohesion.
| Estimate (SE) | 95% CI | |
|---|---|---|
| Fixed Effects | ||
| Cohesion_0 | 5.80** (0.21) | [5.40, 6.20] |
| Cohesion change (δ) | 1.08 (1.05) | [−0.97, 3.13] |
| Treatment → cohesion_0 | 0.16 (0.25) | [−0.33, 0.65] |
| Treatment → δ | 0.93* (0.44) | [0.06, 1.80] |
| Cohesion_0 → δ | −0.249 (0.13) | [−0.49, 0] |
| Random Effects | ||
| σ2 between families | ||
| Cohesion_0 | 3.382** (0.24) | [2.91, 3.86] |
| Cohesion change | 2.78** 1(0.44) | [1.92, 3.64] |
indicates p<.05,
indicates p<0.01
Because the model was just identified, model fit statistics were not computed.
Table 4.
Model fit statistics and estimates of fixed and random effects for latent growth of 1) BPRS and 2) indirect effect from treatment to BPRS via family cohesion
| Model 1: BPRS only | Model 2: BPRS and cohesion | |||
|---|---|---|---|---|
| Model Fit Statistics | ||||
| Chi-square | χ2(3) = 7.093, p=.07 | χ2(8) = 15.214, p=.06 | ||
| RMSEA | 0.065 | 0.053 | ||
| CFI | 0.93 | 0.93 | ||
| SRMR | 0.029 | 0.05 | ||
|
| ||||
| Fixed Effects | Estimate (SE) | 95% CI | Estimate (SE) | 95% CI |
|
| ||||
| BPRS intercept (α) | 52.37** (1.48) | [49.47, 55.27] | 52.39** (1.48) | [49.49, 55.29] |
| BPRS slope (β) | 0.28 (0.45) | [−0.60, 1.16] | 0.141 (0.63) | [−1.09, 1.37] |
| Cohesion_0 | 5.80** (0.21) | [5.40, 6.20] | ||
| Cohesion change (δ) | 0.94 (1.06) | [−1.13, 3.00] | ||
| Treatment → α | 1.22 (1.94) | [−2.57, 5.02] | 1.12 (1.91) | [−2.63, 4.86] |
| Treatment → β | −1.72** (0.57) | [−2.83, −0.60] | −1.73* (0.86) | [−3.41, −0.05] |
| Treatment → cohesion_0 | 0.16 (0.25) | [−0.33, 0.65] | ||
| Treatment → δ | 1.00* (0.45) | [0.12, 1.88] | ||
| Cohesion_0 → δ | −0.24 (0.12) | [−0.48, 0] | ||
| δ → β | −0.29 (0.27) | [−0.81, 0.23] | ||
| Indirect effect (a*b) | −0.29 (0.31) | [−0.89, 0.32] | ||
|
| ||||
| Random Effects | Estimate (SE) | 95% CI | Estimate (SE) | 95% CI |
|
| ||||
| σ2 within families | 101.42** (17.04) | [68.01, 134.82] | 111.32** (17.02) | [77.96, 144.68] |
| σ2 between families | ||||
| BPRS intercept | 127.70** (27.66) | [73.48, 181.91] | 117.31** (26.10) | [66.16, 168.45] |
| BPRS slope | 5.00** (1.87) | [1.32, 8.67] | 6.69** (2.55) | [1.70, 11.69] |
| BPRS intercept, slope | −8.79 (7.00) | [−22.51, 4.93] | −7.629 (7.58) | [−22.49, 7.23] |
| Cohesion_0 | 3.38** (0.24) | [2.91, 3.86] | ||
| Cohesion change | 2.79** (0.45) | [1.91, 3.66] | ||
| BPRS int., cohesion_0 | −3.06 (1.80) | [−6.58, 0.45] | ||
indicates p<.05,
indicates p<0.01.
