Abstract
Flaxseed consumption is associated with reduced oxidative stress and inflammation in lung injury models, and has shown anti-cancer effects for breast and prostate tissues. However, the chemopreventive potential of flaxseed remains unexplored for lung cancer. In this study, we investigated the effect of flaxseed on tobacco smoke carcinogen (NNK)-induced lung tumorigenesis in an A/J mouse model. Mice exposed to NNK were fed a control diet or a 10% flaxseed-supplemented diet for 26 weeks. Flaxseed-fed mice showed reduced lung tumor incidence (78%) and multiplicity, with an average of 2.7 ± 2.3 surface lung tumor nodules and 1.0 ± 0.9 H&E cross-section nodules per lung compared to the control group which had 100% tumor incidence and an average of 10.2 ± 5.7 surface lung tumor nodules and 3.9 ± 2.6 H&E cross-section nodules per lung. Further, flaxseed-fed mice had a lower incidence of adenocarcinomas compared to control-fed mice. Western blotting performed on normal lung tissues showed flaxseed suppressed phosphorylation (activation) of p-AKT, p-ERK, and p-JNK kinases. RNA-Seq data obtained from normal lung and lung tumors of control and flaxeed-fed mice suggested that flaxseed intake resulted in differential expression of genes involved inflammation-mediated cytokine signaling (IL-1,-6,-8,-9, and -12α), xenobiotic metabolism (several CYPs, GSTs, and UGTs), and signaling pathways (AKT and MAPK) involved in tumor cell proliferation. Together, our results indicate that dietary flaxseed supplementation may be an effective chemoprevention strategy for chemically-induced lung carcinogenesis by altering signaling pathways, inflammation, and oxidative stress.
Keywords: Chemoprevention, Cytokines, Lung cancer, RNA sequencing, Signal transduction, Flaxseed, NNK
Introduction
Tobacco smoke contains over 5,000 compounds of which 73 are carcinogenic in laboratory animals and/or humans [1]. Among these, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is one of the most potent carcinogens closely associated with lung carcinogenesis in smokers and in A/J mice independent of the route of administration [1–3]. The occurrence of early onset DNA lesions such as mutations in KRAS oncogene and molecular processes underlying tumor development are similar in humans and NNK-treated A/J mice [4]. Like NNK, benzo [alpha] pyrene (BαP), a polycyclic aromatic hydrocarbon (PAH), is a tobacco carcinogen, and has been extensively used by investigators over the years as the archetypal PAH. It is ubiquitous in the environment and a very potent carcinogen [5]. BαP induces lung cancer in mice, rats, hamsters, and in humans [6, 7]. Therefore, NNK and BαP are commonly studied in the A/J mouse model to assess the chemopreventive potential of various agents for lung cancer.
Several plant-derived phytochemical have shown lung chemopreventive effects. Phytochemicals such as polyphenon E (green tea) [8], PEITC [9, 10] and indole-3-carbinol (cruciferous vegetables) [11], and kavalactones (kava) [12, 13] inhibit development of NNK-induced lung tumors in A/J mice. Their tumor growth-inhibitory properties are attributed to altered expression of phase I cytochrome P450 and phase II detoxifying enzymes involved in NNK activation and detoxification [9, 10]; suppressed NF-κB [12], AKT [11], c-Jun [8] and ERK [8] mediated signaling pathways which regulate aberrant cell proliferation; activated caspase-3 and PARP-mediated apoptosis [12]; and decreased VEGF-mediated angiogenesis [11]. This suggest that plant-derived phytochemicals hold promise for lung cancer chemoprevention.
Flaxseed is a whole-grain oilseed ranging in color from golden yellow to dark brown, and grown across Canada and North Dakota. It is rich in omega-3 fatty acid (α-linolenic acid), lignans (predominantly secoisolariciresinol diglucoside), protein, and fiber. Flaxseed has a nutty taste and can be found in breads, crackers, and other bakery products, or incorporated into the diet by adding to cereal, drinks, yogurt, salads, or soups. The antioxidant and anti-inflammatory components of flaxseed are associated with numerous health benefits including cardiovascular benefits.
The chemopreventive properties of flaxseed are documented in several epidemiological, preclinical, and clinical studies of breast [14–18], colon [19, 20], and prostate cancers [21–23]. Flaxseed-supplemented diets decreases tumor incidence, size, volume, and multiplicity by suppressing cell proliferation [15, 22], inducing cell cycle arrest [23], and inhibiting angiogenesis [14, 18]. However, the chemopreventive potential of flaxseed has not been investigated in lung.
There is evidence that flaxseed intake ameliorates oxidative stress and inflammation, precursors of carcinogenesis, in mouse models of acute and chronic lung injury [24, 25]. In addition, flaxseed significantly alters the expression of genes involved in apoptosis, cell proliferation, inflammation-associated cytokine signaling, oxidative stress, and phase I and phase II detoxification pathways in healthy mouse lung tissue [26]. We have earlier shown that enterolactone (EL), a flaxseed-derived mammalian lignan, inhibits lung cancer cell proliferation by inducing G1-phase cell cycle arrest [27], and suppressing cell motility and invasion by altering FAK-Src signaling [28].
We hypothesized that flaxseed inhibits chemically-induced lung tumorigenesis in A/J mice by modulating genes promoting inflammation and oxidative stress. To test this hypothesis, we studied the effect of 10% flaxseed consumption on inhibition of NNK or BαP-induced lung tumor incidence in A/J mice. We employed RNA sequencing (RNA-Seq) to identify underlying mechanisms associated with the chemopreventive effects of flaxseed in lung.
Material and Methods
Chemicals and diets
NNK was purchased from Toronto Research Chemicals Inc. (North York, Ontario, Canada) and dissolved in saline solution immediately before use. Mouse diets in pellet form were obtained from TestDiet® (St. Louis, MO) and kept under cool (2°C), dry conditions for long-term storage. The control diet was AIN-93G and the treatment diet was AIN-93G supplemented with 10% flaxseed. These isocaloric diets were prepared according to the composition shown in Supplementary Table 1.
A/J mouse model of lung cancer
Five- to six-week-old male A/J mice (n=24) procured from The Jackson Laboratory (Bar Harbor, ME) were housed in the Animal Nutrition and Physiology Center at North Dakota State University (NDSU). The mice were kept in individual solid-bottomed polycarbonate cages with ALPHA-Dri bedding in a pathogen-free, humidified (50–70%), and temperature-controlled (22–25 °C) environment with a 12 h light/dark cycle. The mice were fed ad libitum control diet for one week prior to the start of the experiment. The study protocol was approved by NDSU’s Institutional Animal Care and Use Committee.