Parallel Process Model: BPRS on Treatment via Family Cohesion
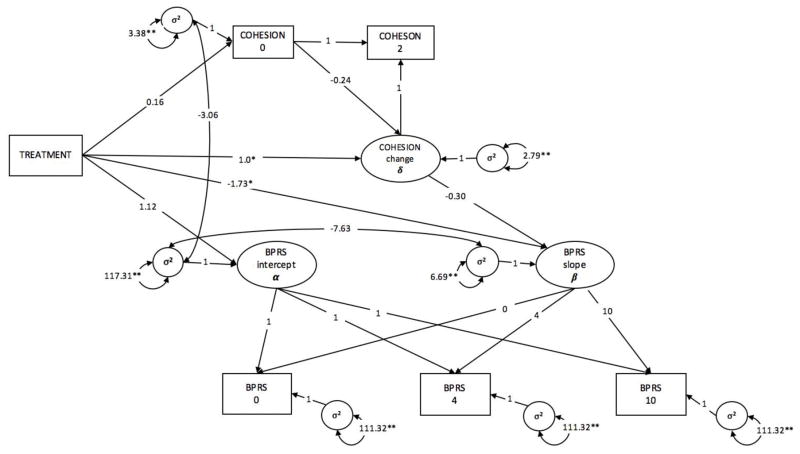
The model including BPRS and the indirect effect of family cohesion exhibited good fit, χ2(8) = 15.214, p = .06. There were no significant differences between treatment groups in baseline BPRS (CIT-S M = 53.51; PSY-ED M = 52.39; p = 0.56) or family cohesion scores (PSY-ED M = 5.80; CIT-S M = 5.96; p = 0.53). The standard PSY-ED treatment did not change BPRS scores over time (γ = 0.141, p = 0.823). However, there was a time by treatment interaction, such that patients in the CIT-S group displayed a significant decrease in BPRS scores over time (γ = −1.91, p = 0.04, d = 0.63). A pattern also emerged for family cohesion: while families in the PSY-ED group did not exhibit significant increases in average family cohesion from baseline to midpoint, families in the CIT-S group displayed an increase of roughly 1 point on the FES cohesion scale (p = .025, d = 0.88). We did not observe a significant indirect effect of treatment on BPRS via family cohesion, as evidenced by the nonsignificant regression of random slope of the outcome (BPRS) on the latent change score of the average family cohesion (a*b = −0.29, p = 0.35). An examination of the variance components revealed a random effect of BPRS at baseline (σ2 = 117.31, p < .001), as well as random variability in change in BPRS over time (σ2 = 6.69, p = 0.009) and at individual time points (σ2 = 111.32, p <.001). Table 4 contains results and indices of model fit, and the full parallel process latent growth model is depicted in Figure 3.
Figure 3.
Parallel process latent growth model of direct and indirect effects of treatment on patient schizophrenia symptoms (BPRS)
Full results of the model are displayed in Table 4. * indicates p<.05, ** indicates p<0.01.
Parallel Process Model: DASS on Treatment via Family Cohesion
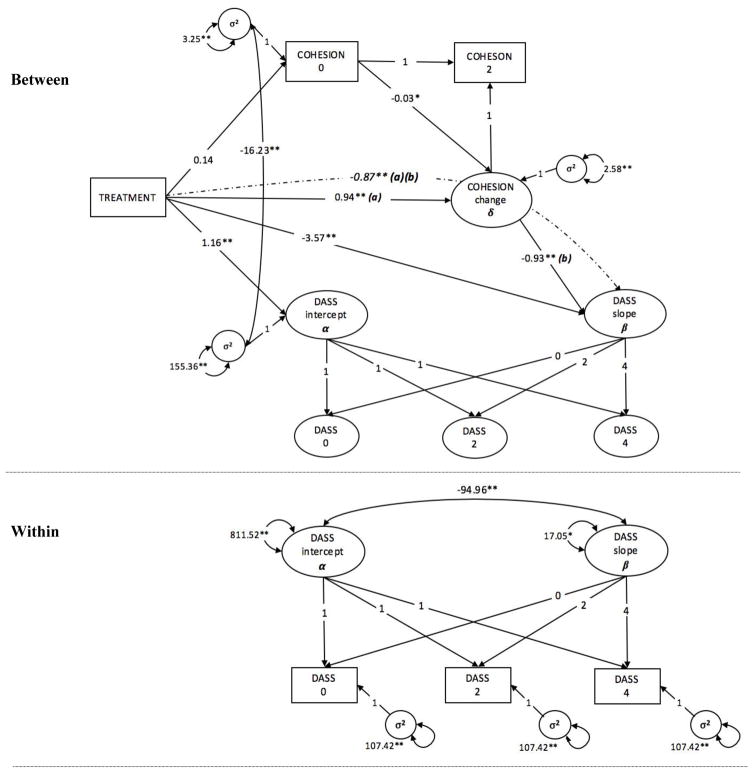
Next, we estimated a multilevel parallel process latent growth/latent change model, with the goal of testing whether increases in family cohesion drove the increases in the treatment-related changes in patient and family member DASS over time. The model including the indirect effect of treatment on DASS via family cohesion exhibited good fit, χ2(12) = 15.42, p = .22. Despite random assignment to groups, the CIT-S group had slightly higher initial DASS scores compared to the PSY-ED group (CIT-S M = 38.582, PSY-ED M = 37.425, p <.001). There was a significant effect of treatment on change in DASS, such that for each month elapsed from baseline, individuals in families in the CIT-S group exhibited a decrease of 3.571 units in DASS (p <.001; d = 0.70), whereas for those in the PSY-ED group DASS did not change significantly over time (γ = -0.60, SE = 0.70, p = 0.32). There was not a significant increase in cohesion from baseline to midpoint for the control group (δ = −0.46, SE = 0.31, p = 0.144), whereas the CIT-S group displayed an average increase of 0.94 units in cohesion over time (p <.001, d = 