Treatment of A/J mice with NNK
After acclimation, mice were assigned into a control group or 10% flaxseed group (n=12) with similar average mouse weights between groups. All mice received weekly intraperitoneal injection of NNK (50 mg/kg body weight) for 4 weeks. Immediately after the first NNK injection, the treatment group mice were switched to 10% flaxseed-supplemented AIN 93G diet, while the control group mice continued feeding on the AIN 93G diet (Fig. 1A). Three mice in the treatment group were removed from the study when they did not readily accept the 10% flaxseed diet. One mouse from the control group was euthanized at 23 weeks to confirm the onset of lung tumor development. The remaining mice, 11 in control group and 9 in 10% flaxseed group, were maintained on their respective diets for 26 weeks. Food consumption was regularly monitored and fresh diet and water was provided ad libitum. Mice were weighed once a month for 6 months. At the end of the study, the mice were euthanized under anesthesia using ketamine/xylazine followed by cervical dislocation. Lung tissues were harvested, weighed, and imaged using a camera attached to an inverted microscope (Zeiss lumar, V12 stereo motorized microscope). Subsequent to imaging, the left lung from each mouse was snap frozen in liquid nitrogen and stored at −80 °C for further molecular analysis, and the right lung was perfused with freshly prepared 10% buffered formaldehyde solution for fixation.
Figure 1.
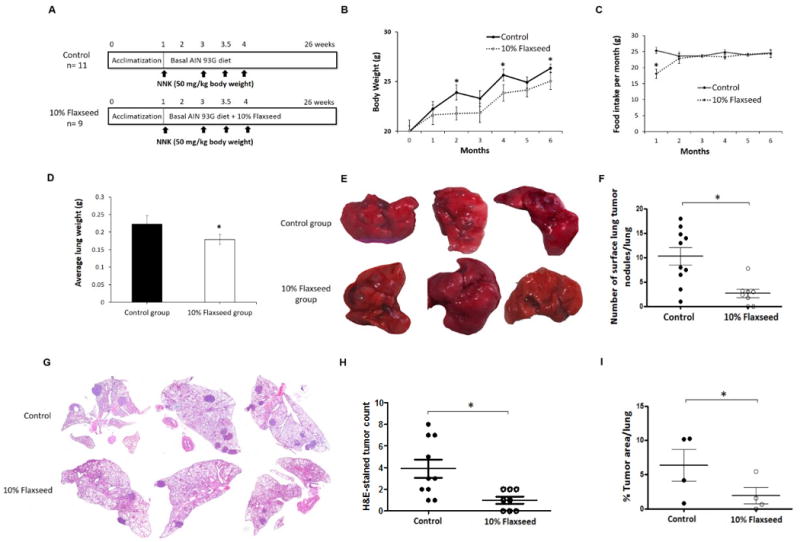
Flaxseed consumption suppresses NNK-induced lung tumor development in an A/J mouse model. A, Schematic representation of the experimental design. A/J mice were injected intraperitoneally with NNK (50 mg/kg) once a week for 4-weeks. After the first NNK injection, control mice were fed the basal-AIN-93G diet and treatment mice were fed AIN-93G supplemented with 10% flaxseed. B, The average body weight (g) ± standard deviation for control and 10% flaxseed-fed mice are shown monthly for the duration of the study. C, Food consumption (g) over the course of a month was recorded for each group of mice, and error bars represent ± standard deviation of monthly food consumption. D, The average lung weight (g) for control and flaxseed-fed mice was deteremined, and error bars represent ± standard deviation of the lung weight for each group. E, Representative images from three control-fed and three flaxseed-fed mice showing surface lung tumor nodules. F, The number of surface tumor nodules per lung was counted by 4 independent observers and averaged per mouse lung for the control and 10% flaxseed-fed animals. Error bars represent ± standard deviation for tumor number. G, Representative images for H&E-stained lung tissue sections obtained from control or flaxseed-fed animals. H, The number of lung tumor nodules per lung from H&E cross-section images. Data represent mean ± standard deviation. I, Tumor morphometry data from H&E-stained lung tissue sections to analyze tumor burden in control and 10% flaxseed group mice (n=4/group). Statistically significant differences are denoted with *, p ≤ 0.05.
Treatment of A/J mice with BaP
A/J mice (n=5 per treatment group) were exposed to a total of 4 mg of benzo [alpha] pyrene (BαP) via sequential 1 mg BαP intraperitoneal injections administered once every 7 days. Control and flaxseed diets were administered either 3 weeks prior to the initial BαP exposure or simultaneously starting with the initial BαP exposure. Mice were euthanized at 5 months following the initial BαP exposure and lungs were histologically evaluated for tumor formation. This study protocol was approved by the University of Pennsylvania’s Institutional Animal Care and Use Committee.
Quantification of flaxseed-derived mammalian lignans enterodiol (ED) and enterolactone (EL) in lung and blood plasma
The ED and EL levels in lung tissue of control-fed (n=11) and 10% flaxseed-fed mice (n= 9), and their levels in circulating blood plasma in control-fed (n=3) and 10% flaxseed-fed mice (n=7) were determined by liquid chromatography tandem mass spectrometry using commercially available standards (Chromadex,Santa Ana, CA) as previous described [24]. Briefly, lignans were separated using the ACQUITY UPLC I-Class System (Waters, Medford, MA) and gradient elution with 0.2% formic acid in acetonitrile and 0.2% formic acid in water. The flow rate was 0.3 mL/min over a C18 column and the runtime was 4 minutes. An AB Sciex 4000 triple quadrupole mass spectrometer (Framingham, MA) equipped with Turbo Ionspray was used for sample analysis. Lignans were monitored by negative ion electrospray tandem mass spectrometry.
Hematoxylin and eosin (H&E) staining
Formaldehyde-fixed paraffin-embedded lung tissue samples were used to prepare 5 μm thick sections on poly-L-lysine-coated slides. Tissue sections were stained with H&E for histopathological observation of the incidence, multiplicity, and size of surface lung tumor nodule in each mouse. Tumor incidence was defined as the number of mice in each group bearing one or more tumor nodules divided by the total number of mice examined. Lung tumor nodules were microscopically examined and quantified by four independent researchers blinded to control and treatment groups. Classification of lung tumors as adenomas or adenocarcinomas was performed by a pathologist according to guidelines previously published [29].
Tumor morphometry
Quantitative morphometric analysis was performed on H&E stained serial lung sections, with an incremental depth of 20 μm, from control and 10% flaxseed-fed mice (n=4/group). Image analysis was performed using the Aperio ScanScope SC (Leica Biosystems Inc; Buffalo Grove IL), Aperio ImageScope, and Aperio Genie Histology Pattern Recognition Software. The glass slides with H&E stained-lung sections were scanned and the images created at 20× magnification. Using the Genie software, a unique algorithm was created based on pattern recognition to separate: i) tumor nodules, ii) benign lung tissue, and iii) glass background. The slides were visualized with Aperio ImageScope for quality control and quantitative measurement of the area occupied by tumor in the digital images. Tumor burden was calculated as % tumor area with respect to total lung area.
Western blotting
Western blotting was performed as described elsewhere [28]. Briefly, 5 milligrams of normal lung tissue from control mice (n=11) and 10% flaxseed-fed mice (n=9) were lysed, proteins were separated on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes before probing with primary antibodies (1:1000; p-AKT, t-AKT, p-ERK, t-ERK, p-JNK, and t-JNK). Anti-rabbit HRP-conjugated secondary antibodies (1:1000) and SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific; Waltham, MA) were used to detect bands, and the images were captured using the MultiImage™ Light Cabinet (Alpha Innotech; San Leandro, CA). The densitometry results were obtained using ImageJ software. p-AKT, p-ERK, and p-JNK protein levels were normalized to their corresponding total proteins.
RNA extraction
RNA from normal lung tissue and lung tumor nodules of control and 10% flaxseed group mice (n=3/group) was extracted using TRIzol reagent (Life Technologies; Carlsbad, CA). Briefly, 5 mg of tissue was homogenized in 1 mL of TRIzol. After incubating the homogenate at room temperature for 15 min, 50 μl of BCP was added. The tube was shaken vigorously for 30 s, incubated again at room temperature for 3 min, and then centrifuged at 12,000×g for 15 min at 4°C. The supernatant was transferred to a fresh tube and total RNA was precipitated by adding 1.5 volumes of 100% ethanol. The precipitated RNA sample was transferred to RNeasy Mini spin column (Qiagen; Valencia, CA) and RNA purification was performed using the manufacturer’s protocol.
RNA-Seq
Three micrograms of total RNA from three mice in each group: normal lung tissue from control group (CN), tumor lung nodule from control group (CT), normal lung tissue from 10% flaxseed group (FN), and tumor lung nodule from 10% flaxseed group (FT) were sent for sequencing to the University of Minnesota Genomics Center. Six barcoded libraries were created and sequenced on Illumina HiSeq 2500. Single-end reads of 50 base pairs (bp) were obtained and mapped to the Mus musculus genome (Build: Mus_musculus.GRCm38) downloaded from Ensembl. The raw fastq were subjected to quality trimming using Sickle, for a minimum length of 50. HISAT2 [30] was used to map the reads on the genome. SAMtools [31] and BAMtools [32] were used to convert and sort the BAM files. HTSeq [33] was used to count the reads.
Regularized log transformed (rlog) data were obtained using the method implemented in the Bioconductor DESeq2 package(v1.14.1). Fragment per kilobase of exon per million fragments mapped (FPKM) were calculated for each gene by normalizing the read count data to both the length of the gene and the total number of mapped reads in the sample. FPKM values were used to plot the heat maps. Differential gene expression analysis was performed using the DESeq2 package. An adjusted P value (q-value) threshold of ≤ 0.1 using the Benjamini & Hochberg’s method and log2 fold change ≥1 were used as the statistical cutoff criteria to further study the differentially expressed genes.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was used to confirm expression changes for select genes found differentially expressed by RNA-Seq. The validation of RNA-Seq results was done using RNA from normal lung tissue of 11 mice in the control group and 9 mice in the flaxseed-fed group, and RNA from lung tumor nodules of 11 mice in the control group and 7 mice in the flaxseed-fed group. Total RNA was isolated from tissues using Fisher SurePrep Kit (Waltham, MA) as per the manufacturer’s instructions. cDNA was synthesized using 50 ng of total RNA and the qScript cDNA synthesis kit (Quanta Biosciences; Beverly, MA). Primers for genes listed in Supplementary Table 2 were designed using Primer Express software (version 2.0, Applied Biosystems; Foster City, CA), and were synthesized by Sigma-Aldrich (St. Louis, MO). Steady-state mRNA levels were evaluated by qPCR using methods described elsewhere [34]. The relative change in gene expression was calculated using 2−ΔΔCt method using housekeeping genes 18S rRNA and β-actin as internal controls.
Results
Flaxseed consumption is associated with reduced body mass in A/J mice
Mice in both diet groups were weighed every 4 weeks, over the 6-month period, and their body mass was determined. Prior to the first NNK administration, the average body weight of mice in the control and flaxseed-fed groups was 20.0 and 20.1 g, respectively. During the study, mice on the 10% flaxseed diet showed a slightly reduced average body mass, with statistically significant differences observed at 2, 4, and 6 months of the study (Fig. 1B). At the end of the study, the average body weight of mice in the control group (26.3 ± 0.8 g) was significantly higher than that of the 10% flaxseed group (25.0 ± 1.6 g; p≤0.05). The flaxseed-fed mice were slow to transition to their new diet, and consumed less food in the first two months compared to the control-fed mice (Fig. 1C). The reduced food consumption in the flaxseed-fed mice likely explains the difference observed in average body weight throughout the study.
Flaxseed-fed mice exhibit reduced lung mass, tumor incidence, and multiplicity
The lungs from NNK-treated control-fed mice (n=11) and flaxseed-fed mice (n=9) were weighed at the conclusion of the study, and the average lung mass was 0.23 g and 0.18 g, respectively. This difference was statistically significant (p≤0.05) (Fig. 1D). The control mice had 100% incidence of lung adenocarcinoma formation with a range of 2 to 36 surface lung tumor nodules per mouse, and an average of 10.2 ± 5.7 lung tumor nodules per lung. Strikingly, the 10% flaxseed diet reduced adenocarcinoma incidence to 78%, and the number of surface lung tumor nodules per mouse was significantly (p≤0.05) less ranging from 0 to 15, with an average of 2.7 ± 2.3 lung tumor nodules per lung (Fig. 1E, F). On H&E-stained lung tissue sections, tumor nodules ranged from 1 to 8, with an average of 3.9 ± 2.6 per lung in control mice. Flaxseed-fed mice exhibited a significant (p≤0.05) decrease in the number of lung tumor nodules ranging from 0 to 2, with an average of 1.0 ± 0.93 per lung (Fig. 1G, H). Pathological classification of H&E-stained lung tumors revealed significantly fewer adenocarcinomas in the flaxseed-fed group (average of 0.25 per lung) compared to the control-fed group (average of 0.41 per lung). The adenoma to carcinoma ratio was 3:1 in flaxseed-fed mice compared to 1.4:1 in control-fed mice (Table 1). Finally, tumor morphometry data showed flaxseed-fed mice had significantly decreased tumor burden as compared to control group mice (0.0–5.4 vs. 0.7–10.2 % of total lung area) (Fig. 1I). These findings suggest that 10% flaxseed intake reduces NNK-induced lung tumor development, multiplicity, and tumor burden in A/J mice, and has the potential to prevent lung tumors from progressing to more advanced disease.
Table 1.