0.79). Furthermore, there was a significant indirect effect of treatment on DASS via family cohesion, such that for every unit increase in cohesion from baseline to midpoint, individuals in the CIT-S group displayed an additional 0.87-unit decrease in DASS on average (p = 0.004, d = 0.17). We observed random effects of intercept and slope at the individual level (intercept σ2 = 811.522, p <.001; slope σ2 = 17.048, p = 0.012), and of the intercept at the family level (σ2 = 155.362, p <.001). Notably, individuals who started at higher levels of DASS exhibited greater decreases in symptoms over time (σ2 = −94.960, p <.001), and initial levels of DASS were negatively associated with baseline family cohesion (σ2 = −16.232, p <.001). The full results and indices of model fit are contained in Table 5, and Figure 4 presents a visualization of the full latent growth model.
Table 5.
Model fit statistics and estimates of fixed and random effects for multilevel latent growth of 1) DASS and 2) indirect effect from treatment to DASS via family cohesion
| Model 1: DASS only | Model 2: DASS and cohesion | |||
|---|---|---|---|---|
| Model Fit Statistics | ||||
| Chi-square | χ2(3) = 8.364, p=.40 | χ2(12) = 15.42, p=.22 | ||
| RMSEA | 0.013 | 0.033 | ||
| CFI | 0.997 | 0.979 | ||
| SRMRwithin | 0.083 | 0.089 | ||
| SRMRbetween | 0.118 | 0.114 | ||
|
| ||||
| Fixed Effects | Estimate (SE) | 95% CI | Estimate (SE) | 95% CI |
|
| ||||
| DASS intercept (α) | 37.66** (3.61) | [30.59, 44.73] | 37.43** (2.22) | [33.07, 41.78] |
| DASS slope (β) | −0.14 (0.83) | [−1.77, 1.48] | −0.60 (0.70) | [−1.80, 0.59] |
| Cohesion_0 | 5.92** (0.22) | [5.48, 6.35] | ||
| Cohesion change (δ) | −0.46 (0.31) | [−1.07, 0.16] | ||
| Treatment → α | 1.07 (4.40) | [−7.55, 9.69] | 1.16** (0.20) | [0.77, 1.54] |
| Treatment → β | −4.39** (1.05) | [−6.44. −2.34] | −3.57** (0.32) | [−4.19, −2.95] |
| Treatment → | 0.14 (0.25) | [−0.36, 0.63] | ||
| cohesion_0 | ||||
| Treatment → δ | 0.94** (0.23) | [0.49, 1.38] | ||
| Cohesion_0 → δ | −0.03* (0.02) | [−0.06, −0.01] | ||
| δ → β | −0.93** (0.10) | [−1.13, −0.73] | ||
| Indirect effect (a*b) | −0.87** (0.31) | [−1.47, −0.27] | ||
|
| ||||
| Random Effects | Estimate (SE) | 95% CI | Estimate (SE) | 95% CI |
|
| ||||
| σ2 within person | 111.92** (40.18) | [33.18, 190.66] | 107.52** (23.93) | [60.61, 154.43] |
| σ2 within families | ||||
| Time-specific variance | 0.01 (4.51) | [0, 0] | 0 (<.001) | [0, 0] |
| DASS intercept | 796.65** (104.25) | [592.33, 1000.97] | 811.52** (88.07) | [638.90, 984.15] |
| DASS slope | 15.71* (7.80) | [0.42, 31.00] | 17.05* (6.81) | [3.71, 30.39] |
| DASS intercept, slope | −85.73** (20.01) | [−124.95, −46.50] | −94.96** (20.74) | [−135.61, −54.31] |
| σ2 between families | ||||
| DASS intercept | 163.15* (90.80) | [−14.81, 341.10] | 155.36** (0.29) | [154.80, 155.92] |
| Cohesion_0 | 3.25** (0.36) | [2.55, 3.96] | ||
| Cohesion change | 2.58** (0.54) | [1.53, 3.63] | ||
| DASS int., cohesion_0 | −16.23** (0.82) | [−17.83, −14.64] | ||
indicates p<.05,
indicates p<0.01.