Number and classification of lung tumors for control and flaxseed-fed mice
| Treatment | Adenomas (A) | Adenocarcinomas (AC) | Ratio A:AC | Average tumors/lung |
|---|---|---|---|---|
| Control (n=10) | 23 | 16 | 1.4:1 | 3.9 |
| Flaxseed (n=8) | 6 | 2 | 3:1 | 1.0 |
The chemopreventive properties of flaxseed were also evaluated in a BαP-induced A/J mouse model. Body weights for control and flaxseed-fed mice were similar for the early time points, but show elevated weight in flaxseed-fed mice at the end of the study (Fig. 2A and B). Lung tumor formation was decreased among mice fed a flaxseed diet that was initiated 3 weeks prior to BαP exposure (31 lung tumor nodules among mice fed a flaxseed diet compared to 43 lung tumor nodules among mice fed a control diet) (Fig. 2C). The average number of tumor nodules per lung (Fig. 2D) was also decreased among mice fed a flaxseed diet (6.2 ± 1.7 lung tumor nodules among mice fed a flaxseed diet compared to 8.6 ± 1.9 lung tumor nodules among mice fed a control diet). Further, the average tumor nodule size (Fig. 2E and F) was significantly decreased (p=0.005) among mice fed a flaxseed diet (12.3 ± 0.4 μM2) compared to a control diet (27.7 ± 3.9 μM2). Total tumor area per lung (Figure 2G) was also significantly decreased (p<0.05) among mice fed a flaxseed diet (121.0 ± 36.6 μM2) compared to a control diet (256.7 ± 58.7 μM2). Finally, the percent of lung area infiltrated by tumor (Fig. 2H) was significantly (p<0.05) reduced in flaxseed-fed mice (1.9 ± 0.8%) compared to control-fed mice (4.6 ± 0.7%).
Figure 2.
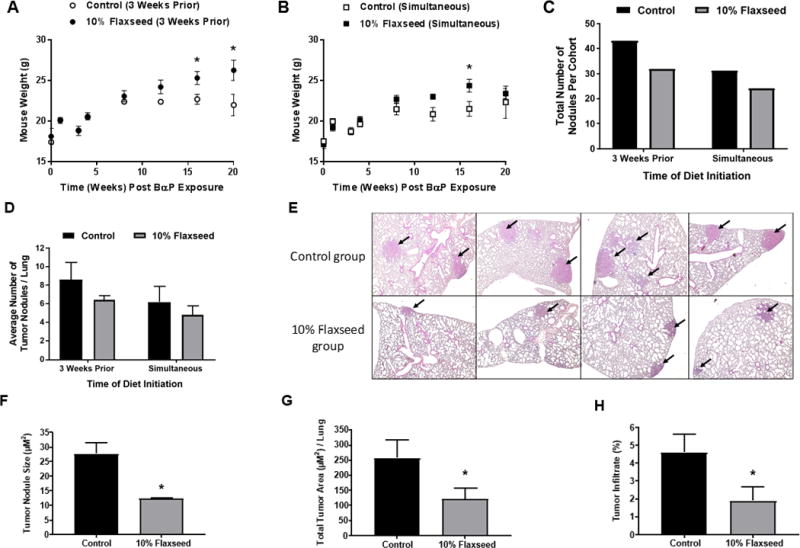
Flaxseed consumption suppresses BαP-induced lung tumor development in an A/J mouse model. Mouse bodyweight is shown for control and 10% flaxseed-fed animals over time (20 weeks) in A, mice fed flaxseed 3 weeks prior to BαP injection and B, mice fed flaxseed at the start of BαP injection. C, The total number of lung tumor nodules and D, average number of tumor nodules per lung are shown for mice fed the control diet or 10% flaxseed diet 3 week prior to or simultaneous with BαP injection. E, Top panels: Lung histology for mice fed control diet and evaluated 5 months post BαP exposure. Lower panels: Lung histology for mice fed 10% flaxseed-supplemented diet. Magnification 400X. Quantitative assessment of H&E-stained lung sections for F) tumor nodule size, G, total tumor area per lung, and the percentage of lung occupied by tumor in control and flaxseed-fed mice. H, Data are presented as mean ± SEM. Sample size = 5 mice per group. Asterisks indicate significant (p<0.05) differences between control and flaxseed fed mice.
Levels of ED and EL in mouse lung tissue and blood plasma
The amount of flaxseed-derived mammalian lignans ED and EL were quantified in lung tissues of control and 10% flaxseed-fed mice. As expected, ED, the first mammalian lignan to appear after consumption of flaxseed, was not detected in lung tissues of control group mice; while, 5 out of 9 flaxseed-fed mice showed ED levels ranging from 19.5–106 ng/g, with an average of 31.70 ng/g of tissue. Surprisingly, EL was not detected in the lung tissues from either diet group.
Circulating levels of ED and EL were detected in blood plasma of both diet groups. Minor amounts of ED, ranging from 1.8–4.7 ng/ml, with an average of 2.8 ng/ml were detected in control group mice, while levels of ED ranging from 16.9–4,360 ng/ml, with an average of 1,539.6 ng/ml were detected in the 10% flaxseed group mice. EL was not detected in blood plasma of control-fed mice, while it was found to be 0–68.4 ng/ml, with an average of 20.8 ng/ml in blood plasma of 10% flaxseed-fed mice.
Flaxseed-supplementation reduced phosphorylation of AKT, ERK, and JNK in normal lung tissue of NNK-treated mice
Sustained phosphorylation leading to activation of PI3K/AKT signaling [35] and mitogen-activated protein kinase (MAPK) signaling such as ERK [36] and JNK [37], which are downstream substrates of KRAS, play a role in NNK-induced lung carcinogenesis in A/J mice. Therefore, we investigated the effect of 10% flaxseed on phosphorylation status of these proteins in normal lung tissue. Reduced phosphorylation of AKT, ERK, and JNK protein kinases was detected in 10% flaxseed-fed mouse lung tissue compared to control group mice (Fig. 3 A–F). These results suggest that 10% flaxseed reduces the incidence of lung tumor development by inhibiting aberrant activation of AKT, ERK, and JNK signaling molecules that play a role in cancer cell survival and proliferation.
Figure 3.
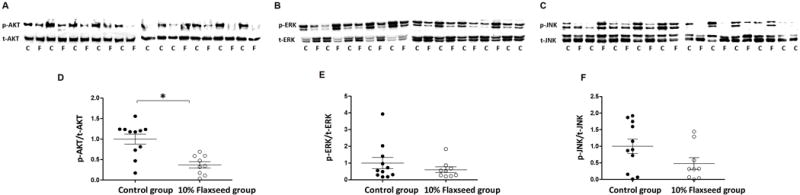
Flaxseed consumption reduces expression of phosphorylated AKT, ERK, and JNK protein kinases. Fifty μg of protein isolated from normal lung tissue of mice fed control (C) or 10% flaxseed (F) diets were subjected to western blotting to analyze expression levels of phosphorylated and total A, AKT, B, ERK, and C, JNK protein kinases. Densitometry measurements of western blot bands were performed using ImageJ software. The ratio of phosphorylated to total D, AKT, E, ERK, and F, JNK are shown, and include error bars representing ± standard deviation for control-fed mice (n=11) and flaxseed-fed mice (n=9). Statistically significant differences are denoted with *, p ≤ 0.05.
Identification of differentially expressed transcripts in normal lung tissue and tumor nodules of control and flaxseed-fed mice using RNA-Seq
To identify the gene expression changes contributing to the chemopreventive effects of flaxseed in mouse lungs, RNA-Seq was performed on RNA isolated from three mice each from CN, CT, FN, and FT tissues (explained in Material and Methods). Principal component analysis (PCA) showed that gene expression analysis for CN and CT tissues clustered close to one another, showing strong inter-group correlation of gene expression. However, the gene expression analysis from FN and FT tissues segregated in two distinct clusters, indicating different inter-group gene expression but a strong intra-group correlation (Fig. 3A). In addition, the PCA plot indicated inter-group variation exists in the gene expression signature between FN vs. CN and FT vs. CT tissues.
We compared the transcriptomes of FN with CN and FT with CT to identify differentially expressed genes. A total of 16,837 gene transcripts were detected in FN vs. CN and FT vs. CT lung tissues. A cutoff criterion, mentioned in Material and Methods, was used to generate a list of significantly differentially expressed transcripts. Compared to the control-group, 10% flaxseed-supplementation resulted in significant differential expression of 5,951 (35.3%; q ≤ 0.10) genes in the normal lung tissue, of which 2,094 (35.1%) transcripts had > 1 log2 fold change (upregulated) while 547 (9.1%) had < 1 log2 fold change (downregulated). Similarly, in the lung tumor nodules, 10% flaxseed-supplementation resulted in significant differential expression of 3,096 (18.3%; q ≤ 0.10) genes, of which 1,329 (42.9%) transcripts had > 1 log2 fold change (upregulated) while 151 (4.8%) had < 1 log2 fold change (downregulated).
Data analysis using Reactome software revealed that the differentially expressed genes are categorized into diverse molecular pathways. The RNA-Seq data analysis for FN vs. CN lung tissues identified genes belonging to pathways regulating, (i) phase I and II xenobiotic metabolism, (ii) cell proliferation involving MAPK and PI3K/AKT signaling pathways, (iii) immune system via cytokines, (iv) detoxification of reactive oxygen species (ROS), (v) cell death, (vi) cellular response to stress and hypoxia, (vii) cell cycle, and (viii) DNA repair (Table 2). Similarly, Table 3 lists pathways differentially regulated between the FT vs. CT lung tissues. The heat maps in Fig. 4 B–C, generated using FPKM values, display differentially expressed genes for FN vs. CN and FT vs. CT lung tissues. A list of these genes along with their average log2 fold change for 3 mice in each group has been provided in Supplementary Tables 3 and 4.
Table 2.
Pathway categorization of significantly (q ≤ 0.1) differentially expressed genes in normal lung tissues from flaxseed-fed mice compared to control-fed mice
| Pathway name | Total genes in the pathway | Identified genes in the pathway | % Significant genes |
|---|---|---|---|
| Xenobiotics | 23 | 12 | 52.17 |
| Phase I | 106 | 33 | 31.13 |
| RAF/MAPK signaling | 253 | 43 | 17.00 |
| SOS-mediated signaling | 253 | 43 | 17.00 |
| IRS-mediated signaling | 329 | 51 | 15.50 |
| EGFR signaling pathway | 373 | 53 | 14.21 |
| VEGF signaling | 347 | 48 | 13.83 |
| PI3K-AKT signaling | 138 | 18 | 13.04 |
| FGFR signaling | 94 | 11 | 11.70 |
| Phase II | 103 | 12 | 11.65 |
| Cytokine signaling in Immune system | 835 | 94 | 11.26 |
| Development biology | 1066 | 111 | 10.41 |
| Detoxification of ROS | 39 | 4 | 10.26 |
| Wnt signaling | 296 | 23 | 7.77 |
| Apoptosis | 174 | 10 | 5.75 |
| Cellular response to hypoxia | 75 | 3 | 4.00 |
| Cellular response to stress | 423 | 15 | 3.55 |
| Cell cycle | 604 | 20 | 3.31 |
| Transcription regulation by TP53 | 364 | 11 | 3.02 |
| Cell cycle checkpoints | 183 | 3 | 1.64 |
| DNA repair | 293 | 4 | 1.37 |
Table 3.
Pathway categorization of significantly (q ≤ 0.1) differentially expressed genes in lung tumor tissues from flaxseed-fed mice compared to control-fed mice
| Pathway name | Total genes in the pathway | Identified genes in the pathway | % Significant genes |
|---|---|---|---|
| ERBB signaling | 46 | 6 | 13.04 |
| MAPK signaling | 298 | 30 | 10.07 |
| IRS-mediated signaling | 302 | 30 | 9.93 |
| Development biology | 1066 | 91 | 8.54 |
| FGFR signaling pathway | 94 | 8 | 8.51 |
| VEGF signaling | 347 | 29 | 8.36 |
| PI3K-AKT signaling | 135 | 11 | 8.15 |
| EGFR signaling pathway | 373 | 30 | 8.04 |
| Mitochondrial biogenesis | 56 | 4 | 7.14 |
| Metabolism | 2,157 | 142 | 6.58 |
| Cytokine signaling in Immune system | 835 | 52 | 6.23 |
| Wnt signaling | 296 | 16 | 5.41 |
| Apoptosis | 174 | 9 | 5.17 |
| JNK Signaling | 22 | 1 | 4.55 |
| Cellular response to hypoxia | 66 | 3 | 4.55 |
| Cellular response to stress | 423 | 12 | 2.84 |
| Transcription regulation by TP53 | 364 | 7 | 1.92 |
| Cell cycle | 499 | 8 | 1.60 |
Figure 4.
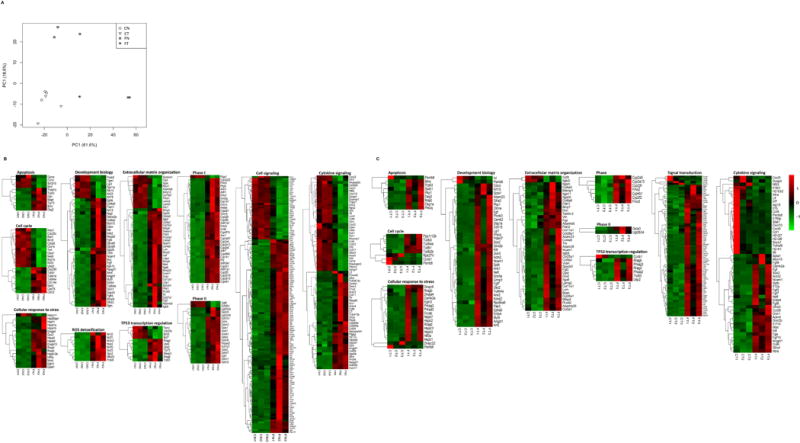
Flaxseed consumption modulates gene expression in normal lung and lung tumor nodules from A/J mice. A, The PCA plot for the RNA-Seq data show transcriptome clustering for normal (CN) and tumor (CT) tissue from control-fed mice, and normal (FN) and tumor (FT) tissues from 10% flaxseed-fed mice. Hierarchical clustering was used to group transcripts into diverse molecular pathways based on expression levels (FPKM). Heat maps for gene expression results obtained from RNA-Seq are shown for B, FN vs. CN (3 mice for each treatment group), and C, FT vs. CT (3 mice for each treatment group). The color gradient represents low (green) to high (red) expression levels for the indicated genes.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
To confirm differential expression by RNA-Seq, we used RT-qPCR to validate selected genes involved in signal transduction, xenobiotic metabolism, transcriptional regulation, and tumor suppression. RT-qPCR results largely supported the RNA-Seq results for the subset of genes we chose to validate (Fig. 5A and B). For example, PPR2, CYP2A5, ELGN3, HIF3a, IL12a, and TP63 were elevated in flaxseed normal and tumor tissue relative to control tissues for both RNA-Seq and RT-qPCR. Western blotting confirmed elevated TP63 protein expression in the majority of flaxseed-fed mouse lungs compared to control-fed animals (Fig. S1). Occasional inconsistencies in the expression of interleukins were observed between RNA-Seq and RT-qPCR, and may be result from a small RNA-Seq sample size (n=3) in our study.