Figure 4.
Parallel process, two-level latent growth model of direct and indirect effects of treatment on patient and caregiver depression, anxiety, and stress (DASS)
Full results of the model are displayed in Table 5. * indicates p<.05, ** indicates p<0.01.
Discussion
Capitalizing on statistical advances to study mechanisms and growth trajectories, the present study builds upon published findings using the same clinical trial data, which indicated that CIT-S was effective in reducing schizophrenia symptom severity and caregiver burden at treatment termination (Weisman de Mamani et al., 2014; Weisman de Mamani & Suro, 2016). We found that the CIT-S treatment effects lasted beyond the 15 weeks of therapy, demonstrating that patients maintained a reduction in BPRS symptom severity at 6-month follow-up. Recognizing the importance of addressing the high rates of depression and anxiety in both patients with schizophrenia and their family caregivers, we addressed whether CIT-S affected patient and caregiver DASS more broadly, a question that had not been previously assessed in the literature. In line with hypotheses, CIT-S yielded significant decreases in depression, anxiety, and stress over time. Because family cohesion constituted a theoretical change mechanism, we evaluated the relationship between treatment-related changes in cohesion and BPRS/DASS over time. Representing tentative evidence for the change theory, increases in cohesion with CIT-S were linked to reductions in patient/caregiver DASS over time, although there was not an indirect effect of treatment via cohesion on patient schizophrenia symptoms on the BPRS.
While prior research demonstrated that CIT-S decreased caregiver burden at treatment termination (Weisman de Mamani & Suro, 2016), the current study is the first to demonstrate that CIT-S also significantly decreased patient and caregiver mood and anxiety symptoms over the course of therapy. Given the elevated risk of emotional distress and mood symptoms associated with caregiving (Magaña et al., 2007), it would be remiss to neglect caregiver depression, anxiety, and stress in family therapy. More than a vehicle to reducing EE and patient relapse risk, caregiver emotional distress represented a key outcome in CIT-S. Similarly, our finding that CIT-S reduced individual DASS is promising with regard to decreasing mood and anxiety symptoms in patients with schizophrenia, since negative and mood symptoms often persist even in the absence of acute positive symptoms (Buckley et al., 2009). Along with the difficult symptoms of psychosis, patients experience complicated emotional distress surrounding stigma and illness-related burden, with evident implications for quality of life (Huppert & Smith, 2005). In spite of striking comorbidity rates of depressive and anxiety disorders in schizophrenia samples (Buckley et al., 2009), however, DASS is seldom a target of family therapy. According to the present study, by infusing cultural components into traditional family psychoeducation, CIT-S led to improvements in patient mental health in a broader sense. The impact of CIT-S on patient and family member DASS dovetails with research on family stress and schizophrenia, which links reductions in family stress with decreased rates of relapse risk for patients with schizophrenia (Liberman, Kopelowicz, Ventura, & Gutkind, 2002), as well as improvements in both patient and caregiver well-being.
Beyond demonstrating effects on DASS, the present study pioneers the investigation of mechanisms of action relevant to CIT-S, with a focus on the relationship between dynamic family behaviors and reductions in negative mental health outcomes. The increase in family cohesion for CIT-S families suggests that the early treatment segments, and the Family Collectivism module in particular, successfully increased average perceptions of family warmth and positive sentiments. Family cohesion, a component introduced in the first treatment module but reinforced throughout the 15 weeks of therapy, represented a key treatment ingredient for CIT-S. Therapists fostered shared family values and experiences, with the idea that this would yield improvements in caregiver mental health, as well as symptoms of psychopathology in patients with schizophrenia (Weisman de Mamani et al., 2014). Given that family cohesion was theoretically central to treatment outcomes, testing changes in cohesion—and the relationship of these changes to treatment results—was a key question in understanding the impact of the culturally modified components that distinguish CIT-S from other family therapies. Furthermore, this project speaks to the utility of parallel process growth modeling for testing dynamic mediation pathways, a practice that is underused in clinical trials (Cheong et al., 2003).
As discussed earlier, a number of cross-sectional studies have reported a significant negative association between family cohesion and schizophrenia symptom severity (González-Pinto et al., 2011; Gurak & Weisman de Mamani, 2014), although this research says little about the ability to cultivate family cohesion in therapy, and whether that would, in turn, yield patient benefits. In line with prior research, we found that there was a significant baseline covariance of family cohesion and DASS (σ2 = −16.232, p <.001). A handful of longitudinal studies have shown that a positive family environment predicts improvements in social functioning and psychiatric symptomatology in high-risk samples (O’Brien, Gordon, Bearden, Lopez, Kopelowicz, & Cannon, 2006). To our knowledge, however, this is the first study to examine whether therapists can actually target family cohesion in treatment, and whether changes in this variable fuel changes in psychiatric treatment outcomes. As part of CIT-S, families spent time discussing perceptions about the illness, engaging in activities to build cohesion, and working through problems with communication skills. Given that CIT-S promoted active problem-solving and fostered team dynamics through shared activities, we expected the latent change in cohesion to predict decreases in depression, anxiety, and stress in patients and caregivers alike, which was supported by the data. By fostering family cohesion in therapy through cultural modifications, clinicians can enhance therapeutic benefits for patients and caregivers alike.
Results of the current analysis should be considered in light of several limitations that point to direction for continued research on CIT-S. In the present trial, treatment dropout was relatively high and led to missing data, unequal groups, and a general decrease in power. A key caveat of this was the lack of power to perform groupwise analyses by ethnicity. Although Weisman de Mamani et al. (2014) reported that CIT-S was equally effective in reducing schizophrenia symptoms across ethnic groups, there remains the possibility that treatment mechanisms operate in a distinct manner according to ethnicity, particularly given that family cohesion relates to a collectivistic orientation. Understanding differential treatment mechanisms according to cultural factors such as collectivism and interdependence will be an important question for future studies on CIT-S. Thus, although growth models present an ideal framework for accounting for missingness (Curran et al., 2010), testing CIT-S with larger samples and using more regular outcome monitoring (e.g., every other session) is necessary to understanding exactly how CIT-S taps into cultural factors. While dropout was not related to primary study variables, we did find that the demographic variables of ethnicity and education related to attrition in the CIT-S group, with participants identifying as Black and reporting fewer years of education terminating treatment sooner. Gurak, Weisman de Mamani, and Ironson (2017) address predictors of attrition in the context of religiosity, noting that religious participants may leave treatment earlier because they find sufficient recourse in spiritual coping or involvement with their religious institution. By attempting to reduce dropout and including a matched-length treatment-as-usual group in subsequent clinical trials of CIT-S, we may achieve greater confidence in the effects of specific CIT-S components.
It is also important to recognize that family cohesion is but one of a number of treatment targets that may be relevant in reducing patient and caregiver symptoms, and within the construct of family cohesion, there may be specific aspects that are more relevant to treatment outcomes than those captured by the Family Environment Scale (FES). As discussed in the introduction, we chose to focus the present study on the mediating role of family cohesion because it was distinctive among potential treatment targets. Not only is family cohesion a key construct in the Family Collectivism module, it is unique in that subsequent modules heavily reinforce the importance of family cohesion in order to achieve success in therapy skills (e.g., family communication, problem solving). However, in future research on CIT-S it will be important to include measures of other key treatment targets in order to test alternative mediators, including communication skills. Relatedly, future studies may reconsider the way in which family cohesion is measured. While the items on the Family Cohesion subscale of the FES capture perceptions of the home environment, they may not measure all aspects of interdependence, particularly those with the strongest theoretical links to patient functioning (Weisman & López, 1996). Employing measures of family cohesion that capture changes in family dynamics in a more active way, such as increased problem solving and engagement in shared activities, may help to illuminate any existing relationships between CIT-S, family functioning, and patient outcomes.