Figure 5.
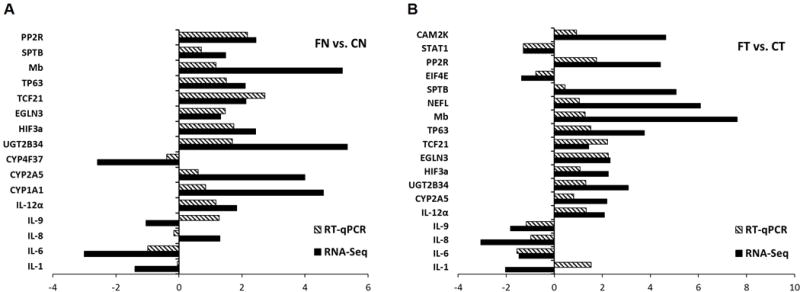
RT-qPCR results confirm flaxseed modulates expression of signal transduction, xenobiotic metabolism, transcriptional regulation, and tumor suppression-associated genes. The graphs show the log2 fold change in expression of 16 different genes quantified by RT-qPCR (striped bars) or RNA-Seq (black bars) for A, normal lung tissue from flaxseed-fed mice (FN) compared to control-fed mice (CN) and B, lung tumor tissue from flaxseed-fed mice (FT) compared to control-fed mice (CT). RNA-Seq expression data were obtained from three mice per treatment group; whereas RT-qPCR data were obtained from eleven mice for CN or CT, nine mice for FN, and seven mice for FT, since only 7 of 9 mice in the flaxseed-fed group developed tumors.
Discussion
Most lung cancers are caused by exposure to tobacco smoke carcinogens. A dietary intervention with potential to protect against chemically-induced lung tumorigenesis would be extremely useful as lung cancer has one of the lowest 5-year survival rates among cancer patients. Previous studies indicate phytochemicals such as polyphenon E [8], PEITC [9, 10], and indole-3-carbinol [11] inhibit development of NNK-induced lung tumors in A/J mice. The present study investigated the effects of flaxseed on formation of chemically-induced lung tumors and the molecular mechanisms associated with the observed lung chemopreventive properties of flaxseed. Our results suggest dietary flaxseed can inhibit NNK or BαP lung tumor development and progression to more advanced disease regardless of when the flaxseed diet was initiated (3 weeks prior to or simultaneous to the initial BαP exposure).
Previous evidence suggests that flaxseed supplementation ameliorates oxidative stress and inflammation in lung injury models [24, 25], and significantly alters gene expression associated with apoptosis, cell proliferation, inflammation-associated cytokine signaling, oxidative stress, and phase I and phase II detoxification pathways in healthy mouse lung tissue [26]. Similarly, we found flaxseed was associated with gene expression changes for signal transduction pathways such as PI3K/AKT and MAPK, apoptosis, cell cycle regulation, cellular response to hypoxia and stress, cytokine response in immune system, development biology, extracellular matrix organization, transcription regulation by TP53, and xenobiotics metabolism. Most transcriptomics studies similar to ours evaluated gene expression changes only in the tumor tissue from control vs. treatment animals. Here, we also compared normal tissue from control and flaxseed-fed animals and found that gene expression patterns were similar for FN vs. CN and FT vs. CT tissues. This suggest that the gene transcription changes happening in normal tissues are a good indicator for what is also happening in the tumor tissues.
At the end of the study, we detected levels of ED and EL in lung tissue and in blood plasma of 10% flaxseed group mice. This suggest that even though initially the flaxseed group mice were reluctant to eat the fortified diet, they became accustomed to it and consumed it as well as the control diet during the majority of the study. The flaxseed-fed mice had lower average body weight at the end of the study which could be attributed to, (i) reduced food consumption in the first two months of the study, (ii) reduced fat digestibility [38], and/or (iii) altered adipogenesis-gene regulation [39]. The decrease in average weight of lungs in the flaxseed fed mice as compared to control group mice suggest tumor burden may be a contributing factor. Flaxseeds contain ~ 30% dietary fibers, consumption of which has been shown to reduce fat digestibility and body weight gain in rats [38]. In addition, flaxseed lignan secoisolariciresinol diglucoside exerts a suppressive effect on body weight gain in mice by altering lipid metabolism [39]. Our result is in line with the effect of flaxseed on body weight gain reported earlier in rodents.
High levels of phosphorylated AKT and ERK are observed in bronchial preneoplastic lesions [40–42], which are precursors of lung adenoma and adenocarcinoma [8]. In addition, NNK-induced murine lung lesions also exhibit increased activation of AKT [35] and ERK [36]; therefore, suppression of these signaling pathways may be critical for improved lung cancer inhibition. We observed decreased phosphorylation of AKT and ERK protein kinases in normal lung tissue from NNK-treated flaxseed-fed mice. Our findings are in line with other reports showing decreased phosphorylation of AKT and ERK protein kinases after treatment with chemopreventive agents such as, degeulin [43], indole-3-carbinol [11], myoinositol [40], and metformin [44] in NNK-induced mouse models of lung tumorigenesis.
AKT is negatively regulated by protein phosphatase-2alpha (PP2A). Our RNA-Seq data showed that the expression levels of PP2A were significantly increased in lung tissues from flaxseed-fed mice. The PP2A gene encodes a major cellular serine-threonine phosphatase and is a potential tumor suppressor due to its negative regulation on kinase-driven intracellular signaling pathways in several cancers, including lung cancer. This suggest that dephosphorylation of AKT observed in healthy lung tissue of flaxseed-fed mice may be due to increased expression of PP2A. Further, enhanced AKT signaling directly correlates with increased rates of aerobic glycolysis in cancer cells [45]. Inhibition of AKT signaling may inhibit glycolysis and elevate reactive oxygen species, leading to preferential killing of cancer cells through oxidative stress [46].