Nevertheless, the use of parallel process latent growth/change models in an MSEM framework conferred a number of significant advantages with regard to characterizing growth at the individual and family level. Given the high proportions of missing data due to dropout, in line with typical rates of dropout in family therapy for schizophrenia (Weisman de Mamani et al., 2014), the growth models allowed for flexibility with regard to unequal groups, observations, and timing of data collection. Moreover, we were able to capitalize on the multivariate multilevel modeling framework to establish temporal precedence of the mechanistic variable of interest, family cohesion. Rather than look at changes between mean levels of cohesion and our outcomes of interest, we analyzed the relationship between the change in DASS and change in cohesion using a procedure that better reflects the dynamic nature of the theory underlying the development of CIT-S.
Ultimately, the results of the present analysis provide increased support for the efficacy of CIT-S in reducing patient and caregiver psychopathology, and in promoting family cohesion, with significant implications for clinical practice with diverse families. With regard to schizophrenia symptoms, CIT-S appears to exert a lasting impact on reducing patient psychosis, as the treatment effect was maintained at 6-month follow-up. Through empirically grounded cultural adaptations of family psychoeducation, CIT-S improved symptoms of depression, anxiety, and stress in patients grappling with serious mental illness, as well as their caregivers. Future studies will help to clarify further the parallel growth processes at work in explaining the dynamic relationships between changes in positive family factors, patient symptoms, and patient and caregiver emotional distress.
Public Health Significance.
This study found that a culturally-informed family therapy for schizophrenia (CIT-S) had a lasting impact on reducing patient symptoms. In addition, by increasing family cohesion, CIT-S decreased patient and caregiver depression, anxiety, and stress. For a family seeking treatment for one member with schizophrenia, therapists may enhance patient gains by incorporating relevant cultural variables into treatment.
Appendix: Data Transparency
Prior manuscripts have been published using these data, which were collected as part of a larger study of randomized clinical trial (RCT) of a culturally informed family therapy for schizophrenia (CIT-S). M1 described predictors of attrition for CIT-S participants, with a focus on religiosity, which we do not address. While another study (M2) examined the relationship between family cohesion and patient symptoms, they only assessed these relationships using a cross-sectional design, before treatment. In the current study, we look at how family cohesion and psychiatric symptoms change dynamically over time with treatment. M4 focused on changes in schizophrenia symptoms on the BPRS from baseline to termination, whereas we examine BPRS scores at 6-month follow-up, which is key to understanding whether CIT-S has a lasting impact on symptoms. Similarly, although M5 tested the effect of CIT-S on caregiver burden and self-conscious emotions, it did not address the direct and indirect effects of CIT-S on DASS, which is a primary focus of the present manuscript. Furthermore, this is the first study to examine treatment mechanisms involved in the observed CIT-S effects over time, with a focus on family cohesion. We assessed these mechanisms using a novel, multilevel, parallel process growth modeling framework, which we feel may serve as a helpful example of an underutilized resource for researchers evaluating mediation processes in clinical trials.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- Bauer DJ, Gottfredson NC, Dean D, Zucker RA. Analyzing repeated measures data on individuals nested within groups: accounting for dynamic group effects. Psychological Methods. 2013;18(1):1. doi: 10.1037/a0030639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrando P, Beltz J, Bressi C, Clerici M, Farma T, Invernizzi G, Cazzullo CL. Expressed emotion and schizophrenia in Italy. A study of an urban population. The British Journal of Psychiatry. 1992;161(2):223–229. doi: 10.1192/bjp.161.2.223. [DOI] [PubMed] [Google Scholar]
- Bry BH, Catalano RF, Kumpfer KL, Lochman JE, Szapocznik J. Scientific findings from family prevention intervention research. Drug Abuse Prevention through Family Interventions. 1998:103–129. [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophrenia Bulletin. 2009;35(2):383–402. doi: 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong J, MacKinnon DP, Khoo ST. Investigation of mediational processes using parallel process latent growth curve modeling. Structural Equation Modeling. 2003;10(2):238–262. doi: 10.1207/S15328007SEM1002_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman EN, Picho K, McArdle JJ, Villagra V, Dierker L, Iordache E. The paired t-test as a simple latent change score model. Frontiers in Psychology. 2013;4:738. doi: 10.3389/fpsyg.2013.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Howard AL, Bainter SA, Lane ST, McGinley JS. The separation of between-person and within-person components of individual change over time: A latent curve model with structured residuals. Journal of Consulting and Clinical Psychology. 2014;82(5):879. doi: 10.1037/a0035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, McGinley JS, Serrano D, Burfeind C. A multivariate growth curve model for three-level data. In: Cooper H, editor. APA Handbook of Research Methods in Psychology. Vol. 3. Washington, DC: American Psychological Association; 2012. pp. 335–358. [Google Scholar]
- Curran PJ, Obeidat K, Losardo D. Twelve Frequently Asked Questions About Growth Curve Modeling. Journal of Cognition and Development: Official Journal of the Cognitive Development Society. 2010;11(2):121–136. doi: 10.1080/15248371003699969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elis O, Caponigro JM, Kring AM. Psychosocial treatments for negative symptoms in schizophrenia: current practices and future directions. Clinical Psychology Review. 2013;33(8):914–928. doi: 10.1016/j.cpr.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falloon IR, Boyd JL, McGill CW. Family care of schizophrenia: A problem- solving approach to the treatment of mental illness. Guilford; 1984. [Google Scholar]
- Feingold A. A regression framework for effect size assessments in longitudinal modeling of group differences. Review of General Psychology. 2013;17(1):111–121. doi: 10.1037/a0030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold A. Confidence interval estimation for standardized effect sizes in multilevel and latent growth modeling. Journal of Consulting and Clinical Psychology. 2015;83(1):157–168. doi: 10.1037/a0037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID- I/P W/PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Freeberg AL, Stein CH. Felt obligation towards parents in Mexican-American and Anglo-American young adults. Journal of Social and Personal Relationships. 1996;13(3):457–471. [Google Scholar]
- González-Pinto A, de Azúa SR, Ibáñez B, Otero-Cuesta S, Castro-Fornieles J, Graell-Berna M, … Baeza I. Can positive family factors be protective against the development of psychosis? Psychiatry Research. 2011;186(1):28–33. doi: 10.1016/j.psychres.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Gurak K, Weisman de Mamani A. Risk and protective factors, perceptions of family environment, ethnicity, and schizophrenia symptoms. The Journal of Nervous and Mental Disease. 2016;204(8):570–577. doi: 10.1097/NMD.0000000000000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurak K, Weisman de Mamani A, Ironson G. Does religiosity predict attrition from a culturally-informed family treatment for schizophrenia that targets religious coping? Journal of Consulting and Clinical Psychology. 2017 doi: 10.1037/ccp0000234. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley JM. Expressed emotion and relapse of psychopathology. Annual Review of Clinical Psychology. 2007;3:329–352. doi: 10.1146/annurev.clinpsy.2.022305.095236. [DOI] [PubMed] [Google Scholar]
- Huppert JD, Smith TE. Anxiety and schizophrenia: the interaction of subtypes of anxiety and psychotic symptoms. CNS Spectrums. 2005;10(09):721–731. doi: 10.1017/s1092852900019714. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Judd CM. Consequences of violating the independence assumption in analysis of variance. Psychological Bulletin. 1986;99(3):422. [Google Scholar]
- Khoo ST. Assessing program effects in the presence of treatment—baseline interactions: A latent curve approach. Psychological Methods. 2001;6(3):234. doi: 10.1037/1082-989x.6.3.234. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. Guilford publications; 2015. [Google Scholar]
- Liberman RP, Kopelowicz A, Ventura J, Gutkind D. Operational criteria and factors related to recovery from schizophrenia. International Review of Psychiatry. 2002;14(4):256–272. [Google Scholar]
- López SR, Nelson Hipke K, Polo AJ, Jenkins JH, Karno M, Vaughn C, Snyder KS. Ethnicity, expressed emotion, attributions, and course of schizophrenia: family warmth matters. Journal of Abnormal Psychology. 2004;113(3):428. doi: 10.1037/0021-843X.113.3.428. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Lovibond SH. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy. 1995;33(3):335–343. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Liberman RP, Nuechterlein KH. Symptom monitoring in the rehabilitation of schizophrenic patients. Schizophrenia Bulletin. 1986;12(4):578–602. doi: 10.1093/schbul/12.4.578. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Evaluation Review. 1993;17(2):144–158. [Google Scholar]