In addition to AKT and ERK signaling pathways, de-regulation of another pivotal kinase, JNK, promotes development of tobacco smoke–induced lung tumors [47]. Genetic ablation of JNK1 alone is associated with reduced tobacco smoke-induced lung tumor multiplicity and size [37]. In this study, we observed that NNK-treated 10% flaxseed-fed mice exhibited decreased phosphorylation of JNK protein kinase in normal lung tissue. These findings, together with our observations suggest that inhibiting AKT, ERK and JNK activity might be a valuable approach to suppress/delay the effects of NNK-induced lung tumorigenesis.
NNK has been shown to up-regulate pro-inflammatory cytokines and down-regulate anti-inflammatory cytokines which contribute to immunosuppression and lung tumor development. NNK up-regulated pro-inflammatory IL-6 [48] and IL-8 [49] leading to increased cell proliferation, neovascularization, and tumor development. IL-1α binding to IL-1 receptor enhanced the production of IL-8 in human airway epithelial cells, indicating that suppression of IL-1α production may reduce IL-8-mediated lung inflammation [50]. Our RNA-Seq data showed a significant decrease in IL-6, IL-1α, and IL-8 mRNA expression in lung tumors in response to flaxseed consumption. NNK inhibits the production of the anti-inflammatory cytokine IL-12α by alveolar macrophages and increases the risk of developing lung cancer by suppressed activation of natural killer cells [51]. We found IL-12α was significantly elevated in flaxseed-fed mice, which may have contributed to reduced lung tumor formation in these animals. Our findings suggest that flaxseed may suppress lung carcinogenesis by mitigating NNK-induced lung immunosuppression by reducing expression of pro-inflammatory cytokines (IL-1α, IL-6, IL-8, and IL-9) and increasing the expression of anti-inflammatory cytokine (IL-12α).
Plant-derived phytochemicals exert chemopreventive effects at the initiation level by (i) inhibiting the expression and/or activity of phase I enzymes (cytochrome P450 (CYPs)) that activate procarcinogens such as NNK, and/or (ii) inducing phase II enzymes (glutathione S-transferases (GSTs) and UDP-glucuronosyltransferase (UGTs)) that detoxify electrophilic metabolites generated by phase I enzymes [9, 10]. In our study, the RNA-Seq data showed that 10% flaxseed altered expression of several CYPs, GSTs, and UGTs. This indicates that chemopreventive property of 10% flaxseed may be dependent on its ability to induce expression of phase II enzymes (GSTs and UGTs) which are responsible for detoxification of NNK.
In summary, we found flaxseed consumption protects against chemically-induced (both NNK and BαP) lung tumor formation in mice. Flaxseed influenced gene expression in normal lung and lung tumor tissues including up-regulating the expression of phase II enzymes, anti-inflammatory cytokines and tumor suppressors, and down-regulating the expression of pro-inflammatory cytokines which may explain the anti-tumor effects of flaxseed in this model. Further work is needed to identify the potential for flaxseed to impact gene expression in airways of a human population exposed to tobacco smoke carcinogens.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. C.C. Solomides for histopathological evaluation of the lung sections.
Financial support: Funding for this pilot project was made possible by NIH Grant Numbers P30 GM103332 and P20 GM109024 (to KMR) from the National Institute of General Medicine (NIGMS). Additional funding to support the work was provided byNIH Grant Number P42 ES023720 (to MCS) from the National Institute of Environmental Health Sciences.
Footnotes
Conflict of Interest: Dr. Christofidou-Solomidou (MCS) has patents No. PCT/US14/41636 and No. PCT/US15/22501 pending and has a founders equity position in LignaMed, LLC. All other coauthors report no actual, potential, or perceived conflict of interest with regard to this manuscript.
References
- 1.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131(12):2724–32. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeifer GP, et al. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21(48):7435–51. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 3.Hecht SS, et al. Rapid single-dose model for lung tumor induction in A/J mice by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and the effect of diet. Carcinogenesis. 1989;10(10):1901–4. doi: 10.1093/carcin/10.10.1901. [DOI] [PubMed] [Google Scholar]
- 4.Belinsky SA, et al. Relationship between the formation of promutagenic adducts and the activation of the K-ras protooncogene in lung tumors from A/J mice treated with nitrosamines. Cancer Res. 1989;49(19):5305–11. [PubMed] [Google Scholar]
- 5.Cavalieri EL, et al. Comparative dose-response tumorigenicity studies of dibenzo[alpha,l]pyrene versus 7,12-dimethylbenz[alpha]anthracene, benzo[alpha]pyrene and two dibenzo[alpha,l]pyrene dihydrodiols in mouse skin and rat mammary gland. Carcinogenesis. 1991;12(10):1939–44. doi: 10.1093/carcin/12.10.1939. [DOI] [PubMed] [Google Scholar]
- 6.Osborne M, Crosby ANT. Benzopyrenes. C. Cambridge University Press; England: p. 1987. [Google Scholar]
- 7.Straif K, et al. Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet Oncol. 2005;6(12):931–2. doi: 10.1016/s1470-2045(05)70458-7. [DOI] [PubMed] [Google Scholar]
- 8.Lu G, et al. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006;66(23):11494–501. doi: 10.1158/0008-5472.CAN-06-1497. [DOI] [PubMed] [Google Scholar]
- 9.Smith TJ, et al. Mechanisms of inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone bioactivation in mouse by dietary phenethyl isothiocyanate. Cancer Res. 1993;53(14):3276–82. [PubMed] [Google Scholar]
- 10.Crampsie MA, et al. Phenylbutyl isoselenocyanate modulates phase I and II enzymes and inhibits 4-(methylnitrosamino)-1-(3-pyridyl)- 1-butanone-induced DNA adducts in mice. Cancer Prev Res (Phila) 2011;4(11):1884–94. doi: 10.1158/1940-6207.CAPR-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian X, et al. Indole-3-carbinol inhibited tobacco smoke carcinogen-induced lung adenocarcinoma in A/J mice when administered during the post-initiation or progression phase of lung tumorigenesis. Cancer Lett. 2011;311(1):57–65. doi: 10.1016/j.canlet.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson TE, et al. Chemopreventive effect of kava on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo[a]pyrene-induced lung tumorigenesis in A/J mice. Cancer Prev Res (Phila) 2008;1(6):430–8. doi: 10.1158/1940-6207.CAPR-08-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayanapillai SC, et al. Dihydromethysticin from kava blocks tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis and differentially reduces DNA damage in A/J mice. Carcinogenesis. 2014;35(10):2365–72. doi: 10.1093/carcin/bgu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabrosin C, et al. Flaxseed inhibits metastasis and decreases extracellular vascular endothelial growth factor in human breast cancer xenografts. Cancer Lett. 2002;185(1):31–7. doi: 10.1016/s0304-3835(02)00239-2. [DOI] [PubMed] [Google Scholar]