- Madianos M, Economou M, Dafni O, Koukia E, Palli A, Rogakou E. Family disruption, economic hardship and psychological distress in schizophrenia: can they be measured? European Psychiatry. 2004;19(7):408–414. doi: 10.1016/j.eurpsy.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Magaña SM, Ramírez García JI, Hernández MG, Cortez R. Psychological distress among Latino family caregivers of adults with schizophrenia: The roles of burden and stigma. Psychiatric Services. 2007;58(3):378–384. doi: 10.1176/appi.ps.58.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Developing treatments for impaired cognition in schizophrenia. Trends in Cognitive Sciences. 2012;16(1):35–42. doi: 10.1016/j.tics.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Miranda AO, Estrada D, Firpo-Jimenez M. Differences in family cohesion, adaptability, and environment among Latino families in dissimilar stages of acculturation. The Family Journal. 2000;8(4):341–350. [Google Scholar]
- Moos RH, Moos B. Family Environment Scale manual. Palo Alto: 1981. [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- O'Brien MP, Gordon JL, Bearden CE, Lopez SR, Kopelowicz A, Cannon TD. Positive family environment predicts improvement in symptoms and social functioning among adolescents at imminent risk for onset of psychosis. Schizophrenia Research. 2006;81(2):269–275. doi: 10.1016/j.schres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Koutra K, Simos P, Triliva S, Lionis C, Vgontzas AN. Linking family cohesion and flexibility with expressed emotion, family burden and psychological distress in caregivers of patients with psychosis: A path analytic model. Psychiatry Research. 2016;240:66–75. doi: 10.1016/j.psychres.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Preacher KJ. Multilevel SEM strategies for evaluating mediation in three-level data. Multivariate Behavioral Research. 2011;46(4):691–731. doi: 10.1080/00273171.2011.589280. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Zyphur MJ, Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychological Methods. 2010;15(3):209. doi: 10.1037/a0020141. [DOI] [PubMed] [Google Scholar]
- Rummel-Kluge C, Pitschel-Walz G, Bäuml J, Kissling W. Psychoeducation in schizophrenia—results of a survey of all psychiatric institutions in Germany, Austria, and Switzerland. Schizophrenia Bulletin. 2006;32(4):765–775. doi: 10.1093/schbul/sbl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suro G, Weisman de Mamani A. Burden, interdependence, ethnicity, and mental health in caregivers of patients with schizophrenia. Family Process. 2013;52:299–311. doi: 10.1111/famp.12002. [DOI] [PubMed] [Google Scholar]
- Tasca GA, Illing V, Joyce AS, Ogrodniczuk JS. Three-level multilevel growth models for nested change data: A guide for group treatment researchers. Psychotherapy Research. 2009;19(4–5):453–461. doi: 10.1080/10503300902933188. [DOI] [PubMed] [Google Scholar]
- Tsai J, Rosenheck RA. Psychiatric comorbidity among adults with schizophrenia: A latent class analysis. Psychiatry Research. 2013;210(1):16–20. doi: 10.1016/j.psychres.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the Brief Psychiatric Rating Scale:" The drift busters.". International Journal of Methods in Psychiatric Research 1993 [Google Scholar]
- Villeneuve K, Potvin S, Lesage A, Nicole L. Meta-analysis of rates of drop-out from psychosocial treatment among persons with schizophrenia spectrum disorder. Schizophrenia Research. 2010;121(1):266–270. doi: 10.1016/j.schres.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Weisman A, Rosales G, Kymalainen J, Armesto J. Ethnicity, family cohesion, religiosity and general emotional distress in patients with schizophrenia and their relatives. The Journal of Nervous and Mental Disease. 2005;193(6):359–368. doi: 10.1097/01.nmd.0000165087.20440.d1. [DOI] [PubMed] [Google Scholar]
- Weisman AG, López SR. Family Values, Religiosity, and Emotional Reactions to Schizophrenia in Mexican and Anglo-American Cultures. Family Process. 1996;35(2):227–237. doi: 10.1111/j.1545-5300.1996.00227.x. [DOI] [PubMed] [Google Scholar]
- Weisman de Mamani A, Suro G. A Randomized Clinical Trial Assessing the Effect of a Culturally-Informed Therapy for Schizophrenia on Self-Conscious Emotions and Burden in caregivers of patients with Schizophrenia. Psychotherapy. 2016;53:156–162. doi: 10.1037/pst0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman de Mamani A, Weintraub MJ, Gurak K, Maura J. A randomized clinical trial to test the efficacy of a family-focused, culturally informed therapy for schizophrenia. Journal of Family Psychology. 2014;28(6):800. doi: 10.1037/fam0000021. [DOI] [PMC free article] [PubMed] [Google Scholar]