- 15.Fabian CJ, et al. Reduction in Ki-67 in benign breast tissue of high-risk women with the lignan secoisolariciresinol diglycoside. Cancer Prev Res (Phila) 2010;3(10):1342–50. doi: 10.1158/1940-6207.CAPR-10-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowcock EC, Cotterchio M, Boucher BA. Consumption of flaxseed, a rich source of lignans, is associated with reduced breast cancer risk. Cancer Causes Control. 2013;24(4):813–6. doi: 10.1007/s10552-013-0155-7. [DOI] [PubMed] [Google Scholar]
- 17.Serraino M, Thompson LU. The effect of flaxseed supplementation on the initiation and promotional stages of mammary tumorigenesis. Nutr Cancer. 1992;17(2):153–9. doi: 10.1080/01635589209514182. [DOI] [PubMed] [Google Scholar]
- 18.Bergman Jungestrom M, Thompson LU, Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 2007;13(3):1061–7. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- 19.Jenab M, Thompson LU. The influence of flaxseed and lignans on colon carcinogenesis and beta-glucuronidase activity. Carcinogenesis. 1996;17(6):1343–8. doi: 10.1093/carcin/17.6.1343. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Salazar M, et al. Flaxseed (Linum usitatissimum L.) and its total non-digestible fraction influence the expression of genes involved in azoxymethane-induced colon cancer in rats. Plant Foods Hum Nutr. 2013;68(3):259–67. doi: 10.1007/s11130-013-0372-y. [DOI] [PubMed] [Google Scholar]
- 21.Demark-Wahnefried W, et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3577–87. doi: 10.1158/1055-9965.EPI-08-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demark-Wahnefried W, et al. Pilot study to explore effects of low-fat, flaxseed-supplemented diet on proliferation of benign prostatic epithelium and prostate-specific antigen. Urology. 2004;63(5):900–4. doi: 10.1016/j.urology.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Lin X, et al. Effect of flaxseed supplementation on prostatic carcinoma in transgenic mice. Urology. 2002;60(5):919–24. doi: 10.1016/s0090-4295(02)01863-0. [DOI] [PubMed] [Google Scholar]
- 24.Kinniry P, et al. Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J Nutr. 2006;136(6):1545–51. doi: 10.1093/jn/136.6.1545. [DOI] [PubMed] [Google Scholar]
- 25.Lee JC, et al. Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L255–65. doi: 10.1152/ajplung.00138.2007. [DOI] [PubMed] [Google Scholar]
- 26.Dukes F, et al. Gene expression profiling of flaxseed in mouse lung tissues-modulation of toxicologically relevant genes. BMC Complement Altern Med. 2012;12:47. doi: 10.1186/1472-6882-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chikara S, et al. Enterolactone Induces G1-phase Cell Cycle Arrest in Nonsmall Cell Lung Cancer Cells by Downregulating Cyclins and Cyclin-dependent Kinases. Nutr Cancer. 2017:1–11. doi: 10.1080/01635581.2017.1296169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chikara S, et al. Enterolactone alters FAK-Src signaling and suppresses migration and invasion of lung cancer cell lines. BMC Complement Altern Med. 2017;17(1):30. doi: 10.1186/s12906-016-1512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikitin AY, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res. 2004;64(7):2307–16. doi: 10.1158/0008-5472.can-03-3376. [DOI] [PubMed] [Google Scholar]
- 30.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker ML, et al. Varying spin state composition by the choice of capping ligand in a family of molecular chains: detailed analysis of magnetic properties of chromium(III) horseshoes. Dalton Trans. 2011;40(12):2725–34. doi: 10.1039/c0dt01243b. [DOI] [PubMed] [Google Scholar]
- 32.Barnett DW, et al. BamTools: a C++ API and toolkit for analyzing and managing BAM files. Bioinformatics. 2011;27(12):1691–2. doi: 10.1093/bioinformatics/btr174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–9. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhillon H, et al. Transcriptome Analysis of Piperlongumine-Treated Human Pancreatic Cancer Cells Reveals Involvement of Oxidative Stress and Endoplasmic Reticulum Stress Pathways. J Med Food. 2016;19(6):578–85. doi: 10.1089/jmf.2015.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West KA, et al. Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3′-kinase/Akt pathway in vitro and in vivo. Cancer Res. 2004;64(2):446–51. doi: 10.1158/0008-5472.can-03-3241. [DOI] [PubMed] [Google Scholar]
- 36.Yamakawa K, et al. Activation of MEK1/2-ERK1/2 signaling during NNK-induced lung carcinogenesis in female A/J mice. Cancer Med. 2016;5(5):903–13. doi: 10.1002/cam4.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi H, et al. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17(1):89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kristensen M, et al. Linseed dietary fibers reduce apparent digestibility of energy and fat and weight gain in growing rats. Nutrients. 2013;5(8):3287–98. doi: 10.3390/nu5083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tominaga S, et al. (−)-Secoisolariciresinol attenuates high-fat diet-induced obesity in C57BL/6 mice. Food Funct. 2012;3(1):76–82. doi: 10.1039/c1fo10166h. [DOI] [PubMed] [Google Scholar]
- 40.Han W, et al. The chemopreventive agent myoinositol inhibits Akt and extracellular signal-regulated kinase in bronchial lesions from heavy smokers. Cancer Prev Res (Phila) 2009;2(4):370–6. doi: 10.1158/1940-6207.CAPR-08-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tichelaar JW, et al. Increased staining for phospho-Akt, p65/RELA and cIAP-2 in pre-neoplastic human bronchial biopsies. BMC Cancer. 2005;5:155. doi: 10.1186/1471-2407-5-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsao AS, et al. Increased phospho-AKT (Ser(473)) expression in bronchial dysplasia: implications for lung cancer prevention studies. Cancer Epidemiol Biomarkers Prev. 2003;12(7):660–4. [PubMed] [Google Scholar]
- 43.Lee HY, et al. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. J Natl Cancer Inst. 2005;97(22):1695–9. doi: 10.1093/jnci/dji377. [DOI] [PubMed] [Google Scholar]
- 44.Memmott RM, et al. Metformin prevents tobacco carcinogen–induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3(9):1066–76. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elstrom RL, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64(11):3892–9. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 46.Gottlob K, et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15(11):1406–18. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15(1):36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 48.Miller A, et al. Differential involvement of gp130 signalling pathways in modulating tobacco carcinogen-induced lung tumourigenesis. Oncogene. 2015;34(12):1510–9. doi: 10.1038/onc.2014.99. [DOI] [PubMed] [Google Scholar]
- 49.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6(5):447–58. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 50.Kim S, et al. Normal CFTR inhibits epidermal growth factor receptor-dependent pro-inflammatory chemokine production in human airway epithelial cells. PLoS One. 2013;8(8):e72981. doi: 10.1371/journal.pone.0072981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Therriault MJ, et al. Immunomodulatory effects of the tobacco-specific carcinogen, NNK, on alveolar macrophages. Clin Exp Immunol. 2003;132(2):232–8. doi: 10.1046/j.1365-2249.2003.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


